Abstract
Previous experiments in the hippocampal CA1 area have shown that corticosterone can facilitate long-term potentiation (LTP) in a rapid non-genomic fashion, while the same hormone suppresses LTP that is induced several hours after hormone application. Here, we elaborated on this finding by examining whether corticosterone exerts opposite effects on LTP depending on the timing of hormone application in the dentate gyrus as well. Moreover, we tested rapid and delayed actions by corticosterone on β-adrenergic-dependent changes in LTP. Unlike the CA1 region, our in vitro field potential recordings show that rapid effects of corticosterone do not influence LTP induced by mild tetanization in the hippocampal dentate gyrus, unless GABAA receptors are blocked. In contrast, the β-adrenergic agonist isoproterenol does initiate a slow-onset, limited amount of potentiation. When corticosterone was applied concurrently with isoproterenol, a further enhancement of synaptic strength was identified, especially during the early stage of potentiation. Yet, treatment with corticosterone several hours in advance of isoproterenol fully prevented any effect of isoproterenol on LTP. This emphasizes that corticosterone can regulate β-adrenergic modulation of synaptic plasticity in opposite directions, depending on the timing of hormone application.
Living organisms like mammals experience stressful situations regularly in their lives. Stress, be it of a physical or psychological nature, potentially disrupts physiological homeostasis. Two systems are subsequently activated, which help to adapt to environmental changes and restore physiological balances: Activation of the autonomous nervous system (ANS) triggers the release of adrenaline from the adrenal medulla; the hypothalamo-pituitary-adrenal (HPA) axis elicits secretion of glucocorticoids (cortisol in humans, corticosterone in rodents) from the adrenal cortex. Although glucocorticoids can cross the blood–brain barrier easily, adrenaline does not readily reach the central nervous system; however, it can stimulate peripheral vagal afferents and lead to central release of noradrenaline from both the nucleus of the solitary tract and the locus coeruleus (Roosevelt et al. 2006). These hormones target many areas, including those involved in the processing of the information about the stressful event, i.e., the hippocampus and the amygdala. Each of these structures is specialized in formulating specific aspects of the ongoing event; yet, their outputs integrate and underlie the memory of an overall picture of the experienced “stress” (Kim and Diamond 2002; McGaugh 2004; Richter-Levin 2004).
Typically, the amygdala and the hippocampus are extensively involved in behavioral tasks like inhibitory avoidance or fear conditioning (Richter-Levin 2004; Roozendaal et al. 2006). These behaviors are affected by corticosterone and noradrenaline. Thus, post-training microinjection of noradrenaline or β-adrenergic agonists into the basolateral amygdala enhances memory consolidation in inhibitory avoidance task (Ferry and McGaugh 1999; Lalumiere and McGaugh 2005), as well as in contextual fear conditioning (LaLumiere et al. 2003) and in spatial water-maze task (Hatfield and McGaugh 1999). Likewise, glucocorticoid receptor (GR) agonists microinfused into the basolateral amygdala (Roozendaal and McGaugh 1997b) or the hippocampus (Roozendaal and McGaugh 1997a; Oitzl et al. 2001) enhance memory retention. It was subsequently shown (Roozendaal et al. 2002) that noradrenergic activation is essential for the memory-enhancing effects, and that glucocorticoids play a permissive role in noradrenergic actions, thus promoting memory formation. Notably, it was suggested that the interaction between the two hormones should occur within an interval of 30 min, which is rather a short time-interval for gene-mediated events to occur (Makara and Haller 2001; Roozendaal 2003).
Yet, glucocorticoids can also suppress noradrenaline-mediated regulation. Thus, adrenalectomy impairs memory in an inhibitory avoidance task; this deficit could be rescued by post-training administration of adrenaline (Borrell et al. 1984). Pretreatment with corticosterone 1 h before adrenaline dramatically reduced the efficacy of adrenaline. A similar result was also observed at the cellular level in the hippocampus (Joels and de Kloet 1989), where noradrenaline increases the excitability of CA1 pyramidal neurons from adrenalectomized animals; treatment of tissue with GR agonists 1–4 h prior to noradrenaline attenuated such increases. Given the delayed action of corticosterone, it is likely that these corticosteroid effects involve gene-mediated mechanisms (Reichardt and Schutz 1998; Vreugdenhil et al. 2001; de Kloet et al. 2005; Zhou and Cidlowski 2005).
Collectively, these observations have led to the hypothesis that corticosterone can enhance memory formation or boost noradrenergic actions when these elements coincide in time, whereas the hormone suppresses these activities when it reaches the same circuits some hours in advance of a learning event (Joels et al. 2006). This is in line with recent observations in the hippocampal CA1 area, showing that corticosterone can enhance long-term potentiation (LTP)—presently the best-described neurobiological substrate of learning and memory (Martin and Morris 2002; Morris 2003)—when the hormone is applied around the time of LTP induction (Wiegert et al. 2006), while it suppresses LTP when given several hours in advance (Alfarez et al. 2002; Wiegert et al. 2005).
The present study aimed to acquire further evidence for this hypothesis in the rat hippocampal dentate gyrus (DG). To this end, we first examined the effects of corticosterone alone on LTP, under conditions where the hormone was either present during tetanic stimulation or several hours in advance. Next, we tested the effects of corticosterone on noradrenergic regulation of LTP, based on the known fact that noradrenergic activation generally facilitates LTP in the dentate gyrus (Stanton and Sarvey 1985, 1987; Dahl and Sarvey 1990; Chaulk and Harley 1998; Bronzino et al. 2001; Frey et al. 2001; Straube and Frey 2003). We specifically tested (1) whether corticosterone administration around the time of LTP induction and application of the β-adrenergic agonist isoproterenol promotes the β-adrenergic action on LTP, and (2) whether corticosterone administration more than 2 h earlier suppresses the isoproterenol-mediated effect.
Results
LTP induction in the dentate gyrus with theta burst stimulation
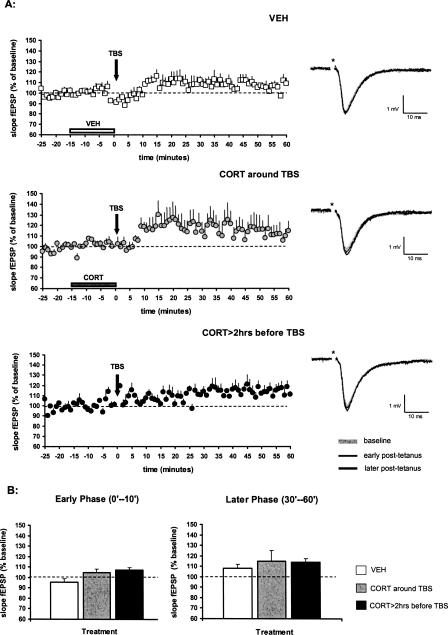
We observed that theta burst stimulation (TBS) alone was insufficient to evoke LTP in the dentate gyrus, as illustrated in the control group where the vehicle medium of corticosterone was perfused for 15 min and coterminated with TBS (Fig. 1A). In this control group, TBS resulted in an insignificant increase in the slope of the field excitatory postsynaptic potentials (mean slope fEPSP ± SEM over the post-tetanus 60 min: 107 ± 3%, n = 10) compared with the pre-TBS baseline response. The early component of potentiation (here defined as the average fEPSP slope over post-tetanus 0–10 min) was 96 ± 3% of the baseline value, while the later component (defined as the average fEPSP slope during post-tetanus 30–60 min) amounted to 108 ± 4%.
Figure 1.
Effect of corticosterone on the slope of the fEPSP evoked in the dentate gyrus by perforant path stimulation. (A) In aCSF (with vehicle perfusion, as indicated by the horizontal bar, n = 10), theta burst stimulation (TBS) does not induce LTP (top). Brief perfusion of 100 nM corticosterone, just before and during TBS, resulted in a slight increase in synaptic responses, but this did not reach statistical significance (middle, n = 11). Pre-incubation with 100 nM corticosterone for 20 min >2 h prior to TBS did not modify post-tetanus synaptic responses (bottom, n = 4). The symbols represent the mean (+ SEM) slope of the fEPSP. (VEH) Vehicle; (CORT) corticosterone. The representative analog traces of recordings are shown on the right, respectively; the asterisk indicates the stimulus artifact. (B) Mean (+ SEM) synaptic responses during the early phase after TBS (here defined as post-tetanus 0–10 min; left) or later phase after TBS (post-tetanus 30–60 min; right) revealed no significant effect of corticosterone treatment compared with vehicle treatment (white bars). The absence of any effect was seen both when the hormone was applied just prior to and during tetanic stimulation (gray bars; using the protocol as indicated in the middle graph in A), and when corticosterone was briefly perfused more than 2 h before TBS (dark bars; using the protocol as indicated in the bottom graph in A).
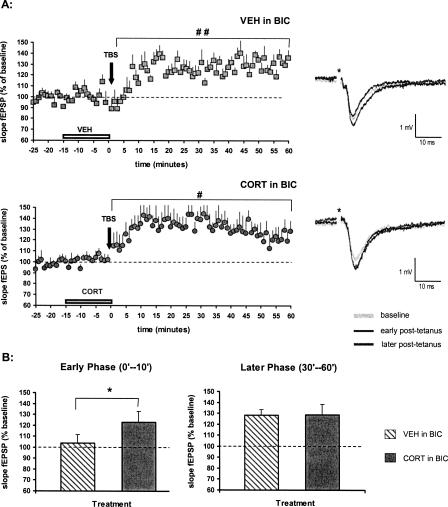
As suggested by earlier reports, TBS can become effective in the dentate gyrus, when GABAA inhibitory activity is prevented (Alfarez et al. 2003; Boekhoorn et al. 2006). This was confirmed in the current study if the vehicle group was tested in the presence of 10 μM (−)-bicuculline methiodide. With bicuculline supplemented to aCSF, the mean fEPSP slope ±SEM over 60 min, subsequent to TBS, was 124 ± 5% (n = 7, P < 0.01) of the baseline level (Fig. 2A). Early phase of potentiation (post-tetanus 0–10 min) amounted to 104 ± 8% and later phase (post-tetanus 30–60 min) to 128 ± 5% (P < 0.01). We conclude that TBS alone is insufficient to induce LTP in vitro in the dentate gyrus unless GABAA receptors are blocked simultaneously.
Figure 2.
Effects of corticosterone on synaptic potentiation in the DG, in the presence of bicuculline. (A) When 10 μM bicuculline (BIC) was continuously present during recording, TBS stimulation did result in a significant and lasting potentiation of the fEPSP slope with vehicle (top, n = 7). Clear synaptic potentiation was also seen with TBS after brief perfusion with 100 nM corticosterone (bottom, n = 8). The symbols represent the mean (+ SEM) slope of the fEPSP. The representative analog traces of recordings are shown on the right, respectively; the asterisk indicates the stimulus artifact. (#) P < 0.05, (##) P < 0.01, compared with the pre-tetanus baseline. (B) The mean (+ SEM) fEPSP slope in the early phase after TBS (post-tetanus 0–10 min, left) was not increased in the vehicle-treated animals (diagonally striped bar) in bicuculline. However, synaptic potentiation was significantly enhanced by perfusion of corticosterone (gray bar). In the later phase after TBS (post-tetanus 30–60 min, right), both groups revealed a comparable degree of synaptic potentiation without between-group difference. (*) P < 0.05, comparison with the vehicle treatment.
Effects of corticosterone on LTP
Application of 100 nM corticosterone (in the absence of bicuculline), just before and during TBS, did not significantly influence synaptic responses after the tetanus. Synaptic responses over the post-tetanus 60 min amounted to 115 ± 9% (mean slope fEPSP ± SEM, n = 11) of the baseline value (Fig. 1A), with 105 ± 3% and 115 ± 10% in the early (post-tetanus 0–10 min) and later (post-tetanus 30–60 min) phases, respectively. In comparison with the vehicle-treated slices, perfusion of corticosterone around the time of TBS did not result in changes in synaptic responses with regard to both early and later components of potentiation (Fig. 1B). Apparently, corticosterone cannot potentiate synaptic responses in the DG when linked to a subthreshold protocol of LTP.
Next, slices were incubated for 20 min with 100 nM corticosterone more than 2 h before TBS. Over the post-synaptic 60 min, synaptic responses amounted to 111 ± 2% (n = 4) of its pre-tetanus value, which is very similar to the values seen in vehicle-treated control slices (Fig. 1A). Also, synaptic responses during both the early phase and later phase after TBS were indistinguishable from those of slices perfused with vehicle only (Fig. 1B).
We considered the possibility that corticosterone can become effective under conditions that GABAergic transmission is suppressed and LTP is induced, i.e., in the presence of 10 μM bicuculline. Previously, we reported that under those conditions a brief pulse of corticosterone 1–4 h in advance of TBS tends to suppress potentiation, but this effect did not reach significance (Alfarez et al. 2003). We presently completed these observations in the presence of bicuculline by examining putative nongenomic effects of corticosterone in the DG, i.e., when corticosterone was administered just before and during TBS. Interestingly, we observed that in the presence of bicuculline, such application of corticosterone results in a clear form of LTP over 60 min (mean slope fEPSP ± SEM: 130 ± 9%, n = 8, P < 0.05) (Fig. 2A) compared with the pre-tetanus values. Early phase of potentiation (post-tetanus 0–10 min) was 123 ± 10% (P = 0.055) of the baseline value and later phase (post-tetanus 30–60 min) was 129 ± 9% (P < 0.05). Compared with the vehicle-treated slices in bicuculline subjected to TBS, a significant enhancement in synaptic response was found in the corticosterone versus vehicle-treated group with respect to the early (P < 0.05), but not the later phase of potentiation (Fig. 2B).
To examine the effect of corticosterone on isoproterenol-mediated actions (next section), we chose to record under conditions that are as close as possible to the “natural” situation, i.e., with intact GABAergic activity. As reported above, corticosterone by itself—when applied shortly before TBS or when given more than 2 h in advance—does not significantly affect synaptic potentiation induced by TBS under those circumstances.
Effect of corticosterone on isoproterenol-mediated actions
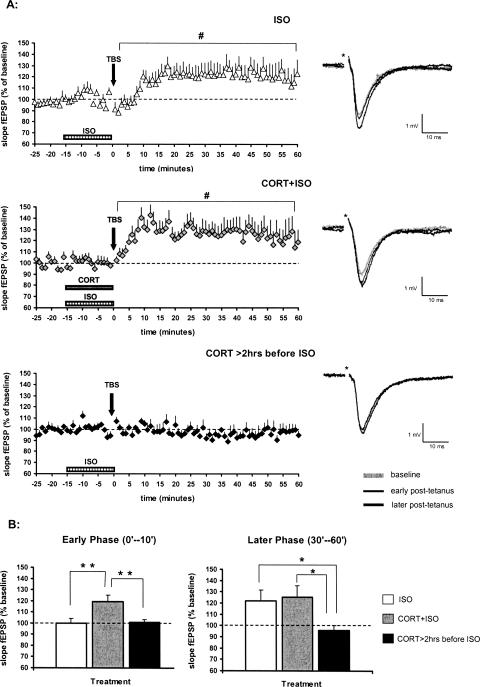
The β-adrenergic agonist (−)-isoproterenol (+)-bitartrate (1.0 μM) was rapidly applied by perfusion for 15 min before and during TBS; this was done to test whether β-adrenoceptor activation could facilitate LTP induction by TBS. Results show that after a brief administration of isoproterenol, mild potentiation was observed over the post-tetanus 60 min (mean slope fEPSP ± SEM: 118 ± 7%, n = 11, P < 0.05) (Fig. 3A). Potentiation was not manifest during the first 10 min after tetanus; however, it slowly developed over time. Early responses (post-tetanus 0–10 min) amounted to 100 ± 5% of the baseline value, and later responses (post-tetanus 30–60 min) to 122 ± 10% (P < 0.05). These data indicate that β-adrenergic activation can mildly facilitate synaptic responses after TBS; this facilitation particularly pertains to the later phase of LTP.
Figure 3.
Time-dependent effect of corticosterone on β-adrenergic actions. (A) Brief perfusion of 1.0 μM isoproterenol (ISO) just before and during TBS (indicated by horizontal bar) induced a slow-onset potentiation of the fEPSP slope (top, n = 11). Concurrent perfusion of isoproterenol and corticosterone (middle, n = 9) immediately evoked LTP after TBS. Brief administration of corticosterone (20 min, CORT) more than 2 h in advance of isoproterenol perfusion completely prevented the occurrence of LTP (bottom, n = 6). All symbols represent the mean (+ SEM) slope of the fEPSP. The representative analog traces of recordings are shown on the right, respectively; the asterisk indicates the stimulus artifact. (#) P < 0.05, compared with the pre-tetanus baseline. (B) Coapplication of corticosterone and isoproterenol (gray bar) during perfusion enhanced the mean (+ SEM) fEPSP slope during the early phase after TBS (post-tetanus 0–10 min, left) when compared with the slices perfused with isoproterenol only (white bar). No differences were found between the groups with respect to the later phase after TBS (post-tetanus 30–60 min, right). Pretreatment of corticosterone more than 2 h in advance of isoproterenol application (dark bar) prevented synaptic potentiation as could be found in the coapplication group during the early phase, and abolished the potentiation as could be found in both coapplication and isoproterenol-alone groups during the later phase after TBS. (*) P < 0.05, (**) P < 0.01, based on between-group comparisons.
Interestingly, when 100 nM corticosterone was co-applied with 1.0 μM isoproterenol, synaptic potentiation was already observed during the early phase after TBS. Synaptic responses over the post-tetanus 60 min were 126 ± 8% of the baseline value (mean slope fEPSP ± SEM, n = 9, P < 0.05) (Fig. 3A). Average fEPSPs were significantly enhanced with respect to the early phase (post-tetanus 0–10 min: 119 ± 6%, P < 0.05) and the later phase (post-tetanus 30–60 min: 125 ± 11%, P < 0.05) in comparison with the baselines. Early phase of potentiation—when corticosterone and isoproterenol were co-applied—was significantly different from that of the slices perfused with isoproterenol only (P < 0.01), with corticosterone only (P < 0.05), or with vehicle only (P < 0.01); however, no significant differences were identified among these groups with regard to the later phase of potentiation (Fig. 3B).
In order to examine whether isoproterenol-mediated effects on LTP can be differently modulated by corticosterone via a delayed action, 100 nM corticosterone was briefly (for 20 min) applied to the slices more than 2 h in advance of isoproterenol perfusion; isoproterenol perfusion coterminated with TBS. An interval of 2 h was chosen to ensure that gene-mediated effects can take place (Karst and Joels 2005; Wiegert et al. 2005; Morsink et al. 2006). Contrary to what was seen with isoproterenol only or with concurrent perfusion of both hormones, isoproterenol did not affect synaptic responses after TBS if corticosterone was applied to the same slice a few hours earlier. In that case, the mean fEPSP slope over the post-tetanus 60 min was 97 ± 4% (mean slope fEPSP ± SEM, n = 6) of the baseline (Fig. 3A), comparable to the control group not subjected to any hormonal treatment (Fig. 1A). During the early post-tetanus phase (post-tetanus 0–10 min), average responses (101 ± 2%) were significantly smaller than those of slices where corticosterone and isoproterenol had been co-applied (P < 0.01), but did not differ from those of slices perfused with isoproterenol only (Fig. 3B). With regard to the later phase (post-tetanus 30–60 min), synaptic responses (96 ± 5%) were significantly reduced in comparison with the slices perfused with both hormones (P < 0.05), or perfused with isoproterenol only (P < 0.05) (Fig. 3B).
Baseline transmission with stress hormones
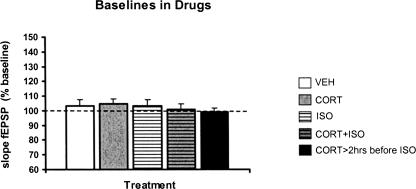
Baseline synaptic responses in response to half-maximal stimulation during the perfusion period with: (1) corticosterone, (2) isoproterenol, (3) isoproterenol together with corticosterone, or (4) corticosterone incubation >2 h before isoproterenol perfusion, neither demonstrated within-group differences compared with their (10 min) pre-perfusion baselines, nor between-group differences compared with the vehicle-perfused group (Fig. 4). These data suggest that synaptic basal transmission was not altered by a brief administration of stress hormone(s) at the present dosages.
Figure 4.
Baseline transmission during drug perfusion was not affected by stress hormones. The mean (+ SEM) slope of the fEPSP induced by half-maximal stimulation of perforant path afferents was not significantly altered by perfusion of corticosterone and/or isoproterenol. This was true when signals were compared with the average of the pretreatment baseline signal for each condition shown below, and when signals recorded during the various drug applications were compared with each other. The observations were based on the following number of animals for each condition: (VEH) white bar, n = 11; (CORT) gray bar, n = 12; (ISO) white-striped bar, n = 11; (CORT+ISO) gray-striped bar, n = 9; (CORT>2hrs before ISO) dark bar, n = 6.
Discussion
In this study we tested the hypothesis that corticosterone time dependently affects LTP and β-adrenergic actions in the rat DG. Based on earlier findings in the hippocampal CA1 area (Wiegert et al. 2005, 2006), we presumed that in the DG, corticosterone—in a rapid nongenomic fashion—promotes LTP and noradrenergic efficacy, while the same hormone has suppressive effects when acting through slow gene-mediated pathways. We observed that in the DG, corticosterone can enhance the early phase of synaptic potentiation only when GABAA-receptor-mediated transmission is blocked; delayed suppression by the hormone, though, was not seen using the present stimulation protocol. Isoproterenol potentiated particularly the later phase of LTP. When corticosterone was coapplied with isoproterenol, a significant enhancement of the early, but not the later LTP phase was observed. Finally, brief administration of corticosterone several hours before application of isoproterenol markedly suppressed the efficacy of isoproterenol to potentiate the later phase of LTP. These observations are largely in line with the hypothesis, although the effects of corticosterone were in some aspects unexpected.
Effect of corticosterone on LTP in the DG
Under in vitro recording conditions, mature DG granule cells exhibit a quite negative resting membrane potential (Liu et al. 1996). Moreover, these neurons are subject to a strong GABAergic inhibitory tone (Nusser and Mody 2002; Chandra et al. 2006). Consequently, it is not easy to induce robust synaptic potentiation in mature DG cells in vitro (Wang et al. 2000). This was confirmed in the present experiments. Under those conditions, corticosterone was unable to facilitate synaptic potentiation after TBS in a rapid nongenomic fashion. However, when the GABAergic inhibitory tone was relieved by applying bicuculline, a rapid facilitatory effect of corticosterone, particularly in the first 10 min after TBS, became apparent. Earlier studies in the CA1 area also revealed that corticosterone applied just before and during high-frequency stimulation has particularly strong effects during the first phase of potentiation, while later phases seem somewhat less affected (Wiegert et al. 2006); it should be noted, though, that in the CA1 area as opposed to the DG, corticosterone administered around the time of high-frequency stimulation still significantly enhanced synaptic potentiation over the entire recording period.
The fact that rapid effects of corticosterone in the DG (as opposed to the CA1 area) can only be seen in the presence of bicuculline could signify that the hormone induces subthreshold actions that do not become evident when cells are far removed from the firing threshold, but can become essential when these neurons are in a more depolarized state. We cannot exclude this possibility with the current extracellular recording approach; yet, the fact that corticosterone by itself did not change the basal synaptic response in any respect seems to argue against it. Similarly, it is unlikely that bicuculline-induced enhancement of endogenous noradrenaline release (Barik and Wonnacott 2006) can explain the findings, in view of the continuous perfusion prior to the start of electrophysiological recording, thus washing out most of the endogenously released transmitters. An interesting possibility is that corticosterone could exert rapid effects on GABAergic transmission, which counteract putative facilitatory effects on TBS-induced synaptic potentiation. In support of this view, recent experiments revealed that neurosteroids potentiate tonic GABAergic inhibition in the DG, an effect that involves GABAA receptor δ-units (Stell et al. 2003). If corticosterone is partly metabolized, the actions of these metabolites could mask putative facilitatory actions of the hormone. This differs from the situation in the CA1 area, where δ-subunits (which play a key role in the steroid-induced enhancement of tonic GABAergic inhibition) are much less expressed (Pirker et al. 2000; Peng et al. 2002).
In contrast to the CA1 area, brief administration of corticosterone several hours in advance of high-frequency stimulation did not affect synaptic potentiation, neither in the absence (this study) nor in the presence of bicuculline (Alfarez et al. 2003). In the former case, this could be simply due to the fact that synaptic potentiation with TBS is so weak that suppressive effects by corticosterone cannot be discerned; however, this cannot explain the findings in the presence of bicuculline, where appreciable LTP is observed. As argued earlier, the apparent lack of gene-mediated effects on LTP may be linked to the TBS protocol (Alfarez et al. 2002, 2003). It should be noted, though, that an apparent lack of gene-mediated corticosteroid effects in the DG—presumably via the low-affinity glucocorticoid receptor (GR)—is not unprecedented, as a similar protocol of corticosterone administration also failed to change calcium currents (van Gemert and Joels 2006) and AMPA-receptor-mediated responses (Karst and Joels 2003) in granule cells, while these parameters are clearly altered by the hormone in the CA1 region (Kerr et al. 1992; Karst et al. 2000; Karst and Joels 2005). As DG granule cells, like CA1 pyramidal cells, abundantly express GRs, this could signify that DG granule cells (1) express different receptor variants, (2) are exposed to lower intracellular levels of corticosterone, or (3) contain a different set of cellular proteins that determine the transcriptional activity of the GR (Joels 2006).
All in all, we conclude that under the present recording conditions, corticosterone does not affect synaptic potentiation in the DG, unless the GABAergic inhibitory tone is relieved to reveal a rapid facilitatory effect by the hormone.
Effects of isoproterenol and its modulation by corticosterone
In contrast to corticosterone, the β-adrenergic agonist isoproterenol does rapidly facilitate synaptic potentiation induced by a weak stimulation protocol in the DG. This is in line with most previous studies in the DG (Stanton and Sarvey 1985, 1987; Dahl and Sarvey 1990; Chaulk and Harley 1998; Bronzino et al. 2001; Frey et al. 2001; Straube and Frey 2003), as well as the CA1 region (Katsuki et al. 1997). Several other studies have identified a form of long-lasting potentiation in the DG by applying β-adrenergic agonists without tetanization, which resembles LTP and is NMDA receptor and protein synthesis dependent (Stanton and Sarvey 1987; Sarvey et al. 1989; Dahl and Sarvey 1990). Another study using in vivo microdialysis established an association between increases in hippocampal noradrenaline level and tetanization of the medial perforant pathway (Bronzino et al. 2001). These studies suggest that noradrenaline can act as an endogenous mediator of DG LTP and suffices to result in long-term enhancement of synaptic strength. In our recording conditions, isoproterenol-mediated regulation of baseline activity was not observed. This may be due to the fact that in our set-up isoproterenol levels peaked only briefly, which may be too short to evoke long-lasting potentiation.
The most remarkable finding of our study is that corticosterone can bidirectionally modulate the isoproterenol-mediated effect. When corticosterone was applied several hours in advance of isoproterenol, the former fully prevented the facilitatory actions of the latter. In view of the time delay and the dose of corticosterone that was applied, it seems likely that these actions are mediated via intracellular GRs. In contrast, when corticosterone was coapplied with isoproterenol, the steroid enhanced the early phase of synaptic potentiation, similar to what was seen when bicuculline was perfused (in the absence of isoproterenol). Possibly, corticosterone enhances the availability of the β-adrenergic agonist through mechanisms that resemble its interference with extrasynaptic catecholamine uptake (Grundemann et al. 1998). Also, it cannot be excluded that corticosterone at the short term increases isoproterenol-induced cAMP accumulation, as has been described in thymocytes (Durant et al. 1983). Both pathways, however, would be expected to enhance isoproterenol effects, not only during the early, but also during the later phase of LTP. Rather, the data seem to indicate that corticosterone and isoproterenol enhance synaptic responses independently and through different pathways. Isoproterenol (like bicuculline) may relieve the inhibitory tone in the DG just enough to enable rapid facilitatory effects of corticosterone to develop.
The dichotomy fits well with earlier studies at the behavioral and cellular level. In an inhibitory avoidance task, corticosterone interacted with β-adrenergic activation to facilitate memory consolidation (Roozendaal et al. 2002). In view of the time window in which corticosterone was active, a rapid non-genomic mechanism seems to be indicated (Roozendaal 2003), although the effectiveness of the selective GR agonist RU 283862 argues against this notion. Delayed effects of corticosterone were examined in another study in adrenalectomized rats (Borrell et al. 1984). Post-training application of adrenaline could rescue the memory deficit in inhibitory avoidance behavior seen after adrenalectomy. If corticosterone was given more than1 h before adrenaline, the dose-response relationship was altered and the efficacy of adrenaline was significantly reduced. Along the same line, at the cellular level, noradrenaline was shown to enhance cellular excitability in hippocampal CA1 pyramidal neurons from adrenalectomized rats; when corticosterone was transiently applied more than 1 h in advance, the β-adrenergic enhancement of activity was suppressed (Joels and de Kloet 1989). While these earlier studies suggest evidence for a time-dependent effect of corticosterone, the present study is the first to demonstrate that in a controlled experimental setting, corticosterone acts in the same direction as a β-adrenergic agonist when the two compounds are present in the circuit around the same time, while corticosterone exerts an opposite effect when cells have been exposed to this hormone several hours before β-adrenoceptors are activated.
A similar bidirectional modulation with time was also found in vivo in studies that explored the role of the amygdala on DG LTP. Thus, basolateral amygdala stimulation enhanced DG LTP when amygdala activation was closely linked in time to DG stimulation, while it impaired LTP when amygdala stimulation preceded DG stimulation by more than1 h (Akirav and Richter-Levin 2002; Vouimba and Richter-Levin 2005; Vouimba et al. 2006). Importantly, in both cases, noradrenaline- and corticosterone-mediated mechanisms appeared to be involved.
Functional implications
The presently observed effects can have consequences for DG-dependent encoding and consolidation of information under stressful circumstances. When an organism is stressed, levels of corticosteroids, noradrenaline, and neuropeptides in (among other areas) limbic regions like the amygdala and hippocampal subfields will be raised, i.e., in areas that are enriched with receptors for these factors. The present data indicate that, at least in the DG patterned input that is transferred along, specific afferent fibers will be strengthened by the hormones such that the ensuing synaptic potentiation (which is weak when hormone levels are not elevated) becomes appreciable shortly after arrival of the patterned input and at least up to 60 min later. This may promote encoding of the information associated with the stressful event.
At that time, however, corticosterone will also initiate a gene-mediated cascade that changes cell function several hours later. Based on the present data, it is expected that unrelated information arriving at the same place some hours after the initial stressful event will become difficult to be encoded, especially when it involves a combination of patterned input and elevated noradrenaline levels. This would preserve the initial information and thus promote the consolidation of that event.
Materials and Methods
Animals
The Animal Committee for Bioethics of the University of Amsterdam approved all of the experiments.
Male Wistar rats (Harlan CPB, the Netherlands) were housed in groups, with food and water ad libitum available. A 12 h:12 h light-dark cycle (light-on at 8:00 am) was maintained, and the temperature kept at 20°C–22°C, the humidity at 55% ± 15%. After arrival, the rats were not disturbed for at least 1 wk before the experiments started. The body weights ranged between 200 g and 300 g at the time of the experiment.
In vitro slice preparation
The animals were decapitated early in the morning, between 9:25 and 10:15 h when plasma corticosterone levels are still quite low. The brain was rapidly removed from the skull and immersed in chilled (4°C) dissection buffer that consists of 120 mM NaCl, 3.5 mM KCl, 5.0 mM MgSO4·7H2O, 1.25 mM NaH2PO4, 0.2 mM CaCl2·2H2O, 10 mM glucose, and 25 mM NaHCO3, oxygenated with 95% O2 and 5% CO2. Then, 400-μm-thick horizontal slices were made with a vibroslicer (Leica VT1000S). Slices were kept in artificial cerebrospinal fluid (aCSF) containing 120 mM NaCl, 3.5 mM KCl, 1.3 mM MgSO4·7H2O, 1.25 mM NaH2PO4, 2.5 mM CaCl2·2H2O, 10 mM glucose, and 25 mM NaHCO3, oxygenated with 95% O2 and 5% CO2. Slices remained in aCSF at room temperature for 2 h before being transferred to the recording chamber.
Electrophysiology
One slice at a time was transferred to the recording chamber, where the temperature was maintained at 30°C–32°C. For recordings of field excitatory postsynaptic potentials (fEPSPs) in the hippocampal dentate gyrus, a bipolar stimulation electrode (60 μm in diameter, stainless steel, insulated except for the tip) was placed in the medial perforant pathway and a glass microelectrode (impedance 2–5 MΩ, filled with aCSF) was positioned in the middle third of the molecular layer in the suprapyramidal blade, serving as the recording electrode.
At the beginning of each experiment, an input–output relationship was established by gradually increasing the stimulus intensity until the maximally evoked responses were obtained. The relationship was fit with a sigmoidal function: R(i) = Rmax/{1 + exp[(i − ih)/−S]}, where R(i) represents the response at the intensity (i), Rmax the maximal response, ih the intensity at which the half maximal response is observed, and S represents an index proportional to the slope of the stimulus-response curve. The intensity that evoked the half-maximal response was applied throughout the experiments. The magnitudes of the responses were assessed by measurement of both the slopes and the amplitudes of the fEPSP signals (Alfarez et al. 2003; Krugers et al. 2005). Both parameters yielded comparable results; here, we only report on the slope of the fEPSP.
In most of the experiments, electrophysiological recordings were done in aCSF. In some experiments, GABAergic transmission was inhibited with 10 μM (−)-bicuculline methiodide (Sigma-Aldrich) added to the aCSF.
Protocols and drug application
In the groups of slices that were tested for rapid drug effects, baseline synaptic transmission at half maximal stimulation intensity was monitored for 10 min, which was followed by the perfusion of either: (1) corticosterone (Sigma-Aldrich) 100 nM, (2) the β-adrenergic agonist (−)-isoproterenol (+)-bitartrate (Sigma-Aldrich) 1.0 μM, (3) a combination of 1.0 μM (−)-isoproterenol (+)-bitartrate with 100 nM corticosterone, or (4) vehicle solution, as the control, into aCSF for 15 min. Perfusion coterminated with the tetanus, which consisted of theta-burst stimulation (TBS): a burst of four pulses at 100 Hz, repeated 200 msec later by another four pulses at 100 Hz; this sequence was repeated five times, with an inter-train interval of 30 sec (Alfarez et al. 2003). After TBS, synaptic responses were further monitored for 60 min.
For the groups of slices that were tested with corticosterone pretreatment, slices were incubated with 100 nM corticosterone at 32°C for 20 min starting 1 h after decapitation. These slices then remained in aCSF at room temperature for at least 2 h before being transferred to the recording chamber. For one group, baseline transmission was monitored during 10 min, followed by a 15-min period of perfusion with 1.0 μM (−)-isoproterenol (+)-bitartrate; this coterminated with TBS, and synaptic responses were further observed during 60 min. For the other group, baseline transmission was monitored during 25 min in total, without any perfusion period, then followed by TBS; after TBS, synaptic responses were monitored during 60 min.
Data analysis
Synaptic potentiation after tetanus was expressed as percentage change from the baseline; the average of the measurements during the 25 min pre-tetanus period served as the baseline value. Changes in synaptic response beyond ±10% from the baseline were considered not related to spontaneous fluctuation and were physiologically meaningful. A two-tailed, paired Student’s t-test was used to compare synaptic responses before versus after TBS within each group. The general linear model for repeated measures (GLM) was performed for between-group comparisons of overall differences in LTP, followed by post hoc least-significant difference (LSD) multiple comparison tests. Two-tailed, unpaired Student’s t-tests were additionally conducted to examine the significances. Between-group comparisons were performed (1) for the entire 60-min post-tetanus period, (2) for the initial post-tetanus phase (0–10 min), and (3) for the later phase after tetanus (30–60 min). All data are expressed as average ± SEM; n indicates the number of animals. P-values < 0.05 were accepted as significantly different.
Acknowledgments
Z.P. is supported by grant no. 912-04-042 from the Dutch Organization for Scientific Research NWO.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.527207
References
- Akirav I., Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J. Neurosci. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarez D.N., Wiegert O., Joels M., Krugers H.J. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience. 2002;115:1119–1126. doi: 10.1016/s0306-4522(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Alfarez D.N., Joels M., Krugers H.J. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur. J. Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Barik J., Wonnacott S. Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: Interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol. Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K., Terwel D., Biemans B., Borghgraef P., Wiegert O., Ramakers G.J., de Vos K., Krugers H., Tomiyama T., Mori H., et al. Improved long-term potentiation and memory in young τ-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J. Neurosci. 2006;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell J., de Kloet E.R., Bohus B. Corticosterone decreases the efficacy of adrenaline to affect passive avoidance retention of adrenalectomized rats. Life Sci. 1984;34:99–104. doi: 10.1016/0024-3205(84)90336-9. [DOI] [PubMed] [Google Scholar]
- Bronzino J.D., Kehoe P., Mallinson K., Fortin D.A. Increased extracellular release of hippocampal NE is associated with tetanization of the medial perforant pathway in the freely moving adult male rat. Hippocampus. 2001;11:423–429. doi: 10.1002/hipo.1057. [DOI] [PubMed] [Google Scholar]
- Chandra D., Jia F., Liang J., Peng Z., Suryanarayanan A., Werner D.F., Spigelman I., Houser C.R., Olsen R.W., Harrison N.L., et al. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk P.C., Harley C.W. Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: A role for both α- and β-adrenoceptor activation. Brain Res. 1998;787:59–70. doi: 10.1016/s0006-8993(97)01460-1. [DOI] [PubMed] [Google Scholar]
- Dahl D., Sarvey J.M. β-adrenergic agonist-induced long-lasting synaptic modifications in hippocampal dentate gyrus require activation of NMDA receptors, but not electrical activation of afferents. Brain Res. 1990;526:347–350. doi: 10.1016/0006-8993(90)91245-c. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joels M., Holsboer F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Durant S., Duval D., Homo-Delarche F. Potentiation by steroids of the β-adrenergic agent-induced stimulation of cyclic AMP in isolated mouse thymocytes. Biochim. Biophys. Acta. 1983;762:315–324. doi: 10.1016/0167-4889(83)90086-1. [DOI] [PubMed] [Google Scholar]
- Ferry B., McGaugh J.L. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Frey S., Bergado-Rosado J., Seidenbecher T., Pape H.C., Frey J.U. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J. Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann D., Schechinger B., Rappold G.A., Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat. Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Hatfield T., McGaugh J.L. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol. Learn. Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M., de Kloet E.R. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989;245:1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Joels M., Pu Z., Wiegert O., Oitzl M.S., Krugers H.J. Learning under stress: How does it work? Trends Cogn. Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Karst H., Joels M. Effect of chronic stress on synaptic currents in rat hippocampal dentate gyrus neurons. J. Neurophysiol. 2003;89:625–633. doi: 10.1152/jn.00691.2002. [DOI] [PubMed] [Google Scholar]
- Karst H., Joels M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. J. Neurophysiol. 2005;94:3479–3486. doi: 10.1152/jn.00143.2005. [DOI] [PubMed] [Google Scholar]
- Karst H., Karten Y.J., Reichardt H.M., de Kloet E.R., Schutz G., Joels M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat. Neurosci. 2000;3:977–978. doi: 10.1038/79910. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Izumi Y., Zorumski C.F. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J. Neurophysiol. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Kerr D.S., Campbell L.W., Thibault O., Landfield P.W. Hippocampal glucocorticoid receptor activation enhances voltage-dependent Ca2+ conductances: Relevance to brain aging. Proc. Natl. Acad. Sci. 1992;89:8527–8531. doi: 10.1073/pnas.89.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Diamond D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Krugers H.J., Alfarez D.N., Karst H., Parashkouhi K., van Gemert N., Joels M. Corticosterone shifts different forms of synaptic potentiation in opposite directions. Hippocampus. 2005;15:697–703. doi: 10.1002/hipo.20092. [DOI] [PubMed] [Google Scholar]
- LaLumiere R.T., McGaugh J.L. Memory enhancement induced by post-training intrabasolateral amygdala infusions of β-adrenergic or muscarinic agonists requires activation of dopamine receptors: Involvement of right, but not left, basolateral amygdala. Learn. Mem. 2005;12:527–532. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere R.T., Buen T.V., McGaugh J.L. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J. Neurosci. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.B., Lio P.A., Pasternak J.F., Trommer B.L. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J. Neurophysiol. 1996;76:1074–1088. doi: 10.1152/jn.1996.76.2.1074. [DOI] [PubMed] [Google Scholar]
- Makara G.B., Haller J. Non-genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog. Neurobiol. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Martin S.J., Morris R.G. New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Morris R.G. Long-term potentiation and memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:643–647. doi: 10.1098/rstb.2002.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink M.C., Steenbergen P.J., Vos J.B., Karst H., Joels M., De Kloet E.R., Datson N.A. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J. Neuroendocrinol. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z., Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Reichardt H.M., Joels M., de Kloet E.R. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Hauer B., Mihalek R.M., Homanics G.E., Sieghart W., Olsen R.W., Houser C.R. GABAA receptor changes in δ subunit-deficient mice: Altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. GABAA receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Schutz G. Glucocorticoid signaling––Multiple variations of a common theme. Mol. Cell. Endocrinol. 1998;146:1–6. doi: 10.1016/s0303-7207(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G. The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist. 2004;10:31–39. doi: 10.1177/1073858403259955. [DOI] [PubMed] [Google Scholar]
- Roosevelt R.W., Smith D.C., Clough R.W., Jensen R.A., Browning R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997a;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol. Learn. Mem. 1997b;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Quirarte G.L., McGaugh J.L. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor–cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Okuda S., de Quervain D.J., McGaugh J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Sarvey J.M., Burgard E.C., Decker G. Long-term potentiation: Studies in the hippocampal slice. J. Neurosci. Methods. 1989;28:109–124. doi: 10.1016/0165-0270(89)90016-2. [DOI] [PubMed] [Google Scholar]
- Stanton P.K., Sarvey J.M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J. Neurosci. 1985;5:2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P.K., Sarvey J.M. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res. Bull. 1987;18:115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Stell B.M., Brickley S.G., Tang C.Y., Farrant M., Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Frey J.U. Involvement of β-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: The critical role of the LTP induction strength. Neuroscience. 2003;119:473–479. doi: 10.1016/s0306-4522(03)00151-9. [DOI] [PubMed] [Google Scholar]
- van Gemert N.G., Joels M. Effect of chronic stress and mifepristone treatment on voltage-dependent Ca2+ currents in rat hippocampal dentate gyrus. J. Neuroendocrinol. 2006;18:732–741. doi: 10.1111/j.1365-2826.2006.01472.x. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M., Richter-Levin G. Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cereb. Cortex. 2005;15:1815–1821. doi: 10.1093/cercor/bhi058. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M., Yaniv D., Richter-Levin G. Glucocorticoid receptors and β-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology. 2006;52:244–252. doi: 10.1016/j.neuropharm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E., de Kloet E.R., Schaaf M., Datson N.A. Genetic dissection of corticosterone receptor function in the rat hippocampus. Eur. Neuropsychopharmacol. 2001;11:423–430. doi: 10.1016/s0924-977x(01)00119-5. [DOI] [PubMed] [Google Scholar]
- Wang S., Scott B.W., Wojtowicz J.M. Heterogenous properties of dentate granule neurons in the adult rat. J. Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Wiegert O., Pu Z., Shor S., Joels M., Krugers H. Glucocorticoid receptor activation selectively hampers N-methyl-D-aspartate receptor dependent hippocampal synaptic plasticity in vitro. Neuroscience. 2005;135:403–411. doi: 10.1016/j.neuroscience.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Wiegert O., Joels M., Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn. Mem. 2006;13:110–113. doi: 10.1101/lm.87706. [DOI] [PubMed] [Google Scholar]
- Zhou J., Cidlowski J.A. The human glucocorticoid receptor: One gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]