Abstract
The present study investigated the articulatory implementation deficits of Broca’s and Wernicke’s aphasics and their potential neuroanatomical correlates. Five Broca’s aphasics, two Wernicke’s aphasics, and four age-matched normal speakers produced consonant-vowel-(consonant) real word tokens consisting of [m, n] followed by [i, e, a, o, u]. Three acoustic measures were analyzed corresponding to different properties of articulatory implementation: murmur duration (a measure of timing), amplitude of the first harmonic at consonantal release (a measure of articulatory coordination), and murmur amplitude over time (a measure of laryngeal control). Results showed that Broca’s aphasics displayed impairments in all of these parameters, whereas Wernicke’s aphasics only exhibited greater variability in the production of two of the parameters. The lesion extent data showed that damage in either Broca’s area or the insula cortex was not predictive of the severity of the speech output impairment. Instead, lesions in the upper and lower motor face areas and the supplementary motor area resulted in the most severe implementation impairments. For the Wernicke’s aphasics, the posterior areas (superior marginal gyrus, parietal, and sensory) appear to be involved in the retrieval and encoding of lexical forms for speech production, resulting in increased variability in speech production.
Keywords: aphasic, speech, nasal consonants, murmur, acoustic, articulatory, laryngeal, lesion extent
Introduction
It has long been noted that aphasic patients with anterior brain damage have deficits affecting the sound structure of their speech output. What has been less clear is whether the nature of the deficits reflects an impairment in phonological selection and planning, or whether it reflects an impairment in articulatory implementation. To explore this question, a number of studies have conducted quantitative acoustic analyses of the speech production of these patients with the goal of inferring the articulatory configurations giving rise to the obtained acoustic patterns. Investigations of the phonetic patterns of speech have shown that patients with anterior lesions including Broca’s aphasics have deficits in articulatory implementation, affecting the timing and coordination of two independent articulators, as well as deficits in laryngeal control.
The conclusions drawn from these studies have been based on examination of the phonetic dimensions of voicing in stop consonants (as measured by the acoustic parameter of voice onset time or VOT) and nasality. VOT, the timing relation between the release of the stop closure (tongue or lips) and the onset of glottal vibration (larynx) distinguishes voiced and voiceless stop consonants (Lisker and Abramson, 1964). The frequent overlap of VOT distributions for voiced and voiceless stop consonants seen in anterior aphasic productions suggests a deficit in temporal coordination of these articulatory gestures (Blumstein, Cooper, Zurif, and Caramazza, 1977; Freeman, Sands, and Harris, 1978; Blumstein, Cooper, Goodglass, Statlender and Gottlieb, 1980; Gandour and Dardarananda, 1984; Shewan, Leeper, and Booth, 1984).
In the case of nasality, the spectral structure of nasal consonants is formed by the resonances of the pharyngeal, nasal, and oral cavities. Production of a nasal consonant requires coordinating the lowering of the velum (thereby opening the velo-pharyngeal port) with the closing of the oral cavity to allow sound to be propagated through the nasal cavity, followed by coordinating the raising of the velum in conjunction with the release of the oral closure. The acoustic consequence of having sound pass through the nasal cavity is the occurrence of a nasal murmur prior to the release of the oral closure. The nasal murmur which contains spectral information for both manner and place of articulation is the definitive attribute of this consonant class.
Studies of patients with anterior lesions show various deficits in temporal coordination in nasal consonant production including marked variability in velar gestures affecting velum height and slope of velar lowering (Itoh, Sasanuma, and Ushijima, 1979; Itoh, Sasanuma, Hirose, Yoshioka and Ushijima, 1980), premature occlusion of the velar port (Ziegler and Cramon, 1985), and inappropriate velar lowering and a high degree of velar movement variation (Katz, Machetanz, Orth, and Schonle, 1990).
Patients with anterior lesions also have deficits in laryngeal control apart from laryngeal timing or coordination with the supralaryngeal vocal tract. These deficits emerge in the production of stop consonants where there is weaker high frequency energy (Shinn and Blumstein, 1983), in the production of fricative consonants where there is a weak energy throughout the frequency spectrum in the fricative noise (Harmes, Daniloff, Hoffman, Lewis, Kramer, and Absher, 1984), and a weaker amplitude of voicing for voiced fricatives resulting in greater overlap in the amplitudes of glottal excitation for voiced and voiceless fricatives (Kurowski, Hazen, and Blumstein, 2003). Deficits in the production of prosody also occur and are characterized by restrictions in fundamental frequency range (Cooper, Soares, Nicol, Michelow, and Goloskie, 1984; Ryalls, 1982; Kent and Rosenbek, 1983; Harmes et al., 1984), and a reduced proficiency in implementing lexical tone (Packard, 1986; Gandour. Ponglorpisit, Khunadorn, Dechongkit, Boongird, Boonklam, and Potisuk, 1992).
Although these studies show that patients with anterior lesions have deficits in articulatory implementation involving temporal coordination and laryngeal control, there have been no studies that have explored the extent to which such impairments co-occur in the same patients. That is, do impairments involving temporal coordination, timing, and laryngeal control co-occur or do such impairments surface independently in individual patients? To explore this question, the present study will investigate three articulatory parameters that contribute to the production of nasal consonants: the role of timing, laryngeal control, and coordination of articulatory gestures. Nasals as a consonant class provide a unique opportunity to explore these three factors in the utterance of a single segment. As discussed earlier, nasal consonant production requires appropriate timing of the murmur with the release. It requires the coordination of independent articulators, namely movement of the tongue or lips, to achieve oral closure relative to the lowering of the velum, and the raising of the velum relative to the release of the oral closure into the following vowel. In addition, the production of nasal consonants in English requires laryngeal control to maintain voicing throughout the nasal murmur and into the vowel.
To examine each of these elements (timing, laryngeal control, and articulatory coordination), three acoustic measures were utilized. The duration of the nasal murmur was used to evaluate timing. Changes in durational patterns compared to normals would be indicative of a timing impairment. The amplitude of the first harmonic in the murmur was used as a measure of laryngeal control. Abnormal amplitude patterning would suggest irregularities in laryngeal source characteristics. The nature of the release of the nasal consonant was used as a measure of articulatory coordination. In this measure, the amplitude of the murmur was compared to that of the vowel in the vicinity of the consonantal release. Abnormal patterns would implicate difficulties coordinating movement of the velum relative to release of the oral closure.
Although it is generally agreed that Broca’s aphasics have impairments in articulatory implementation, it is not clear what the neural substrates are that contribute to such impairments. It has been proposed that Broca’s area is involved in motor programming of speech (Hillis, A.E., Work, M., Barker, P.B., Jacobs, M.A., Breese, E.L., and Maurer, K., 2004), while others (Dronkers, 1996) have suggested that the insula plays a crucial role in the coordination of speech articulation. Baum, S., Blumstein, S., Naeser, M., and Palumbo, C. (1990) showed different patterns of impairment depending on the lesion sites in a group of patients who were clinically diagnosed as Broca’s aphasics. In particular, patients with lesions involving Broca’s area, the anterior limb of the internal capsule, and the lowest motor cortex areas for tongue and larynx showed impairments in the production of voice-onset time (VOT). Other Broca’s aphasics without this lesion distribution did not show such impairments. Thus, there was a dissociation between clinical diagnosis and underlying neuropathology in accounting for the speech output deficit of these patients.
In addition to investigating the production of nasal consonants in Broca’s aphasics, the present study included analysis of the production of two Wernicke’s aphasics. While it is generally believed that posterior patients do not exhibit the same deficits in timing and coordination as seen in anterior aphasics, there is growing evidence that Wernicke’s aphasics display a subclinical phonetic deficit (cf. Baum et al., 1990; Vijayan and Gandour, 1995, for a review). For example, Wernicke’s aphasics fail to show normal changes in VOT change as a function of rate of speech and place of articulation in the production of stop consonants (Baum and Ryan, 1993). In fricative consonant production, Wernicke’s aphasics, like anterior aphasics, demonstrate increased variability in fricative duration and do not maintain the voicing contrast utilizing fricative noise duration (Baum et al., 1990). Wernicke’s aphasics have also shown longer than normal vowel durations (Ryalls, 1987; Gandour, Ponglorpisit, Khunadorn, Dechongkit, Boongird, and Boonklam, 1992) and problematic use of vowel duration as a cue to syllable-final voicing (Duffy and Gawle, 1984; Tuller, 1984). Although the inclusion of only two patients does not allow for statistical group comparisons, it does provide a means of qualitatively comparing the parameters of nasal production to both normal controls and Broca’s aphasics.
The aims of the current study are to investigate the acoustic patterns of speech production of nasal consonants and the accompanying lesion sites giving rise to those patterns. The following questions will be addressed: (1) do Broca’s aphasics show articulatory implementation impairments in nasal consonant production. ; (2) which factor or combination of factors (timing, coordination, laryngeal control) are impaired in the production of nasal consonants; and (3) what are the neuroanatomical correlates of such impairments. With regard to questions (1) and (2), we hypothesize that Broca’s aphasics will demonstrate deficits in all three parameters contributing to nasal consonant production. With regard to question (3), we hypothesize that, as shown by Baum et al. (1990), Broca’s aphasics who have lesions involving Broca’s area, the anterior limb of the internal capsule, and the lowest motor cortex areas for tongue and larynx will show deficits in the production of nasal consonants, whereas damage to the anterior insular cortex will not be predictive of speech output impairments.
Method
Subjects
Seven aphasic patients and four age-matched normal controls participated. The four normal speakers were male, native speakers of English. Their ages ranged from 54 to 68 years with a mean age of 61.5. All of the aphasic participants were right-handed males and native speakers of English with no prior history of stroke, traumatic head injury, seizures or a hearing disorder. All had unilateral left hemisphere lesions. Six of the aphasics had lesions due to a cerebral vascular accident (CVA); the seventh patient had a left intracerebral hematoma. The age range of the aphasic patients was 54–69 years, with a mean age of 60.1. On the basis of clinical examination using the Boston Diagnostic Aphasia exam (Goodglass and Kaplan, 1983), five of the patients were diagnosed as Broca’s aphasics and two were diagnosed as Wernicke’s aphasics. Table 1 provides a clinical profile of the patients. All of the patients were considered to be chronic aphasics at the time of their recording.
Table 1.
Clinical profile of Broca’s (B) and Wernicke’s (W) aphasics based on the Boston Diagnostic Aphasia Exam
| Patients | Articulation Rating | Verbal Agility | Auditory Comp (Z-score) | Repetition High | Repetition Low |
|---|---|---|---|---|---|
| B1 | 5.0 | 10 | +0.83 | 7 | 3 |
| B2 | 5.5 | 7 | +1.10 | 6 | 5 |
| B3 | 2.0 | 7 | +0.80 | 7 | 3 |
| B4 | * | * | −1.29 | * | * |
| B5 | 5.5 | 0 | +0.70 | 2 | 2 |
| W1 | 7.0 | 14 | +0.18 | 9 | 1 |
| W2 | 6.5 | 8 | −0.35 | 1 | 0 |
At the time of BDAE testing, B4 had been rated as having no usable speech.
In addition to clinical diagnosis, extent of lesion analysis was performed on chronic retrospective scans (range 9 – 16 years post onset) using the procedures described in Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, and Albert (1989). Specific neuroanatomical areas were identified on CT scan slices including B, B/W, W, SM, and SM+1. Each of these areas was then assessed visually for extent of lesion within that area and a numerical value (from 0–5) was given for each anatomical area. A value of 0 indicates no lesion; 3 indicates half of the area has lesion; 3.5 indicates that the lesion is patchy with more than half of it lesioned; 4 indicates more than half of the area has lesion; and a value of 5 indicates that the whole area has solid lesion. Broca’s area was defined as consisting of the pars triangularis (Brodmann’s area 45) and pars opercularis (Brodmann’s area 44). Wernicke’s area was defined as the posterior two-thirds of the superior temporal gyrus (Brodmann’s area 22). We acknowledge that this method of lesion analysis is a rather simplistic user-dependent image analysis; however, the scans were obtained retrospectively, and therefore were not collected in a manner that would allow the use of computer-based tools to quantify lesion distribution and extent. Nonetheless, using this method, inter-rater reliability is +.948 (p<0.001) (Palumbo, 1999). It should be noted that, on average, the aphasics were recorded within three years of the CT scans used in the lesion analysis. Subjects were tested three to ten years post stroke and were assessed to be chronic aphasics at the time of the recording.
Stimuli
The stimuli consisted of CV(C) real word monosyllables beginning with a nasal consonant [m, n] and followed by one of the vowels [i, e, a, o, u]. Since it is easier for patients to produce stimuli that form real words, the final consonant, if present, varied so as to create the following English words: me, may, mop, mow, moo, knee, nay, not, no, new. Each target syllable was produced in citation form five times in randomized blocks of tokens. Thus, each subject produced a total of 50 CV(C) syllables. The stimuli were printed in orthographic form on 3×5 cards and the subjects were instructed to read each token, speaking clearly but naturally. If subjects were unable to correctly read a token, they repeated it after the examiner.
Because the focus of this study was to examine nasal consonant production, the acoustic analyses were limited to those utterances that were perceived by the examiners as exemplars of the phonemic target. Thus, tokens that contained manner errors or place of articulation errors were not included in the analyses. However, those tokens perceived by the examiner(s) as phonetically distorted versions of the test token, such as syllables uttered in a creaky voice, as well as tokens preceded by an epenthetic vowel, were included. Only 9 stimuli were eliminated from the analysis using these criteria, leaving a range of token responses for each aphasic participant from a low of 45 tokens to the full 50 tokens.
Procedure
The stimuli were recorded onto magnetic tape using a Sony Walkman Professional tape recorder and a Sony stereo microphone. The recorded CV(C) syllables were then digitized at a sampling rate of 10 kHz with a 4.5 kHz low-pass filter and a 12-bit quantization for acoustic analysis.
Using a software waveform editor (BLISS, Mertus, 2003), cursors were placed at the beginning of the murmur and at the point of release of the oral closure into the vowel for each token. Figure 1 (top panel) shows this cursor placement for a baseline [ma] token produced by one of the normal speakers (N1). Generally, visual inspection of the waveform display was sufficient to ascertain the beginning of the nasal murmur. In some utterances (nine tokens from three Broca’s aphasics), patients produced an epenthetic vowel preceding the beginning of the nasal murmur. In such cases, linear predictive coding (LPC) analysis of the waveform was used to determine the beginning of the murmur, thereby excluding the epenthetic vowel from the analysis. To locate the point of discontinuity or release into the vowel, the results of visual inspection were corroborated by means of LPC analysis. Using a full-Hamming window sized to fit individual pitch periods, LPC spectra were obtained for five contiguous pitch periods: the last two pitch periods of the murmur, the pitch period containing the point of discontinuity, and two vowel pitch periods immediately following it. LPC patterning for the pitch period containing the point of discontinuity was distinct from the patterning of both the murmur pulses preceding it and the vowel pulses following it.
Figure 1.
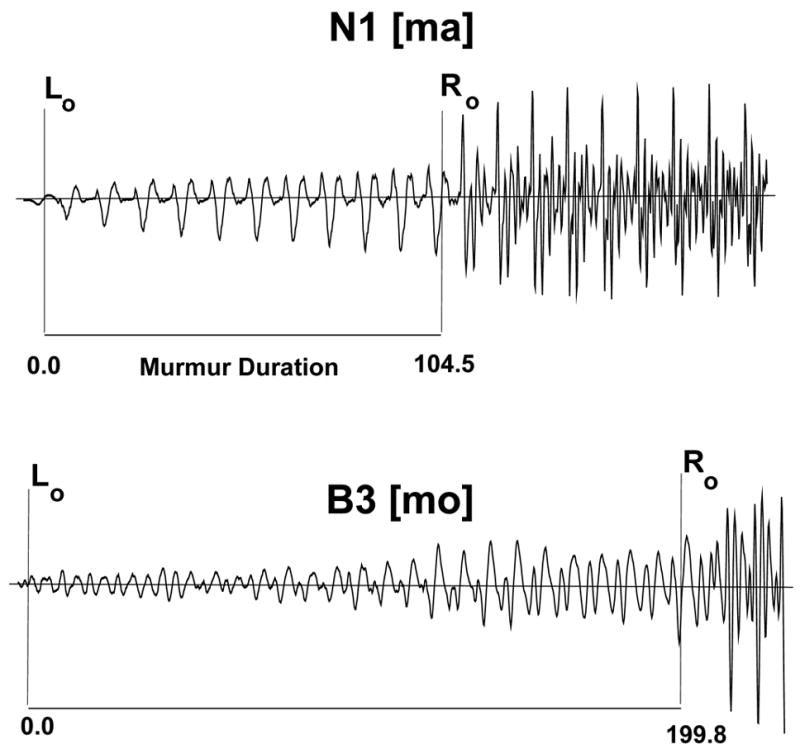
The top panel shows cursor placement identifying the beginning (L0) and end (R0) of the murmur in a [ma] token uttered by one of the normal speakers (N1). Subtracting L0 from R0 yields the murmur duration (104.5 msec). R0 also indicates the start of the pitch period containing the point of release of the oral closure into the vowel. The bottom panel shows murmur aberrations in the waveform of one of the Broca’s aphasics (B3), indicating a failure to maintain a single, smooth murmur patterning.
Analyses
Duration Measure
The duration of the nasal consonant was measured from the onset of the nasal murmur to the point of release of the oral closure into the vowel. In some cases, the beginning of the nasal consonant did not always coincide with the first pulse of the nasal murmur. Difficulty in initiating some speech tokens produced aperiodic waveform areas with extremely low amplitude that appeared prior to the weak but more regular murmur pulses. Generally, these areas ranged in duration from 5 to 20 msec. In these cases, the aperiodic areas were included in the measures of the duration of the nasal consonants. Duration means, standard deviations, and frequency distributions were subsequently computed.
Amplitude Measures
Two amplitude measures were undertaken. The first analysis tracked amplitude changes in the laryngeal source throughout the duration of the murmur by measuring the amplitude of the first harmonic. The second analysis examined the release characteristics of the nasal consonant, focusing on the changes in amplitude from the end of the murmur to the release of the oral closure.
The analysis of the amplitude of the murmur measured changes throughout the murmur by examining potential changes in amplitude in three areas: initial, medial, and final or end-of-murmur. In principle, the nasal murmur reflects closure of the oral cavity with continuing vocal cord vibration. As a result, a normally produced nasal consonant in initial position may show little amplitude change throughout the duration of the murmur. Alternatively, there may be a gradual incremental change in the amplitude of the murmur from its onset, because the initiation of voicing started from silence. In order to be able to compare amplitude changes in the murmur quantitatively across tokens which varied extensively in their absolute durations, the murmur portion of the stimulus was divided into thirds. Wherever possible (i.e., given the duration of the murmur), four contiguous pitch periods were analyzed in each of the three parts, for a total of twelve amplitude measurements. The initial portion of the murmur started at the beginning of the first murmur pulse, even if that pitch period was very weak. The middle portion of the murmur was measured from the pitch period at the midpoint of the murmur duration. The final portion or end-of-murmur was the last four murmur pitch periods before the discontinuity corresponding to the consonant release. The majority of the tokens (83% for the aphasics and 80% for the normal controls) had murmurs with at least 12 pitch periods allowing for analysis over the range of the murmur duration. For the remaining stimuli which contained 8 pitch periods or less, the murmur was divided into an initial and final portion.
For this analysis, a full-Hamming window, sized to fit the last murmur pulse before the discontinuity, was placed in turn over each of the twelve pitch periods corresponding to the initial, middle, and final portions of the nasal murmur. At each of these points in the murmur, a Discrete Fourier Transform (DFT) analysis was used to locate the first harmonic in the spectral envelope and to measure its amplitude. While normal speakers’ murmurs are characterized by glottal pulses that are regular in both shape and amplitude, as seen in Figure 1 (top panel), some of the Broca’s aphasics produced nasal murmurs whose waveforms were much less smooth and whose pitch periods failed to maintain a single patterning (i.e. shape) over the length of the murmur. Figure 1 (bottom panel) provides an example of such a murmur from subject B3, whose nasal productions were the most frequently irregular. To accommodate the pitch to pitch variations between contiguous murmur pulses, the window size was adjusted as needed.
In addition to the amplitude values obtained in the murmur, a measure of the vowel amplitude was obtained, using a 25.6 msec full-Hamming window placed at the beginning of the third pitch period into the vowel (counting from the point of discontinuity). As in the case of the murmur, a DFT analysis was used to ascertain the location of the first harmonic in the vowel envelope, providing a single vowel amplitude measure for that CV(C) token. The vowel amplitude was then used to normalize the murmur amplitude data within the same token by subtracting the vowel amplitude from the amplitude measurement taken at each of the twelve targeted murmur pulses. This yielded twelve data points which, taken together, represented global amplitude change across the duration of the murmur.
To quantify and evaluate local amplitude change over the course of the murmur for each token, difference scores were obtained between each of the twelve data points corresponding to consecutive pitch periods of the murmur. The size and direction of the difference between consecutive pitch periods would indicate either a normal patterning (characterized by either small incremental increases in amplitude toward the amplitude level of the vowel or minimal change in consecutive pitch periods) or the presence of areas where the amplitude change was disproportionately large or moving away from the amplitude of the vowel. Finally, to provide a more global measure of amplitude change, for each token, the normalized amplitude value of the first pitch period of the murmur was subtracted from that of the last pitch period of the murmur, yielding an overall measure of amplitude change for that token.
The second amplitude analysis charted amplitude changes from the murmur to the release of the nasal into the following vowel. It is the case that the spectrum at consonantal release differs as a function of place of articulation owing to differences in the point of closure in the supralaryngeal tract. Consequently, in the analysis of the amplitude changes at the release of the consonant, we took into consideration these differences. In particular, we compared band energy (BEN) changes from murmur to vowel with a center frequency and bandwidth appropriate to each place of articulation. For [m], a center frequency of 585 Hz and a bandwidth of 370 Hz were used; for [n], the center frequency was 1785 Hz with a bandwidth of 1030 Hz. Using a full-Hamming window sized to fit the last two pitch periods of the murmur for each token, BEN measures were taken immediately preceding and following the consonantal release. Specifically, the same-sized window was first positioned over the last two pitch periods of the murmur and then over the next two pitch periods, the first of which contained the point of release into the vowel. The BEN energy measurements were made using a rectangular filter with the pre-emphasis set to zero. Difference scores were obtained between the BEN energy of the nasal murmur and that of the release into the vowel.
Results
Lesion results
The results of the lesion analyses are shown in Table 2. As can be seen, the Broca’s aphasics have extensive lesions (including anterior as well as temporal-parietal areas) that are both cortical and deep. Although all of the Broca’s aphasics have damage in Broca’s area and the insular cortex, patient B2 has the least involvement of lower motor areas, pre-motor cortex, motor areas for larynx, mouth, and face, as well as sparing of the SMA undercut. However, along with patient B5, B2 has the greatest involvement of the insular cortex. Thus, based on the previous work of Baum et al. (1990) as described earlier, B2 should be minimally impaired in the production of nasal consonants. In contrast, based on the proposal by Dronkers (1996), B2, with extensive damage of the anterior insular cortex, should have a severe motor programming deficit.
Table 2.
Extent of lesion analysis using ratings based on a scale of 0–5. A score of 3 or above means that more than half of the area is lesioned.
| Anterior Cortical areas
|
Anterior Subcortical areas
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Broca’s area | Insular cortex | Pre-Motor | Motor-larynx | Motor-mouth | Motor-upper face | total of lower motor* | SMA undercut | MScF | ALIC | Anterior PVWM | Middle PVWM | |
| B 1 | 3.50 | 3.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | yes | 4.75 | 2.00 | 5.00 | 4.75 |
| B 2 | 4.00 | 5.00 | 2.00 | 4.50 | 4.00 | 3.00 | 3.80 | no | 4.75 | 5.00 | 4.50 | 4.75 |
| B 3 | 4.25 | 4.00 | 3.00 | 5.00 | 5.00 | 5.00 | 5.00 | yes | 4.75 | 3.60 | 4.75 | 4.25 |
| B 4 | 4.00 | 3.60 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | yes | 2.50 | 3.00 | 4.50 | 0.00 |
| B 5 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | yes | 4.50 | 5.00 | 4.88 | 4.88 |
|
| ||||||||||||
| W 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | no | 0.00 | 0.00 | 0.00 | 0.00 |
| W 2 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | no | 0.00 | 0.00 | 0.00 | 0.00 |
|
| ||||||||||||
|
Posterior Cortical areas
|
Posterior Sub cortical areas
|
|||||||||||
| Wernicke’s area | Sensory | Anterior SMG | Posterior SMG | Angular gyrus | Superior parietal lobule | temporal isthmus | Posterior PVWM | |||||
|
| ||||||||||||
| B 1 | 0.00 | 5.00 | 2.75 | 0.00 | 0.00 | 2.00 | 0.00 | 3.75 | ||||
| B 2 | 4.00 | 4.00 | 4.50 | 2.00 | 0.00 | 2.00 | 5.00 | 4.75 | ||||
| B 3 | 0.00 | 4.50 | 1.50 | 1.50 | 0.00 | 0.00 | 0.00 | 3.00 | ||||
| B 4 | 2.00 | 4.00 | 0.00 | 1.50 | 3.50 | 4.00 | 4.50 | 3.50 | ||||
| B 5 | 0.00 | 5.00 | 5.00 | 5.00 | 4.00 | 2.00 | 0.00 | 4.88 | ||||
|
| ||||||||||||
| W 1 | 2.00 | 0.00 | 0.00 | 3.50 | 0.00 | 3.00 | 2.00 | 2.00 | ||||
| W 2 | 0.00 | 0.00 | 0.00 | 5.00 | 5.00 | 4.00 | 3.00 | 4.00 | ||||
SMA = supplementay motor area
SMG = supramarginal gyrus
MScF = Medial Subcallosal fasciculus
ALIC = Anterior limb internal capsule
PVWM = periventricular white matter
Total of lower motor = average of the three motor areas that make up the lower motor cortex: larynx, mouth, upper face.
SMA undercut: there is no real difference between the SMA being undercut versus lesioned. The SMA is in the anterior cerebral artery distribution and thus is rarely lesioned in aphasic patients. In contrast, the white matter deep to it is supplied by the middle cerebral artery and lesions in this area effectively cut-off connections between the SMA and other areas such as the medial subcallosal fasciculus, which is implicated in speech output (Naeser et al., 1989)
Compared to the lesions of Broca’s aphasics, the lesions of the Wernicke’s aphasics are almost exclusively posterior, although a small lesion in the insular cortex can be seen for W2. Only W1 has a lesion in Wernicke’s area and it is not extensive. For both Wernicke’s patients, the most extensive cortical lesions are in the posterior supramarginal gyrus (SMG) and the superior parietal lobule, with subcortical areas involving the temporal isthmus and the posterior periventricular white matter.
Analysis of Parameters of Nasal Production
In order to relate potential patterns of deficits in the parameters of nasal production to clinical type and to the neuroanatomical findings, we conducted two sets of analyses for each parameter of nasal production. In the first, we examined the patterns of production in relation to clinical type, irrespective of lesion differences within a clinical group, and in the second, we examined the patterns of production for each patient in a clinical group in relation to the lesion data. In this way, we examined the question using a combined multi-case and clinical group approach.
Murmur Duration
Figure 2 (a,b,c) shows the duration distributions for each subject and Table 3 summarizes the means and standard deviations of the murmur durations of the aphasic patients as well as normal participants. As can be seen in Figure 2, the distribution of duration values for the murmur is both shorter and more tightly clustered for the normal subjects compared to the Broca’s aphasics. As predicted, only B2 had mean durations that were similar to normals. As for the Wernicke’s aphasics, although one patient (W2) had murmur duration values similar to normals, the other Wernicke’s aphasic produced murmurs that were similar in duration to that of the Broca’s aphasics. As Table 3 shows, both the Broca’s and Wernicke’s aphasics had larger standard deviations than did normal controls.
Figure 2.
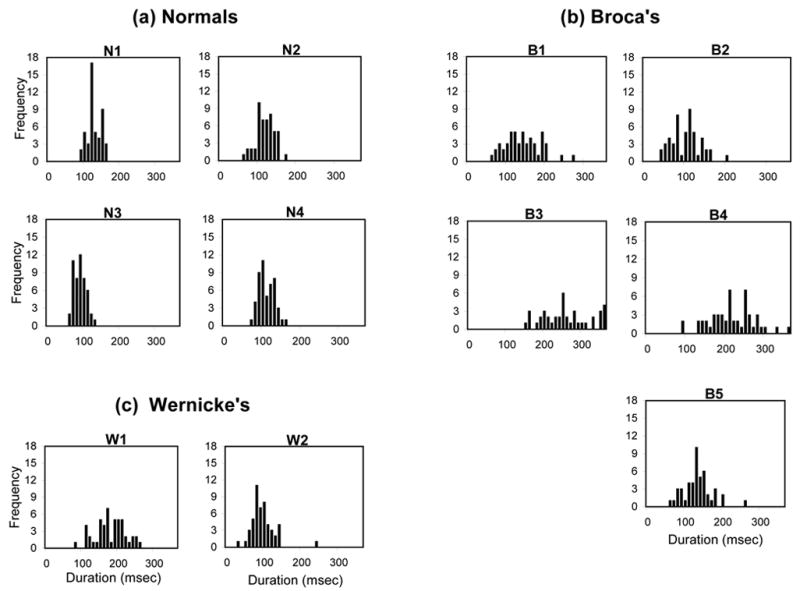
The frequency distribution of murmur durations for (a) Normals, (b) Broca’s aphasics, and (c) Wernicke’s aphasics.
Table 3.
Mean murmur duration (and standard deviation) in msec for normals and aphasic patients
| Subject | Normal | Broca | Wernicke |
|---|---|---|---|
| 1 | 113 (19) | 136 (48) | 163 (56) |
| 2 | 112 (25) | 95 (40) | 81 (42) |
| 3 | 84 (16) | 255 (80) | |
| 4 | 105 (20) | 200 (64) | |
| 5 | 119 (68) | ||
|
| |||
| X̄(sd) | 103 (20) | 161 (60) | 122 (49) |
Two Mann-Whitney U tests were performed on the murmur durations comparing the Broca’s aphasics to the normal controls. The first analysis included all of the patients clinically diagnosed as Broca’s aphasics and failed to show a significant difference between the normals and Broca’s aphasics (Z= −1.715, p=.09). However, based on the lesion data for B2 and the predictions that he would not show the same patterns as the other patients in the Broca’s group, a second analysis was conducted excluding his data. This second Mann-Whitney U test showed significant murmur duration differences between age-matched controls and Broca’s aphasics (Z= −2.309, p<.02), with longer murmur durations for the Broca’s aphasics than for the age-matched controls.
Amplitude Measures
Murmur Analysis: Amplitude Changes over Time
Figure 3 (a,b,c) displays amplitude changes over the course of the murmur for each participant. As Figure 3 shows, there were a number of patterns of amplitude change across subjects. However, similar patterns occurred in both normal and aphasic productions. Table 4 provides a summary of the measure of local amplitude change in the murmur corresponding to the largest mean difference between consecutive pitch periods within the murmur for each speaker. The smallest amplitude differences occurred in the four normal speakers and W2. A Mann-Whitney U test comparing all five Broca’s aphasics to normal controls was significant (Z= −2.449, p<.01) for this parameter, as was a second Mann-Whitney U test without B2 (Z= −2.309, p<.02). The similar results in the two analyses reflect the fact that B2’s data for this parameter fell within the range of the other Broca’s aphasics. Table 4 also shows the means and standard deviations of the amplitude difference between the first and the last pitch period of the murmur for each subject. As with the duration analysis, all of the patients had larger standard deviations compared to the control subjects for this parameter of global amplitude change across the murmur. A Mann-Whitney U test for the mean change in amplitude comparing the five Broca’s aphasics and four age-matched controls was not significant (Z= −0.980, p= .33). Eliminating B2 did not yield a significant result (Z= −.866, p= .39), even though B2’s mean global amplitude difference was closer to the normal range than that of the other Broca’s aphasics.
Figure 3.
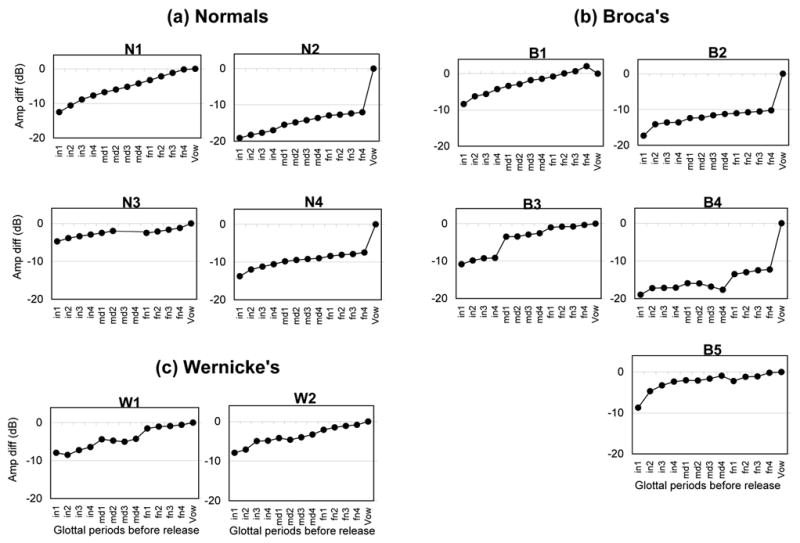
Patterns of amplitude change over the time course of the murmur for (a) Normals, (b) Broca’s aphasics, and (c) Wernicke’s aphasics. On the abscissa are the twelve murmur pulses and the single vowel area targeted in each token. The ordinate indicates the mean amplitude difference in dB between the first harmonic of each murmur pulse and that of the vowel.
Table 4.
Amplitude changes (in dB) for Broca’s (B), Wernicke’s (W), and Normal (N) subjects: the maximal local amplitude difference in the murmur and the global amplitude change across the murmur
| Speakers | Local Difference | Global Difference | |
|---|---|---|---|
| Means | Means | Standard Deviation | |
| N1 | 1.91 | 12.37 | 2.36 |
| N2 | 1.51 | 7.10 | 2.75 |
| N3 | 0.88 | 3.57 | 1.58 |
| N4 | 1.81 | 6.30 | 2.04 |
| X̄ | 1.53 | 7.34 | 2.18 |
| B1 | 2.14 | 10.45 | 4.99 |
| B2 | 3.18 | 7.18 | 3.79 |
| B3 | 5.66 | 10.44 | 4.00 |
| B4 | 4.12 | 6.68 | 5.25 |
| B5 | 4.02 | 8.43 | 6.23 |
| X̄ | 3.82 | 8.64 | 4.85 |
| W1 | 2.74 | 7.39 | 4.39 |
| W2 | 2.14 | 7.04 | 4.56 |
| X̄ | 2.44 | 7.22 | 4.48 |
Release Characteristics of the Nasal Consonant
Distribution of the BEN energy changes from the nasal murmur into the release are shown for each subject in Figure 4 (a,b,c). As the distribution patterns show, for four of the five Broca’s aphasics (B1, B3, B4, B5), the amplitude changes at the release of the nasal consonant tend to be weaker than those for either the normal subjects or the Wernicke’s aphasics. As in the previous analyses, B2 showed a normal pattern of production. To confirm these patterns statistically, a Mann-Whitney U test was performed on the mean amplitude difference across all tokens comparing the Broca’s aphasics as a group to the normal control group. When the U test included B2, no significant difference emerged between the two groups (Z= −1.470, p= .14). However, when B2’s data was excluded, there was a significant effect for Group (Z= −2.021, p<.04).
Figure 4.
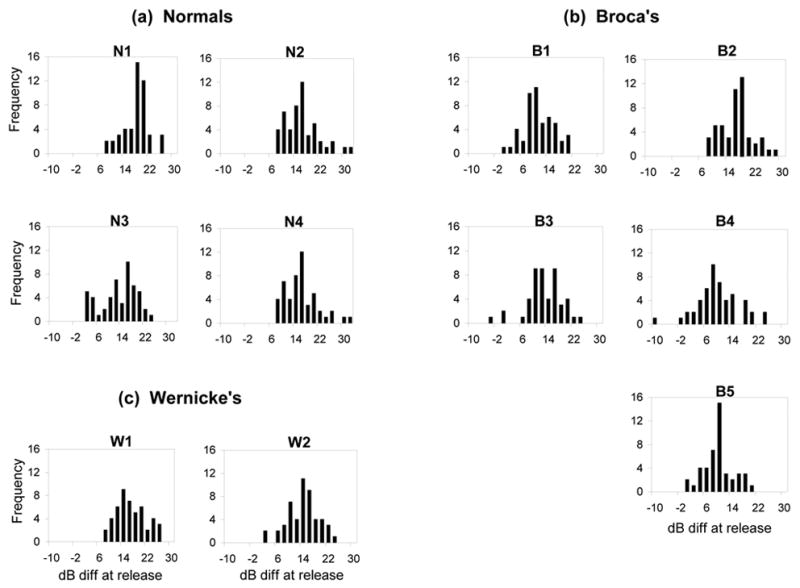
Frequency distribution of the band energy (BEN) difference (in dB) around the release of the nasal murmur into the vowel for (a) Normals, (b) Broca’s aphasics and (c) Wernicke’s aphasics (see text).
Patterns of Impairment
Figure 5 shows a bubble chart displaying the results of the three acoustic measures of nasal consonant production for each subject: murmur duration, BEN energy at release, and maximal local amplitude difference. The x-axis corresponds to duration, the y-axis to BEN amplitude change, and the diameter of the circle reflects the quantitative value of the maximum local amplitude difference. As can be seen, the data generally cluster by group with the control subjects forming one cluster, and the Broca’s aphasics another cluster. The data from a young normal pilot subject (YN) is also included to illustrate that the pattern of results for this participant and the age-matched control subjects is similar. The exceptions to the patient cluster are B2 and W2, both of whom show similar patterns of production on the murmur duration and BEN measures to that of the control subjects. Nonetheless, B2 and W2 are not normal in all parameters of production. Considering the third parameter, maximal local amplitude change, represented by the actual size of the circles, both B2 and W2 are abnormal in this parameter compared to normals.
Figure 5.
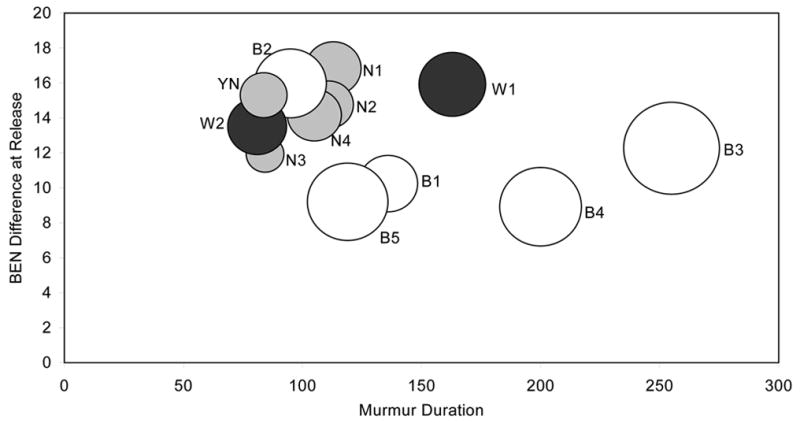
Comparison of performance between normal speakers (grey) and that of Wernicke’s (black) and Broca’s (white) aphasics on three acoustic measures. The normal speakers include the four old normals (N1, N2, N3, N4) and one young normal pilot speaker (YN). In the bubble graph, each speaker’s circle is located as a point along the abscissa (representing their mean murmur duration across all tokens) and the ordinate (indicating their mean BEN amplitude difference in dB at the release of the murmur into the vowel). The size of the circle represents the maximal local amplitude difference for each speaker: the larger the circle diameter, the more abnormal is the local amplitude difference.
Figure 5 along with Table 4 provide a means of determining what deficits in the parameters of production may co-occur in the aphasic speakers. Table 4 showed that laryngeal control, as measured by maximal local amplitude difference (shown as circle diameter in Fig 6), was abnormal in all aphasic speakers. Four of the Broca’s aphasics (B1, B3, B4, B5) also have abnormal murmur durations consistent with a timing deficit. Thus, deficits of laryngeal control and timing may co-occur. In addition, these four Broca’s aphasics (B1, B3, B4, B5) exhibit a third deficit, as shown by the BEN energy difference, suggesting an impairment in articulatory coordination at consonantal release. Only B2 shows a single deficit, namely, the implementation of laryngeal control as seen in local amplitude adjustments across time. For this patient, articulatory coordination at consonantal release (amplitude into the vowel) and timing (murmur duration) are within normal limits. With regard to the Wernicke’s aphasics, as shown in Figure 5, W1 has abnormal murmur durations, suggesting a timing deficit, whereas W2 is normal in that measure. However, both of the Wernicke’s aphasics showed impaired local amplitude adjustments over time (as shown by circle diameters larger than those of normal controls).
Figure 6.
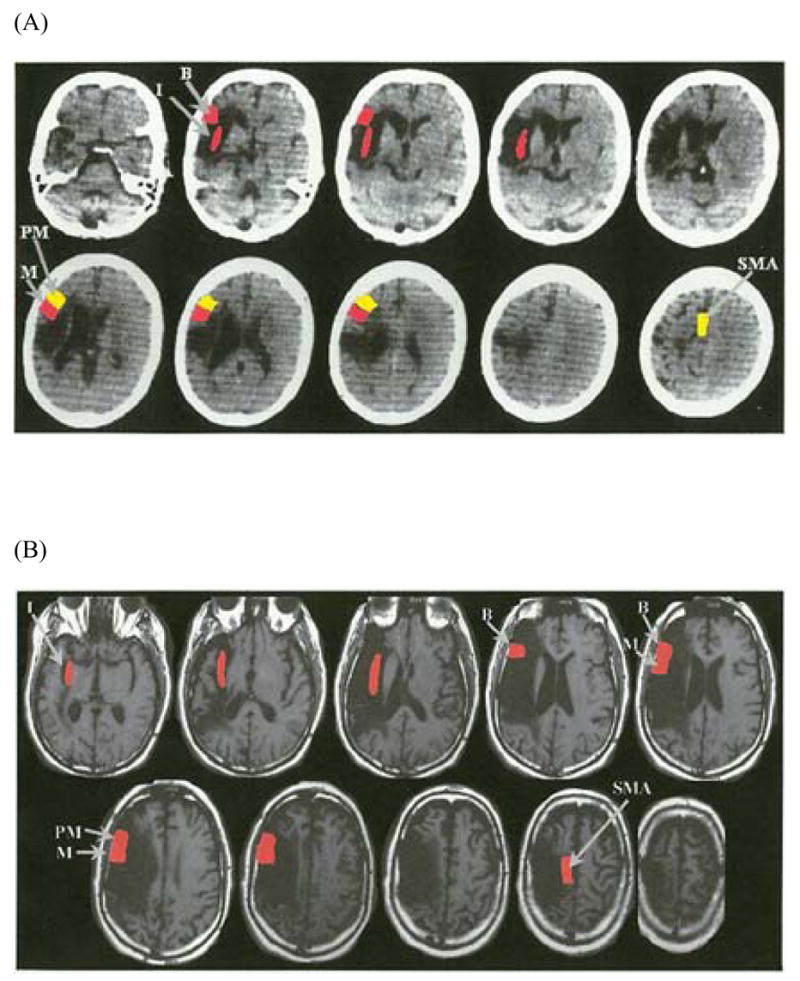
(a, b) CT Scans of subjects B2 and B5. The red and yellow colors reflect the extent of lesion present in the areas of interest, as listed in Table 2. The areas in red denote extensive lesion (i.e., there is lesion in more than half of the neuroanatomical area of interest). The areas in yellow denote areas of minimal lesion extent where the lesion is present in less than half of the area of interest or no lesion is present. Both scans are axial images with left hemisphere on the left side of the figure. Figure 6a is the CT scan of subject B2 who was the least impaired of the Broca’s aphasics. This scan (slice thickness = 7mm) was performed 10 years post onset. There is extensive lesion in Broca’s area (B), the insular region (I), and the lower motor cortex area for the face (M). Extent of lesion is minimal in the lower pre-motor cortex (PM) and there is sparing of the supplementary motor area (SMA). Figure 6b is the MRI scan of subject B5 who was among the most impaired of the Broca’s patients. This scan (slice thickness = 5mm) was performed 13 years post onset. All areas examined had extensive lesion including Broca’s area (B), the insular region (I), the lower motor cortex area for the face (M), the lower pre-motor cortex (PM), and the supplementary motor area (SMA).
Neuroanatomical Correlates
One of the goals of the current study was to relate the patterns of articulatory production as discussed in the previous section to their underlying neural substrates. To that end, we examined the patterns of production for each subject as shown in the bubble chart of Figure 5 in relation to the lesion extent data shown in Table 2. Specifically, we attempted to determine what lesion patterns gave rise to the near normal performance of B2 compared to the other Broca’s aphasics in that group. First, we compared B2 who was the least impaired of all of the Broca’s aphasics, as measured by the three acoustic parameters, to B5, one of the most impaired patients in the production measures. As shown in Table 2 and in the structural scans in Figure 6, B2 has extensive lesions in the insular cortex, Broca’s area, and the lower motor cortex for face. He has minimal lesion in the pre-motor cortex and the supplementary motor area (SMA). In contrast, B5 has extensive lesions in the insula, Broca’s area and the lower motor cortex for face. What distinguishes these two patients is the extent of lesion in the pre-motor cortex, motor upper face area, and SMA, with B5 showing more extensive damage in these areas. In fact, Table 2 shows that these areas of damage (pre-motor cortex, motor upper face area, and SMA) best distinguish B2 from the other Broca’s aphasics as well.
With respect to the Wernicke’s aphasics, the cortical areas that are implicated in their speech output problems, as manifested especially by increased variability in producing the articulatory parameters of nasal consonants, include the posterior SMG and superior parietal lobule and subcortical structures. It appears that there do not need to be lesions in Wernicke’s area to result in such speech output patterns.
Discussion
It was the goal of this study to explore the production of nasal consonants in Broca’s aphasic patients in order to assess the contribution of various articulatory parameters to the aphasics’ speech production deficit and to identify potential neural substrates of the deficit.
Deficits in Nasal Consonant Production
As discussed below, with the exception of B2, all other Broca’s aphasics showed deficits in parameters of murmur duration (timing) and the amplitude difference in the vicinity of the consonant release (articulatory coordination). A failure to control the amplitude energy around the point of consonantal release is consistent with a deficit in coordinating the release of the closure in the oral cavity with the closing of the velum. More specifically, the weaker amplitude displayed at the release could be the result of the velum being too open at the release of the murmur or closing too slowly at the release. In either case, the consequence is that there are energy losses through the nose, weakening the magnitude of amplitude change at the nasal release. Deficits in articulatory coordination are consistent with studies showing that Broca’s aphasics have impairments in the production of voice-onset time, i.e. the timing relation between the release of closure in stop consonants and the onset of vocal cord vibration.
All of the patients clinically diagnosed as Broca’s aphasics also showed impairments in the value of the maximum local amplitude difference. These patterns of impairment in the amplitude of the murmur are consistent with a deficit in laryngeal control. Similar conclusions have been made in analyses of voicing in fricative consonants (Kurowski et al., 2003) where Broca’s aphasics were unable to sustain normal amplitude levels of voicing during the frication noise, and in analyses of prosody and tone (Ryalls, 1982; Kent and Rosenbek, 1983; Cooper et al., 1984; Harmes et al., 1984; Packard, 1986; and Gandour, Potisuk et al., 1992), where Broca’s aphasics showed abnormal pitch modulation.
It is worth considering whether any of the speech output deficits identified in the current study reflect physiological changes with age that may affect the articulatory implementation of speech. Most measures of the patterns of speech production have used young normal subjects. The few studies that focused on the effects of normal aging on the production of nasal consonants have shown weak or no effects of age on the patterns of speech output (Hutchinson, Robinson, and Nerbonne, 1978; Hoit, Watson, Hixon, McMahon, and Johnson, 1994). Given the inconclusive evidence in the literature, the inclusion of four age-matched normal speakers in the present study provided an important baseline from which to consider the aphasics’ performance. As the results of the current study showed, the pathological patterns displayed by the aphasic patients differed from the old normal subjects and the young normal pilot subject, suggesting that the patterns of deficits shown for the aphasic patients reflect the underlying neuropathology and not the effects of age.
Neural Substrates of the Deficit
The lesion extent data provide some insights into the neural systems underlying the speech output deficits of Broca’s aphasics. Previous studies implicate anterior brain structures including Broca’s area, the pre-motor and motor regions for face and mouth areas, the white matter deep to these structures, the basal ganglia, and the insula (Hillis et al., 2004; Damasio, 1998; Cummings, 1993; Naeser et al., 1989). Moreover, Naeser et al. showed increased severity of speech deficit when two neuroanatomical areas were affected by lesion: (1) the most medial and rostral portion of the subcallosal fasciculus (deep to Broca’s area) and (2) the periventricular white matter near the body of the lateral ventricle deep to the lower motor/sensory cortex for mouth (affecting motor execution and feedback). All five of the Broca’s aphasics had lesion extension in these areas. However, although it is the case that all of these areas may be involved in speech output, the normal pattern of results for B2 who has extensive damage in both Broca’s area and the insular cortex, suggest that the extent of damage in either Broca’s area or the insular cortex, two areas previously identified as central to the articulatory implementation of speech, does not appear to be predictive of the severity of the speech output impairment. Instead, the extent of lesions in other frontal areas including the upper and lower motor face areas and the SMA as shown by all patients except B2, result in the most severe articulatory implementation impairments. These results are consistent with those of Baum et al. (1990) who showed the most severe impairments in the production of voice-onset time emerging when these same areas were damaged. They are also consistent with neuroimaging results with normal subjects showing increased activation of sensorimotor and motor cortex during speech articulation (Murphy, Corfield, Guz, Fink, Wise, Harrison, and Adams, 1997; Huang, Carr, and Cao, 2001; Blank, Scott, Murphy, Warburton, and Wise, 2002).
There is increasing evidence to suggest that Broca’s area does not play a significant role in the articulatory implementation of speech. This evidence comes not only from lesion data (cf. Bates, Wilson, Saygin, Dick, Sereno, Knight, and Dronkers, 2003), but also from functional neuroimaging studies (Wise, Greene, Buchel, and Scott, 1999; Huang et al., 2001; Murphy et al., 1997). It appears that the role of Broca’s area may relate more to accessing phonetic-phonological representations than to the processes involved in motor programming and in the implementation of the articulatory parameters of speech (cf. also Huang et al., 2001).
What is less clear is the role of the insula. Recent lesion studies (Dronkers, 1996; Bates et al., 2003; Dronkers, Ogar, Willock, and Wilkins, 2004) as well as functional neuroimaging studies (Wise et al., 1999; Ackermann and Riecker, 2004, Shuster and Lemieux, 2005) have identified the anterior insula as a critical area subserving articulatory planning or implementation of speech. However, Hillis et al. (2004) using diffusion-weighted and perfusion-weighted imaging found no association between apraxia of speech and the left insula but strongly implicated Broca’s area in apraxia of speech. The results of the current study do suggest that the insula plays an important role in the coordination of articulatory movements. However, it implicates other frontal areas as potentially more critical in determining the severity of the output disorder. Namely, the primary motor and supplementary motor areas appear to be crucially involved in the implementation of the articulatory parameters of speech.
The results of this research underscore the importance of examining patterns of deficits not simply by clinical diagnosis but also by examination of the location and extent of the lesions associated with the clinical diagnosis. Moreover, much as quantitative lesion analyses have provided greater insight into the neural systems underlying language, similarly quantitative analyses of the parameters of speech (either based on acoustic or physiological measures) will be required to map out the nature of speech production deficits in aphasia. The integration of this data with quantitative lesion analyses ultimately will allow for determining the functional anatomy of speech production. As the results of this study show, it is critical in such analyses to determine lesion extent rather than relying solely on identifying areas of lesion overlap. That is, the presence of a lesion in an area does not indicate the extent of the damage to an area, a factor which is essential in delineating the functional role a particular neural area may play (cf. Dronkers, 1996; for discussion see Hillis et al., 2004).
The results of the current study add to the increasing body of evidence suggesting that the neural mechanisms underlying the speech production system are not limited to the frontal areas, but rather involve a more distributed system including posterior areas as well. It is the case that all of the Broca’s aphasics had lesions that extended to posterior areas. One question is whether the severity of their speech output deficits is exacerbated by damage to these areas. The lesion analysis of the Wernicke’s aphasics suggests that this is not the case; instead, the contribution of posterior structures to speech output appears to be of a qualitatively different nature than that of the anterior structures. In particular, the most consistent pattern of disorder that emerged for the two Wernicke’s aphasics was increased variability in the production of each of the articulatory parameters. However, neither patient displayed an impairment in articulatory coordination. The cortical lesion that was common to both patients included the SMG and the superior parietal lobule.
The qualitatively different pattern of production of the Wernicke’s aphasics from that of the Broca’s suggests a different functional role for these posterior structures. The Wernicke’s aphasics showed impairments in the duration of the nasal murmur. W1 showed abnormal murmur durations, and, both W1 and W2 had larger standard deviations than normals in the duration of the murmur. Additionally, both Wernicke’s aphasics showed larger than normal local amplitude differences. Finally, the Wernicke’s aphasics showed significantly larger standard deviations than normals for the global difference amplitude measure. That Wernicke’s aphasics show greater variability has been shown in a number of studies exploring a range of acoustic parameters in consonant and vowel production (Baum et al., 1990; Ryalls, 1987). Additionally, they have shown deficits in a number of duration parameters of speech, consistent with the view that the posterior aphasics’ deficit resides in “more global, durational patterns of speech” (Baum et al., 1990, p. 54). Taken together, these results add to the body of literature suggesting that Wernicke’s aphasics have a subclinical phonetic impairment, subclinical in the sense that it is not perceptible to the examiner, but nonetheless is pathological as shown by acoustic analysis.
Recent neuroimaging results are consistent with the findings that posterior structures are part of a network that subserves speech production. In particular, in a PET study with normal subjects, Wise, Scott, Blank, Mummery, Murphy, and Warburton, (2001) showed activation in the posterior part of the left superior temporal gyrus during speech production. This activation occurred in the absence of auditory speech input, but rather was observed during overt articulation when subjects were retrieving words and then producing them. Wise et al. (2001) suggest that the medial left temporal-parietal junction serves as an interface between posterior temporal cortex and motor cortex for speech. Huang et al. (2001) showed a distributed area of activation in silent and overt speech production which included the SMG and superior parietal lobule as well as other posterior structures including the angular gyrus and the posterior superior temporal and middle temporal gyrus.
Of interest, a number of neuroimaging studies have shown SMG activation in the perception of speech (cf. Binder and Price, 2001 for a review). The results of these studies have been interpreted to suggest that the SMG and parietal lobe structures may be involved in the access of the phonological form of a word (Gelfand and Bookheimer, 2003; Celsis, Boulanouar, Doyon, Ranjeva, Berry, Nespoulous, and Chollet, 1999; Prabhakaran, Blumstein, Myers, Hutchison, and Britton, 2006). Assuming that there is a common lexical (phonological) representation for both production and perception, it is possible that these posterior structures are involved in the retrieval and encoding of lexical forms for speech production. Such a proposal is in keeping with recent hypotheses regarding the interface between auditory and motor speech systems (Hickok and Poeppel, 2004, 2000). Damage to these areas would presumably add ‘noise’ to the system, resulting in increased variability of a global nature. In contrast, the conversion of the phonetic structure of speech to the appropriate motor programs for speech output appears to occur in the frontal areas. Damage to these areas affects those articulatory parameters that require articulatory precision and fine motor control of the larynx and vocal tract structures.
Acknowledgments
This research was supported in part by NIH Grants NIDCD00314 to Brown University and NIDCD0081 to the Boston University School of Medicine. This material is the result of work supported with resources and the use of facilities at the Department of Veterans Affairs Medical Centers in Boston, MA and Providence, RI. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain and Language. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion –symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Baum S, Ryan L. Rate of speech effects in aphasia: Voice onset time. Brain and Language. 1993;44:431–445. doi: 10.1006/brln.1993.1026. [DOI] [PubMed] [Google Scholar]
- Baum S, Blumstein S, Naeser M, Palumbo C. Temporal dimensions of consonant and vowel production: An acoustic and CT scan analysis of aphasic speech. Brain and Language. 1990;39:33–56. doi: 10.1016/0093-934x(90)90003-y. [DOI] [PubMed] [Google Scholar]
- Binder J, Price CJ. Functional neuroimaging of language. In: Cabeza R, Kingstone A, editors. Handbook of Neuroimaging. Cambridge; MIT Press: 2001. [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Blumstein S, Cooper W, Goodglass H, Statlender S, Gottlieb J. Production deficits in aphasia: A voice-onset time analysis. Brain and Language. 1980;9:153–170. doi: 10.1016/0093-934x(80)90137-6. [DOI] [PubMed] [Google Scholar]
- Blumstein S, Cooper W, Zurif EB, Caramazza A. The perception and production of voice-onset time in aphasia. Neuropsychologia. 1977;15:371–383. doi: 10.1016/0028-3932(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet F. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. Neuroimage. 1999;9:135–144. doi: 10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cooper WE, Soares C, Nicol J, Michelow D, Goloskie S. Clausal intonation after unilateral brain damage. Language and Speech. 1984;27:17–24. doi: 10.1177/002383098402700102. [DOI] [PubMed] [Google Scholar]
- Cummings J. Frontal-subcortical circuits and human behavior. Neurological Review. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Damasio H. Neuroanatomical correlates of the aphasias. In: Sarno MT, editor. Acquired Aphasia. New York: Academic Press; 1998. pp. 43–70. [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;38:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Ogar J, Willock S, Wilkins DP. Confirming the role of the insula in coordinating complex but not simple articulatory movements. Brain and Language. 2004;91:23–24. [Google Scholar]
- Duffy J, Gawle C. Apraxic speakers’ vowel duration in consonant-vowel-consonant syllables. In: Rosenbek JC, McNeil MR, Aronson AE, editors. Apraxia of Speech. San Diego: College-Hill Press; 1984. [Google Scholar]
- Freeman FJ, Sands ES, Harris KS. Temporal coordination of phonation and articulation in a case of verbal apraxia: A voice-onset time analysis. Brain and Language. 1978;6:106–111. doi: 10.1016/0093-934x(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Gandour J, Dardarananda R. Voice-onset time in aphasia: Thai. II: Production. Brain and Language. 1984;18:389–410. doi: 10.1016/0093-934x(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Gandour J, Ponglorpisit S, Khunadorn F, Dechongkit S, Boongird P, Boonklam R. Timing characteristics of speech after brain damage: Vowel length in Thai. Brain and Language. 1992;42:337–345. doi: 10.1016/0093-934x(92)90105-n. [DOI] [PubMed] [Google Scholar]
- Gandour J, Ponglorpisit S, Khunadorn F, Dechongkit S, Boongird P, Boonklam R, Potisuk S. Lexical tones in Thai after unilateral brain damage. Brain and Language. 1992;43:275–307. doi: 10.1016/0093-934x(92)90131-w. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating Neural Mechanisms of Temporal Sequencing and Processing Phonemes. Neuron. 2003;38:831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Harmes SR, Daniloff R, Hoffman P, Lewis J, Kramer M, Absher R. Temporal and articulatory control of fricative articulation by speakers with Broca’s aphasia. Journal of Phonetics. 1984;12:367–385. [Google Scholar]
- Hickock G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickock G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hoit J, Watson P, Hixon K, McMahon P, Johnson C. Age and velopharyngeal function during speech production. Journal of Speech and Hearing Research. 1994;37:295–302. doi: 10.1044/jshr.3702.295. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech use using event-related fMRI. Human Brain Mapping. 2001;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J, Robinson K, Nerbonne M. Patterns of nasalance in a sample of normal gerontologic subjects. Journal of Communication Disorders. 1978;11:469–481. doi: 10.1016/0021-9924(78)90021-7. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasanuma S, Hirose H, Yoshioka H, Ushijima T. Abnormal articulatory dynamics of a patient with apraxia of speech. Brain and Language. 1980;11:66–75. doi: 10.1016/0093-934x(80)90110-8. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasanuma S, Ushijima T. Velar movements during speech of a patient with apraxia of speech. Brain and Language. 1979;7:227–239. doi: 10.1016/0093-934x(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Katz W, Machetanz J, Orth U, Schonle P. A kinematic analysis of anticipatory coarticulation in the speech of anterior aphasic subjects using electromagnetic articulography. Brain and Language. 1990;38:555–575. doi: 10.1016/0093-934x(90)90137-6. [DOI] [PubMed] [Google Scholar]
- Kent R, Rosenbek J. Acoustic patterns of apraxia of speech. Journal of Speech and Hearing Research. 1983;26:231–249. doi: 10.1044/jshr.2602.231. [DOI] [PubMed] [Google Scholar]
- Kurowski K, Hazen E, Blumstein S. The nature of speech production impairments in anterior aphasics: An acoustic analysis of voicing in fricative consonants. Brain and Language. 2003;84:353–371. doi: 10.1016/s0093-934x(02)00555-2. [DOI] [PubMed] [Google Scholar]
- Lisker L, Abramson A. A cross-language study of voicing in initial stops: Acoustical measurements. Word. 1964;20:384–422. [Google Scholar]
- Mertus JA. BLISS: The Brown Lab Interactive Speech System. Brown University; Providence, RI: 2002. [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJS, Harrison J, Adams L. Cerebral areas associated with motor control of speech in humans. Journal of Applied Physiology. 1997;83:1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Naeser M, Palumbo C, Helm-Estabrooks N, Stiassny-Eder D, Albert M. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Packard J. Tone production deficits in nonfluent aphasic Chinese speech. Brain and Language. 1986;29:212–223. doi: 10.1016/0093-934x(86)90045-3. [DOI] [PubMed] [Google Scholar]
- Palumbo C. PhD Dissertation, Department of Behavioral Neuroscience, Boston University School of Medicine and Graduate School. 1999. Predicting outcome in aphasia with CT scans performed before two months poststroke versus two- to six-months poststroke. [Google Scholar]
- Prabhakaran R, Blumstein SE, Myers EB, Hutchison E, Britton B. An event-related fMRI investigation of phonological-lexical competition. Neuropsychologia. 2006;44:2209–2221. doi: 10.1016/j.neuropsychologia.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Ryalls J. Intonation in Broca’s aphasia. Neuropsychologia. 1982;20:355–360. doi: 10.1016/0028-3932(82)90110-5. [DOI] [PubMed] [Google Scholar]
- Ryalls J. Vowel production in aphasia: Towards an account of the consonant-vowel dissociation. In: Ryalls J, editor. Phonetic approaches to speech production in aphasia and related disorders. Boston: College-Hill Press; 1987. pp. 23–43. [Google Scholar]
- Shewan CM, Leeper H, Booth J. An analysis of voice-onset time (VOT) in aphasia and normal subjects. In: Rosenbek J, McNeil M, Aronson A, editors. Apraxia of speech. San Diego: College-Hill Press; 1984. pp. 197–220. [Google Scholar]
- Shinn P, Blumstein S. Phonetic disintegration in aphasia: Acoustic analysis of spectral characteristics for place of articulation. Brain and Language. 1983;20:90–114. doi: 10.1016/0093-934x(83)90035-4. [DOI] [PubMed] [Google Scholar]
- Shuster L, Lemieux S. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain and Language. 2005;93:20–31. doi: 10.1016/j.bandl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Tuller B. On categorizing aphasic speech errors. Neuropsychologia. 1984;22:547–557. doi: 10.1016/0028-3932(84)90019-8. [DOI] [PubMed] [Google Scholar]
- Vijayan A, Gandour J. On the notion of a ‘subtle phonetic deficit in fluent/posterior aphasia. Brain and Language. 1995;48:106–119. doi: 10.1006/brln.1995.1004. [DOI] [PubMed] [Google Scholar]
- Watson B. Fundamental frequency during phonetically governed devoicing in normal young and aged speakers. Journal of the Acoustical Society of America. 1998;103:3642–3647. doi: 10.1121/1.423068. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. The Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank C, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within “Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Ziegler W, von Cramon D. Anticipatory coarticulation in a patient with apraxia of speech. Brain and Language. 1985;26:117–130. doi: 10.1016/0093-934x(85)90032-x. [DOI] [PubMed] [Google Scholar]


