Abstract
The possible antihyperalgesic and antiallodynic activity of loperamide, an opioid agonist which does not readily penetrate the blood-brain barrier, were examined in the spinal nerve ligation model of experimental neuropathic pain. Intraperitoneal (i.p.) injection of loperamide effectively reversed thermal hyperalgesia. In contrast, loperamide had minimal effects on cold allodynia and no effects on mechanical allodynia. The antihyperalgesic action of loperamide against noxious heat was antagonized by naltrindole, a δ-opioid receptor selective antagonist, but not by pretreatment with β-funaltrexamine, a μ-opioid receptor selective antagonist, or administration of nor-binaltorphimine, a κ-opioid receptor selective antagonist. Furthermore, i.p. injection of [D-Ala2, Glu4]-deltorphin II, a δ-opioid receptor selective peptide agonist, also reversed thermal hyperalgesia. The present results suggest that thermal hyperalgesia in experimental neuropathic pain can be reduced through activation of peripheral δ-opioid receptors. The data suggest the possible application of peripherally restricted and δ-opioid receptor selective agonists in the treatment of some aspects of neuropathic pain without many of the side effects associated with centrally acting opioids and without the peripheral side effects of opioid agonists acting at μ-receptors.
Keywords: loperamide, neuropathic pain, peripheral δ-opioid receptors, thermal hyperalgesia
Introduction
Opioids are the most powerful drugs for treatment severe acute and chronic pain, but their use is limited by undesired side effects such as sedation, respiratory depression, or drug abuse [1]. To date, three opioid receptors (μ-, δ-, and κ-opioid receptor) have been cloned. All three receptors are expressed throughout the central nervous system (CNS) and the peripheral nervous system (PNS) [2]. Activation of opioid receptors in CNS results in potent analgesia in part via inhibition of ascending excitatory nociceptive transmissions and activation of descending inhibitory systems [3]. These beneficial actions are often limited, however, by a lack of separation between doses required to produce analgesia through activation of opioid receptors in CNS and doses which produce severe side effects. Activation of opioid receptors in PNS has also been suggested to produce antinociception in various pain models [3, 4], though this may require conditions of inflammatory injury [5].
Experimental evidence suggests that peripherally selective opioids, may have therapeutic potential as analgesics and antihyperalgesics without the side effects associated with activation of opioid receptors in CNS [6]. Additionally, while it is known that activation of μ-opioid receptors in PNS can result in significant gastrointestinal side effects, opioids which do not act preferentially through μ-receptors are not associated with inhibition of gastrointestinal motility or alteration of fluid balance which can lead to constipation [7, 8].
Damage to nervous system sometimes causes a chronic pain state referred to as neuropathic pain which is characterized most often by spontaneous burning pain and sometimes by allodynia (pain evoked by nonpainful stimuli), and hyperalgesia (an increased response to painful stimuli) [9]. Presently available therapeutic approaches are not sufficient for treatment of these symptoms due to the side-effects associated with currently available therapies [10]. Although it has been suggested that neuropathic pain may be attenuated by opioids at higher doses than those effective in acute pain [11], this is accompanied by aggravation of central side effects and studies show that even neuropathic pain patients that acknowledge medical benefit with opioids drop out of therapy within months due to such side effects [12]. Although recent studies have demonstrated local administration of μ-opioid agonists reduced mechanical allodynia in models of rat neuropathic pain [13-16], possible beneficial effects of systemically administered opioids which are peripherally restricted have not been well studied.
Recently, loperamide, an opioid agonist which does not readily cross the blood-brain barrier and is antagonized by peripherally restricted opiate antgonists [17], has been shown to produce analgesia in various models of pathological pain [17-20]. However, its effects have not been investigated in a model of neuropathic pain. In this study, we investigated potential beneficial actions of loperamide in a model of rat neuropathic pain. Our study assessed if the systemic administration of loperamide could elicit antihyperalgesia and antiallodynia through activation of opioid receptors in PNS. Additionally, we sought to characterize the type of opioid receptor involved in the antihyperalgesic actions of loperamide.
Material and methods
All experiments were performed according to the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for handling and use of laboratory animals and received approval from the Institutional Care and Animal Use Committee at the University of Arizona. Male Sprague-Dawley rats, weighing 200-350 g, were used in all experiments. The spinal nerve ligation injury was performed under isoflurane anesthesia according to the method described by Fukuoka et al [21]. The skin was incised over the caudal lumber region and muscles were retracted. The L5 spinal nerve was isolated and tightly ligated with 4-0 silk thread. In sham-operated rats, the L5 spinal nerve was isolated without ligation.
Behavioral testing was performed on 13-15 days after surgery. Thermal hyperalgesia was assessed using the plantar test (Ugo Basile, Varese, Italy) [22]. The rats were placed in Plexiglas enclosures on a clear glass plate. With the rat standing relatively still, a radiant heat source beneath the glass floor was aimed at the plantar surface of the hind paw, and the withdrawal latency was measured. Before assessment of thermal hyperalgesia, the intensity of the radiant heat was adjusted to yield a baseline latency of ∼20 seconds from naïve rats with the cutoff of automatically set at 30 seconds to avoid tissue damage. Cold allodynia was estimated by measuring brisk foot withdrawal in response to acetone application [23]. Approximately 30 μL of acetone was applied 5 times to the hind paw. The frequency of foot withdrawal was expressed as a percent. Mechanical allodynia was determined by measuring the paw withdrawal threshold to probing with von Frey filaments [24]. Calibrated filaments were applied to the plantar surface of the hind paw of a rat through wire-mesh floor. The paw withdrawal threshold was determined by applying Dixon's nonparametric test [25].
All reagents were dissolved with 10% DMSO in distilled water. Loperamide was injected intraperitoneally (i.p.) 60 min before test. Naltrindole was injected subcutaneously (s.c.) at a dose of 2 mg/kg, 30 min before loperamide. β-Funaltrexamine (β-FNA) and nor-binaltorphimine (nor-BNI) were injected (s.c.) 24 hours before loperamide at doses of 20 mg/kg and 3 mg/kg, respectively. These doses and time-courses for antagonists, especially for β-FNA and nor-BNI were chosen based on previous reports from our laboratory [26,27]. [D-Ala2, Glu4]-deltorphin II was injected (i.p.) at a dose of 6 mg/kg, 15 min before testing.
Results
Spinal nerve injury resulted in appearance of symptoms of neuropathic pain (thermal hyperalgesia, cold allodynia, and mechanical allodynia, Fig 1A∼1C). I.p. injection of loperamide reduced thermal hyperalgesia, and at a dose of 10 mg/kg, the withdrawal latency was longer than that in sham rats suggesting the presence of antinociceptive activity (Fig 1A). In sham rats, 3 mg/kg of loperamide did not change withdrawal latency. On the other hand, antiallodynic effects were not observed in cold allodynia or mechanical allodynia (Fig 1B and 1C). In order to characterize which subtypes of opioid receptor are involved in loperamide-induced anti-thermal hyperalgesia, subtype selective antagonists were examined. S.c. injection of naltrindole (1 mg/kg), a δ-opioid receptor selective antagonist, inhibited the antihyperalgesic effects of loperamide, while s.c. injection of β-FNA (20 mg/kg), a μ-opioid receptor selective antagonist, or nor-BNI (3 mg/kg), a κ-opioid receptor selective antagonist did not show any effects (Fig. 2). Naltrindole, β-FNA, or nor-BNI had no effect when given alone on the withdrawal latencies (data not shown). For further investigation of functions of peripheral δ-opioid receptors, the antihyperalgesic effects of i.p. injection of [D-Ala2, Glu4]-deltorphin II (6 mg/kg), a δ-opioid receptor selective peptide agonist, was tested. Fifteen min after injection, [D-Ala2, Glu4]-deltorphin II reversed thermal hyperalgesia in nerve injury rats (Fig. 3).
Fig. 1.
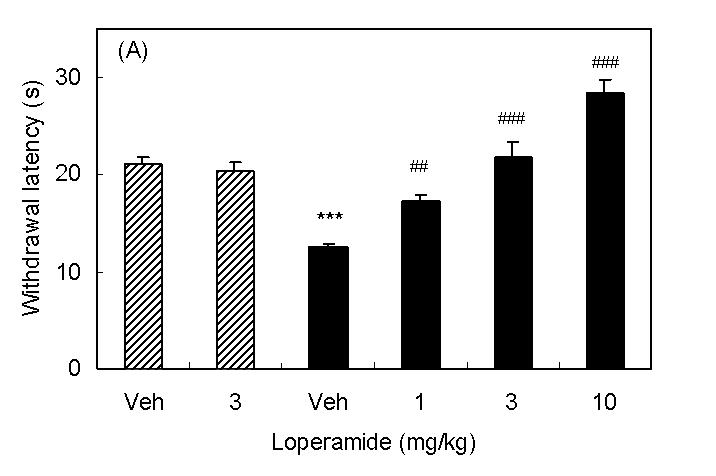
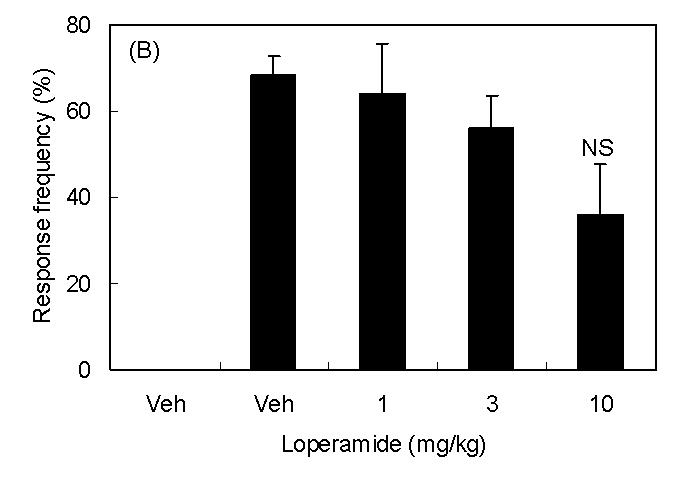
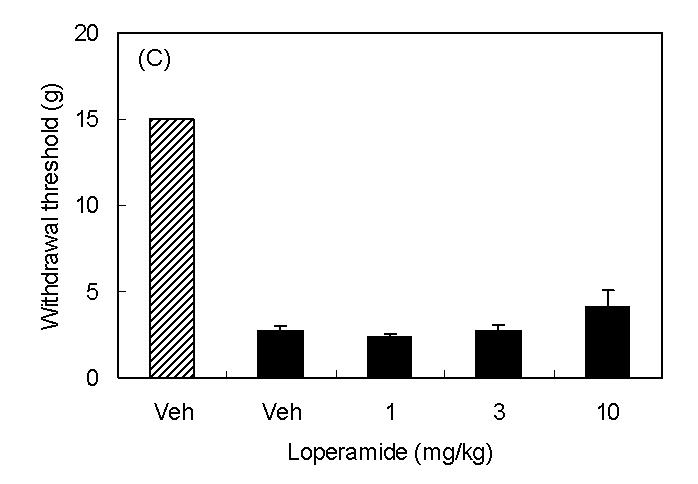
Antihyperalgesic effects of i.p. injection of loperamide. Results are means with S.E.M. of n=5-6 experiments/group. (A) Paw withdrawal latencies to noxious thermal stimulation to sham rats (hatched bar) and nerve injured rats (black bar). ***P<0.001 vs. sham, Student's t-test, ##P<0.01 vs. vehicle, one-way ANOVA Dunnett test, ###P<0.001 vs. vehicle, one-way ANOVA Dunnett test. (B) Response frequencies to innoxious cold stimulation. NS, not statistically different vs. vehicle, Student's t-test. (C) Paw withdrawal thresholds to innoxious mechanical stimulation.
Fig. 2.
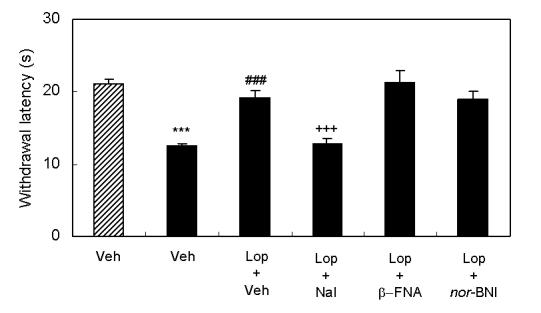
Effects of s.c. injection of subtype selective opioid antagonists on the antihyperalgesic effects induced by i.p. injection of loperamide. Results are means with S.E.M. of n=6-7 experiments/group. Paw withdrawal latencies to noxious thermal stimulation to sham rats (hatched bar) and nerve injured rats (black bar). ***P<0.001 vs. sham, Student's t-test, ###P<0.001 vs. vehicle, Student's t-test, +++P<0.001 vs. loperamide, Student's t-test.
Fig. 3.
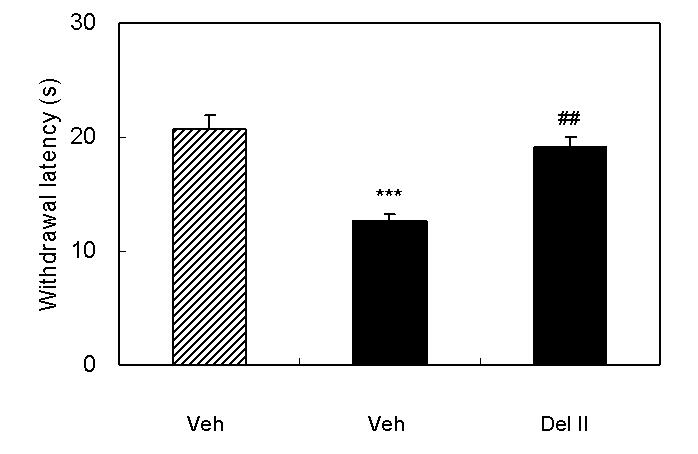
Antihyperalgesic effects of i.p. injection of [D-Ala2, Glu4]-deltorphin II. Results are means with S.E.M. of n=6-7 experiments/group. Paw withdrawal latencies to noxious thermal stimulation to sham rats (hatched bar) and nerve injured rats (black bar). ***P<0.001 vs. sham, Student's t-test, ##P<0.01 vs. vehicle, Student's t-test.
Discussion
The present study demonstrates that systemically administration of loperamide reversed thermal hyperalgesia in a model of rat neuropathic pain. Loperamide-induced anti-thermal hyperalgesia was inhibited by pretreatment of naltrindole, a δ-opioid receptor selective antagonist, while pretreatment of either β-FNA, a μ-opioid receptor selective antagonist, or nor-BNI, a κ-opioid receptor selective antagonist, did not change effects of loperamide. These doses and time courses of β-FNA and nor-BNI have been previously demonstrated to block the activation of μ- and κ-opioid receptors, respectively [26,27]. Additionally, the dose and time course of naltrindole has previously been shown to be ineffective in blocking the analgesic effects of morphine [28]. These results indicate that thermal hyperalgesia in neuropathic pain could be reduced through activation of peripheral δ-opioid receptors. Loperamide shows a high affinity to μ-opioid receptors (Ki = 3.3 nmol/L), but it also shows high affinity to δ-opioid receptors (Ki = 48 nmol/L) at recombinant human receptors [17]. Furthermore, systemically administered [D-Ala2, Glu4]-deltorphin II, a δ-opioid receptor selective peptide agonist, also reduced thermal hyperalgesia in neuropathic rats. Therefore, the data suggests that the antihyperalgesic effects of loperamide in neuropathic rats are mediated through activation of peripheral δ-opioid receptors. It is noteworthy that loperamide failed to produce antinociceptive effects in sham rats at a dose of 3 mg/kg. At this dose, loperamide reversed thermal sensitivity of neuropathic rats to normal levels. This result is generally consistent with the view that analgesic effects of opioids on acute pain are primarily mediated through receptors located in the brain and spinal cord [18]. Higher doses of loperamide (10 mg/kg, i.p.) produced significant antinociception in neuropathic rats suggesting penetration to the CNS at this dose. Although the reason why loperamide was effective only in neuropathic rats is not known, it is possible that expression of δ-opioid receptors in PNS may be altered after nerve injury. Recent reports showed that inflammatory stimulation, capsaicin treatment, and morphine treatment increased cell surface δ-opioid receptors in the dorsal root ganglia neurons or primary culture of sensory neurons [5, 29]. Although δ-opioid receptors in the spinal cord were shown to decrease after nerve injury [30,31], it is possible that peripheral δ-opioid receptors may increase after nerve injury.
In our tests, the effects of loperamide were more potent in thermal hyperalgesia than those in cold allodynia or mechanical allodynia. Previously, particular analgesic drugs were reported to show a preferential ability to inhibit one of these symptoms. Since the peripheral nociceptors involved in the transmission of thermal, cold, or mechanical stimuli are different, it seems that their sensitization could be differently modulated [32]. Thus, our results seem to suggest that δ-opioid receptors may be expressed in afferent neurons which are involved in transmission of thermal stimuli in nerve injured rats.
In summary, the present study demonstrates that systemically administered loperamide can reverse thermal hyperalgesia induced by nerve injury, through activation of peripheral δ-opioid receptors which could be upregulated during development this pathology. Although further studies are necessary to reveal the mechanism through which peripheral δ-opioids show antihyperalgesic effects, they seem to have therapeutic potential for treatment for some aspects of neuropathic pain (e.g., burning pain) without central side effects. Additionally, the peripheral activation of δ-opioid receptors is not known to alter gastrointestinal function, suggesting added benefit for the treatment of such pain states.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 2.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- 3.Yaksh TL. Spinal systems and pain processing: development of novel analgesic drugs with mechanically defined models. Trends Pharmacol Sci. 1999;20:329–337. doi: 10.1016/s0165-6147(99)01370-x. [DOI] [PubMed] [Google Scholar]
- 4.Stein C, Hassan AHS, Lehrberger K, Stein C, Giefing J, Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342:321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- 5.Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the δ-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower V. New paths to pain relief. Nat Biotechnol. 2000;18:387–391. doi: 10.1038/74438. [DOI] [PubMed] [Google Scholar]
- 7.Shook JE, Pelton JT, Hruby VJ, Burks TF. Peptide opioid antagonist separates peripheral and central opioid antitransit effects. J Pharmacol Exp Ther. 1987;243:492–500. [PubMed] [Google Scholar]
- 8.Broccardo M, Improta G, Tabacco A. Central effect of SNC 80, a selective and systemically active δ-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur J Pharmacol. 1998;342:247–251. doi: 10.1016/s0014-2999(97)01470-2. [DOI] [PubMed] [Google Scholar]
- 9.Ossipov MH, Porreca F. Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx. 2005;2:650–661. doi: 10.1602/neurorx.2.4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. Eur J Pharmacol. 2001;429:1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- 11.Gordon DB, Love G. Pharmacologic management of neuropathic pain. Pain Manag Nurs. 2004;5(Suppl 1):19–33. doi: 10.1016/j.pmn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Dellemijn PLI, van Duijn H, Vanneste JAL. Prolonged transdermal fentanyl in neuropathic pain. J Pain Symptom Manage. 1998;16:220–229. doi: 10.1016/s0885-3924(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 13.Pertovaara A, Wei H. Peripheral effects of morphine in neuropathic rats: role of sympathetic postganglionic nerve fibers. Eur J Pharmacol. 2001;429:139–145. doi: 10.1016/s0014-2999(01)01315-2. [DOI] [PubMed] [Google Scholar]
- 14.Martinez V, Christensen D, Kayser V. The glycine/NMDA receptor antagonist (+)-HA966 enhances the peripheral effect of morphine in neuropathic rats. Pain. 2002;99:537–545. doi: 10.1016/S0304-3959(02)00270-1. [DOI] [PubMed] [Google Scholar]
- 15.Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. μ Opioid receptors and the site of a peripheral nerve injury. Ann Neurol. 2003;53:366–375. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- 16.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of μ-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: Interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525:83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Dehaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Ther Exp. 1999;289:494–502. [PubMed] [Google Scholar]
- 19.Menendez L, Lastra A, Hidalgo A, Meana A, Garcia E, Baamonde ACA. Peripheral opioids act as analgesics in bone cancer pain in mice. Neuroreport. 2003;14:867–869. doi: 10.1097/00001756-200305060-00018. [DOI] [PubMed] [Google Scholar]
- 20.Menendez L, Lastra A, Meana A, Hidalgo A, Baamonde A. Analgesic effects of loperamide in bone cancer pain in mice. Pharmacol Biochem Behav. 2005;81:114–121. doi: 10.1016/j.pbb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 24.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 25.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 26.Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F. Opioid antinociception in a rat model of visceral pain: systemic administration, versus local drug administration. J Pharmacol Exp Ther. 1995;275:1535–1542. [PubMed] [Google Scholar]
- 27.Horan R, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 28.Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 29.Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic δ-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson B, Schulte G, Elde R, Grant G. Effects of sciatic nerve injuries on δ-opioid receptor and substance P immunoreactivities in the superficial dorsal horn of the rat. Eur J Pain. 1999;3:115–129. doi: 10.1053/eujp.1998.0104. [DOI] [PubMed] [Google Scholar]
- 31.Stone LS, Vulchanova L, Riedl MS, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on delta opioid receptor (DOR) immunoreactivity in the rat spinal cord. Neurosci Lett. 2004;361:208–211. doi: 10.1016/j.neulet.2003.12.067. [DOI] [PubMed] [Google Scholar]
- 32.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–209. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]


