Abstract
The 5-HT1A receptor agonist 8-OH-DPAT (0.5 mg/kg) enhances behavioral recovery when administered 15 min after experimental traumatic brain injury (TBI). To determine if benefits are still attainable at clinically relevant times, treatment was delayed 1 and 2 hr post-TBI and motor/cognitive performance was compared to early (i.e., 15 min) administration. No differences were observed among the vehicle and 8-OH-DPAT groups treated at 1 and 2 hr, but all three were significantly impaired vs. early 8-OH-DPAT. The data suggest that an early and narrow critical period exists for the behavioral recovery afforded by a single 8-OH-DPAT treatment paradigm. The critical window corresponds to the well documented TBI-induced glutamate increase, suggesting that 8-OH-DPAT may be conferring neuroprotection by attenuating this acute deleterious surge.
Keywords: beam-walk, beam-balance, controlled cortical impact, functional recovery, learning and memory, Morris water maze, neurobehavior, serotonin receptor agonists, traumatic brain injury
Introduction
The pathophysiological responses to traumatic brain injury (TBI) result from either primary or secondary insults [15]. The effects of the primary insult are immediate and irreversible, such as cell death at the trauma site, while the consequences of the secondary injury evolve over a period of min to hrs and thus afford the opportunity for pharmacological interventions that may attenuate or prohibit secondary damage, and perhaps limit functional deficits. One area of research that has received considerable attention as a secondary sequela of brain injury is the acute and rapid increase in extracellular levels of the excitatory amino acid glutamate [3,7,8,10,20,23,25]. Glutamate activates the N-methyl-D-aspartate (NMDA) receptor, which in turn leads to post-synaptic calcium influx and the induction of deleterious events that contribute to neuronal dysfunction or death [5] and ultimately to neurobehavioral impairment.
Several studies utilizing various CNS injury models have reported that serotonin1A (5-HT1A) receptor agonists confer neuroprotection [1,6,11-14,24]. Specifically, the administration of the 5-HT1A receptor agonist repinotan HCL (formerly BAYx3702) after cerebral ischemia [6,24] or subdural hematoma [1] results in decreased lesion volumes vs. vehicle-treated controls. Furthermore, early treatment with repinotan HCL after cortical impact attenuates histopathology and improves neurobehavioral performance [12]. Additionally, a single and early (i.e., 15 min post injury) systemic administration of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) also attenuates TBI-induced functional deficits [11,13,14]. The protective effect of acute 5-HT1A receptor agonists following brain insult is purported to result from a decrease in glutamate release, which is mediated, in part, via activation of pre-synaptic 5-HT1A receptors located on glutamatergic terminals [16,21]. Moreover, electrophysiological studies reveal that 5-HT1A receptor agonism induces neuronal hyperpolarization [2,17,18,22]. These mechanisms may reduce the acute deleterious surge of glutamate after TBI thus effectively attenuating the post-injury excitotoxic cascade.
The goal of the current study was to empirically determine whether delaying 8-OH-DPAT treatment after TBI by 1 or 2 hrs, which are clinically relevant intervention time points, results in beneficial effects. The findings could potentially have significant implications for clinical intervention strategies.
Sixty male Harlan Sprague-Dawley rats weighing 300–325 g on the day of surgery were housed in standard steel-wire mesh cages in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with free access to food and water. After one week of acclimatization, the rats were prepared for surgery as previously described [11]. Briefly, anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. After endotracheal intubation the rats were placed in a stereotaxic frame and ventilated mechanically. Utilizing aseptic techniques a midline scalp incision was made, the skin and fascia were reflected and a craniectomy was made in the right hemisphere with a hand held trephine. TBI was produced by impacting the exposed cortex 2.8 mm at 4 m/sec. After the impact, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and placed in a temporary cage until the effects of anesthesia waned, as evidenced by ambulation, before being returned to the colony. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Following surgery, the rats were randomly assigned to two sham (n=6 per group) and four TBI groups (n=12 per group). Both sham groups and two TBI groups received either saline vehicle (1.0 mL/kg) or a single intraperitoneal (i.p.) administration of 8-OH-DPAT (0.5 mg/kg; prepared daily by dissolving in sterile saline) at 15 min after surgery while the remaining two TBI groups received the same dose of 8-OH-DPAT at 1 hr or 2 hr after TBI. Specifically, the group conditions were as follows: TBI + 8-OH-DPAT (15 min), TBI + VEHICLE (15 min), Sham + 8-OH-DPAT (15 min), Sham + VEHICLE (15 min), TBI + 8-OH-DPAT (1 hr), TBI + 8-OH-DPAT (2 hr). The dose, timing, and route of 8-OH-DPAT administration were selected based on previous studies from our laboratory showing this regimen to be neuroprotective and to promote behavioral recovery after TBI [11,13,14].
All experimental procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). Every attempt was made to limit the number of subjects used and to minimize suffering.
An established beam-balance task was utilized to assess motor function [9,11-14]. The task consists of placing the rat on an elevated (90 cm) narrow wooden beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. Testing was conducted immediately before surgery (to establish a baseline measure), as well as on post-operative days 1–5, and consisted of three trials (60 sec allotted time with an intertrial interval of 30 sec) per day. The average daily scores for each subject were used in the statistical analyses.
Spatial learning was assessed in a Morris water maze (MWM) task demonstrated to be sensitive to cognitive function/dysfunction after TBI [9,11-14]. Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning acquisition began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses. The data were obtained using a spontaneous motor activity recording & tracking (SMART) system (San Diego Instruments, San Diego, CA).
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures ANOVA. When the overall ANOVA revealed a significant effect, the data were further analyzed with the Bonferroni/Dunn post-hoc test to determine specific group differences. The data are presented as the mean ± standard error (SE) and are considered significant when corresponding P values are < 0.05 or as determined by the Bonferroni/Dunn statistic after adjusting for multiple comparisons.
One rat did not survive the surgical procedures and thus the statistical analyses are based on fifty-nine rats. Furthermore, no significant differences were revealed between the 8-OH-DPAT and vehicle-treated sham groups in any behavioral measure and thus the data were pooled and analyzed as one group (denoted as SHAM).
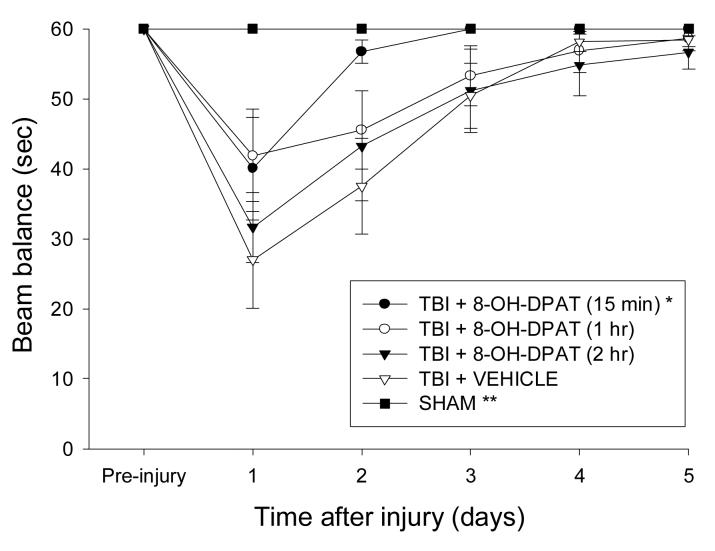
All rats were capable of balancing on the beam and as such no pre-surgical differences were observed among groups. However, significant impairments were detected in all TBI groups vs. SHAM on the first day after surgery, regardless of treatment or time of administration (Fig. 1). Although beam balance performance gradually improved in all TBI groups over the 5 days of testing, the TBI + 8-OH-DPAT (15 min) improved at a significantly faster rate vs. the TBI + VEHICLE group [P < 0.05]. This facilitated recovery also resulted in the TBI + 8-OH-DPAT (15 min) group not being different overall vs. SHAM controls (P = 0.12). No significant difference was revealed between the TBI + 8-OH-DPAT (1 hr) and TBI + 8-OH-DPAT (2 hr) groups [P = 0.21], and neither differed from the TBI + VEHICLE group [P's > 0.05].
Fig. 1.
Mean (± SE) vestibulomotor ability as measured by time (sec) to balance on an elevated wooden beam prior to, and after, TBI or SHAM injury. *P < 0.05 vs. TBI + VEHICLE. **P < 0.05 vs. TBI + VEHICLE, TBI + 8-OH-DPAT (1 hr), and TBI + 8-OH-DPAT (2 hr).
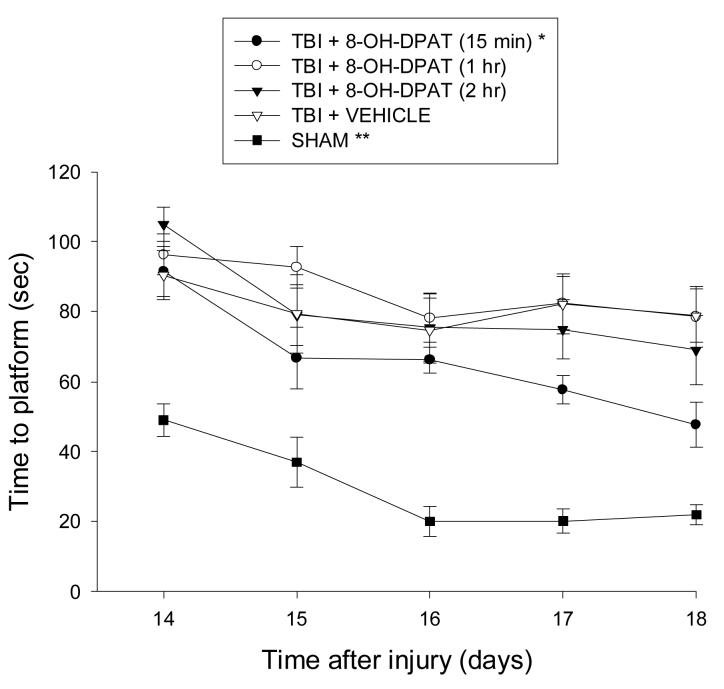
TBI produced significant cognitive impairment as evidenced by subjects in each group often requiring the full 120 sec on some trials to locate the escape platform on the first day of training. However, as depicted in Fig. 2, despite the initial deficits, the TBI + 8-OH-DPAT (15 min) group performed progressively better vs. the TBI + VEHICLE, TBI + 8-OH-DPAT (1 hr), and TBI + 8-OH-DPAT (2 hr) groups over the course of training [P = 0.0018, P < 0.0001, and P = 0.0029, respectively]. No significant differences were revealed among the TBI + VEHICLE and delayed 8-OH-DPAT-treated groups [all P's > 0.05]. All injured groups remained significantly impaired relative to the SHAM group on each day of training [P < 0.0001].
Fig. 2.
Mean (± SE) time (sec) to locate escape platform in the Morris water maze on post-TBI days 14-18. *P < 0.05 vs. TBI + VEHICLE, TBI + 8-OH-DPAT (1 hr), and TBI + 8-OH-DPAT (2 hr). **P < 0.05 vs. all TBI groups.
This study sought to determine the duration of the therapeutic window for successful treatment with the 5-HT1A receptor agonist 8-OH-DPAT after experimental brain trauma. The results replicated prior reports from our laboratory demonstrating that a single systemic administration of 8-OH-DPAT at 15 min attenuates TBI-induced neurobehavioral deficits [11,13,14]. Furthermore, the study provided novel findings by indicating for the first time that delaying 8-OH-DPAT by even 1 hr after TBI renders this treatment paradigm ineffective as neither the 1 hr nor 2 hr delayed groups exhibited improvement relative to the vehicle-treated controls in either motor (i.e., beam balance) or cognitive (i.e., spatial learning and memory) performance. Furthermore, there was no significant difference between 1 hr and 2 hr treatments. These data suggest that the therapeutic efficacy of a single post-TBI administration of 8-OHDPAT is confined to a very early critical window of opportunity and that administering a single treatment beyond that clearly defined period is of no behavioral value. Because histopathology was not assessed, a comment regarding the efficacy of delayed treatment on this outcome is prohibitive.
The narrow therapeutic window suggests that a single administration of 8-OH-DPAT is producing its beneficial effects by acting on acute pathophysiological responses of TBI such as the attenuation of glutamate-induced excitotoxicity. Numerous TBI studies have revealed a temporal course of glutamate that is marked by a sharp rise within the first 20-30 min after injury followed by a subsequent return to normal levels by 1 hr [7,20,23]. Furthermore, several studies have shown that 5-HT1A receptor agonists decrease glutamate release in experimental models of brain insult [4,6,19,24]. These published findings in concert with our experimental paradigm supports the notion that administration of 8-OH-DPAT at 15 min after trauma is temporally in phase with the TBI-induced glutamate surge and thus affords an opportunity for neuroprotection by blocking or reducing the deleterious rise. This hypothesis also provides an explanation for why a single administration of 8-OH-DPAT at time points following the normalization of glutamate levels is of no behavioral value.
An acknowledged limitation of the current study is that we speculate on a mechanism of action (i.e., decreased glutamate levels) for acute 8-OH-DPAT treatment based on data from other CNS injury models [6,24]. The next logical step is to empirically determine whether 8-OH-DPAT is indeed attenuating the acute rise in TBI-induced glutamate or whether alternative mechanisms are mediating the observed effects. A second limitation concerns the
In conclusion, the results reveal that the beneficial effect on motor and cognitive recovery conferred by a single systemic administration of the 5-HT1A receptor agonist 8-OH-DPAT is restricted to an early period after experimental TBI. The critical window corresponds to the well documented TBI-induced glutamate increase, suggesting that 8-OH-DPAT may be, in part, conferring neuroprotection by attenuating this acute deleterious surge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Support: This work was supported by National Institutes of Heath grants HD043851 and HD046700 awarded to AEK
There are no conflicts of interest to report
References
- 1.Alessandri B, Tsuchida E, Bullock RM. The neuroprotective effect of a new serotonin receptor agonist, BAY X3702, upon focal ischemic brain damage caused by acute subdural hematoma in the rat. Brain Res. 1999;845:232–235. doi: 10.1016/s0006-8993(99)01948-4. [DOI] [PubMed] [Google Scholar]
- 2.Andrade R. Electrophysiology of 5-HT1A receptors in the rat hippocampus and cortex. Drug Dev Res. 1992;26:275–286. [Google Scholar]
- 3.Bullock R, Zauner A, Myseros JS, Marmarou A, Woodward JJ, Young HF. Evidence of prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann NY Acad Sci. 1995;765:290–297. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- 4.Calcagno E, Carli M, Invernizzi W. The 5-HT1A receptor agonist 8-OH-DPAT prevents prefrontal glutamate and serotonin release in response to blockade of cortical NMDA receptors. J Neurochem. 2006;96:853–860. doi: 10.1111/j.1471-4159.2005.03600.x. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vry J, Dietrich H, Glaser T, Heine H-G, Horváth E, Jork R, Maertins T, Mauler F, Opitz W, Scherling D, Schohe-Loop R, Schwartz T. BAY x 3702. Drugs of the Future. 1997;22:341–349. [Google Scholar]
- 7.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 8.Globus MYT, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 10.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increase in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 11.Kline AE, Dixon CE, Zafonte RD, Bolinger BD. The therapeutic efficacy conferred by the 5HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma. 2004;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- 12.Kline AE, Yu J, Horvath E, Marion DW, Dixon CE. The selective 5-HT1A receptor agonist repinotan HCL attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 13.Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE. Protective effects of the 5HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci Lett. 2002;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- 14.Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochanek PM, Clark RS, Ruppel RA, Adelson PD, Bell MJ, Whalen MJ, Robertson CL, Satchell MA, Seidberg NA, Marion DW, Jenkins LW. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: lessons learned from the bedside. Pediatr Crit Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama S, Nei K, Tanaka C. Regulation of glutamate release via NMDA and 5-HT1A receptors in guinea pig dentate gyrus. Brain Res. 1996;728:175–180. doi: 10.1016/0006-8993(96)00395-2. [DOI] [PubMed] [Google Scholar]
- 17.Mauler F, Fahrig T, Horváth E, Jork R. Inhibition of evoked glutamate release by the neuroprotective 5-HT1A receptor agonist BAY x 3702 in vitro and in vivo. Brain Res. 2001;888:150–157. doi: 10.1016/s0006-8993(00)03074-2. [DOI] [PubMed] [Google Scholar]
- 18.Melena J, Chidlow G, Osborne NN. Blockade of voltage-sensitive Na+ channels by the 5-HT1A receptor agonist 8-OH-DPAT: possible significance for neuroprotection. Eur J Pharmacol. 2000;406:319–324. doi: 10.1016/s0014-2999(00)00688-9. [DOI] [PubMed] [Google Scholar]
- 19.Mignon LJ, Wolf WA. 8-Hydroxy-2-(di-n-propylamino)tetralin reduces striatal glutamate in an animal model of Parkinson's disease. Neuroreport. 2005;16:699–703. [Google Scholar]
- 20.Palmer AM, Marion DW, Botscheller ML, Swedlow PF, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 21.Prehn JH, Backhauss C, Karkoutly C, Nuglisch J, Peruche B, Rossberg C, Krieglstein J. Neuroprotective properties of 5-HT1A receptor agonists in rodent models of focal and global cerebral ischemia. Eur J Pharmacol. 1991;203:213–222. doi: 10.1016/0014-2999(91)90717-5. [DOI] [PubMed] [Google Scholar]
- 22.Raiteri M, Maura G, Barzizza A. Activation of presynaptic 5-hydroxytryptamine1-like receptors on glutamatergic terminals inhibits N-methyl-D-aspartate-induced cyclic GMP production in rat cerebellar slices. J Pharmacol Exp Ther. 1991;257:1184–1188. [PubMed] [Google Scholar]
- 23.Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, Kochanek PM, Graham SH. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;943:15–22. doi: 10.1016/s0006-8993(02)02471-x. [DOI] [PubMed] [Google Scholar]
- 24.Semkova I, Wolz P, Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, Bay x 3702, demonstrated in vitro and in vivo. Eur J Pharmacol. 1998;359:251–260. doi: 10.1016/s0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]
- 25.Stover JF, Morganti-Kossmann MC, Lenzlinger PM, Stocker R, Kempski OS, Kossmann T. Glutamate and taurine are increased in ventricular cerebrospinal fluid of severely brain-injured patients. J Neurotrauma. 1999;16:135–142. doi: 10.1089/neu.1999.16.135. [DOI] [PubMed] [Google Scholar]