Abstract
Information that is spaced over time is better remembered than the same amount of information massed together. This phenomenon, known as the spacing effect, was explored with respect to its effect on learning and neurogenesis in the adult dentate gyrus of the hippocampal formation. Because the cells are generated over time and because learning enhances their survival, we hypothesized that training with spaced trials would rescue more new neurons from death than the same number of massed trials. In the first experiment, animals trained with spaced trials in the Morris water maze outperformed animals trained with massed trials, but there was not a direct effect of trial spacing on cell survival. Rather, animals that learned well retained more cells than animals that did not learn or learned poorly. Moreover, performance during acquisition correlated with the number of cells remaining in the dentate gyrus after training. In the second experiment, the time between blocks of trials was increased. Consequently, animals trained with spaced trials performed as well as those trained with massed, but remembered the location better two weeks later. The strength of that memory correlated with the number of new cells remaining in the hippocampus. Together, these data indicate that learning, and not mere exposure to training, enhances the survival of cells that are generated 1 wk before training. They also indicate that learning over an extended period of time induces a more persistent memory, which then relates to the number of cells that reside in the hippocampus.
New neurons are generated continuously over time in the dentate gyrus of the hippocampal formation, a brain region that is important for learning and memory. While thousands of neurons are generated there each day, many of these cells die within a few weeks of their birth (Cameron and McKay 2001). Previously, it was found that learning could enhance the survival of cells if animals were trained on a learning task just as the cells were about to die. The effect was evident after training with several learning tasks, including spatial learning with the Morris water maze (Gould et al. 1999; Leuner et al. 2004, 2006). In the maze task, the animal is placed in a tank of water, which is made opaque, and swims to a platform that is hidden just below the surface of the water. The starting location on each trial is randomized so that the animal learns to use spatial cues in the room to navigate to the platform. The hippocampus is necessary for learning as well as retaining the memory of the platform location (Riedel et al. 1999). There was no effect on cell survival whether animals were trained on a hippocampal-independent version of the task or whether they were placed in the water with no platform for a similar amount of time. Therefore, the increase in cell survival was not induced by motor activity or the stress of the training procedure. Since the initial report, this phenomenon and related ones have been reported (Kempermann and Gage 2002; Dobrossy et al. 2003; Drapeau et al. 2003; Hairston et al. 2005; Kee et al. 2007).
For over a century, it has been recognized that learning and/or memory is enhanced when information is distributed over time when compared with the same amount of information massed together in time (Ebbinghaus 1885; translation Ebbinghaus 1913). The “distribution of practice” (McGaugh 1966) or “the spacing effect” has been demonstrated in a variety of learning models, including word-pair associates in humans (Hser and Wickens 1989), appetitive conditioning in rodents (Lattal 1999), and olfactory avoidance in Drosophila (Yin et al. 1995). The effect is observed in one of the most frequently tested animal learning tasks, the Morris water maze (Klapdor and Van Der Staay 1998; Gerlai 2001). In one study, animals trained with spaced trials performed better than animals trained with massed trials, and as expected, had a better memory after training (Commins et al. 2003). In yet another study, animals trained with massed versus spaced trials performed similarly during training and remembered the platform location equally well when tested shortly after training. However, those trained with spaced trials remembered the location of the platform for a longer period of time than those trained with the same number of massed trials (Spreng et al. 2002). This observation illustrates one of the hallmarks of the spacing effect; memory in animals trained with massed trials gradually decays, while that in animals trained with spaced trials persists, allowing them to express a memory for the stored information (Hintzman 1974).
The functional role of new neurons in spatial learning is not yet clear, but the effects of learning on their survival seem to depend on the age of the cells at the time of training—apparently during a 1 to 2 wk period after they are born. When new neurons are selectively destroyed using either a cytostatic drug, or more recently, highly focused irradiation, spatial learning is not impaired (Shors et al. 2002; Madsen et al. 2003; Saxe et al. 2006). However, a deficit in the expression of a spatial memory emerged 2 wk after training in animals subjected to irradiation (Snyder et al. 2005). Others have found increases in gene activity in the cells as the animals experience a spatial environment some weeks after they are born (Ramirez-Amaya et al. 2006; Kee et al. 2007). Still others report that spatial training decreases cell proliferation and increases apoptosis (Dobrossy et al. 2003; Ambrogini et al. 2004). Together then, the various studies suggest that newly generated cells in the dentate gyrus are sensitive to spatial information, but that they are not used directly in the acquisition of spatial memories. Rather, if anything, they appear to play a role in the retention and/or retrieval of those memories.
In the following study, we examine the potential effect of trial spacing on the survival of newly generated cells in the hippocampus. In the first experiment, we hypothesized that trials distributed across 4 d would enhance learning compared with the same number of trials massed in 1 d. We further hypothesized that spaced training over days would rescue a greater proportion of cells from a single population compared with training that occurs in 1 d. To test this hypothesis, animals were injected with a single dose of BrdU 1 wk before the start of training. Training consisted of either trials spaced over 4 d or massed together in 1 d (Fig. 1A). We expected that training with spaced trials would rescue a greater number of cells than training with the same number of massed trials. In the second experiment, we hypothesized that animals trained with spaced trials would remember the platform location for a longer period of time than those trained with massed trials, and that the new cells would be more likely to survive. To test this hypothesis, animals were injected with BrdU 1 wk before the start of training with either massed or spaced trials. In this study, a 1-h interval was inserted between blocks of massed trials in order to reduce motor fatigue associated with swimming. The memory for the platform location was assessed 2 d and again 2 wk after the end of training. The number of new neurons that remained in the dentate gyrus after the training experiences was determined, as well as the percentage of cells that differentiated into neurons.
Figure 1.
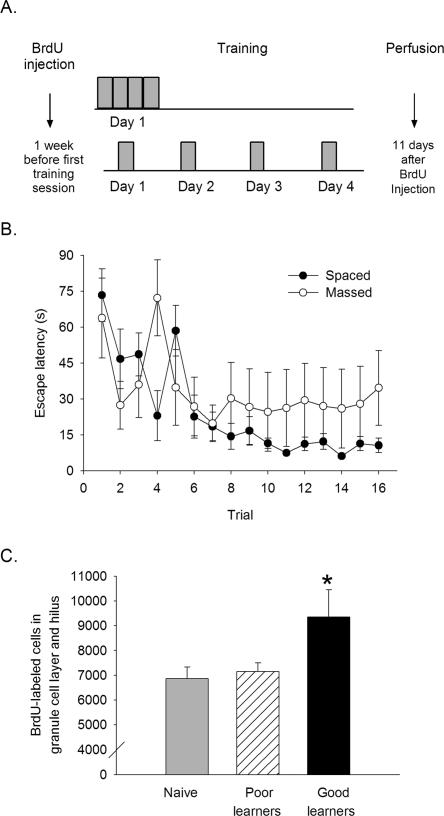
Learning increases cell survival in the hippocampus. (A) BrdU was injected 1 wk before the start of training. Massed subjects were trained with four consecutive blocks of four trials in 1 d. Spaced subjects were trained with one block of four trials for four consecutive days. Animals from both groups (plus a naïve group with no training) were perfused 11 d after BrdU injection. (B) Mean escape latency for massed (○) and spaced (●) animals across 16 training trials. Animals trained with spaced trials were able to navigate to the platform in less time than animals trained with massed trials. (C) The number of BrdU cells that were observed 1 d after the end of spaced training is shown. Animals that were designated as good learners possessed more new cells in their dentate gyrus than did the poor learners and the naïve controls. (*) P < 0.05.
Results
Experiment 1: Acquisition of spatial memories and neurogenesis
Spatial learning in the water maze was assessed by measuring the time for the rat to navigate to the platform (escape latency) for each trial. Data were analyzed using an ANOVA with training condition (massed vs. spaced trials) as the independent measure, escape latency as the dependent measure, and escape latency across trials as the repeated measure. Across training trials, escape latency decreased, indicating that the rats were able to find the hidden platform using the spatial cues surrounding the maze (F(15,165) = 4.68, P < 0.01) (Fig. 1B). By the end of training, animals in the spaced condition outperformed those in the massed condition, i.e., they required less time to locate the platform (F(15,165) = 1.74, P < 0.05) (Fig. 1B). However, the number of BrdU-labeled cells in the dentate gyrus obtained from rats exposed to the massed trials (7540 ± 740) versus spaced trials (8556 ± 874) was not significantly different between groups, nor were numbers different from the numbers of cells in the group of naïve controls (6868 ± 463) (F(2,16) = 1.34, P > 0.05). Therefore, mere exposure to training did not significantly alter the number of cells that survived in the massed or spaced condition. Importantly, some animals in the spaced condition learned poorly and performed similarly to those in the massed condition. Thus, despite training with different trial distribution, individual differences in learning were still observed.
To assess the potential impact of learning on cell survival, we examined the escape latency for individual animals. Animals were grouped into those that learned to find the platform during the last four trials and did so in <10 sec. Using this criterion, five of the eight rats that were trained with spaced trials learned, only one of the five animals trained with massed trials learned, and three trained with spaced trials did not learn. Using this criterion, animals were categorized as good learners (n = 6) or poor learners (n = 7). The numbers of BrdU-labeled cells in these two groups were then compared. Good learners possessed significantly more BrdU-labeled cells (9348 ± 1099) than did the poor learners (7152 ± 347) and naïve controls (Figs. 1C, 2A–D). The difference in the number of BrdU-labeled cells among the three groups was significant (F(2,16) = 3.76, P < 0.05). Post-hoc analysis with Newman-Keuls indicated that the good learners possessed more BrdU-labeled cells than the poor learners (P = 0.04) and more than the naïve controls (P = 0.05). The number of cells in the poor learners was not different from the number of cells in the naïve controls (P = 0.77). Using the Spearman rank-order correlation coefficient there was a positive correlation between the mean escape latency of the last four trials and number of BrdU-labeled cells in the dentate gyrus (rs = −0.66, P = 0.01).
Figure 2.
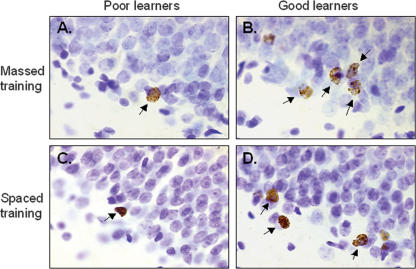
Number of BrdU-labeled cells in the dentate gyrus depends on how well the animal learned. An example of an animal trained with massed trials that learned poorly (A), massed trials that learned well (B), spaced trials that learned poorly (C), and spaced trials that learned well (D).
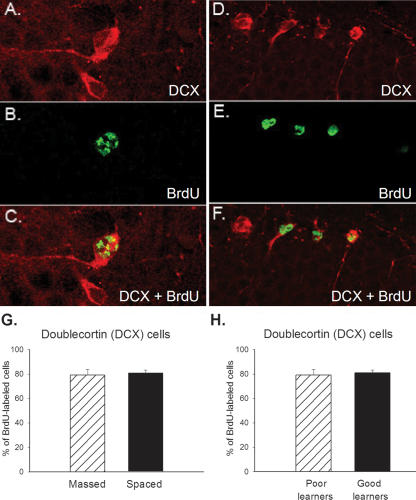
A subset of animals was assessed for the percentage of cells that differentiated into neurons at the time of sacrifice. In this experiment, we were interested in determining the relationship between acquisition and cell survival immediately after training. Because cells were labeled 1 wk before training, and training in the spaced group lasted 4 d, all animals were sacrificed after training was complete, 11 d after BrdU injection. At this time point, cells are still maturing. At this relatively early stage, it can be determined whether a cell will differentiate into a neuron based on the expression of doublecortin (DCX) (Nacher et al. 2001; Brown et al. 2003; Couillard-Despres et al. 2005). Using confocal microscopy, we analyzed the number of BrdU-labeled cells that colocalized DCX, a reliable marker of young neurons (Fig. 3A–F). The percentage of double-labeled cells in animals trained with massed trials (n = 4) was ∼79% and not different from the percentage in animals trained with spaced trials (n = 4) at 81% (F(1,6) = 0.23; P > 0.05) (Fig. 3G). There was no difference between the proportion of cells that differentiated into neurons in poor versus good learners (P > 0.05) (Fig. 3H).
Figure 3.
Majority of BrdU-labeled cells become neurons. Most BrdU-labeled cells had begun to differentiate into neurons 11 d after they were born. Representative cells from the dentate gyrus that express doublecortin (A), BrdU (B), DCX and BrdU (C) are shown. A similar sequence is shown for D–F. (G) Graph depicts percentage of BrdU-labeled cells that expressed DCX in animals trained with massed versus spaced training. No difference was observed. (H) Similarly, there was no difference in the percentage of BrdU-labeled cells that expressed DCX between good and poor learners.
Experiment 2: Retention and neurogenesis
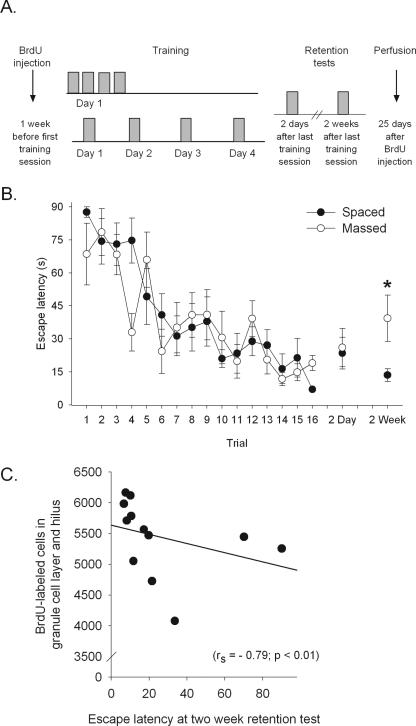
Animals in the spaced condition were trained as before, four trials per day for four consecutive days, with a 1-min intertrial interval (ITI). In order to determine whether the deficit in performance during training may be affected by motor fatigue, rats in the massed condition were trained in a slightly different manner. In this experiment, trials were delivered in blocks of four (1 min ITI). All animals still received the same number of trials (16 trials = four blocks × four trials), but were given a 1-h interval between blocks (Fig. 4A). Animals trained with spaced trials were given a 24-h interval between blocks. All animals were returned to their home cage during the time between blocks. As in Experiment 1, both massed and spaced trained animals learned to find the hidden platform using spatial cues surrounding the maze (F(15,150) = 9.59, P < 0.01) (Fig. 4B). However, in this experiment, there was no difference in performance between the two groups (F(1,10) = 1.92, P > 0.05) (Fig. 4B). After the end of training, all animals were returned to their home cages and left undisturbed for 2 d. To assess memory of the platform location, animals were returned to the water maze for a retention test, and time to find the platform was recorded. The platform remained in the maze to prevent extinction and any effects that extinction might have on cell survival. For the retention test, both massed and spaced trained animals are only exposed to a single trial. Any deficits observed during the retention test may be attributed to memory impairments, and not fatigue. Animals trained with either massed or spaced trials reached asymptotic performance during training, and did so at a similar rate across trials. Two days after the end of training, both groups remembered the platform location; their escape latency for this retention test was similar to their mean performance on the last four trials of training and not different between the two groups (t(10) = 0.10; P > 0.05). Two weeks later, a second retention test was presented to the animals. The group trained with massed trials required an average of 40 sec to find the platform, and the group trained with spaced trials required only 14 sec to find the platform. Although the difference between the means was not statistically significant (t(10) = 1.98; P = 0.07), there was a significant difference in the variance between groups. The animals trained with massed trials expressed a greater variance than those trained with spaced trials (F ratio = 13.66; P < 0.05) (Fig. 4B). Thus, 2 wk after training, the majority of spaced-trained animals remembered the platform location, whereas only a few of the massed-trained animals remembered. We then correlated the memory of the platform location during the 2-wk retention test with the number of BrdU-labeled cells in both groups. The escape latency for the retention test 2 wk after training correlated with the number of BrdU-labeled cells (rs = −0.79, P < 0.01). Because animals trained with spaced trials had a better memory of the platform location than those trained with massed trials, we also tested the correlation in spaced and massed-trained animals separately. In the spaced condition, there was a significant correlation between memory and the number of BrdU-labeled cells (rs = −0.89, P = 0.003), whereas in the massed condition, no significant correlation was observed (rs = −0.70, P = 0.09).
Figure 4.
Memory predicts cell survival. (A) BrdU was injected 1 wk before the beginning of training. Massed subjects were trained with four blocks of four trials in 1 d, with a 1-h intertrial interval between blocks. Spaced subjects were trained with one block of four trials for four consecutive days. Both groups were tested with a single trial 2 d and 2 wk after the end of training. Animals from both groups were perfused 25 d after the one BrdU injection. (B) Escape latency for animals trained with either massed (○) or spaced (●) trials is shown. There was no difference in the time to escape between the two groups. However, 2 wk after the end of training, more animals trained with spaced trials remembered the platform location. (*) P < 0.05. (C) Using Spearman’s rank-order correlation coefficient, the number of BrdU-labeled cells in the dentate gyrus correlated with the performance on the retention test 2 wk later.
In the second experiment, all animals were sacrificed after the 2-wk retention test and 25 d after the one BrdU injection. At this point, cells are fully mature and express neuronal-specific nuclear protein (NeuN). To determine how many of the new cells differentiated into neurons, the number of BrdU-labeled cells that also expressed NeuN was determined; 83% of BrdU-labeled cells expressed NeuN in animals trained with massed trials and 88% in those trained with spaced trials. Percentages were not different between groups and are consistent with those reported in previous studies (Gould et al. 1999; Leuner et al. 2004).
Discussion
The beneficial effect of spacing information over time on learning and memory is well established (Ebbinghaus 1885, translation Ebbinghaus 1913). The spacing effect has been successfully applied in the classroom as well as in patients with cognitive deficits (Schacter et al. 1985; Dempster 1989). Consistent with the literature, we report here that animals trained with spaced trials tended to learn faster and remember longer than animals exposed to the same amount of massed trials. Because new neurons are continuously generated over time, our initial hypothesis was that training with spaced trials would rescue more new neurons from death than the same number of massed trials. Such an outcome would suggest a direct relationship between length of training and cell survival. The data were inconsistent with the hypothesis, at least to the extent that the animals trained with spaced trials did not possess significantly more cells than animals trained with massed trials. Rather, the manner in which the trials were distributed affected learning, which then related to the number of new cells that were maintained in the dentate gyrus after the training experience. Thus, the relationship between trial distribution and cell survival is an indirect one mediated by learning itself. From these data, we were able to conclude that learning, and not only training, is an important variable when it comes to assessing the effects of training on the survival of new neurons in the dentate gyrus. Moreover, we report a correlation between the strength of a memory and the number of cells that remain in the dentate gyrus of the hippocampal formation several weeks after the cells were born and 2 wk after the animals were trained. These data suggest a possible role for the new cells in the retrieval and/or retention of a memory. Each of these findings is discussed in turn.
In the first experiment, animals were trained with either massed or spaced trials and cell survival was assessed. There was no effect of massed versus spaced trials, per se, on the number of new cells that survived, presumably because some animals trained with spaced trials did not learn. When animals were categorized either as good learners or poor learners, the good learners had retained a greater number of the new cells than did the poor learners. Interestingly enough, the poor learners retained a similar number of cells as the naïve controls that had remained in their home cage during the training procedure. For all groups, the majority of BrdU-labeled cells differentiated into neurons, i.e., ∼80% of BrdU-labeled cells were also labeled with doublecortin (DCX) 11 d after they were born. There were no differences in the proportion of cells that differentiated into neurons between animals trained with massed or spaced trials, or animals categorized as good or poor learners. Thus, 1 d after training, most cells had differentiated into neurons and more of them survived in animals that learned to find the platform using spatial cues in the environment. It is noted that the numbers of surviving BrdU-labeled cells are considerably higher than those presented in the initial report (Gould et al. 1999). This may be a result of procedural differences, including the age of the animals, the housing conditions, or antibody used for BrdU detection. However, the findings are generally consistent with our previous study and others as well (Dobrossy et al. 2003; Drapeau et al. 2003; Hairston et al. 2005; Kee et al. 2007). One study did report a reduction in cell number after spatial training (Mohapel et al. 2006), but they used multiple injections of BrdU, making it difficult to assess how many cells are generated versus how many survive after training. In our studies, animals are injected only once, and thus the data reflect the survival of one population of cells generated within a couple of hours 1 wk before training. Again, the important finding reported here is that learning is a critical factor; animals that were trained but did not learn did not possess any more cells than animals that were not trained at all.
In the second experiment, we assessed the more intriguing consequence of the spacing effect, that is, that training with spaced trials does not necessarily enhance learning relative to training with massed trials, but rather increases the persistence and strength of the memory (Spreng et al. 2002). To accomplish this, we introduced a longer interval of time between blocks of trials, while still keeping the trials massed together in 1 d. The change in experimental design eliminated the differences in performance during training, probably because the animals trained with massed trials were not as fatigued during the training process. Despite similar rates of acquisition, most animals trained with spaced trials remembered the location of the hidden platform 2 wk later, whereas few of the animals trained with massed trials remembered. Somewhat surprisingly, we found no group difference in the number of cells that survived after training with massed versus spaced trials. However, as shown in Figure 4B, the variability in the group trained with massed trials was significantly greater than the variability of animals trained with spaced trials. Based on this, we correlated individual differences in memory with the number of surviving cells in the dentate gyrus. Using a Spearman’s rank-order test, we detected a positive correlation between performance on the second retention test and the number of surviving cells, the vast majority of which differentiated into neurons. Thus, the animals with an accurate memory for the platform location 2 wk later tended to have more cells than animals with a poor memory of that location. Admittedly, the sample size is relatively small for a regression analysis. Nevertheless, the correlation between performance and cell survival was evident in the first experiment when neurons were <2 wk of age, and again in the second experiment, when neurons were mature at nearly 4 wk of age. The data presented here are consistent with a recent report in which individual differences in learning related to the number of cells that survive after training (Dalla et al. 2007). In that study, animals were exposed to either trace eyeblink conditioning or contiguous trace conditioning. Regardless of the task parameters, good learners retained more of the new cells after training than did poor learners.
In general, the results from the two experiments indicate that learning, and not simply training, determines how many new neurons survive in the hippocampus after training on a spatial memory task. There are at least two explanations of how this might happen—one is quantitative, and the other qualitative. In the quantitative version, training with trials that are spaced over time would influence more cells by virtue of the fact that the training experience is prolonged. If this explanation were valid, then animals trained with spaced trials should have more new cells surviving than animals trained with massed trials, regardless of how well they learned the task. However, in Experiment 1 we found that this was not the case, because only animals that learned had more cells, irrespective of whether the trials were spaced or massed. Animals that were trained with spaced trials, but performed only as well as those trained with massed trials, had fewer surviving cells. Because we only labeled one population of new cells, i.e., one injection of BrdU, it remains possible that the quantitative explanation is valid when multiple populations of new cells are labeled or when training is extended over a longer period of time. The qualitative explanation asserts that training with spaced trials affected the cells in a qualitatively different way than training with massed trials. Differences could include changes in gene expression and protein synthesis, which extend the life of cells that are engaged during the learning process. This type of explanation is consistent with the data presented in the second experiment. Animals trained with massed or spaced trials learned similarly, but more of those trained with spaced trials remembered the location of the platform 2 wk later, and they tended to possess more of the new cells at that time. Presumably, some process altered the cells differently in animals that were trained with spaced trials such that they survived. It is noted that such a distinction cannot be applied in a strict sense, because there was no overall differences in cell survival between animals trained with massed versus space trials. Rather, it would appear that survival of immature neurons is enhanced in animals that learn well and remember the spatial information, which is facilitated by training over longer periods of time.
It is worth considering the potential impact of sleep on the effects reported here. In the first experiment, animals exposed to spaced trials were able to sleep between blocks and those that were trained with massed trials did not sleep, or did not sleep for long. Many studies have suggested that sleep, or even a brief period of rest, is important for consolidation to occur (Karni et al. 1994) as well as for the spacing effect to emerge (Hintzman 1974). Interestingly enough, Hairston et al. (2005) reported that spatial learning enhanced cell survival, but not if animals were deprived of sleep in between days of training. Similarly, in our studies, animals that were trained with massed trials had minimal opportunity to sleep. However, the direction of the relationship cannot be determined, because the animals that were sleep deprived also did not learn or remember well. As we show here, training without learning is insufficient to rescue new cells from death. In the second experiment, a 1-h intertrial interval was given between blocks of trials. This attenuated the slight difference in performance observed between animals that were trained with massed trials versus those trained with spaced trials in the first experiment. Nonetheless, the long-term memory for the platform location was relatively weak in most of the animals trained with massed trials. It is conceivable that neuronal events necessary for the long-term storage or retrieval of the memory were not induced in the animals trained with the massed trials.
The mechanism whereby learning increases cell survival has not been identified, but would presumably involve some type of activity-dependent process. That is, cells that are ‘activated’ in a specific way during the learning process preferentially survive. In the first experiment, we found that good learners had more surviving cells than poor learners. Importantly, the good learners were predominantly from the group trained with spaced trials; only one animal trained with massed trials was designated as a good learner. The distribution of trials across days may be more effective, because the cells are activated over a longer period of time, allowing changes in gene expression and protein synthesis to be induced and expressed. This is certainly the case for other demonstrations of the spacing effect. In Drosophila, training with spaced trials extends the memory, which depends on cAMP-responsive element-binding protein (CREB) and protein synthesis (Yin et al. 1995). Others find that spaced tetanic stimulation induces a more persistent expression of LTP (Scharf et al. 2002) and that the consequences of spaced application of serotonin in Aplysia is more profound than when massed (Mauelshagen et al. 1998). Overall, it seems likely that prolonged increase in cell excitability and/or a more refined pattern of activation over time leads to the increase in cell survival in response to learning. Glutamatergic and GABAergic mechanisms are probably involved because of their role in learning and cell survival (Tsien et al. 1996; Tashiro et al. 2006). For example, application of glutamate to hippocampal neural progenitor cells increased the proportion of cells that differentiated into neurons in vitro (Deisseroth et al. 2004), and GABA activation of hippocampal progenitor cells promotes neuronal differentiation (Tozuka et al. 2005). A single stimulation of the perforant pathway does not evoke GABA currents in hippocampal progenitor cells, whereas repeated stimulation (using a theta burst protocol) does (Tozuka et al. 2005). Moreover, tetanic stimulation enhances survival of new cells in the dentate, one of a relatively few manipulations other than learning to do so (Bruel-Jungerman et al. 2006).
There are numerous explanations for why training with spaced information is more effective than training with massed information. Certainly, the increase in time between trials is the most important variable because it provides more opportunities for rehearsal and retrieval. Perhaps related to this model is the multiple trace theory (Nadel and Moscovitch 1997; Moscovitch et al. 2005). This theory poses that memories become more stable with time because more and different traces of the initial memory are made. The time between spaced trials would provide a means of distinguishing one learning event from another because the contexts are more likely to be different across time. As a consequence, more and different traces are produced, which in the end provide a more stable memory representation in the brain. An increase in numbers of traces would enhance the ability to remember events over progressively longer periods of time. As noted, a recent study found that animals in which neurogenesis is prevented expressed a memory deficit for the platform location 2 wk after learning—the same time point as the relationship reported here (Snyder et al. 2005). Thus, it is possible that the inability to rescue the new cells from death during the learning experience disrupts the circuitry used in the retrieval of the spatial memory and/or the context in which the event occurred. Whatever the explanation, the data presented here indicate that training with spaced trials induces a more persistent memory and the strength of that memory relates to the number of new cells that survive in the adult hippocampus.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats were ∼70 d of age at the time of the 5-bromo-2′-deoxyuridine (BrdU) injection. They were bred in the Department of Psychology at Rutgers University, and at 60 d of age, were housed individually in hanging wire cages with unlimited access to food and water and maintained on a 12:12 light/dark cycle with light onset at 8:00 am.
Spatial navigation training in the Morris water maze
A circular metal tank (170 × 60 cm) was filled with room-temperature water and made opaque with white, nontoxic paint. Complex cues, i.e., small posters, lab equipment, and wall shelves, were set distal to the maze in a dimly lit room. During the trial, the experimenter stood behind a curtain to avoid acting as a visible cue. Rats were given 90 sec to find the hidden platform (15 × 15 cm), which was located ∼3 cm below the surface of the water. If they did not find it within 90 sec, the rat was guided to the platform by the experimenter. Rats remained on the platform for 30 sec. Upon removal from the platform, they were placed in a deep, opaque bucket (preventing visual access to cues), which was lined with a towel and lit overhead with a lamp, where they remained for 60 sec. The starting quadrant was pseudo-randomized across all trials, excluding the quadrant with the platform. The quadrant containing the platform was not included, to prevent artificially low escape latencies that occur when the animal swims into it at the start of the trial.
Experiment 1: Acquisition of spatial memories and neurogenesis
In the spaced condition (n = 8), training consisted of four trials per day for four consecutive days. In the massed condition (n = 5), training consisted of 16 trials in 1 d. In both conditions, the intertrial interval (ITI) was 1 min. BrdU, 200 mg/kg (Sigma-Aldrich), was injected into the intraperitoneal cavity 1 wk before the first day of training. A third group of naïve rats (n = 6) was also injected with BrdU, but were not exposed to training. Animals from all groups were sacrificed 11 d after the BrdU injection.
BrdU-injections and immunohistochemistry
BrdU (200 mg/kg, i.p.) was mixed at 15 mg/mL in 0.9% saline (pH 7.4). Animals were deeply anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde. Brains were stored in 4% paraformaldehyde at 4°C for at least 2 d, then transferred to phospho-buffered saline (0.1 M PBS at pH 7.4) before cutting. The right hemisphere was mounted onto an oscillating tissue slicer and sections were cut at 40 μm. Every 12th section of the hippocampus was mounted onto slides and used for BrdU-immunohistochemistry using peroxidase methods. Briefly, citrate buffer (pH 6.0) was microwaved until boiling. Sections were placed into citrate buffer, reheated for another 5 min, allowed to cool at room temperature for 15 min, and rinsed with 0.1 M PBS. Trypsin was used to permeabilize cell membranes; 2N HCl in PBS was used to denature DNA. Sections were incubated in primary antibody, mouse anti-BrdU, (1:100, Becton Dickinson) overnight at 4°C. The next day, sections were incubated in secondary biotinylated anti-mouse (1:200, Vector) followed by avidin–biotin complex (Vectastain ABC Kit, Vector), then 3–3′-diaminobenzidene (DAB SigmaFast tablets, Sigma-Aldrich). BrdU-labeled cells were counted at 1000X with the experimenter blind to condition. To estimate the total number of BrdU-labeled cells in the dentate gyrus, cell counts were multiplied by a factor of 24 (two hemispheres × 12 serial sections).
For double labeling, free-floating sections were rinsed with tris-buffered saline (0.1 M TBS at pH 7.5). DNA was denatured with 2N HCl in TBS. Sections were incubated in primary antibodies, goat anti-doublecortin (1:100, Santa Cruz Biotechnology), and mouse anti-BrdU (1:200, Becton-Dickinson) with 0.5% Tween-20 (Sigma-Aldrich) in TBS for 48 h at 4°C. Sections were then incubated in secondary antibodies, Rhodamine Red-X anti-goat (1:200, Jackson Immunoresearch) and Fluro 488 anti-mouse (1:200, Molecular Probes) in TBS for 30 min. Tissue was mounted onto slides and coverslipped with glycerol:TBS (1:3). Number of colocalized cells was determined with a Zeiss (Oberkochen) LSM 510 confocal laser scanning microscope. Sections were scanned using a Plan-Neofluar 25× water-immersion objective and dual-channel excitation with argon (488 nm) and helium-neon (543 nm). Forty cells per subject (n = 8) were counted on random sections throughout the hippocampus. Colocalization analysis included visual inspection of size and shape of cell throughout a z-stack, orthogonal planes, and a profile of excitation intensity of the cell.
Experiment 2: Retention and neurogenesis
In the second experiment, we further assessed the potential impact of spaced (n = 7) versus massed (n = 5) training on spatial maze learning and the survival of new cells in the adult hippocampus. The procedures in the second experiment are similar to those in the first experiment with two exceptions. First, we assessed retention as well as acquisition. The memory for the platform location was assessed 2 d after the end of training and again 2 wk after the end of training. For the retention test, the platform remained in the same location as during training. This procedure provided a direct measure of time to escape without introducing an extinction trial prior to the second retention test, which was conducted 2 wk later. To reduce the potential for fatigue during massed training, the time between blocks was increased from 1 min to 1 h, during which the animals were returned to their home cages. This procedural change also provided both groups with a change of context between the four blocks of four training trials. Animals from both groups were sacrificed 25 d after BrdU injection.
BrdU-injections and immunohistochemistry
Similar procedures as Experiment 1 were followed. However, a subset of brains (n = 4) were labeled with BrdU and neuron-specific nuclear protein (NeuN), a marker of mature neurons, instead of DCX. Double-labeling procedures resembled those in Experiment 1; however, the following antibodies and dilutions were used instead: primary antibodies were rat anti-BrdU (1:50, Accurate Chemicals & Scientific Corporation) and mouse anti-NeuN (1:200, Chemicon). BrdU signal was amplified with donkey anti-rat biotin-SP (1:200, Jackson Immunoresearch). Secondary antibodies were fluorescin-DTAF-streptavidin (1:200, Jackson Immunoresearch) and donkey anti-mouse rhodamine red X (1:1000, Jackson Immunoresearch). Confocal microscopy was conducted as in Experiment 1.
Statistical analysis
Performance in the water maze was assessed by mean escape latency (time to reach platform) and the number of BrdU-labeled cells used to assess cell survival. One-way analysis of variance (ANOVA) with repeated measures was used to detect differences between groups and across trials. Post-hoc analysis was done with Newman-Keuls. Spearman’s rank-order correlation coefficient, rs, was used to detect correlations between the numbers of surviving cells and escape latencies. This statistical test was selected instead of the parametric Pearson’s r correlation for two reasons. First, parametric statistical tests include the assumptions that (1) values have a Gaussian, or normal, distribution, and (2) number of subjects represents a sufficiently large sample size. When mean escape latencies were plotted, they did not follow a Gaussian distribution. Second, there were a relatively small number of animals from each experiment, 13 in the first experiment and 12 in the second experiment.
Acknowledgments
This work was supported by NIH (NIMH 59970) and NSF (IOB-0444364) (T.J.S.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.488707
References
- Ambrogini P., Orsini L., Mancini C., Ferri P., Ciaroni S., Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci. Lett. 2004;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Brown J.P., Couillard-Despres S., Cooper-Kuhn C.M., Winkler J., Aigner L., Kuhn H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E., Davis S., Rampon C., Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J. Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H.A., McKay R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Commins S., Cunningham L., Harvey D., Walsh D. Massed but not spaced training impairs spatial memory. Behav. Brain Res. 2003;139:215–223. doi: 10.1016/s0166-4328(02)00270-x. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S., Winner B., Schaubeck S., Aigner R., Vroemen M., Weidner N., Bogdahn U., Winkler J., Kuhn H.G., Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Dalla C., Bangasser D.A., Edgecomb C., Shors T.J. Neurogenesis and learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol. Learn. Mem. 2007 doi: 10.1016/j.nlm.2007.02.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K., Singla S., Toda H., Monje M., Palmer T.D., Malenka R.C. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dempster F.N. Spacing effects and their implications for theory and practice. Educ. Psychol. Rev. 1989;1:309–330. [Google Scholar]
- Dobrossy M.D., Drapeau E., Aurousseau C., Le Moal M., Piazza P.V., Abrous D.N. Differential effects of learning on neurogenesis: Learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P.V., Abrous D.N. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H. Uber das Gedachtnis. Dover; New York: 1885. [Google Scholar]
- Ebbinghaus H. Memory. A contribution to experimental psychology. Teachers College Columbia University; New York: 1913. [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: Simple paradigms complex problems. Behav. Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Gould E., Beylin A., Tanapat P., Reeves A., Shors T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hairston I.S., Little M.T., Scanlon M.D., Barakat M.T., Palmer T.D., Sapolsky R.M., Heller H.C. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J. Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hintzman D. Theoretical implications of the spacing effect. In: Solso R.L., editor. Theories in cognitive psychology: The Loyola Symposium. John Wiley & Sons; Potomac, MD: 1974. pp. 77–97. [Google Scholar]
- Hser Y., Wickens T. The effects of the spacing of test trials and study trials in paired-association learning. Educational Psych. 1989;9:99–120. [Google Scholar]
- Karni A., Tanne D., Rubenstein B.S., Askenasy J.J., Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kee N., Teixeira C.M., Wang A.H., Frankland P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F.H. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur. J. Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Klapdor K., Van Der Staay F.J. Repeated acquisition of a spatial navigation task in mice: Effects of spacing of trials and of unilateral middle cerebral artery occlusion. Physiol. Behav. 1998;63:903–909. doi: 10.1016/s0031-9384(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Lattal K.M. Trial and intertrial durations in Pavlovian conditioning: Issues of learning and performance. J. Exp. Psychol. Anim. Behav. Process. 1999;25:433–450. doi: 10.1037/0097-7403.25.4.433. [DOI] [PubMed] [Google Scholar]
- Leuner B., Mendolia-Loffredo S., Kozorovitskiy Y., Samburg D., Gould E., Shors T.J. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B., Waddell J., Gould E., Shors T.J. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J. Neurosci. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T.M., Kristjansen P.E., Bolwig T.G., Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J., Sherff C.M., Carew T.J. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn. Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- McGaugh J.L. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Mohapel P., Mundt-Petersen K., Brundin P., Frielingsdorf H. Working memory training decreases hippocampal neurogenesis. Neuroscience. 2006;142:609–613. doi: 10.1016/j.neuroscience.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Moscovitch M., Rosenbaum R.S., Gilboa A., Addis D.R., Westmacott R., Grady C., McAndrews M.P., Levine B., Black S., Winocur G., et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. J. Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J., Crespo C., McEwen B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nadel L., Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V., Marrone D.F., Gage F.H., Worley P.F., Barnes C.A. Integration of new neurons into functional neural networks. J. Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G., Micheau J., Lam A.G., Roloff E.L., Martin S.J., Bridge H., de Hoz L., Poeschel B., McCulloch J., Morris R.G. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat. Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Saxe M.D., Battaglia F., Wang J.W., Malleret G., David D.J., Monckton J.E., Garcia A.D., Sofroniew M.V., Kandel E.R., Santarelli L., et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Rich S.A., Stampp M.S. Remediation of memory disorders: Experimental evaluation of the spaced-retrieval technique. J. Clin. Exp. Neuropyschol. 1985;7:79–96. doi: 10.1080/01688638508401243. [DOI] [PubMed] [Google Scholar]
- Scharf M.T., Woo N.H., Lattal K.M., Young J.Z., Nguyen P.V., Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J. Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Shors T.J., Townsend D.A., Zhao M., Kozorovitskiy Y., Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Hong N.S., McDonald R.J., Wojtowicz J.M. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Spreng M., Rossier J., Schenk F. Spaced training facilitates long-term retention of place navigation in adult but not in adolescent rats. Behav. Brain Res. 2002;128:103–108. doi: 10.1016/s0166-4328(01)00266-2. [DOI] [PubMed] [Google Scholar]
- Tashiro A., Sandler V.M., Toni N., Zhao C., Gage F.H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tsien J.Z., Huerta P.T., Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]