Abstract
Sex steroid hormones regulate various neural functions that regulate vertebrate sociosexual behavior. A number of sex steroids can be synthesized de novo in the brain, including estrogens by the enzyme aromatase. Aromatase, the neuropeptides arginine vasotocin/vasopressin, and the monoamine neurotransmitter dopamine have all been implicated in the control of male sexual and aggressive behavior in a variety of vertebrates. This study examined the expression of brain aromatase in a teleost fish, the bluehead wrasse (Thalassoma bifasciatum), a teleost fish that exhibits socially-controlled behavioral and gonadal sex change. We used immunocytochemistry (ICC) to characterize distributions of aromatase-immunoreactive (ir) cells, and to examine their relationship with AVT-ir neurons, and tyrosine hydroxylase-ir (TH-ir) neurons in the key sensory and integrative areas of the brain of this species. Aromatase-ir appeared to be in glial cell populations, and was found in the dorsal and ventral telencephalon, the preoptic area of the hypothalamus, and the lateral recess of the third ventricle, among other brain areas. Aromatase-ir fibers are closely associated with AVT-ir neurons throughout the preoptic area, indicating the potential for functional interactions. Aromatase-ir cell bodies and fibers were also co-regionalized with TH-ir neurons, suggesting possible interaction between the dopaminergic system and neural estrogen production. The presence of aromatase in brain regions important in the regulation of sexual and aggressive behavior suggests local estrogen synthesis could regulate sex change through effects on signaling systems that subserve reproductive behavior and function.
Keywords: aromatase, arginine vasotocin, dopamine, estrogen, teleost, hypothalamus
1. Introduction
In all vertebrate taxa including mammals, mating behavior is a complex sequence of behavioral responses requiring the ability to integrate endogenous hormonal and neurochemical changes with environmental information. The most important environmental information for many species can come from conspecifics. These social signals are often sexual in nature and have profound effects on both neural function and behavioral profiles. The mechanisms underlying behavioral adaptations to changing social conditions have not been comprehensively identified as yet and understanding the molecular basis of this transduction of social information is a key challenge for social neuroscience.
Sex hormones play key roles in neural modulation of behavioral processes. Both testosterone (T) and estradiol 17β (E2) stimulate male sexual behavior in a variety of vertebrates (Cross and Roselli 1999). While ‘classical’ genomic pathways are clearly important for many of these effects, increasing evidence also points to rapid steroid actions on neurons and in the mediation of sexual behavior (Revankar et al. 2005; Remage-Healey and Bass 2004;). For example, changes in the conversion of androgens to estrogens by aromatase can be seen within minutes in the quail brain. This suggests estrogen production in the brain could potentially be regulated over short time courses and such rapid alterations would be consistent with observed estrogen effects on behavior (Balthazart et al. 2001, Balthazart and Ball, 2006). Rapid alterations in neural estrogen production have also been documented in sex changing fishes (see below).
We are focusing on modulation of neural estrogen through aromatase as a potential mechanism underlying rapid adaptation to changing social conditions in a sex changing coral reef fish, the bluehead wrasse (Thalassoma bifasciatum). Several studies have highlighted the importance of estrogens and the aromatase pathway in the gonadal sex change processes (Cardwell and Liley 1991; Cochran and Grier 1991; Godwin and Thomas 1993; Chang et al. 1994, 1995; Kroon and Liley 2000; Kroon et al. 2003) and have suggested that it is a decrease in E2 levels that permits male development (Kroon et al. 2005). Similarly, we found that when the estrogen synthesis blocker 1,4,6-androstatrien-3,17-dione (ATD) is given alone or coadministered with T, complete color and gonadal sex change is induced in female T. bifasciatum (Austin et al. unpublished).
Many sex-changing fishes, including the bluehead wrasse, show very rapid behavioral changes during the sex change process. Male behaviors are often expressed within minutes or hours as an individual assumes social dominance (Robertson 1972; Warner and Swearer 1991; Godwin et al. 1996; Black et al. 2005). This short time scale of behavioral change is consistent with the rapid steroid actions on behavior in other systems discussed above. The neural form of aromatase (cytochrome P450b or AROb) is abundantly expressed in the brains of teleost fishes (Callard et al. 2001), including in key regions regulating sexual behavior (Schlinger et al., 1999; Forlano et al. 2001, 2005a, Forlano et al. b; Chang et al. 2005; Kishida and Callard 2001; Menuet et al. 2003, 2005). As with the important role of aromatase in gonadal sex change processes, neural estrogen synthesis via AROb appears to be critical in transducing social signals regulating male-typical sexual behavior under changing social conditions. In Lythrypnus dalli, another gobiid species with socially controlled sex change, neural aromatase activity in dominant females decreases significantly within hours of male removal (Black et al. 2005). This decrease in aromatase activity is correlated with rapid increases in aggressive and territorial behavior in the transitional females.
Other neural signaling systems have also been implicated in the process of behavioral and gonadal sex change. Arginine vasotocin (AVT), the neuropeptide found in fishes that is homologous to tetrapod arginine vasopressin (AVP), is of particular interest in the bluehead wrasse system. AVT affects reproductive behaviors in a broad range of vertebrates (Thompson and Moore 2003; Moore 1992; Moore and Lowry 1998; Goodson and Bass 2001), including several fishes (Salek et al., 2001; reviewed by Moore 1992; Moore and Lowry 1998; Goodson and Bass 2001). Expression of AVT is higher in dominant male bluehead wrasses than females and increases rapidly with sex change (Godwin et al., 2000). This increased expression of AVT is driven by social dominance and is independent of gonads (Semsar and Godwin 2003). Additionally, administration of AVT increases aggressive and courtship behavior typical of dominant males (Semsar et al. 2001) while an AVT receptor antagonist blocks territorial acquisition in large males and behavioral sex change in females (Semsar and Godwin 2004).
Monoamine neurotransmitters represent other neurochemical systems that can influence and be influenced by steroid hormone signaling. Behavioral studies in mice, quail, teleost fishes, and primates have demonstrated rapid steroid actions involving neurotransmitters (Hull et al. 2004). The dopamine system regulates sexual behavior and function in a variety of vertebrates and is often responsive to the steroid hormone environment (see Hull et al. 2004). Both human and non-human animal studies strongly suggest that dopamine facilitates male sexual behavior (Dominguez and Hull 2005). Levels of dopamine and other monoamines change over the course of female-to-male sex change in a congener of the bluehead wrasse (Thalassoma duperrey, Larson et al. 2003a) and manipulations of dopaminergic signaling can influence this process (Larson et al. 2003b). In quail, estradiol rapidly modulates male sexual behavior and this correlates with changes in levels of dopamine in the brain (Cornil et al. 2006). Dopamine has also been shown to down-regulate neural aromatase activity in quail (Balthazart et al. 2002; Balthazart and Ball, 2006), providing strong evidence for the involvement of monoamines in the neurochemical pathway controlling male sexual behavior.
The bluehead wrasse (Thalassoma bifasciatum) offers an experimentally tractable model in which to investigate the neurochemical pathways that underlie behavioral adaptation to changing social conditions. This common Caribbean reef fish has three sexual phenotypes: large, brightly colored terminal phase (TP) males and smaller, yellow and brown striped, initial phase (IP) males and females. TP males develop from either sex-changing females or role-changing IP males. Most TP males maintain territories on reefs and court and mate with females within these territories, although there is variation among males in these behaviors (see Semsar et al., 2001). Females display the yellow and brown coloration and live within groups on reefs that normally include a dominant TP male. IP males are non-territorial, usually mate in large aggregations (‘group spawns’), and may also mimic females to ‘sneak‘ or ‘streak‘ spawn with TP male/female pairs. When the TP males are removed from a social group, the largest female or IP male present will change sex and/or role to become a TP male (Warner and Swearer, 1991). Gonadal change takes place over 8–10 days, but male-typical behavior is often exhibited by sex changing females within minutes of removal of the TP male (Godwin et al. 1996).
This unpredictable social system requires rapid adaptation to changing dominance hierarchies. The first step in determining how estrogen might influence these behaviors and other neural signaling systems in the bluehead wrasse brain is an assessment of the sites of neural estrogen synthesis and neuroanatomical potential for interactions. Specifically, our objectives in this study are to: 1) examine the distribution of aromatase expression in the brain of the bluehead wrasse, and 2) assess the potential colocalization of aromatase with AVT and tyrosine hydroxylase to determine the neuroanatomical potential for functional interactions between these systems. Full descriptions of AVT and tyrosine hydroxylase distributions in the bluehead wrasse brain are beyond the scope of this study.
2. Results
Aromatase immunolocalization
Aromatase-labeled cell bodies were visualized throughout the brain and found in greatest abundance in the forebrain. The antibody used in this study was made against a conserved amino acid sequence of known teleost aromatases, and was first used in the plainfin midshipman (Forlano et al. 2001). An identical pattern of labeling was consistently seen in the same brain regions between animals. The antibody showed cytoplasmic labeling of aromatase-ir cell bodies and also labeled fibers. In control assays, omission of the primary antibody eliminated labeling throughout the brain, and serial dilutions of the primary antibody produced progressive decreases in labeling. In general, aromatase-ir distribution was similar to distribution patterns seen in other teleosts (Fig. 1). These distributions are described below for bluehead wrasses. The morphology of the aromatase-ir cells suggests that aromatase expression in the bluehead wrasse brain is of glial origin, and this is consistent with that seen in other teleosts (Forlano et al. 2001, Menuet et al. 2003, 2005). No differences in distribution of labeling were observed between estrogen-implanted fish and wild-caught fish.
Figure 1.
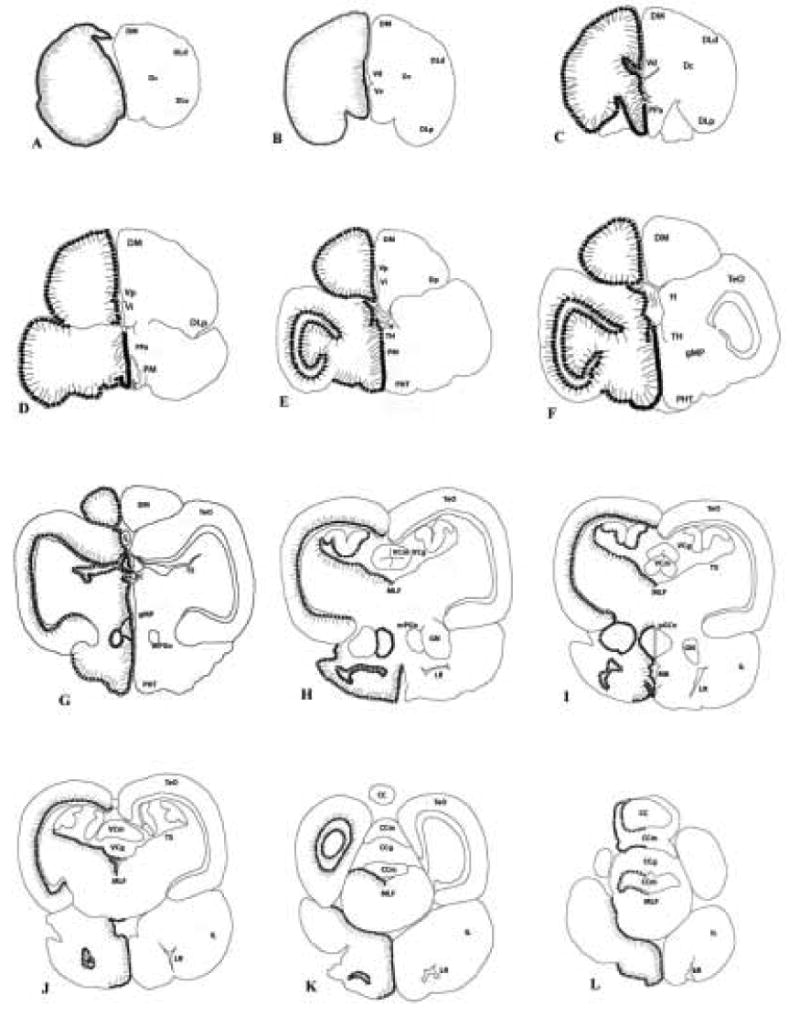
Diagram of neuroanatomical distribution of aromatase-ir positive structures on representative coronal sections (A–L) of the brain of the bluehead wrasse. Relative numbers, sizes, and locations of aromatase-ir cells and fibers are on the left side of the midline, with structures labeled on the right. See abbreviations in Table 1.
Aromatase-ir was observed along nearly all ventricular surfaces in the brain. Aromatase-ir labeling in the telencephalon was seen along the lateral margins of both hemispheres, and medially along the walls of the telencephalic ventricle (Fig. 2A) and the third ventricle in the diencephalon. Aromatase positive cells were also found along the intermediate zone and postcommissural nucleus of the ventral telencephalon (Fig. 2B), along the ventricle in the habenula, and in the suprachiasmatic nucleus (SCN). In the preoptic area of the hypothalamus, aromatase-ir populations were found in the anterior parvocellular preoptic nucleus (PPa), the posterior parvocellular preoptic nucleus (PPp), and both the magnocellular and gigantocellular preoptic nuclei (Fig. 2C). Further caudally in the diencephalon, cells were labeled in both the lateral and anterior tuberal nuclei and in the dorsal hypothalamus (specifically the posterior periventricular nucleus or NPPv).
Figure 2.

Aromatase immunoreactivity in the telencephalon and diencephalon. All images are fluorescently labeled with anti-aromatase in red. Arrows point to long fibers that suggest labeled cells are radial glia. Panel A, lining the telencephalic ventricle in the medial zone of the dorsal telencephalic area; B, postcommissural nucleus of ventral telencephalon; C, magnocellular preoptic area. Scale bars: 200μm.
In the midbrain, aromatase labeling was found in several areas. In the optic tectum, aromatase-ir was seen along the internal edge in the stratum periventriculare, with long processes extending outwards dorsally and laterally, suggesting that the cells are radial glia (Fig. 3A). In other areas of the midbrain, aromatase-ir cells are found in the periventricular gray zone, in the vicinity of the posterior commissure, and both the torus longitudinalis and torus semicircularis. Aromatase-ir cells also line the ventricular wall on the medial side of the thalamus and are found in the preopticohypophysial tract and tectal ventricle.
Figure 3.

Aromatase-immunoreactivity in the optic tectum and granule cell layer of the valvula of the cerebellum. All images are of aromatase immunoreactivity fluorescently labeled with anti-aromatase in red. Panel A, cell bodies in the stratum periventriculare (SPV) with fibers extending dorsally through the optic tectum (TeO); B, cell bodies and fibers in the granule cell layer of the valvula of the cerebellum; C, closeup of lower central portion of B. Scale bars: A and C, 100μm; B, 400 μm.
In the hindbrain, lower levels of aromatase-ir were apparent. Cells were found lining the fourth ventricle and cerebral aqueduct (CA) and lateral recess, as well as the medial longitudinal fasciculus (MLF) and inferior lobe of the hypothalamus. In the cerebellum, aromatase-ir populations were seen both above the molecular cell layer and throughout the granule cell layer (Fig. 3B–C).
Aromatase co-regionalization with AVT and Tyrosine Hydroxylase
Consistent with a previous study in our laboratory (Semsar and Godwin 2003), AVT-ir cells were found in three distinct populations: the parvocellular, magnocellular, and gigantocellular preoptic nuclei. Aromatase-ir cells and fibers in these regions were found in very close association with AVT-ir neurons (Fig. 4A–B). Tyrosine hydroxylase-ir fibers were also co-regionalized with AVT-ir neurons in the gigantocellular, and magnocellular preoptic areas (Fig. 4C–D). The source of these TH fibers is uncertain, but strongly staining TH-ir cell bodies are found in the posterior parvocellular preoptic area, just ventral to the magnocellular AVT-ir neuron populations. More generally, tyrosine hydroxylase-ir labeled fibers were found in areas that also showed aromatase-ir cells, including the dorsal and ventral telencephalon, the anterior and posterior parvocellular preoptic nucleus (Fig. 5A), the optic tectum and the torus semicircularis (Fig. 5B). Tyrosine hydroxylase staining in the stratum periventriculare (SPV) of the optic tectum was verified with a single fluorescent label (Fig. 5C) that was absent when the primary antibody was eliminated (Fig. 5D).
Figure 4.

Arginine vasotocin-immunoreactivity co-regionalized with aromatase-immunoreactivity and with tyrosine hydroxylase-immunoreactivity. Panels A and B show anti-aromatase in red and anti-arginine vasopressin in green. Panel A, magnocellular preoptic area and preoptico-hypophysial tract; B, gigantocellular preoptic area. The arrows in A show ‘beading’ of arginine vasotocin-immunoreactive fibers. Panels C and D show anti-arginine vasopressin in red and anti-tyrosine hydroxylase in green. Panel C, arginine vasotocin-ir neurons in magnocellular preoptic (PM) area and TH-ir neurons ventral to the PM; D, inset of C showing TH-ir fibers co-regionalized with arginine vasotocin-ir neurons. Scale bars: A, 400μm; B, 100μm; C 200μm; D, 50 μm.
Figure 5.

Aromatase-immunoreactivity (red) co-regionalized with tyrosine hydroxylase-immunoreactivity (green) in the top panel, and tyrosine hydroxylase-immunoreactivity alone (green) in the bottom panel. Panel A, posterior parvocellular preoptic nucleus; B, aromatase-ir fibers in the torus semicircularis (TS) and anti-tyrosine hydroxylase cell bodies in the stratum periventriculare (SPV) with fibers extending ventrally through the TS; C, anti-tyrosine hydroxylase cell bodies in the SPV with fibers extending dorsally into the TeO; D, absence of primary antibody eliminates staining in section adjacent to C. Scale bars: A, 200 μm; B-D, 100 μm.
Discussion
In this study, we have demonstrated widespread localization of aromatase immunoreactivity in the bluehead wrasse brain. The distribution pattern was generally similar to that of aromatase-ir cells described for other teleost species (Gelinas and Callard, 1997; Forlano et al., 2001; Menuet et al. 2003, 2005, Goto-Kazeto et al. 2004, Strobl-Mazzulla et al. 2005). Importantly, aromatase-ir populations are seen in key brain areas associated with the integration of social cues and with the regulation of male-typical sociosexual behavior. Below, we compare the distribution of aromatase-ir with that described from other teleosts and consider potential functional interactions between aromatase-expressing glia and AVT and dopaminergic neurons.
Aromatase Distribution: Comparisons Across Species
The pattern of aromatase-ir found here for bluehead wrasses is generally consistent with that described for other teleosts, but there are some interesting differences. The similarities include labeling in the preoptic and ventral areas of the telencephalon which are important integrative areas for social behavior and reproductive function. These ventral telencephalic nuclei are considered homologous to amygdalar and septal areas in mammals and other tetrapods (Wullimann and Mueller 2004). Two major differences seen among the teleost species that have been investigated is the expression pattern of aromatase found in the hindbrain and in the optic tectum. The plainfin midshipman (Porichthys notatus) shows strong expression in the hindbrain, especially in the sonic motor nucleus (SMN), but not in the optic tectum. The plainfin midshipman is a vocalizing species and aromatase activity in the hindbrain differs across sexual morphs that vary in vocalizing behavior in this species (Schlinger et al. 1999). Hindbrain aromatase expression has not been well characterized in a species where communication is primarily based on visual cues instead. In contrast to the midshipman, aromatase-ir cells were abundant in several layers of the optic tectum in the bluehead wrasse. Both rainbow trout and zebrafish also show aromatase-ir cells in the optic tectum (Menuet et al. 2003, 2005). As with bluehead wrasses, these are species that rely more on visual cues in their environment. Callard and coworkers (2001) reported aromatase mRNA and protein in the goldfish (Carassius auratus) retina and optic tectum and suggested neurally-derived estrogen may modulate the visual system.
Potential interactions with the AVT and Dopamine systems
From studies in our laboratory on sites of AVT production in the bluehead wrasse brain, we have located three populations of AVT neurons within the preoptic area (parvo-, magno-, and gigantocellular; Semsar and Godwin 2003), as well as one population in the ventral tuberal hypothalamus (Elkins and Godwin, unpublished). Immunocytochemical labeling in this study is consistent with these findings, and shows co-regionalization of AVT-ir neurons in the preoptic area with aromatase-ir cells. Based on cell morphology these aromatase-ir cells appear to be glial. This finding is in contrast with findings from other vertebrates, in which aromatase is normally expressed neurons and in glia only after brain injury. However, aromatase expression in glia is consistent with results in the plainfin midshipman based on both morphology and GFAP labeling (Porichthys notatus; Forlano et al. 2001) and other teleost species based on either labeling with glial markers or on cellular morphology (rainbow trout and zebrafish, Menuet et al. 2003, 2005; Pellegrini et al. 2005).
Distributions of aromatase-ir cells also overlapped with both fibers and somata of TH-ir neurons. The distribution pattern of TH-ir staining in the bluehead wrasse is consistent with that seen in several other species of teleosts including the zebrafish, Senegalese sole, rainbow trout, and goldfish (Rink and Wullimann, 2002, Rodriguez-Gomez et al. 2000, Vetillard et al. 2002, Hornby et al. 1987). Most notably, TH-ir cells and fibers in the bluehead wrasse were found in areas that also show aromatase-ir cell populations, including in the dorsal and ventral telencephalon and the preoptic area. We tentatively interpret these findings to indicate dopaminergic rather than noradrenergic innervation in these regions based on findings in zebrafish (Rink and Wullimann 2002) and the lack of staining found using a dopamine β-hydroxylase antibody in these areas of the bluehead wrasse brain (unpublished data). The co-regionalization of aromatase-ir cells and TH-ir neurons suggests dopamine signaling could affect or be affected by neural estrogen synthesis, particularly in the preoptic area. Dopamine plays an important role in regulating aromatase expression and/or activity in the vertebrate brain and this interaction is particularly well studied in the Japanese quail (for reviews, see Balthazart et al. 2002; Balthazart and Ball 2006).
Neural Aromatase and Sex Change
We postulate that neural estrogen synthesis via aromatase plays a critical role in regulating sex change in the bluehead wrasse. Specifically, we hypothesize that neurally-produced estrogen blocks behavioral sex change under socially inhibitory conditions as recently proposed for a sex changing goby (Black et al., 2005). Aromatase localization in brain regions that show intrasexual variation in AVT expression could allow transduction of social cues and initiation of sex change through modifications in estrogen signaling. We further propose that this effect is at least partly mediated through interactions with the AVT system and possibly the dopaminergic system. This pathway would be consistent with evidence of a key role for neurally produced estrogen affecting sexual differentiation and function in a variety of vertebrates including fishes (Black et al. 2005) and with recent findings regarding estrogen signaling and AVP neuron function in mammals (Plumari et al. 2002). An alternative model for steroid hormone regulation of behavioral sex change focused on the potential role of corticosteroids and inhibitory effects on AVT neurons has been proposed and is described in detail by Perry and Grober (2003).
Blocking aromatase activity with inhibitors can induce gonadal sex change under inhibitory social conditions (Kroon and Liley 2000; Kroon et al., 2005; Bhandari et al. 2004) and this is also true in the bluehead wrasse (Austin et al., unpublished). However, it is not clear whether these effects of aromatase inhibition on gonadal sex change are mediated in the gonads or the brain. However, patterns in other species suggest neural aromatization has important effects on behavior. The plainfin midshipman (Porichthys notatus) exhibits two distinct male phenotypes that show pronounced differences in behavior which are correlated with differences in aromatase expression in various brain regions involved in sexual and aggressive behavior (Schlinger et al. 1999; Forlano et al. 2001; Forlano et al., 2005a, b). Recently, Black and coworkers (2005) documented similar sex differences in neural aromatase activity in the female-to-male sex changing goby Lythrypnus dalli and found that neural aromatase activity rapidly declined at the onset of socially induced sex change.
Estrogen (E2) critically affects sexual differentiation and the display of male sexual behavior in many vertebrates. However, there is considerable diversity in the nature of these effects both among species and in terms of their biochemical mediation. The best understood example of estrogen effects on male reproductive behavior is seen in rodents where estrogen plays a critical role in both the organization during development and activation in adulthood of male sexual behavior (Meisel and Sachs 1994). However, not all systems are masculinized by treatment with estrogen and estrogen effects on male-typical sexual behavior can be very rapid. Estradiol masculinizes the vasotocin system of Japanese quail during development (Panzica et al., 1998) and can influence male sexual behavior rapidly in adulthood in mice, quail, and the plainfin midshipman (Cross and Roselli 1999; Balthazart and Ball 2006, Remage-Healey and Bass 2004). These rapid effects suggest estrogen actions may occur non-genomically, consistent with other rapid estrogen effects documented in neurons (for review, see Balthazart and Ball 2006). The estrogens active in the brain may be derived from either gonadal sources or local conversion through neural aromatization.
How might rapid alterations in aromatase activity occur? We examined the dopaminergic system here in addition to the vasotocin system in part because of recent findings in Japanese quail (Coturnix japonica) showing that aromatase activity can be decreased within minutes by calcium-dependent phosphorylation processes (Balthazart et al. 2001, reviewed in Balthazart and Ball, 2006). Based on dopaminergic innervation, the presence of DA receptors in the preoptic area, and induction of changes in aromatase activity through activation of DA receptors, these authors suggest that dopaminergic input to aromatase-positive neurons (Cornil et al. 2004) rapidly affects estrogen production in the preoptic area in response to environmental cues. No comparable information is available for fishes, but dopamine is a key regulator of reproductive processes in some species via its inhibitory effects on gonadotropins (Devlin and Nagahama 2002). Extensive TH-ir innervation of the preoptic area, as found here, has also been found in other species of fishes (e.g., Vetillard et al. 2002). Interestingly, these TH-ir neurons also express estrogen receptors (ERs) in the rainbow trout (Oncorhyncus mykiss; Linard et al. 1996).
The close association of aromatase-ir and AVT-ir cells in the bluehead wrasse preoptic area suggests the possibility of direct estrogen effects on AVT signaling. In mice, ERβ has been co-localized with AVP in the paraventricular nucleus (PVN), the putative homologue of the magnocellular preoptic area in teleosts (Hrabovszky et al. 1998; Nomura et al. 2002; Kapsimali et al. 2001) and ERβ regulates AVP expression in the mouse PVN (Nomura et al. 2002). Several studies in fishes show expression of at least one ER subtype in areas with aromatase-ir cells including the preoptic region (fishes have three ER subtypes ERa, ERβa, and ERβb; Hawkins et al., 2001, 2005; Forlano et al. 2005c2005a,b; Menuet et al. 2003; Tchoudakova and coworkers, 1999). Large neurons in the preoptic area of the Atlantic croaker express an ERβ subtype (ERβb; Hawkins et al. 2001, 2005). The neurochemical phenotype of these neurons is not yet characterized, but their size and location are consistent with that of AVT and isotocin neurons. If some of these Erβb-expressing neurons are AVT neurons, it would suggest possible direct estrogenic regulation of AVT similar to the situation with AVP in mammals.
Here we demonstrate the close association of aromatase-ir, AVT-ir, and TH-ir cells in areas of the brain known to regulate male sexual behavior and reproductive function. These findings suggest modulation of neural estrogen synthesis through aromatase expression and/or activity could mediate rapid behavioral adaptation to changing social conditions in the bluehead wrasse. Consistent with this hypothesis, we have recently found that estrogen implants can block behavioral sex change under socially permissive conditions (unpublished data). In order to further investigate estrogenic regulation of sociosexual behavior in this species, our future goals are to compare aromatase mRNA expression across phenotypes of the bluehead wrasse and over the course of sex change and explore the potential role of estrogen receptor subtypes (ERα, βa, and βb) in mediating the behavioral effects of estrogens.
Experimental Procedure
All experimental methods described here were approved by and are in compliance with the guidelines of the Institutional Animal Care and Use Committee of North Carolina State University (NCSU).
For hormone administration, female and IP male bluehead wrasses were obtained from (a commercial dealer (FL), shipped to NCSU, and held in 107 l glass aquaria on a recirculating seawater system while being fed daily with commercial flake food.. After a two day acclimation period, surgeries were performed as in previous studies (Godwin et al., 1996; Semsar and Godwin 2003, 2004; Austin et al. in prep). Fish were implanted abdominally with 8mm Silastic implants (Silastic tubing, 1.47 mm ID, 1.96 mm OD, Dow Corning, Midland, MI; approximately 20ul total volume) containing either estradiol benzoate (1μg/g, n=2 of each phenotype) dissolved in peanut or peanut oil alone as a vehicle control (n=2 of each phenotype). Individuals were active and feeding the next day and grew new scales over the surgical wound within a week, suggesting full recovery. After 10 days, the fish were killed using an overdose of MS-222 (tricaine methanesulfonate, Sigma, St. Louis, MO) and their brains dissected out, fixed in 4% paraformaldehyde for 24 hours, then transferred to a 30% sucrose solution for cryoprotection for 24 hours, and sunk in freezing medium (OCT, TissueTek, Elkart, IN) for cryosectioning.
In order to assess potential effects of estradiol benzoate implants on aromatase-ir distributions, we also examined unmanipulated animals. We captured adult female, IP male, and TP male wrasses (n=5 each) directly from patch reefs in Teague Bay, St. Croix, U.S. Virgin Islands. The fish were captured by liftnet in June 2005 during daylight hours and were killed within 2 minutes of capture using an overdose of MS-222 (tricaine methanesulfonate, Sigma, St. Louis, MO). Their brains were processed as described above, and all cryoprotected brains were held at −80° C until cryosectioning.
Immunocytochemistry
For immunocytochemistry (ICC) analysis of aromatase-ir, AVT-ir, and TH-ir cells, brains were coronally cryosectioned into six adjacent series of 20 μm sections, meaning adjacent sections on a slide were separated by 100 μm, and then stored at −80° C until utilized in assays. Double-labeling of the selected antigens was performed simultaneously. Slides were allowed to come to room temperature (RT), dehydrated in a series of ethanol washes (70, 95, 100% EtOH; 1 minute each), air dried and outlined in PAP pen (Fisher Scientific, St. Louis, MO). The slides were then rinsed in phosphate-buffered saline (0.1M PBS; 2 X 10 minutes), blocked in 10% normal goat serum (NGS, Vector Laboratories, Burlingame, CA) in PBS (30 minutes, RT), and placed in primary antisera diluted in PBS containing 1% NGS (16–20 hours, RT). The antisera and working dilutions used were: aromatase, a rabbit polyclonal used at a dilution of 1:50 (Forlano et al. 2001); arginine vasopressin (AVP), a guinea pig polyclonal (1:1,000; DiaSorin, Stillwater, MN); tyrosine hydroxylase (TH), a mouse monoclonal (1:1000; Immunostar, Hudson, WI), and glial fibrillary acidic protein (GFAP), a guinea pig monoclonal (1:100; Molecular Probes, Portland, OR). The aromatase polyclonal antibody (kindly provided by A.H. Bass, Cornell University) was developed against a conserved amino acid sequence of known teleost aromatases and the specificity of labeling was confirmed using an antibody designed against a specific teleost aromatase within that group, the plainfin midshipman (Porichthys notatus; Forlano et al. 2001). All other antisera used in this study were obtained commercially. Following the 16–20 hour incubations of the slides in the primary antisera, sections were rinsed in PBS (2 X 10 minutes) and placed in fluorescent secondary antibodies (both 1:100, Alexa-fluor 594 nm for anti-aromatase, 488nm for both anti-AVT and anti-TH; Molecular Probes, Portland, OR). After a 2 hour incubation in the dark (RT), sections were rinsed in PBS (2 X 10 minutes), allowed to air dry in the dark, and coverslipped using Gel Mount (Fisher Scientific, St. Louis, MO). Elimination and serial dilutions of the primary antibody from incubation were used as controls. Fluorescent images were taken using a Leica DMR digital microscope (Leica Microsystems, Bannockburn, IL) with a Hamamatsu Orca-ER cooled CCD camera (Hamamatsu Corporation, Hamamatsu City, Japan) and Openlab 3.5.1 software (Improvision, Inc., Lexington, MA). The images were sharpened and adjusted for light and contrast in Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Neuroanatomical nomenclature follows that of Wullimann et al. (1996).
Table 1.
Abbreviations
| AC | anterior commissure |
| CC | crista cerebellaris |
| CCg | granule cell layer of the cerebellum |
| CCm | molecular cell layer of the cerebellum |
| DC | central nucleus of telencephalic area dorsalis |
| DLp | posterior part of lateral zone of telencephalic area dorsalis |
| DLd+v | dorsal and ventral division of lateral zone of area dorsalis |
| DM | medial zone of telencephalic area dorsalis |
| DP | posterior zone of telencephalic area dorsalis |
| gMP | gigantocellular portion of the magnocellular preoptic nucleus |
| Gn | glomerular nucleus |
| H | habenula |
| IL | inferior lobe of the hypothalamus |
| LR | lateral recess |
| MB | mammillary body |
| MLF | medial longitudinal fascicle |
| mPGn | medial preglomerular nucleus |
| TeO | optic tectum |
| PC | posterior commissure |
| PHT | preopticohypophysial tract |
| PGCn | preglomerular commissural nucleus |
| PM | magnocellular preoptic nucleus |
| PPa | anterior parvocellular preoptic nucleus |
| PPp | posterior parvocellular preoptic nucleus |
| Th | thalamus |
| TLo | torus longitudinalis |
| TS | torus semicircularis |
| VCg | granule cell layer of the valvula cerebelli |
| VCm | molecular cell layer of the valvula cerebelli |
| Vd | dorsal zone of telencephalic area ventralis |
| Vi | intermediate zone of telencephalic area ventralis |
| Vp | postcommissural nucleus of telencephalic area ventralis |
| Vv | ventral zone of telencephalic area ventralis |
Acknowledgments
We would like to thank Andrew H. Bass and Margaret A. Marchaterre of Cornell University for the gift of the aromatase antiserum and advice on immunocytochemistry procedures. Additionally, we are extremely grateful to Thomas Miller and Mike Harris for assistance collecting and processing the fish and to Brad Ring for fish care at NCSU. Also, this manuscript improved thanks to comments from two anonymous reviewers. This work was supported by a University of North Carolina system genomics training grant fellowship to KEM, NIH-MH 5827, NSF 0212449 (JG), and NSF 0416926 (JG and MBH). This is a contribution of the W.M. Keck Center for Behavioral Biology at North Carolina State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;16:483–490. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 3.Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J, Ball GF. Is brain estradiol a hormone or neurotransmitter? Trends Neurosci. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari RK, Higa M, Komuro H, Nakamura S, Nakamura M. Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra) Zool Sci. 2003;20:1399–1403. doi: 10.2108/zsj.20.1399. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari R, Higa M, Nakamura S, Nakamura M. Aromatase inhibitor induces complete sex change in the protogynous honeycomb grouper (Epinephelus merra) Mol Reprod Dev. 2004;67:303–307. doi: 10.1002/mrd.20027. [DOI] [PubMed] [Google Scholar]
- 7.Black MP, Baillien M, Balthazart J, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc Biol Sci. 2005;272(1579):2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callard GV, Tchoudakova AV, Kishida M, Wood E. Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish. J Ster Biochem Molec Biol. 2001;79:305–314. doi: 10.1016/s0960-0760(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 9.Cardwell JR, Liley NR. Androgen control of social status in males of a wild population of stoplight parrotfish, Sparisoma viride (Scaridae) Horm Behav. 1991;25:1–18. doi: 10.1016/0018-506x(91)90035-g. [DOI] [PubMed] [Google Scholar]
- 10.Chang CF, Lee MF, Chen GR. Estradiol-17beta associated with the sex reversal in protandrous black porgy, Acanthopagrus schlegeli. J Expt Zool. 1994;268:53–58. [Google Scholar]
- 11.Chang CF, Lau EL, Lin BY. Estradiol-17beta suppresses testicular development and stimulates sex reversal in protandrous black porgy Acanthopagrus schlegeli. Fish Physiol Biochem. 1995;14:481–488. doi: 10.1007/BF00004348. [DOI] [PubMed] [Google Scholar]
- 12.Chang X, Kobayashi T, Senthilkumaran B, Kobayashi-Kajura H, Sudhakumari CC, Nagahama Y. Two types of aromatase with different encoding genes, tissue distribution and developmental expression in Nile tilapia (Oreochromis niloticus) Gen Comp Endocrinol. 2005;141:101–115. doi: 10.1016/j.ygcen.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Cochran RC, Grier HJ. Regulation of sexual succession in the protogynous black sea bass, Centropristis striatus (Osteichthyes: Serranidae) Gen Comp Endocrinol. 1991;82:69–77. doi: 10.1016/0016-6480(91)90297-j. [DOI] [PubMed] [Google Scholar]
- 14.Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Res. 2004;1029:224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Cornil CA, Dalla C, Papadopoulou-Datifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Cross E, Roselli CE. 17β-Estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 17.Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- 18.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Forlano PM, Deitcher DL, Myers DM, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21(22):8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: Divergence across sex and vocal phenotypes. J Neurobiol. 2005a;65(1):37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- 21.Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: Female preoptic and vocal motor nuclei. J Neurobiol. 2005b;65(1):50–58. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- 22.Forlano PM, Deitcher DL, Myers DM, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005c;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 23.Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen Comp Endocrinol. 1997;106:155–168. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- 24.Godwin JR, Thomas P. Sex change and steroid profiles in the protandrous anemonefish Amphiprion melanopus (Pomacentridae, Teleostei) Gen Comp Endocrinol. 1993;91:144–157. doi: 10.1006/gcen.1993.1114. [DOI] [PubMed] [Google Scholar]
- 25.Godwin J, Crews D, Warner RR. Behavioral sex change in the absence of gonads in a coral reef fish. Proc Roy Soc B. 1996;263(1377):1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- 26.Godwin J, Sawby R, Warner RR, Crews D, Grober MS. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav Evol. 2000;55:77–84. doi: 10.1159/000006643. [DOI] [PubMed] [Google Scholar]
- 27.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 28.Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM. Localization and expression of aromatase mRNA in adult zebrafish. Gen Comp Endocrinol. 2004;139:72–84. doi: 10.1016/j.ygcen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. PNAS. 2000;97(20):10751–10756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins MB, Thomas P. The unusual binding properties of the third distinct teleost estrogen receptor subtype ERβa are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinol. 2004;145(6):2968–2977. doi: 10.1210/en.2003-0806. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins MB, Godwin J, Crews D, Thomas P. The distributions of the duplicate oestrogen receptors ERβa and ERβb in the forebrain of the Atlantic croaker (Micropogonias undulates): evidence for subfunctionalization after gene duplication. Proc Royal Soc London B. 2005 doi: 10.198/rspb.2004.3008. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higa M, Ogasawara K, Sakaguchi A, Nagahama Y, Nakamura M. Role of steroid hormones in sex change in protogynous wrasse. Fish Physiol Biochem. 2003;28:149–150. [Google Scholar]
- 33.Hornby PJ, Piekut DT, Demski LS. Localization of immunoreactive tyrosine hydroxylase in the goldfish brain. J Comp Neurol. 1987;261:1–14. doi: 10.1002/cne.902610102. [DOI] [PubMed] [Google Scholar]
- 34.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-β mRNA in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinol. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 35.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Phsyiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Kapsimali M, Bourrat F, Vernier P. Distribution of the orphan nuclear receptor Nurr1 in medaka (Oryzias latipes): cues to the definition of homologous cell groups in the vertebrate brain. J Comp Neurol. 2001;431:276–292. doi: 10.1002/1096-9861(20010312)431:3<276::aid-cne1070>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Kroon FJ, Liley NR. The role of steroid hormones in protogynous sex change in the blackeye goby, Coryphopterus nicholsii (Teleostei: Gobiidae) Gen Comp Endocrinol. 2000;118:273–283. doi: 10.1006/gcen.2000.7459. [DOI] [PubMed] [Google Scholar]
- 38.Kroon FJ, Munday PL, Pankhurst NW. Steroid hormone levels and bidirectional sex change in Gobiodon histrio. J Fish Biol. 2003;62:153–167. [Google Scholar]
- 39.Kroon FJ, Munday PL, Westcott DA, Hobbs J-P, Liley NR. Aromatase pathway mediates sex change in each direction. Proc Royal Soc Lond B. 2005;272(1570):1399–1405. doi: 10.1098/rspb.2005.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larson ET, Norris DO, Summers CH. Monoaminergic changes associated with socially induced sex reversal in the saddleback wrasse. Neurosci. 2003a;119:251–263. doi: 10.1016/s0306-4522(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 41.Larson ET, Norris DO, Grau EG, Summers CH. Monoamines stimulate sex reversal in the saddleback wrasse. Gen Comp Endocrinol. 2003b;130:289–298. doi: 10.1016/s0016-6480(02)00622-6. [DOI] [PubMed] [Google Scholar]
- 42.Lee YH, Wu J-L, Yueh W-S, Lin B-Y, Huang J-D, Lee C-Y, Lee M-F, Lau E-L, Lee F-Y, Morrey C, Nagahama Y, Chang C-F. Sex change in the protandrous black porgy, Acanthopagrus schlegeli: a review in gonadal development, estradiol, estrogen receptor, aromatase activity and gonadotropin. J Exp Zool. 2001;290:715–726. doi: 10.1002/jez.1122. [DOI] [PubMed] [Google Scholar]
- 43.Linard B, Anglade I, Corio M, Navas JM, Pakdel F, Saligaut C, Kah O. Estrogen receptors are expressed in a subset of tyrosine hydroxylase-positive neurons of the anterior preoptic region in the rainbow trout. Neuroendocrinology. 1996;63:156–165. doi: 10.1159/000126952. [DOI] [PubMed] [Google Scholar]
- 44.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Raven Press; New York: 1994. pp. 3–105. [Google Scholar]
- 45.Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J Comp Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- 46.Menuet A, Pellegrini E, Brion F, Gueguen M-M, Anglade I, Pakdel I, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J Comp Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- 47.Moore FL. Evolutionary precedents for behavioral actions of oxytocin and vasopressin. Ann NY Acad Sci. 1992;652(1):156–165. doi: 10.1111/j.1749-6632.1992.tb34352.x. [DOI] [PubMed] [Google Scholar]
- 48.Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol Part C: Pharmacol Toxicol Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 49.Morrey CM, Nakamura M, Kobayashi T, Grau EG, Nagahama Y. P450scc-like immunoreactivity throughout gonadal restructuring in the protogynous hermaphrodite Thalassoma duperrey. Int J Dev Biol. 1998;42:811–816. [PubMed] [Google Scholar]
- 50.Nakamura M, Hourigan TF, Yamauchi K, Nagahama Y, Grau EG. Histological and ultrastructural evidence for the role of gonadal steroid hormones in sex change in the protogynous wrasse Thalassoma duperrey. Env Biol Fishes. 1989;24(2):117–136. [Google Scholar]
- 51.Nakamura M, Bhandari RK, Higa M. The role estrogens play in sex differentiation and sex changes of fish. Fish Physiol Biochem. 2003;28:113–117. [Google Scholar]
- 52.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Molec Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 53.Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemcani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrini E, Menuet A, Lethimonier C, Adrio1 F, Gueguen M-M, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen Comp Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Perry AN, Grober MS. A model for social control of sex change: interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm Behav. 2003;43(1):31e–8. doi: 10.1016/s0018-506x(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 56.Plumari L, Viglietti-Panzica C, Allieri F, Honda S, Harada N, Absil P, Balthazart J, Panzica GC. Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. J Neuroendocrinol. 2002;14(12):971–978. doi: 10.1046/j.1365-2826.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 57.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resko JA, Pereyra-Martinez AC, Stadelman HL, Roselli CE. Region-specific regulation of cytochrome P450 aromatase messenger ribonucleic acid by androgen in brains of male rhesus monkeys. Biol Reprod. 2000;62:1818–1822. doi: 10.1095/biolreprod62.6.1818. [DOI] [PubMed] [Google Scholar]
- 59.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;305:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 60.Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull. 2002;57(3–4):385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 61.Robertson DR. Social control of sex reversal in a coral-reef fish. Science. 1972;177:1007–1009. doi: 10.1126/science.177.4053.1007. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Gomez FJ, Rendon-Unceta MC, Sarasquete C, Munoz-Cueto JA. Localization of tyrosine hydroxylase-immunoreactivity in the brain of the Senegalese sole, Solea senegalensis. J Chem Neuroanat. 2000;19:17–32. doi: 10.1016/s0891-0618(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 63.Salek SJ, Sullivan CV, Godwin J. Courtship behavior of male white perch, Morone americana: evidence for control by androgens. Comp Biochem Physiol A. 2001;130:731–740. doi: 10.1016/s1095-6433(01)00405-6. [DOI] [PubMed] [Google Scholar]
- 64.Schlinger BA, Greco C, Bass AH. Aromatase activity in the hindbrain vocal control region of a teleost fish: divergence among males with alternative reproductive tactics. Proc Roy Soc Lond B. 1999;266:131–136. [Google Scholar]
- 65.Semsar K, Kandel FLM, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- 66.Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neurosci. 2003;23(10):4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Strobl-Mazzulla PH, Moncaut NP, López GC, Miranda LA, Canario AVM, Somoza GM. Brain aromatase from pejerrey fish (Odontesthes bonariensis): cDNA cloning, tissue expression, and immunohistochemical localization. Gen Comp Endocrinol. 2005;143:21–32. doi: 10.1016/j.ygcen.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Tchoudakova A, Pathak A, Callard GV. Molecular cloning of an estrogen receptor β subtype from the goldfish, Carassius auratus. Cen Comp Endocrinol. 1999;113:388–400. doi: 10.1006/gcen.1998.7217. [DOI] [PubMed] [Google Scholar]
- 71.Thompson RR, Moore FL. The effects of testosterone and vasotocin on behavioral responses to visual and olfactory female sexual stimuli in ovariectomized female roughskin newts. Horm Behav. 2003;44:311–318. doi: 10.1016/s0018-506x(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 72.Warner RR, Swearer SE. Social control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae) Biol Bull. 1991;181:199–204. doi: 10.2307/1542090. [DOI] [PubMed] [Google Scholar]
- 73.Warner RR, Schultz ET. Sexual selection and male characteristics in the bluehead wrasse, Thalassoma bifasciatum: Mating site acquisition, mating site defense, and female choice. Evolution. 1992;46(5):1421–1442. doi: 10.1111/j.1558-5646.1992.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 74.Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain: a topological atlas. Berkhauser Verlag; Boston, MA: 1996. [Google Scholar]
- 75.Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- 76.Vetillard A, Bennani S, Saligaut C, Jego P, Bailhache T. Localization of tyrosine hydroxylase and its messenger RNA in the brain of rainbow trout by immunocytochemistry and by in situ hybridization. J Comp Neurol. 2002;449:374–389. doi: 10.1002/cne.10296. [DOI] [PubMed] [Google Scholar]


