Abstract
Animals must be able to find and evaluate food to ensure survival. The ability to associate a cue with the presence of food is advantageous because it allows an animal to quickly identify a situation associated with a good, bad, or even harmful food. Identifying genes underlying these natural learned responses is essential to understanding this ability. Here, we investigate whether natural variation in the foraging (for) gene in Drosophila melanogaster larvae is important in mediating associations between either an odor or a light stimulus and food reward. We found that for influences olfactory conditioning and that the mushroom bodies play a role in this for-mediated olfactory learning. Genotypes associated with high activity of the product of for, cGMP-dependent protein kinase (PKG), showed greater memory acquisition and retention compared with genotypes associated with low activity of PKG when trained with three conditioning trials. Interestingly, increasing the number of training trials resulted in decreased memory retention only in genotypes associated with high PKG activity. The difference in the dynamics of memory acquisition and retention between variants of for suggests that the ability to learn and retain an association may be linked to the foraging strategies of the two variants.
Learned associations such as identifying odors as indicators of food, or showing preferences for food-related odors, are conserved across diverse taxa from humans and mice to slugs, honeybees, and fruit flies (Ache and Young 2005). The fruit fly Drosophila melanogaster can use odors as cues for the presence of both a food reward and an aversive stimulus (Tempel et al. 1983; Scherer et al. 2003; Schwaerzel et al. 2003; Margulies et al. 2005; McGuire et al. 2005; Kim et al. 2007). Identifying genes underlying these natural learned responses is essential to understanding this ability. Here, we use classical reward conditioning to investigate how natural variation in the foraging (for) gene affects acquisition and decay of memory in D. melanogaster larvae.
for encodes a cGMP-dependent protein kinase (PKG) (Osborne et al. 1997). In mammals, PKG is important for proper induction of long-term potentiation (LTP) and long-term depression (LTD) (Hartell 1994; Zhuo et al. 1994; Lev-Ram et al. 1997; Arancio et al. 2001; Fiel et al. 2003, 2005; Kleppisch et al. 2003; Hofmann et al. 2006). Although LTP and LTD are thought to be important mechanisms underlying learning and memory (Whitlock et al. 2006), few studies have shown a direct role for PKG in learning and memory. This study aims to do this by investigating how learning differs between allelic variants of for in Drosophila larvae.
for is an ideal candidate to study how natural genetic variation can affect learning and memory, since natural for variants exist that have subtle but significant variations in PKG activity (Osborne et al. 1997). The rover variants of for (forR) have higher for transcript levels and higher PKG activities compared with the sitter variants of for (fors) (Osborne et al. 1997). These variations in PKG activity account for major behavioral differences between the variants (Osborne et al. 1997). Rover variants move more within a food patch and are more likely to leave one food patch in search for another, whereas sitter variants move less within and between food patches (Sokolowski 1980, 2001). Thus, establishing how the variants of for associate a cue with a taste reward may consequently reveal more about how associative reward conditioning is modulated in a natural population, as well as clarify the relationship between learning, memory, and foraging behavior in a natural environment.
Results
The role of for in olfactory reward conditioning
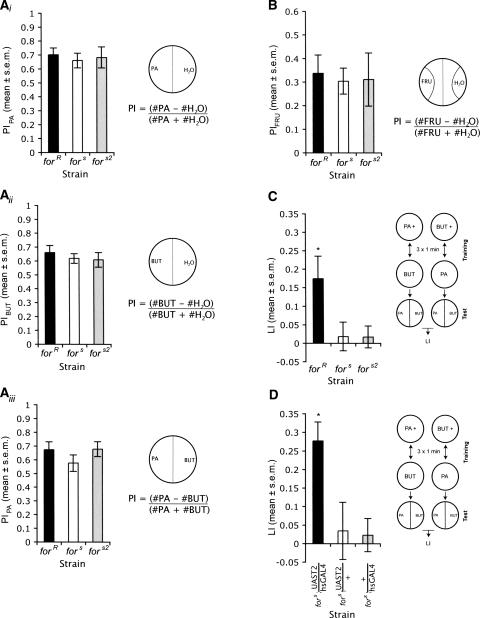
We evaluated the role of for in mediating the relative preference for olfactory or light stimuli as a function of their previous association with a positive taste reward (Garcia et al. 1968) by comparing the role of for in olfactory and visual conditioning. To investigate the role of for in olfactory conditioning, we used a reciprocal training protocol, where a group of 30 larvae received three 1-min pairings of fructose–agarose with propyl acetate (PA) and plain agarose with butanol (BUT), and simultaneously, another group of 30 larvae received three 1-min pairings of fructose–agarose with BUT and plain agarose with PA (Scherer et al. 2003; Neuser et al. 2005). Each group was then given a 3-min choice test between PA and BUT, and a preference index (PI) for PA was calculated by subtracting the number of larvae on the BUT side of the plate from the number of larvae on the PA side of the plate and dividing this by the total number of larvae on the plate (Scherer et al. 2003; Neuser et al. 2005). A learning index (LI) was calculated by subtracting the preference index of the larvae that received BUT paired with fructose from the preference index of the larvae that received PA paired with fructose and dividing that number by two (Gerber et al. 2004a; Neuser et al. 2005). Thus, a learning index of zero indicated that no associations were formed, whereas a learning index significantly greater than zero indicated a positive association between conditioned stimuli and reward. PA and BUT were chosen, as they were unlikely to be odor equivalents; in larvae they are detected and processed by different combinations of peripheral and central structures (Kreher et al. 2005). In addition, we found that larvae of the natural rover (forR), sitter (fors), and sitter mutant (fors2) strain, which was generated on a rover genetic background (de Belle et al. 1989; Pereira and Sokolowski 1993), do not differ significantly in their response to these odors (below).
As an olfactory control, we measured the ability of rover (forR) and sitter (fors and fors2) larvae to differentiate between an odor (PA or BUT) and dH2O to determine whether for played a role in sensitivity to the odors used in our olfactory conditioning assays. We found that forR, fors, and fors2 larvae all preferred PA over dH2O, with no significant strain differences in this preference (Fig. 1A; Wilcoxon forR X2(1,24) = 19.76, P < 0.0001, fors X2(1,24) = 19.75, P < 0.0001, fors2 X2(1,24) = 19.74, P < 0.0001, ANOVA F(2,33) = 0.13, P = 0.88). Larvae also preferred BUT over dH2O with no significant strain differences in preferences (Fig. 1A, ii) (Wilcoxon forR X2(1,24) = 19.74, P < 0.0001, fors X2(1,24) = 19.74, P < 0.0001, fors2 X2(1,24) = 19.78, P < 0.0001, ANOVA F(2,33) = 0.40, P = 0.68). All strains preferred PA over BUT, and once again, there were no significant strain differences in their preference (Fig. 1A, iii) (Wilcoxon forR X2(1,24) = 19.74, P < 0.0001, fors X2(1,24) = 19.75, P < 0.0001, fors2 X2(1,24) = 19.74, P < 0.0001, ANOVA F(2,33) = 1.07, P = 0.35). These results show that PA and BUT are appropriate odors to use for the olfactory conditioning paradigm, since there are no significant differences between strains in preference for either odorant.
Figure 1.
for plays a role in olfactory conditioning. (A) for does not affect preference for propyl acetate or butanol. (i) There are no significant differences in the preference of forR, fors, and fors2 larvae for PA over water (n = 12/strain). (ii) There are no significant differences in the preference of forR, fors, and fors2 larvae for BUT over water (n = 12/strain). (iii) There are no significant differences in the preference of forR, fors, and fors2 larvae for PA over BUT (n = 12/strain). (B) for does not affect a preference for 2.0 M fructose (FRU). forR, fors, and fors2 larvae all prefer the presence of 2.0 M FRU over water (n = 12/strain). (C) forR larvae show significantly greater learning than fors and fors2 larvae when trained with three 1-min pairings between odor and reward (n = 16/strain). (D) Increasing expression of for-T2 in fors larvae significantly increases LI (12<n>13).
We further determined whether responses to the fructose reward used in these olfactory conditioning assays differed between rovers and sitters. Specifically, we tested whether for plays a role in sensitivity to our fructose reward (2.0 M fructose) using a choice test between 2.0 M fructose and dH20, and found that both forR and fors larvae preferred 2.0 M fructose to dH20 (Fig. 1B) (Wilcoxon forR X2(1,28) = 17.02, P < 0.0001, fors X2(1,28) = 23.17, P < 0.0001). fors2, a sitter mutant made on a rover genetic background, behaved similarly to fors in the fructose preference test (Fig. 1B) (Wilcoxon fors2 X2(1,28) = 23.16, P < 0.0001). Importantly, there were no significant differences between strains for the 2.0 M fructose reward used in our conditioning assays (Fig. 1B) (ANOVA F(2,39) = 0.37, P = 0.69).
Once we established that the larvae did not differ in their response to the olfactory cues or the fructose reward, we tested whether rovers and sitters differed in their ability to associate an olfactory stimulus with the presence of fructose. We found that rover (forR) larvae had a significantly higher LI than fors (Fig. 1C) (ANOVA F(2,45) = 4.32, P = 0.019, SNK P = 0.015) and fors2 larvae (SNK P = 0.014). There were no significant differences in LI between fors and fors2 larvae (SNK P = 0.98), suggesting that the increase in LI in rovers is specific to the for locus. Intriguingly, the difference in LI between rovers (forR) and sitters (fors and fors2) is not due to an extremely high LI in rovers, but seems to be due to no significant LI in sitters (Fig. 1C; Wilcoxon forR X2(1,32) = 8.66, P = 0.0033, fors X2(1,32) = 0.42, P = 0.52, fors2 X2(1,32) = 0.11, P = 0.74). The results therefore suggest that natural variation in for affects olfactory conditioning and that sitters have a decreased ability to form an association between an olfactory cue and fructose reward compared with rovers when trained with a small number of training trials.
Further evidence of for’s role in olfactory conditioning was found in our transgenic experiments. We increased the expression of the T2 transcript of for in fors larvae using a hsGAL4 at 23°C (K.R. Kaun, C.A.L. Riedl, M. Chakaborty-Chatterjee, A.T. Belay, S. Douglas, A. Gibbs, and M.B. Sokolowski, in prep.) and found an increase in LI (Fig. 1D). We found that fors;UAS-T2/hsGAL4 larvae show significantly greater LI than control larvae, fors;UAS-T2/+ and fors;+/hsGAL4 larvae (Fig. 1D; ANOVA F(2,34) = 6.19, P = 0.0051, SNK fors;UAS-T2/hsGAL4 vs. fors;UAS-T2/+ P = 0.0034, fors;UAS-T2/hsGAL4 vs. fors;+/hsGAL4 P = 0.0057). There were no significant differences between fors;UAS-T2/+ and fors;+/hsGAL4 larvae (SNK P = 0.89). Once again, we found that larvae with increased expression of for (fors;UAS-T2/hsGAL4 larvae) showed a LI greater than zero, whereas sitter larvae (fors;UAS-T2/+ and fors;+/hsGAL4) did not (Fig. 1D; Wilcoxon fors;UAS-T2/hsGAL4 X2(1,24) = 19.73, P < 0.0001, fors;UAS-T2/+ X2(1,25) = 0.12, P = 0.73, fors;+/hsGAL4 X2(1,24) = 2.19, P = 0.14).
The results of our genetic and transgenic studies show that natural variation in the for gene affects olfactory reward learning, thereby directly implicating PKG in learning. for plays a role in olfactory conditioning, where only relatively higher levels of activity of PKG allow association between an odor and taste reward when larvae are trained with three 1-min trials.
The role of for in visual reward conditioning
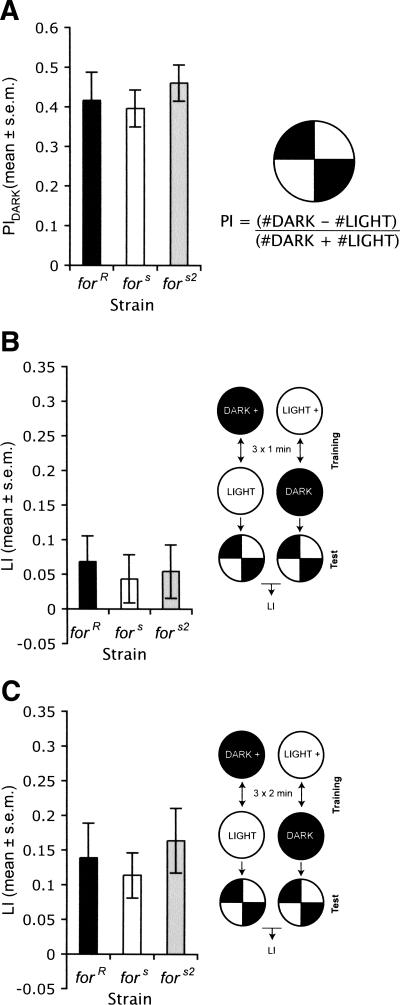
To investigate the role of for in visual conditioning, we gave larvae three 1-min pairings of light and fructose, and alternate three 1-min pairings of plain agarose with dark in one group of animals. Simultaneously, another group of 30 larvae received three 1-min pairings of fructose–agarose with dark and plain agarose with light (Gerber et al. 2004a). The LI was calculated as above.
We first tested whether for played a role in sensitivity to light. We found that forR, fors, and fors2 larvae all preferred dark over light (Fig. 2A; Wilcoxon forR X2(1,24) = 11.55, P = 0.0007, fors X2(1,24) = 13.74, P = 0.0002, fors2 X2(1,24) = 6.99, P = 0.0082), and there were no significant differences between strains for the preference to dark (Fig. 2A; ANOVA F(2,33) = 0.29, P = 0.75).
Figure 2.
No evidence for a role of for in larval visual conditioning. (A) for does not affect preference for presence for darkness. There are no significant differences in the preference of forR, fors, and fors2 larvae for darkness over light (n = 14/strain). (B) forR, fors, and fors2 larvae do not differ significantly in their visual conditioning learning index when trained with three 1-min pairings between visual stimulus and reward (n = 18/strain). (C) Three 2-min pairings between visual stimulus and reward (10–11).
Once we established that the larvae did not differ in their response to the fructose reward (see above) or the light stimuli, we tested the ability of larvae to associate the presence of light or dark with fructose. We found no significant differences in LI between rovers (forR) and sitters (fors, fors2) (Fig. 2B; ANOVA F(2,51) = 0.12, P = 0.89). Interestingly, we found forR showed a LI greater than zero, but fors did not (Fig. 2B; Wilcoxon forR X2(1,36) = 9.26, P = 0.0023, fors X2(1,36) = 1.48, P = 0.22). However, fors2 also showed a LI greater than zero (Fig. 2B; Wilcoxon fors2 X2(1,36) = 5.92, P = 0.015). We speculated that this lack of effect might be due to the small LI values that we obtained using three 1-min pairings between the visual stimulus and fructose reward. In an attempt to increase the value of the LIs, we increased the time in which either the reward or plain agarose plate was paired with the presence of light and dark from 1 to 2 min. When we did this, we once again found no significant differences in LI between strains, although with this longer pairing time, all larvae showed LIs significantly greater than zero (Fig. 2C; ANOVA F(2,29) = 0.38, P = 0.68, Wilcoxon forR X2(1,21) = 9.26, P = 0.0023, fors X2(1,21) = 6.80, P = 0.0091, fors2 X2(1,21) = 10.44, P = 0.0012).
Although these results do not rule out the possibility that for plays a role in visual conditioning, they suggest that for does not play a robust role in larval visual reward conditioning. As a result, we decided to focus the remainder of our studies on olfactory conditioning.
for acts through the mushroom bodies to mediate olfactory conditioning
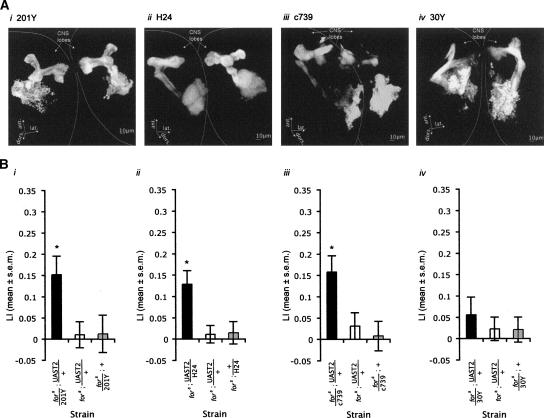
The mushroom bodies are important in mediating olfactory conditioning in Drosophila (Heisenberg et al. 1985; Gerber et al. 2004b; Davis 2005; Honjo and Furukubo-Tokunaga 2005; Margulies et al. 2005; McGuire et al. 2005). Previous research has suggested mushroom-body independent pathways for visual conditioning, but not olfactory conditioning (Wolf et al. 1998; Lui et al. 2006). Since we did not find a robust role for for in larval visual conditioning and the mushroom bodies are necessary for olfactory conditioning, we hypothesized that for may play a role in mushroom-body mediated olfactory conditioning. FOR is expressed in the mushroom-body neuropiles and calyx, providing further indication that for may affect memory acquisition via the mushroom bodies (Belay et al. 2007).
We tested this by quantifying olfactory conditioning when increasing expression of for-T2 in the mushroom bodies in fors larvae using the mushroom-body GAL4 drivers 201Y, H24, c739, and 30Y (Yang et al. 1995; Melzig et al. 1998). Figure 3A depicts the expression pattern in the mushroom bodies of each of these drivers in mid-third instar larvae. Increasing expression of for-T2 in a 201Y, H24, and c739 pattern significantly increased LI in larvae trained with three 1-min intervals (Fig. 3B; ANOVA 201Y: F(2,48) = 4.39, P = 0.018; H24: F(2,51) = 6.59, P = 0.0029; c739: F(2,45) = 5.84, P = 0.0056). Increasing expression of for-T2 in a 30Y pattern partially restored the effect, because the LI was significantly greater than zero, whereas the sitter controls were not (Wilcoxon fors/30Y; UAST2/+: X2(1,35) = 4.14, P = 0.04; fors;UAS-T2/+: X2(1,37) = 0.76, P = 0.38; fors/30Y;+: X2(1,35) = 0.10, P = 0.75); however, the LI was not significantly increased when compared with the sitter controls (F(2,52) = 0.36, P = 0.70). The partial rescue of 30Y is interesting because 30Y, c739, and 201Y have all been reported to be expressed in a subset of Kenyon cells (KC) in each of four clusters, i.e., calyx, pedunculus, β/λ lobe, and circumference of the α/δ lobe of third-instar larvae (Tettamanti et al. 1997). The difference in LI between sitters with increased expression of T2 in a 30Y pattern compared with 201Y, c739, or H24 pattern may potentially be due to differences in expression in different subsets of KCs. Alternatively, this difference may be due to weaker expression of 30Y during early larval instars; 30Y is uniquely expressed in four KC clusters and the calyx in first instars, and additionally in the pedunculus, β/λ lobe, and circumference of the α/δ lobe in second and third instar larvae (Tettamanti et al. 1997). Overall, these results suggest that driving expression of for in the mushroom bodies is sufficient to increase olfactory conditioning.
Figure 3.
The mushroom bodies play a role in for-dependent learning. (A) Expression of GAL4 lines 201Y (i), H24 (ii), c739 (iii), and 30Y (iv) in third-instar larvae were visualized using UAS-GFP. All lines expression in subset of the Kenyon cells staining the calyx, pedunculus, vertical lobe (α/δ lobe), and medial lobe (β/λ lobe) of the larval mushroom body (201Y, c739, 30Y larval expression patterns published previously in Tettamanti et al. 1997). (B) Increasing expression of for-T2 in the mushroom bodies using the GAL4 lines 201Y (n = 17/strain) (i), H24 (17–19) (ii), and c739 (iii) (n = 16/strain) in fors larvae is sufficient to significantly increase LI. (iv) Increasing expression of for-T2 in a 30Y pattern in fors larvae significantly increased LI above zero, but did not significantly increase LI compared with the LIs of fors heterozygous control larvae (17–19).
for affects the timescale of memory acquisition
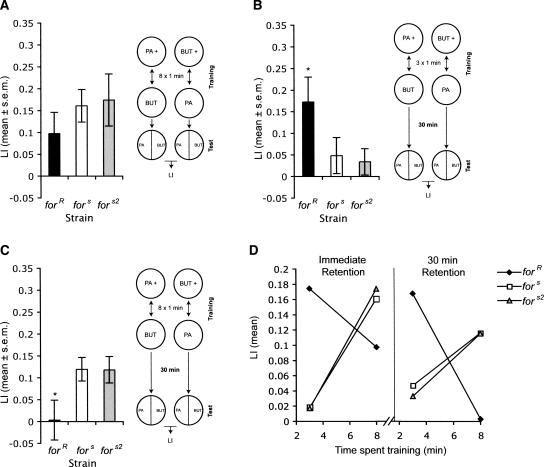
Taken together, the above results suggest that rovers are more able to associate an odor with a fructose reward compared with sitters. Recall that sitters, unlike rovers, did not show a conditioned response with three 1-min pairings. Since learned associations must be flexible in nature to take into account a complex and constantly changing environment, we hypothesized that the variants of for may react differently to manipulations of the training paradigm. Specifically, as for affects the time that an animal spends on a food patch, we hypothesized that sitter variants of for might show increased performance, given an increase in the length of training. To test this, we asked whether more odor–reward pairings were required for sitters to show a conditioned response. We found that eight 1-min pairings between odor and reward did result in a significant LI in fors and fors2 larvae (Fig. 4A; Wilcoxon fors: X2(1,34) = 14.71, P = 0.0001; fors2: X2(1,33) = 7.59, P = 0.0059), abolishing between-genotype differences (F(2,50) = 0.62, P = 0.54). Interestingly, the LI of forR larvae may have decreased slightly compared with the LI when the larvae were trained with three odor-reward pairings; however, this difference was not significant. These results suggest that sitters are indeed able to associate an odor with a reward, and that larvae with the different alleles of for respond differently to a change in training trial number to affect LI. The ability of sitters to learn as well as rovers after increasing the number of training trials may be linked to the different foraging strategies of the two variants. Sitters are more likely to spend a longer time on a food patch than rovers (Sokolowski 2001), and thus may have evolved a learning style that involves more training in order to associate a smell with a food reward accordingly. This brings into question whether different foraging strategies of the two variants are also linked to the ability to retain an association between a smell and taste reward.
Figure 4.
The role of for in olfactory learning is dependent on training experience. (A) Increasing the number of pairings between odor and reward from three to eight 1-min pairings significantly increases LI of sitter larvae such that forR, fors, and fors2 do not show significant differences in LI (17–18). (B) forR larvae show significantly greater LI than fors and fors2 larvae 30 min after training when trained with three 1-min pairings between odor and reward (16–17). (C) forR larvae show significantly smaller LI than fors and fors2 larvae 30 min after training when trained with eight 1-min pairings between odor and reward (n = 17/strain). (D) When the data from Figures 1C and 4A–C are replotted, it becomes apparent that forR larvae show higher learning indices when trained with three training trials, whereas fors and fors2 larvae show higher learning with eight training trials: this effect is seen both for immediate retentions (left) and 30-min retention (right). This data suggests that for plays a role in the timescale of memory acquisition and retention.
Previous work suggests that larvae can remember for up to 30 min post-training (Neuser et al. 2005). We tested whether rovers (forR) and sitters (fors and fors2) were able to retain their LI after 30 min when given three 1-min pairings between an odor and fructose reward. We found that rovers showed significantly greater LI than sitters when tested 30 min after training (Fig. 4B; F(2,47) = 3.61, P = 0.035). This is expected, since sitters do not show LI significantly greater than zero when tested directly after being trained with three 1-min pairings. However, when the larvae are trained with eight 1-min pairings between an odor and fructose, then sitters show significantly greater LI compared with rovers 30 min after training (Fig. 4C; F(2,48) = 3.59, P = 0.035). In this case, forR larvae did not show significant LI greater than zero, whereas fors and fors2 larvae did (Wilcoxon; forR: X2(1,33) = 0.09, P = 0.76; fors: X2(1,33) = 11.34, P = 0.0008; fors2: X2(1,33) = 15.83, P < 0.0001). Thus, sitters show better memory retention than rovers after having been trained with more trials, but rovers show better memory retention than sitters after using few training trials (Fig. 4D). This may also be linked to the different foraging strategies of the two variants. Since rovers move more while foraging, they are more likely to come across a greater number of food patches than sitters (Sokolowski 2001). Thus, as they usually leave a food patch quickly, they need to also form new associations quickly and retain that information for immediate use when searching for the next food patch.
Discussion
While many studies have suggested the importance of cAMP signaling in associative learning (Micheau and Riedel 1999; Fiel et al. 2005; Margulies et al. 2005; McGuire et al. 2005; Hofmann et al. 2006), our results implicate the cGMP signaling pathway in mediating reward learning and memory. Specifically, our results suggest a novel role for the foraging gene in larval learning and memory. We found that for plays a more robust role in larval olfactory reward conditioning than larval visual reward conditioning. We also found that the role of for in larval olfactory reward conditioning involves the mushroom bodies. Our results suggest that the role of for in olfactory memory is dependent on training experience, suggesting that for may be involved in multiple mechanisms that influence formation of olfactory associations. Our evidence suggests that these mechanisms affect the training experience necessary for memory acquisition and retention. For example, fors larvae respond to an increase in the number of training trials compared with forR larvae. Interestingly, for also plays a role in short- and long-term memory in Drosophila adults, suggesting that these mechanisms may be conserved throughout development (F. Mery, A.T. Belay, M.B. Sokolowski, and T. Kawecki, in prep.).
for may also play a role in the mechanisms that affect the time-course of training necessary to produce memory acquisition and retention. Our results suggest that increased PKG activity is associated with faster learning, and decreased PKG activity is associated with slower learning. As mentioned above, these differences may be related to the alternative foraging strategies of forR and fors larvae (Sokolowski 1980). forR larvae move more when foraging, and thus, potentially come across many food patches, whereas sitters move less and potentially stay close to a smaller number of food patches. Thus, it may be advantageous for forR larvae to quickly form an association between a new food source and odor and then use this new information to assess the quality of future food sources. Decreased memory retention may be advantageous for rovers, as previous studies suggest that formation and retention of formed associations can be energetically costly (Dukas 1999; Mery and Kawecki 2005; Barnard et al. 2006). Conversely, staying longer in a single food patch may result in a delay in the ability to form an association between a food source and odor in fors larvae. However, it may aid in the ability of fors larvae to remember which nearby food patches had been depleted.
Intriguingly, rover adult flies show a slower rate of habituation compared with sitter adult flies upon repetitive presentation of stimuli (Engel et al. 2000; Scheiner et al. 2004). forR flies show less habituation and generalization of habituation to repeated sucrose stimuli compared with fors and fors2 flies (Scheiner et al. 2004). This is consistent with a more rapid response decrement of the giant-fiber escape circuit in fors and fors2 flies compared with forR flies as measured electrophysiologically (Engel et al. 2000). As repetitive stimuli in the form of odor-reward pairings are used in the current study, the decrease in LI seen after increasing the amount of odor–reward pairings may, in part, represent a response decrement to the stimuli.
The differences in olfactory learning and memory due to for may be inextricably linked to the different foraging strategies of the two natural variants. This leads to the question of whether for may play a role in other behaviors associated with foraging and food such as path-integration, territoriality, aggression, and courtship. If so, for may play a role in higher-order reward pathways that mediate these individual behaviors. Interestingly, the mushroom bodies play a role in such complex behaviors, including courtship, courtship conditioning, spatial learning, aggression, and sleep (Zars 2000; Baier et al. 2002; Joiner et al. 2006; Pitman et al. 2006). Accordingly, the role of the mushroom bodies in for-dependent larval reward learning hints at a role of for in more complex behaviors involving higher-order reward pathways. Future research will provide insight as to whether a role of for in a variety of complex behaviors exists.
Materials and Methods
Strains
Rover and sitter strains are isogenic for chromosomes 2 and 3 and homozygous for the forR or fors alleles, respectively (de Belle and Sokolowski 1987). fors2 is a sitter mutant generated on the rover genetic background (de Belle et al. 1989; Pereira and Sokolowski 1993). The fors2 mutation has been mapped to for and it decreases PKG enzyme activity and for transcript levels (de Belle et al. 1989; Osborne et al. 1997). In order to increase the expression of for, a DNA fragment encoding the complete forT2 amino acid sequence (Kalderon and Rubin 1989) was subcloned into the transformation vector pUAST and transformed into w1 embryos using standard methods (Spradling and Rubin 1982). Transgenic flies were crossed into a sitter background, creating w1;fors;UASforT2, which were then crossed to w1;fors;hsGAL4 (K.R. Kaun, C.A.L. Riedl, M. Chakaborty-Chatterjee, A.T. Belay, S. Douglas, A. Gibbs, and M.B. Sokolowski, in prep.). We relied on leaky expression of the hs promoter (Osborne et al. 1997); experiments were done at 23°C. Mushroom body drivers 201Y, 30Y, c739 (Yang et al. 1995) (donated by Joel Levine, University of Toronto at Mississauga, Canada) were backcrossed nine generations into a w1;fors background. H24 (Melzig et al. 1998) (also donated by Joel Levine) was crossed into a w1;fors background via third chromosome substitution. UAS-GFP (Bloomington Stock Center) was crossed onto a w1;fors background via third chromosome substitution.
Flies were maintained in 170-mL plastic culture bottles with 40 mL of standard culture medium at 23 ± 1°C and a 12L:12D photocycle. Standard culture medium contained 50 g of Baker’s yeast, 100 g of sucrose, 16 g of agar, 0.1 g of KPO4, 8 g of sodium potassium tartarate, 0.5 g of NaCl, 0.5g MgCl2, and 0.5g Fe2(SO4)3/L of tap water. Larvae were reared at 25°C from egg-hatch to mid-third instar (96 ± 3h post-hatch) at densities of 100 larvae/35 mL of medium in 100 × 15-mm Petri dishes. All tests took place under red light at room temperature (23 ± 2°C) in an otherwise dark fume hood.
Olfactory sensitivity
As in Scherer et al. (2003), larvae were placed along the midline of a plain agarose plate, on which a 5-mm diameter Teflon odorant cup filled with either 2 μL of pure odorant or dH2O was placed opposite an identical 5-mm diameter Teflon odorant cup filled with either 2 μL of pure odorant or dH2O. Odorants used in this study were propyl acetate (PA, VWR) and butanol (BUT, Sigma). Larvae were left on the plate for 3 min. In cases where larvae were given a choice test between an odorant and water, a preference index (PI) was calculated by subtracting the number of larvae on the dH2O side from the number of larvae on the odorant side and dividing that number by the total number of larvae on the plate. In cases where larvae were given a choice test between PA and BUT, a preference index (PI) was calculated by subtracting the number of larvae on the BUT side from the number of larvae on the PA side and dividing that number by the total number of larvae on the plate.
Fructose sensitivity
Fructose preference tests were performed on 1% agarose plates with samples of water at one side and fructose solution at the opposite side. Plates were prepared by spreading 100 uL of dH2O on one side of a plate and 100 μL of 2.0 M fructose (FRU) solution on the opposite side of the plate, then letting the plate dry in a fume hood for 10 min (see Fig. 1B). Thirty larvae were placed along the midline of the plate and left for 15 min at 25°C. A preference index (PI) was calculated by subtracting the number of larvae in the dH2O semicircle from the number of larvae in the FRU semicircle and dividing that number by the total number of larvae on the plate.
Olfactory conditioning
Olfactory conditioning was performed similarly to Scherer et al. (2003) with modifications for an en masse protocol outline by Neuser et al. (2005). Larvae were reared at densities of 100 larvae/35 mL of medium in 100 × 1-mm Petri dishes and tested at mid-third instar (96 ± 3 h post-hatch). A total of 2.0 μL of pure odorant was used for all training and tests. Larvae were trained in groups of 30, with reciprocal groups trained simultaneously (30 larvae receiving PA paired with 2.0 M fructose–agarose reward were trained at the same time as 30 larvae receiving BUT paired with 2.0 M fructose–agarose reward). In the PA+FRU condition, larvae were given three 1-min pairings of PA with fructose–agarose, alternated with three 1-min pairings of BUT with plain agarose. In the BUT+FRU condition, larvae were given three 1-min pairings of BUT with fructose–agarose, alternated with three 1-min pairings of PA with plain agarose (Fig. 2A). When testing for the effects of increasing the length of training, larvae were given the above protocol with eight pairings instead of three (Fig. 4A). Preference indexes (PI) were calculated by subtracting the number of larvae on the PA side from the number of larvae on the BUT side, and dividing that number by the total amount of larvae on the plate. The learning indexes (LI) were calculated by subtracting the PI(BUT+FRU) from the PI(PA+FRU) and dividing that number by two to yield values between 1 and −1. Orientation of the PA and BUT training plates and sides of PA and BUT during the test were rotated to control for side and placement preferences. The sequence of pairings between odor and reward was also randomized to ensure no effect receiving reward before non-reward plate or vice versa.
Visual sensitivity
Visual sensitivity tests were modified from Sawin-McCormack et al. (1995). Larvae were placed along the midline of a plain agarose plate, which was divided into two dark quadrants and two light quadrants, each opposite each other, for 45 sec. A preference index (PI) was calculated by subtracting the number of larvae on the dark quadrants from the number of larvae in the light quadrants and dividing that number by the total number of larvae on the plate.
Visual conditioning
Visual conditioning was performed similarly to Gerber et al. (2004a) with the following exceptions. Larvae were reared at densities of 100 larvae/35 mL of medium in 100 × 15-mm Petri dishes and trained and tested at mid-third instar (96 ± 3 h post-hatch). Larvae were trained in groups of 30 with reciprocal groups trained simultaneously (30 larvae receiving light paired with 2.0 M fructose–agarose reward were trained at the same time as 30 larvae receiving dark paired with 2.0 M fructose–agarose reward). In the light+FRU condition, larvae were given three 1-min pairings of light with fructose–agarose, alternated with three 1-min pairings of dark with plain agarose. In the dark+FRU condition, larvae were given three 1-min pairings of dark with fructose–agarose, alternated with three 1-min pairings of light with plain agarose (Fig. 1A). In a revised test, larvae were given the above protocol with 2-min pairings (Fig. 1B). Larvae were tested on a plain agarose plate with two dark quadrants and two light quadrants (each opposite each other) for 45 sec. Preference indexes (PI) were calculated by subtracting the number of larvae on the light quadrants from the number of larvae on the dark quadrants, then dividing that number by the total number of larvae on the plate. The learning indexes (LI) were calculated by subtracting the PI(Light+FRU) from the PI(Dark+FRU) and dividing that number by two. Orientation of dark and light training plates and dark and light quadrants in the test plate were rotated to control for side and placement preference. The sequence of pairings between odor and reward was also randomized to ensure no effect receiving reward before nonreward plate or vice-versa.
Fluorescent imaging and microcopy
Brains of third instar w1;fors;UAS-GFP/H24, w1;fors/201Y;UAS-GFP, w1;fors/30Y;UAS-GFP, or w1;fors/c739;UAS-GFP larvae were dissected and fixed for 5 min in a phosphate-buffered saline solution (PBS, 2.5 mM NaH2PO4, 8.5 mM Na2HPO4, and 175 mM NaCl at pH 7.4) with 4% paraformaldehyde. After rinsing in PBS and soaking in 60% glycerol for 5 min, samples were mounted and immediately imaged using the Argon-2 laser in a Zeiss Axioplan 2.0 confocal microscope. Images were acquired using a 20× objective and digital zoom. Images were scanned at resolution of 1024 × 1024 pixels. Z-stacks were acquired with scans taken every 4 μm. Adobe Photoshop CS was used to tile images and to enhance contrast on whole images for Figure 3A.
Statistical analysis
JMP/IN 5.1 was used for all statistical analyses (SAS Institute, Inc.). LI were used for all statistical analysis. One-way analyses of variances (ANOVA) were performed in addition to nonparametric Wilcoxon tests where necessary using P < 0.05 as significant. Levene and Bartlett tests were used to test for unequal variances. Pairwise comparisons were performed with Student Neuman-Keuls post-hoc comparisons using P < 0.05 as significant. Wilcoxon tests were used to test significance of LI values against zero to indicate whether larvae showed a significant learning index (using P < 0.05 as significant).
Acknowledgments
We thank Joel Levine for the mushroom-body drivers, and Matthew Cobb for suggesting propyl acetate and butanol as odorants for the olfactory conditioning assay. We also thank Evan Ardiel, Jason Asselin, Nicholas Dube, and Reza Azanchi for their help in the larval olfactory conditioning experiments. We thank Joel Levine, Scott Douglas, Frederic Mery, and Tadeusz Kawecki for their comments on the manuscript. This work was funded by a Canadian Institute of Health Research Grant, the Canada Research Chair Program Grant to M.B.S., and by a Heisenberg fellowship of the Deutsche Forschungsgemeinschaft to B.G. A Natural Sciences and Engineering Research Council CGS-D grant supported K.R.K.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.505807
References
- Ache B.W., Young J.M. Olfaction: Diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Arancio O., Antonova I., Gambaryan S., Lohmann S.M., Wood J.S., Lawrence D.S., Hawkins R.D. Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation. J. Neurosci. 2001;21:143–149. doi: 10.1523/JNEUROSCI.21-01-00143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier A., Wittek B., Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Barnard C.J., Collins S.A., Daisley J.N., Behnke J.M. Odour learning and immunity costs in mice. Behav. Processes. 2006;72:74–83. doi: 10.1016/j.beproc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Belay A.T., Scheiner R., So A.K.-C., Chakaborty-Chatterjee M., Levine J.D., Sokolowski M.B. The foraging gene of Drosophila melanogaster: Spatial-expressing analysis and sucrose responsiveness. J. Comp. Neurol. 2007 doi: 10.1002/cne.21466. (in press) [DOI] [PubMed] [Google Scholar]
- Davis R.L. Olfactory memory formation in Drosophila: From molecular systems neuroscience. Annu. Rev. Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- de Belle J.S., Sokolowski M.B. Heredity of rover-sitter alternative foraging strategies of Drosophila melanogaster larvae. Heredity. 1987;59:73–84. [Google Scholar]
- de Belle J.S., Hilliker A.J., Sokolowski M.B. Genetic localization of foraging (for): A major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123:157–163. doi: 10.1093/genetics/123.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R. Costs of memory: Ideas and predictions. J. Theor. Biol. 1999;197:41–50. doi: 10.1006/jtbi.1998.0856. [DOI] [PubMed] [Google Scholar]
- Engel J.E., Xie X.J., Sokolowski M.B., Wu C.F. A cGMP-dependent protein kinase gene, foraging, modifies habituation-like response decrement of the giant fiber escape circuit in Drosophila. Learn. Mem. 2000;7:341–352. doi: 10.1101/lm.31600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiel R., Hartmann J., Luo C., Wolfsgruber W., Schilling K., Fiel S., Barski J.J., Meyes M., Konnerth A., De Zeeuw C.I., et al. Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase 1. J. Cell Biol. 2003;163:295–302. doi: 10.1083/jcb.200306148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiel R., Hofmann F., Kleppisch T. Function of cGMP-dependent protein kinases in the nervous system. Rev. Neurosci. 2005;16:23–41. doi: 10.1515/revneuro.2005.16.1.23. [DOI] [PubMed] [Google Scholar]
- Garcia J., McGowan B.K., Ervin F.R., Koelling R.A. Cues: Their relative effectiveness as a function of the reinforcer. Science. 1968;160:794–795. doi: 10.1126/science.160.3829.794. [DOI] [PubMed] [Google Scholar]
- Gerber B., Scherer S., Neuser K., Michels B., Hendel T., Stocker R.F., Heisenberg M. Visual learning in indivually assayed Drosophila larvae. J. Exp. Biol. 2004a;207:179–188. doi: 10.1242/jeb.00718. [DOI] [PubMed] [Google Scholar]
- Gerber B., Tanimoto H., Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr. Opin. Neurobiol. 2004b;14:737–744. doi: 10.1016/j.conb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Hartell N.A. cGMP acts within cerebellar Purkinje cells to produce long term depression via mechanisms involving PKC and PKG. Neuroreport. 1994;5:833–836. doi: 10.1097/00001756-199403000-00024. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Borst A., Wagner S., Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J. Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Feil R., Kleppisch T., Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- Honjo K., Furukubo-Tokunaga K. Induction of camp response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J. Neurosci. 2005;25:7905–7913. doi: 10.1523/JNEUROSCI.2135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner W.J., Crocker A., White B.H., Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Rubin G.M. cGMP-dependent protein kinase genes in Drosophila. J. Biol. Chem. 1989;264:10738–10748. [PubMed] [Google Scholar]
- Kim Y.C., Lee H.G., Han K.A. Classical reward conditioning in Drosophila melanogaster. Genes Brain Behav. 2007;6:201–207. doi: 10.1111/j.1601-183X.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Kleppisch T., Wolfsgruber W., Feil S., Allmann R., Wotjkak C.T., Goebbels S., Nave K.A., Hoffmann F., Feil R. Hippocampal cGMP-dependent protein kinase 1 supports an age- and protein synthesis-dependent component of long-term potentiation but is not essential for spatial reference and contextual memory. J. Neurosci. 2003;23:6005–6012. doi: 10.1523/JNEUROSCI.23-14-06005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher S.A., Kwon J.Y., Carlson J.R. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V., Jiang T., Wood J., Lawrence D.S., Tsien R.Y. Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron. 1997;36:1079–1089. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- Lui G., Seiler H., Wen A., Zars T., Ito K., Wolf R., Heisenberg M., Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Margulies C., Tully T., Dubnau J. Deconstructing memory in Drosophila. Curr. Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S.E., Deshazer M., Davis R.L. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog. Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Melzig J., Rein K.H., Pfister H., Jackle H., Heisenberg M., Raabe T. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr. Biol. 1998;8:1223–1226. doi: 10.1016/s0960-9822(07)00514-3. [DOI] [PubMed] [Google Scholar]
- Mery F., Kawecki T.J. A cost of long-term memory in Drosophila. Science. 2005;308:1148. doi: 10.1126/science.1111331. [DOI] [PubMed] [Google Scholar]
- Micheau J., Riedel G. Protein kinases: Which one is the memory molecule? Cell. Mol. Life Sci. 1999;55:521–524. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuser K., Husse J., Stock P., Gerber B. Appetitive olfactory learning in Drosophila larvae: Effects of repetition, reward strength, age, gender, assay type and memory span. Anim. Behav. 2005;69:891–898. [Google Scholar]
- Osborne K.A., Robichon A., Burgess E., Butland S., Shaw R.A., Coulthard A., Pereira H.S., Greenspan R.J., Sokolowski M.B. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Pereira H.S., Sokolowski M.B. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc. Natl. Acad. Sci. 1993;90:5044–5046. doi: 10.1073/pnas.90.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman J.L., McGill J.J., Keegan K.P., Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- Sawin-McCormack E.P., Sokolowski M.B., Campos A.R. Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J. Neurogenet. 1995;10:119–135. doi: 10.3109/01677069509083459. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stocker R., Gerber B. Olfactory conditioning in individually assayed Drosophila larvae. Learn. Mem. 2003;10:217–225. doi: 10.1101/lm.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R., Sokolowski M.B., Erber J. Activity of cGMP-dependent protein kianse (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learn. Mem. 2004;11:303–311. doi: 10.1101/lm.71604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Freggi-Grelen F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversize and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M.B. Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behav. Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Sokolowski M.B. Drosophila: Genetics meets behaviour. Nat. Rev. Genet. 2001;2:879–890. doi: 10.1038/35098592. [DOI] [PubMed] [Google Scholar]
- Spradling A.C., Rubin G.M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Tempel B., Bonini N., Dawson D.R., Quinn W.G. Reward learning in normal and mutant Drosophila. Proc. Natl. Acad. Sci. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M., Armstrong J.D., Endo K., Yang M.Y., Furukubo-Tokunaga F., Kaiser K., Reichert H. Early development of the Drosophila mushroom bodies, brain centres for associative learning and memory. Dev. Genes Evol. 1997;207:242–252. doi: 10.1007/s004270050112. [DOI] [PubMed] [Google Scholar]
- Whitlock J.R., Heynen A.J., Shuler M.G., Bear M.F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wolf R., Wittig T., Liu L., Wiestmann G., Eyding D., Heisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learn. Mem. 1998;5:166–178. [PMC free article] [PubMed] [Google Scholar]
- Yang M.Y., Armstrong J.D., Vilinsky I., Strausfeld N.J., Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions in the insect mushroom bodies. Curr. Opin. Neurobiol. 2000;10:790–795. doi: 10.1016/s0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Hu Y., Schultz C., Kandel E.R., Hawkins R.D. Role of guanylyl cylase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368:635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]