Abstract
Sleep architecture as well as memory function are strongly age dependent. Slow wave sleep (SWS), in particular, decreases dramatically with increasing age, starting already beyond the age of 30. SWS normally predominates during early nocturnal sleep and is implicated in declarative memory consolidation. However, the consequences of changes in sleep across the life span for sleep-associated memory consolidation have not been evaluated so far. Here, we compared declarative memory consolidation (for word-pair associates) during sleep in young and middle-aged healthy humans. The age groups (18–25 vs. 48–55 yr) did not differ with regard to learning performance before retention periods that covered, respectively, the first and second half of nocturnal sleep. However, after early retention sleep, where the younger subjects showed distinctly more SWS than the middle-aged (62.3 ± 3.7 min vs. 18.4 ± 7.2 min, P < 0.001), retrieval of the word pairs in the middle-aged was clearly worse than in the young (P < 0.001). In contrast, declarative memory retention did not differ between groups after late sleep, where retention was generally worse than after early sleep (P = 0.005). Retention of declarative memories was the same in both age groups when sleep periods containing equal amounts of SWS were compared, i.e., across late sleep in the young and across early sleep in the middle-aged. Our results indicate a decline in sleep-associated declarative memory consolidation that develops already during midlife and is associated with a decrease in early nocturnal SWS.
Many studies have demonstrated the importance of sleep for memory consolidation (Smith 2001; Maquet et al. 2003; Stickgold 2005; Born et al. 2006; Ellenbogen et al. 2006b). Sleep after a learning period enhances both declarative memories (i.e., for episodes and facts) and procedural memories for skills (Fischer et al. 2002; Walker et al. 2002; Ellenbogen et al. 2006a; Gais et al. 2006). Notably, both types of memory appear to benefit from a differing extent from the different sleep stages. Based on the fact that in humans slow wave sleep (SWS) mainly occurs during early nocturnal sleep and rapid eye movement (REM) sleep, mainly during late sleep, several studies have revealed marked differences in memory consolidation by comparing retention performance across periods of early and late night-time sleep (Yaroush et al. 1971; Plihal and Born 1997). Using this approach, it was found that early sleep with high amounts of SWS supports consolidation in paired associate tasks, a typical form of declarative memory, whereas the late part of sleep with predominant REM sleep seems particularly effective in consolidating procedural memory for skills (Plihal and Born 1997). Up until now, evidence has also been converging from studies using other paradigms to support the notion that declarative memory consolidation profits from SWS and lighter stages of non-REM sleep in particular (Peigneux et al. 2004), whereas REM sleep seems to be more critical for the consolidation of procedural and emotional memories (Plihal and Born 1997; Wagner et al. 2001; Fischer et al. 2002), although SWS can also contribute to the consolidation of such memories (Ficca et al. 2000; Gais et al. 2000; Stickgold et al. 2000; Ambrosini and Giuditta 2001). A recent study shows that the experimental bilateral fronto-temporal induction of slow oscillation-like potential fields during early non-REM sleep facilitates SWS and increases the retention of word pairs in a hippocampus-dependent declarative memory task (Marshall et al. 2006). Daytime naps with low amounts of SWS did not affect declarative memory consolidation, but significantly improved procedural memory (Backhaus and Junghanns 2006).
An intriguing approach to assessing the function of sleep stages for declarative memory formation is offered by examining specific sleep disturbances occurring under pathological conditions or during the course of aging. Thus, patients with primary insomnia exhibit a specific and prominent reduction in SWS, and, in comparison to age-matched controls, these patients indeed displayed a markedly impaired consolidation of declarative memory during sleep (Backhaus et al. 2006). Although there has been a strong focus in recent research on the memory function of sleep, changes in sleep architecture across the life span have been neglected in this context. Beyond the age of 30, the amount of SWS is already in rapid decline with lighter non-REM sleep stages increasing, whereas REM sleep is affected less obviously during this middle-age period (Van Cauter et al. 2000). At the same time memory functions, and declarative memory, in particular, declines (Prull et al. 2000). This age-associated decline of declarative memory performance is accompanied by structural and functional changes in the hippocampus, prefrontal cortex, and frontal white matter (Daselaar et al. 2003; Driscoll et al. 2003; Tisserand and Jolles 2003; Hedden and Gabrieli 2004; Hof and Morrison 2004). If declarative memory consolidation depends on SWS, both should be affected in parallel by degenerative processes of aging with a concomitant decline across the life span. Here, we expected an age-dependent decline of memory consolidation, especially during retention periods covering early nocturnal sleep, but less so during late sleep, where the amount of SWS is low even in young people. In order to test these hypotheses, we compared retention of declarative memories (word pair associates) after periods of early and late nocturnal sleep in two distinct age groups of healthy volunteers and related memory performance to parameters of sleep architecture. A list of 40 word pairs was learned to a criterion of 60% correct responses (with cued recall) immediately before the 3.5-h sleep intervals, and cued recall was tested again 15 min after awakening. Verbal intelligence was measured and performance on the digit span task was examined at learning and recall testing to control for changes in general cognitive function that might have confounded memory performance.
Results
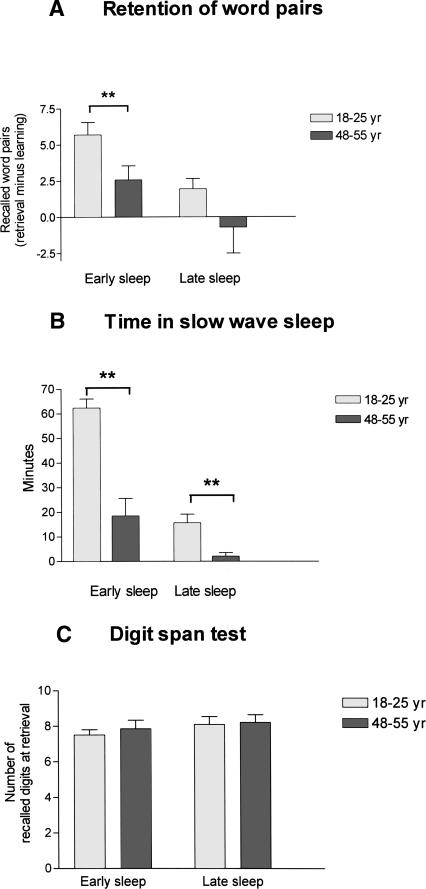
The age groups did not differ with regard to education (young: 12.9 ± 0.3 yr of school education, middle-aged 13.3 ± 0.4, t = −0.76, P = 0.45) and subjective sleep quality (Pittsburgh Sleep Quality Index; young: 3.1 ± 0.4; middle-aged: 3.0 ± 0.5, t = −0.2, P = 0.8). After early sleep, the younger subjects retained a significantly greater number of previously learned word pairs than the middle-aged subjects (Table 1; Fig. 1A). This difference was confirmed in an ANOVA that revealed a significant before/after sleep × age group interaction effect (F(1,28) = 10.3, P = 0.003). The groups showed comparable performance on the last trial at learning before the early retention sleep interval (Table 1), although the middle-aged subjects needed more trials to criterion than the young subjects (young: 1.3 ± 0.1; middle-aged: 2.5 ± 0.3, t = −2.9, P = 0.01). After early retention sleep, the groups differed in absolute recall (Table 1) and in their retention rate (difference between post-sleep retrieval and learning performance before sleep; Fig. 1A). Regression analysis on the retention rate for word pairs during early sleep as dependent variable and time in SWS, REM sleep, and age as independent variables revealed a highly significant effect for time in SWS (β = 0.51, P = 0.004; Fig. 2), but not for time in REM sleep (β = 0.19, P = 0.24) or age (β = 0.28, P = 0.22). Age and retention of word pairs did not correlate in a partial correlation analysis that controlled for the amount of SWS (r = −0.224, P = 0.122).
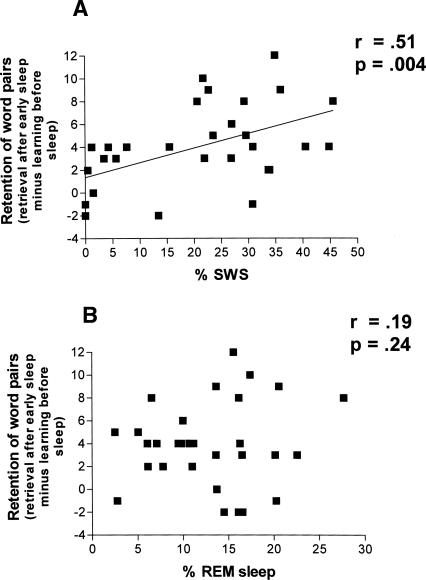
Table 1.
Number of words correctly recalled on the declarative word pair associate learning task at learning before sleep (immediate recall) and at retrieval testing after sleep
t-test pairs early/late young: learning: t = −0.505, P = 0.62; retrieval: t = 2.18, P = 0.04.
t-test pairs early/late middle-aged: learning: t = −0.455, P = 0.65; retrieval: t = 1.36 P = 0.19.
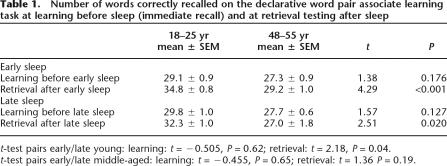
Figure 1.
Retention of word pairs (A), time in slow wave sleep (B), and performance in the digit span test (C) during the early and late sleep condition in groups of young and middle-aged subjects. In the early sleep condition, where the young subjects had more than a threefold higher amount of slow wave sleep compared with the middle-aged subjects, retention of word pairs was significantly better in the young than it was in the middle-aged subjects. With comparable amounts of slow wave sleep (late-sleep condition in the young subjects and early-sleep condition in the middle-aged subjects), groups did not differ in their retention of word pairs. Since the age groups performed equally well on the digit span test, differences in retrieval of declarative memory were not due to differences in working memory. P < 0.005.
Figure 2.
Retention of word pairs after early sleep plotted against the percentages of SWS (A) and REM sleep (B).
Learning performance at the last trial before the late-sleep condition was also comparable in both age groups (Table 1). The young subjects needed 1.5 ± 0.6 trials to reach the criterion, the middle-aged 2.0 ± 0.3 (t = −1.3, P = 0.187).
Retrieval after sleep was better in the young than in the middle-aged subjects, but in contrast to the early sleep condition, groups did not differ significantly with regard to retention of word pairs in the late sleep condition (F(1,28) = 2.7, P = 0.11 for before/after × age interaction and F(1,28) = 0.8, P = 0.36 for main effect before/after). Furthermore, retention was generally worse in the late compared with the early sleep condition (F(1,28) = 9.2, P = 0.005 for the interaction effect of early/late × before/after).
Differences in declarative memory retention were not due to differences in working memory between the age groups at learning or retrieval. Performance in the digit span test on the four testing occasions was closely comparable in the younger and middle-aged subjects (F(1,28) = 0.01, P = 0.91 for main effect of age; F(3,26) = 0.95, P = 0.43 for time × age interaction; means ± SEM at learning before early sleep—young: 7.7 ± 0.3, middle-aged: 7.2 ± 0.4, t = 0.84, P = 0.4; performance at learning before late sleep—young: 7.6 ± 0.4, middle-aged: 7.8 ± 0.4, t = −0.35, P = 0.7; for respective values at retrieval testing, refer to Fig. 1C). Age groups also did not differ in performance on all subtests of verbal intelligence from the Intelligence-Structure-Test (I-S-T 2000R.: “sentence completion”—young: 13.5 ± 0.6, middle-aged: 14.0 ± 0.9, t = −0.38, P = 0.71; “analogies”: 11.3 ± 0.7 vs. 10.0 ± 1.2, t = 0.91, P = 0.38; “similarities”: 11.8 ± 0.6 vs. 10.8 ± 1.1, t = 0.81, P = 0.42) or in total verbal IQ (young: 101.2 ± 1.4, middle-aged: 106.2 ± 2.7, t = −1.63, P = 0.13). Furthermore, groups did not differ in immediate and delayed recall of a word-list task and in a word fluency task, which they performed during a separate session before the experiment proper (first immediate recall of a word-list in number of recalled words: young: 7.3 ± 0.2, middle-aged: 6.7 ± 0.2, t = 1.68, P = 0.13; second immediate recall: young: 8.6 ± 0.2, middle-aged: 8.3 ± 0.2, t = 0.81, P = 0.42; delayed recall: young: 8.1 ± 0.2, middle-aged: 7.7 ± 0.5, t = 0.77, P = 0.45; word fluency in number of words: young: 24.8 ± 1.4, middle-aged: 27.5 ± 1.0, t = −1.47, P = 0.15).
Age groups did not differ in tiredness at the time of memory encoding in the early and late sleep condition (Stanford Sleepiness Scale, F(1,28) = 0.98, P = 0.757 for the early/late main effect and F(1,28) = 0.98, P = 0.757 for early/late × age interaction. Early sleep condition: young: 3.1 ± 0.1, middle-aged: 3.7 ± 0.5, t = −1.12, P = 0.27; late sleep condition: young: 3.1 ± 0.2, middle-aged: 3.6 ± 0.3, t = −1.07, P = 0.29).
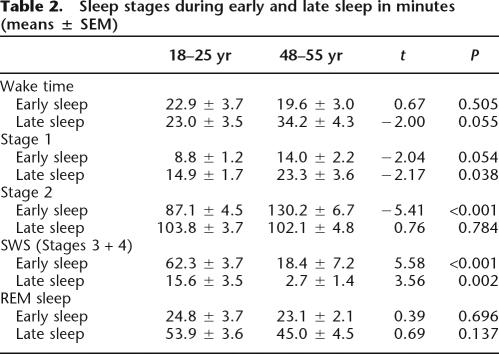
Time in bed was the same for the early and late sleep condition and both age groups (early sleep: young: 206 ± 2.3 min, middle-aged: 205.5 ± 2.5 min, t = 0.21, P = 0.83; late sleep: 211.5 ± 1.0 min and 206.9 ± 3.9 min, t = 1.2, P = 0.24). As expected, sleep architecture differed distinctly both between early and late sleep and between the age groups. SWS in both groups prevailed during early sleep (F(1,28) = 80.7, P < 0.001 for main effect of early/late sleep). The young subjects spent more time in SWS during early as well as during late sleep than the middle-aged subjects (F(1,28) = 31.9, P < 0.001 for main effect of age; see Figure 1B).
The lower amount of SWS in the middle-aged subjects was replaced mainly with sleep stage 2 during early sleep (Table 2). In contrast to SWS, REM sleep did not differ between the groups either during early or late sleep (F(1,28) = 0.65, P = 0.42 for early/late × age interaction; F(1,28) = 1.92, P = 0.17 for the main effect of the age factor), but was, as expected, predominant during late sleep (F(1,28) = 43.0, P < 0.001 for sleep interval; Table 2).
Table 2.
Sleep stages during early and late sleep in minutes (means ± SEM)
For exploratory purposes, retrieval was tested a second time in the early sleep condition the next morning after subjects had slept for another 3.5-h period during the late night. This Supplemental analysis aimed to answer the question of whether late sleep might add to the supportive effect on memory once subjects had a period of early retention sleep. However, the recall performance (absolute number of recalled word pairs) did not differ between the first and second retrievals (F(1,28) = 2.6, P = 0.12, for main effect of first/second retrieval; F(1,28) = 0.6, P = 0.44 for retrieval × age interaction; young: first retrieval: 34.8 ± 0.8, second retrieval: 35.0 ± 0.7; t-test pairs: t = −0.65, P = 0.52; middle-aged: 29.2 ± 1.0 and 29.9 ± 1.0, t = −1.5, P = 0.15). The difference between age groups also remained the same on both retrieval tests (F(1,28) = 0.02, P = 0.87 for main effect of age across first and second retrieval).
Since the time that middle-aged subjects spent in SWS during early retention sleep was well comparable with the time the younger subjects spent in SWS during late sleep (see Fig. 1B for means and SEM, t = −0.35, P = 0.72), we compared early sleep retention rates in the middle-aged subjects with late sleep retention rates in the younger subjects. There was no significant difference in the retention rate between the age groups (see Fig. 1A for means and SEM, t = 0.5, P = 0.62).
Discussion
The central finding of the study is that retrieval of memories after sleep was worse in the middle-aged than in the young group, although the age groups did not differ in their learning performance on the word-pair associate task at the last criterion trial prior to sleep. The young subjects in both sleep conditions spent significantly more time in SWS, with highest amounts during early sleep. Retention of word pairs, as defined by the difference in performance at retrieval testing after sleep and learning before sleep, was significantly better in the young group for the early sleep condition, where the young subjects had a mean of 62 min of SWS compared with 18 min in the middle-aged subjects. The distinctly reduced retention of word pairs after early sleep in the middle-aged subjects suggests a compromised consolidation of declarative memories in this age group.
There were no hints that the two age groups substantially differed in general cognitive function, including working memory, or tiredness at any of the testing occasions. Also immediate and delayed memory retrieval function tested on a separate occasion was comparable in both groups. The only relevant difference was that, at learning before early sleep, the middle-aged subjects needed more trials to reach the study criterion than the young controls. However, at the criterion, trial performance was very similar for both groups in this condition, which assured that the amount of acquired declarative information as well as the depth of encoding was well comparable between age groups. Since our study focused on age differences during sleep-associated memory consolidation, a comparable amount of declarative information before sleep had to be ensured by having the subject in both groups learning the list to the same criterion. On this background, it seems justified to attribute the differences between the age groups in retrieval of word pairs after sleep to differences in memory consolidation during sleep.
There was no additional gain in memory recall when subjects after early sleep slept for an additional 3.5 h during late sleep, i.e., a period of sleep with generally reduced amounts of SWS. Moreover, retention periods of sleep with similar amounts of SWS (i.e., early sleep in the middle-aged and late sleep in the young subjects) were associated with similar retention scores for the middle-aged and younger subjects, indicating that SWS rather than age itself is associated with the changes in sleep-related declarative memory consolidation. However, the comparison between consolidation during early sleep in the middle-aged and late sleep in the young subjects must be interpreted with caution because of differences possibly confounding this comparison, such as the different times of encoding and retrieval.
A central role of SWS for memory consolidation has been proposed previously, although with two principally different conceptualizations, of which both, nonetheless, fit the present findings. Based mainly on findings of a globally reduced activity within neocortical networks during SWS, Tononi and Cirelli (2003, 2006) proposed that SWS acts via a general downscaling of synaptic connections that were potentiated during encoding of information during the preceding wake phase. Synaptic downscaling by erasure of all weak synaptic connections below a certain threshold improves signal-to-noise ratio, and in this way can indirectly enhance recently acquired memory traces. Alternatively, the consolidation specifically relating to declarative memories known to depend on hippocampal function might take place in the framework of a dialogue between the neocortex and hippocampus. This view assumes that the sleep-dependent consolidation process relies on a covert reactivation of newly encoded memories in hippocampal networks (Wilson and McNaughton 1994; Buzsaki 1996, 1998). The hippocampal memory replay activity during SWS is driven by <1 Hz slow oscillations that are generated within neocortical networks, partially as a function of the prior use of the networks for encoding information during wakefulness. The reactivation stimulates the transfer of the recently encoded information from hippocampal back to neocortical networks, where they are then stored for the long term. A recent study demonstrated that transcranially applied oscillating potentials at a frequency of 0.75 Hz can both facilitate SWS and retention of declarative memory, thus underscoring the importance of this physiological process for declarative memory consolidation (Marshall et al. 2006).
Whereas a decline in SWS and slow oscillations was demonstrated earlier in middle-aged subjects (Carrier et al. 2001; Landolt and Borbely 2001), we show here that this decline is correlated with a parallel decline in declarative memory consolidation. According to the concepts outlined above, this outcome points toward a disturbance in hippocampal memory replay during sleep, which, remarkably, starts already well before old age. Structural and functional change in hippocampal regions identified during this midlife period fit this view (Hedden and Gabrieli 2004).
Our data suggest a critical role of sleep in the age-dependent decline of declarative memory consolidation. The cause of the parallel decline in SWS and hippocampus-dependent memory consolidation is, however, obscure. Several factors have been identified that might coincidentally affect sleep regulation and hippocampal function during the course of aging. Thus, a low central nervous cholinergic activity, as well as a minimal glucocorticoid activity during SWS, are prerequisites for an effective consolidation of hippocampus-dependent declarative memories (Plihal and Born 1999; Gais and Born 2004; Rasch et al. 2006). There is corresponding evidence from animal and human studies that both hippocampal cholinergic tone and glucocorticoid activity during the rest period are comparatively enhanced in the middle-aged as opposed to young individuals (Mizuno et al. 1994; Kern et al. 1996; Mitsushima et al. 1996; Van Cauter et al. 2000). The same factors can impair phenotypic sleep via an interconnected network (Steiger 2002; Steriade 2004; Jones 2005), and as such, may impair in parallel both SWS and hippocampal memory replay activity, resulting in a consolidation deficit for declarative memory. Whatever the basic mechanism is that links SWS to hippocampal memory consolidation, the early onset of substantial decay in this system during the course of aging may render it a target for preventive approaches in the therapy of cognitive aging.
Materials and Methods
Subjects
Sixteen young healthy subjects aged between 18 and 25 yr (20.4 ± 0.6 yr) and 14 middle-aged healthy subjects aged between 48 and 55 yr (50.0 ± 0.6 yr), all right-handed and non-smokers, with a body mass index within the normal range (22.6 ± 0.4 kg/m2), participated in the study. Subjects were recruited by advertisement and had a regular sleep-wake rhythm, were not shift-working and had no sleep disorder. They estimated their sleep using the Pittsburgh Sleep Quality Index (Buysse et al. 1989; Backhaus et al. 2002). Participants had neither a psychiatric nor a somatic disorder and did not take any psychoactive drugs or medications that might affect sleep or memory. They underwent a thorough physical and psychiatric assessment, including the Structured Clinical Interview for the Diagnosis of Psychiatric Disorders according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (Wittchen et al. 1997; American Psychiatric Association 2000). The study was approved by the local ethics committee and was conducted according to the Declaration of Helsinki. After a complete description of the study to the subjects, their written informed consent was obtained.
Procedure and tasks
Each subject spent three nights in the sleep laboratory. The first night served to adapt the subjects to the sleep laboratory, and to exclude sleep apnea (apnea/hypopnea index >5/h) and periodic leg movements with EEG-arousal (>5/h). Each subject then participated in two experimental nights, i.e., early and late sleep conditions, respectively, which were spaced one week apart. Subjects were randomly assigned to the order of these experimental nights. A word-pair associate task was used for testing declarative memory.
To exclude the possibility that differences in declarative memory performance were due to differences in verbal intelligence or working memory, subjects on a separate occasion and before entering the study performed all verbal subtests of the Intelligence-Structure-Test (I-S-T 2000R; Amthauer et al. 2001) and the word-list task as well as the word-fluency task of the DemTect test (Calabrese and Kessler 2000). The word-list task included three retrievals: (1) After subjects heard a word-list of 10 words for the first time, they were to recall all of the words they could remember. (2) Immediately thereafter, all subjects heard the same word-list for a second time and immediate recall was tested again. (3) After a delay of about 5 min—during which the subjects performed the word fluency task—subjects were unexpectedly asked to recall again as many of the words of the word-list as possible. Moreover, during the experimental sessions, before the learning phase and after retrieval testing, subjects performed a working memory task (digit span) to ensure that age groups did not differ in working memory function at the time of encoding or retrieval.
In the early sleep condition, the learning phase for the declarative memory task (word-pair associates) took place between 22:00 and 22.25 h before subjects went to bed at 22:30 h. After 3.5 h in bed, they were woken up at 2.00 h, and delayed retrieval of the word-pair associate task was tested 15 min later to avoid confounding effects of sleep inertia. Afterward, subjects performed again the digit span task to ensure that age groups did not differ in working memory at delayed retrieval testing.
For the late sleep condition, subjects first slept 3.5 h and were woken up at 2:00 h. Fifteen minutes later, they performed the digit span test and the declarative memory task (word-pair associates) before they went to bed for another 3.5 h during the late night. The next morning, 15 min after awakening, delayed retrieval of the word-pair associate task was tested, followed by the digit span test.
To investigate whether sleep during the second half of the night further adds to the effects of early sleep on declarative memory consolidation, we extend the early sleep condition: following the first delayed retrieval phase after early sleep, subjects slept again 3.5 h during the second half of the night. Fifteen minutes after awakening in the morning, delayed retrieval was tested a second time.
Declarative memory was tested using a word-pair associate task consisting of 40 word pairs of German nouns that were standardized with respect to word frequency, length, emotionality, meaningfulness, and concreteness (Plihal and Born 1997). Two additional word pairs at the beginning and end of the test served to buffer primacy and recency effects, and were not included in the analysis. The word pairs were presented visually for 5 sec each. Immediately after presentation of all word pairs, the subjects were asked to recall orally the second word in a pair upon presentation of the first word (cued recall). The list was presented repeatedly in different order until the subject had correctly recalled at least 24 words (60% criterion). During learning, subjects were given feedback so that the subject’s response was always followed by a presentation of the correct response word for 1 sec. Upon retrieval testing after retention sleep, subjects were again asked to recall the word pairs using the same cued recall procedure that was applied during the learning phase, but this time without feedback. Retention of word pairs was defined by the difference between retrieval performance after sleep and learning performance before sleep. Subjects were tested using parallel tests, which were given in a randomly sequenced order. Tiredness was measured directly after the learning phase for the word-pair associate task using the Stanford Sleepiness Scale (Hoddes et al. 1973).
Polysomnographic recordings
Standard polysomnographic recordings were obtained using EEG electrodes positioned at C3 and C4, referenced against A2 and A1, respectively (as defined by the international 10-20 system). Electromyographic activity was recorded from submental electrodes. Vertical and horizontal eye movements were recorded from two horizontal and one vertical electrodes. Furthermore, electromyographic recordings of the legs and recordings of breathing (chest and abdominal excursions, nose-mouth airflow) together with oxygen monitoring were performed on the first night to rule out sleep apnea or periodic leg movements. Sleep stages were scored according to standard criteria (Rechtschaffen and Kales 1968) by experienced staff blind to the experimental condition.
Statistical analyses
Differences between the sleep conditions and group differences were analyzed by analyses of variance (ANOVA) on the raw numbers for word recall. Post hoc t-tests were used to specify significant main and interaction effects. Regression analysis was performed on data from the early sleep condition to separate influences of age, SWS, and REM sleep on sleep-related declarative memory consolidation. Socio-demographic and questionnaire data as well as other data not repeatedly measured were analyzed using two-tailed t-tests for independent samples. A P-value of <0.05 was considered significant. Data were analyzed using SPSS for Windows, Version 12. Variability is expressed in the form of standard errors of the mean (SEM).
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to J. Backhaus and K. Junghanns (BA 2022/2-2, SFB 645 “Plasticity and Sleep”). We thank Jolanta Chwalko for assisting in the study.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.470507
References
- Ambrosini M.V., Giuditta A. Learning and sleep: The sequential hypothesis. Sleep Med. Rev. 2001;5:477–490. doi: 10.1053/smrv.2001.0180. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Amthauer R., Brocke B., Liepmann D., Beauducel A. I-S-T 2000 R. Intelligenz-Struktur-Test 2000 R. Hogrefe Verlag; Göttingen: 2001. [Google Scholar]
- Backhaus J., Junghanns K. Daytime naps improve procedural motor memory. Sleep Med. 2006;7:508–512. doi: 10.1016/j.sleep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Backhaus J., Junghanns K., Broocks A., Riemann D., Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Backhaus J., Junghanns K., Born J., Hohaus K., Faasch F., Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol. Psychiatry. 2006;60:1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Born J., Rasch B., Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cereb. Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: A neurophysiological perspective. J. Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Calabrese P., Kessler J. DemTect. Eisai; Frankfurt/Karlsruhe, Germany: 2000. [Google Scholar]
- Carrier J., Land S., Buysse D., Kupfer D.J., Monk T.H. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiology. 2001;38:232–242. [PubMed] [Google Scholar]
- Daselaar S.M., Veltman D.J., Rombouts S.A., Raaijmakers J.G., Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Driscoll I., Hamilton D.A., Petropoulos H., Yeo R.A., Brooks W.M., Baumgartner R.N., Sutherland R.J. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb. Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Ellenbogen J.M., Hulbert J.C., Stickgold R., Dinges D.F., Thompson-Schill S.L. Interfering with theories of sleep and memory: Sleep, declarative memory, and associative interference. Curr. Biol. 2006a;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Ellenbogen J.M., Payne J.D., Stickgold R. The role of sleep in declarative memory consolidation: Passive, permissive, active or none? Curr. Opin. Neurobiol. 2006b;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ficca G., Lombardo P., Rossi L., Salzarulo P. Morning recall of verbal material depends on prior sleep organization. Behav. Brain Res. 2000;112:159–163. doi: 10.1016/s0166-4328(00)00177-7. [DOI] [PubMed] [Google Scholar]
- Fischer S., Hallschmid M., Elsner A.L., Born J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc. Natl. Acad. Sci. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Plihal W., Wagner U., Born J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gais S., Lucas B., Born J. Sleep after learning aids memory recall. Learn. Mem. 2006;13:259–262. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T., Gabrieli J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hoddes E., Dement W.C., Zarcone V. The development and use of the Stanford Sleepiness Scale (SSS) Psychophysiology. 1973;10:421–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hof P.R., Morrison J.H. The aging brain: Morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Jones B.E. From waking to sleeping: Neuronal and chemical substrates. Trends Pharmacol. Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kern W., Dodt C., Born J., Fehm H.L. Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of aging. J. Gerontol. A Biol. Sci. Med. Sci. 1996;51:M3–M9. doi: 10.1093/gerona/51a.1.m3. [DOI] [PubMed] [Google Scholar]
- Landolt H.P., Borbely A.A. Age-dependent changes in sleep EEG topography. Clin. Neurophysiol. 2001;112:369–377. doi: 10.1016/s1388-2457(00)00542-3. [DOI] [PubMed] [Google Scholar]
- Maquet P., Smith C., Stickgold R. Sleep and brain plasticity. Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- Marshall L., Helgadottir H., Molle M., Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:559–560. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Mitsushima D., Mizuno T., Kimura F. Age-related changes in diurnal acetylcholine release in the prefrontal cortex of male rats as measured by microdialysis. Neuroscience. 1996;72:429–434. doi: 10.1016/0306-4522(95)00572-2. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Arita J., Kimura F. Spontaneous acetylcholine release in the hippocampus exhibits a diurnal variation in both young and old rats. Neurosci. Lett. 1994;178:271–274. doi: 10.1016/0304-3940(94)90776-5. [DOI] [PubMed] [Google Scholar]
- Peigneux P., Laureys S., Fuchs S., Collette F., Perrin F., Reggers J., Phillips C., Degueldre C., Del Fiore G., Aerts J., et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Plihal W., Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Plihal W., Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport. 1999;10:2741–2747. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- Prull M.W., Gabrieli J.D.E., Bunge S.A. Age-related changes in memory: A cognitive neuroscience perspective. In: Craik F.I.M., Salthouse T.A., editors. The handbook of aging and cognition. Lawrence Earlbaum Associates; Mahwah, NJ: 2000. pp. 91–153. [Google Scholar]
- Rasch B.H., Born J., Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J. Cogn. Neurosci. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A., Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Government Printing Office; Public Health Service, Washington, DC: 1968. [Google Scholar]
- Smith C. Sleep states and memory processes in humans: Procedural versus declarative memory systems. Sleep Med. Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- Steiger A. Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med. Rev. 2002;6:125–138. doi: 10.1053/smrv.2001.0159. [DOI] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking–sleep cycle. Prog. Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R., Whidbee D., Schirmer B., Patel V., Hobson J.A. Visual discrimination task improvement: A multi-step process occurring during sleep. J. Cogn. Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Tisserand D.J., Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Van Cauter E., Leproult R., Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Wagner U., Gais S., Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.P., Brakefield T., Morgan A., Hobson J.A., Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Wilson M.A., McNaughton B.L. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Zaudig M., Fydrich T. SKID, Strukturiertes Klinisches Interview für DSM-IV. Hogrefe Verlag; Göttingen: 1997. [Google Scholar]
- Yaroush R., Sullivan M.J., Ekstrand B.R. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J. Exp. Psychol. 1971;88:361–366. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]