Abstract
The propensity of individual trabeculae to fracture (microfracture) may be important clinically since it could be indicative of bone fragility. Whether or not an overloaded trabecula fractures is determined in part by its structural ductility, a mechanical property that describes how much deformation a trabecula can sustain. The overall goal of this study was to determine the structural ductility of individual trabeculae and the degree to which it is influenced by pyridinium and non-enzymatic collagen cross-links. Vertically oriented rod-like trabeculae were taken from the thoracic vertebral bodies of 32 cadavers (16 male and 16 female, 54–94 years of age). A total of 221 trabeculae (4–9 per donor) were tested to failure in tension using a micro-tensile loading device. A subset of 76 samples was analyzed to determine the concentration of hydroxylysyl-pyridinoline (HP) and lysyl-pyridinoline (LP) cross-links as well as pentosidine, a marker of non-enzymatic glycation. Structural ductility (defined as the ultimate strain of the whole trabecula) ranged from 1.8–20.2% strain (8.8 ± 3.7%, mean ± S.D.) and did not depend on age (p=0.39), sex (p=0.57) or thickness of the sample at the point of failure (p = 0.36). Pentosidine was the only marker of collagen cross-linking measured that was found to be correlated with structural ductility (p = 0.01) and explained about 9% of the observed variance. We conclude that the ductility of individual trabeculae varies tremendously, can be substantial, and is weakly influenced by non-enzymatic glycation.
Keywords: Bone biomechanics, cancellous bone, collagen, microfracture
Introduction
Collagen cross-linking is thought to be a potentially important aspect of bone quality (1,2) and as such may play a role in the etiology of osteoporotic fractures. The propensity of individual trabeculae to fracture (microfracture) when a whole bone is overloaded but not overtly fractured may be important for understanding the multi-scale failure mechanics of cancellous bone. Fractured trabeculae can weaken bone by diminishing the load-carrying capacity of the cancellous structure (3) and are more likely to be completely resorbed by osteoclasts (4,5). Because resorbed trabeculae may not be replaced during tissue repair (4,6,7), a microfracture may have a permanent detrimental effect on bone volume fraction, microarchitecture, and the mechanical properties of the cancellous structure. Whether or not a loaded trabecula fractures is determined in part by its ductility1, a failure property that is related to the amount of deformation that the trabecula can sustain (8).
The ductility of individual trabeculae may be influenced by a number of factors. One such factor is collagen cross-linking (2,9–17). A number of different kinds of collagen cross-links exist in bone (18,19) including reducible (immature) cross-links, and the non-reducible pyrrolic, pyridinium and non-enzymatic glycation mediated cross-links. With respect to their effects on bone biomechanics, the immature cross-link dihydroxylysinonorleucine (DHLNL) and pyrrolic cross-links were both found to be correlated with the bending strength of cortical bone in avian models (20). Pyrrolic cross-link concentration has also been associated with two-dimensional measures of trabecular thickness (21). Recent studies, however, did not find any immature or pyrrolic cross-links to be related to cancellous bone mechanical properties in middle-aged and elderly humans (2,22). The mature pyridinium cross-links hydroxylysyl-pyridinoline (HP) and lysyl-pyridinoline (LP) and the ratio of the two (HP/LP) have been shown to be correlated with bone strength, stiffness and/or ductility (2,10,17), although, since other studies have not observed some of these relationships (12,14), the role of pyridinium cross-links remains unclear. Non-enzymatic glycation (NEG) mediated cross-links have been consistently correlated to cortical bone toughness both in samples treated experimentally to increase glycation (13,16) as well as in untreated bone samples (14). While these studies characterize cortical bone tissue and cancellous bone, none have addressed the role of collagen cross-linking at a more fundamental structural level such as individual trabeculae. A number of studies have biomechanically tested individual trabeculae (23–26) and uniformly shaped (micromachined) specimens derived from trabeculae (27–29) to measure either elastic modulus or fatigue properties of the trabecular bone tissue, however they but did not report on ductility or collagen intermolecular status. Thus, at this juncture we are unaware of quantitative reports on the ductility of trabecular tissue or individual trabeculae and the potential influence of collagen cross-links.
To provide a more mechanistic link between collagen cross-linking and its possible influence on cancellous bone strength, the overall goal of this study was to determine the structural ductility of individual trabeculae and the degree to which it is influenced by pyridinium and non-enzymatic collagen cross-links. To do this, we measured the ductility of whole trabeculae, which we refer to as the structural ductility, since it is more closely related to microfracture than the ductility of the trabecular tissue material itself. Specifically our objectives were to: 1) measure the structural ductility of individual trabeculae from cadaveric vertebral bodies of older adults; and 2) determine whether structural ductility is related to the amount of pyridinium or NEG mediated collagen cross-links. This study is unique in its focus on structural ductility of individual trabeculae as opposed to analysis of cortical bone tissue or larger specimens of cancellous bone.
Materials and Methods
Specimen Collection
Thirty-two thoracic vertebral bodies (T12 or T8) were harvested from fresh frozen cadaver spines, 16 each from age-matched men (age 55–94 years, mean 76 ± 13) and women (age 54–92 years, mean 78 ± 10). Sagittal slices (1 mm thick) of each vertebral body were obtained under constant irrigation with a low speed diamond saw (Isomet 1000, Buehler). Using a dissecting microscope and scalpel, 10–12 vertical rod-like trabeculae were excised from the center region of each slice (Figure 1). Specimens were typically 3–4 mm in length and included a single trabecula with portions of additional trabeculae on either end to assist with handling and glue application. Trabeculae were not machined further after dissection so that the mechanics of the whole trabecula were apparent. Specimens were immersed in saline at room temperature for up to three hours prior to mechanical testing.
Figure 1.
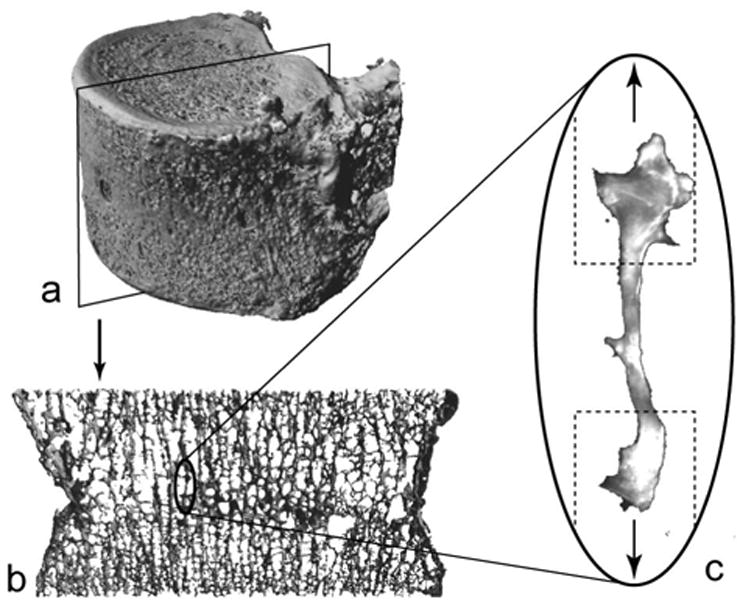
Vertebral bodies (a) were cut into 1 mm thick sagittal sections (b) in order to dissect individual, rod-like, vertical trabeculae (c). The approximate placement of sample holders is indicated with a dashed line and the direction of loading is indicated by arrows.
Mechanical Testing
Mechanical testing of the individual trabeculae was performed using a motorized tensile substage (Electroscan E-3, Ernest F. Fullam Inc., Latham, NY, USA). Small rectangular brass holders were placed within the test clamps of the substage and aligned with the substage crossheads using a placement jig. Each holder contained grooves made from 1.2 mm diameter tapped holes that were cut in half longitudinally to create trenches for sample alignment. Trabeculae were set within the trenches spanning two holders and potted in cyanoacrylate glue (Loctite 401, Henkel Loctite Corp, Rocky Hill, CT, USA). After the glue was dry (about 10 minutes), a drop of saline was applied to the trabecula in the gage region to maintain hydration of the specimen during testing. Surface staining confirmed that the cyanoacrylate glue was not present within the sample gage length using this protocol.
Tensile loading was performed five minutes after saline application at a controlled displacement rate of 0.1 mm/s. The applied force was measured using a 10-pound (43 N, sensitivity ±0.02 N) miniature load cell (Model 31, Sensotec, Columbus, OH, USA). Displacement between the testing clamps was measured using a linear variable displacement transducer (LBB series, Measurement Specialities, Fairfield, NJ, USA, sensitivity ± 0.10 μm) attached to the crossheads of the substage. The initial gage length of each specimen was measured from digital photographs (resolution 2304 x 1536 pixels, ~72 pixels/mm, Figure 2) and was used in the strain calculation. Intra-observer variation in measurement of initial gage length was 6% on average. Force and displacement data were collected onto a desktop computer at 200 Hz using a 16-bit data acquisition card (PCI-6023E, National Instruments, Austin, TX, USA). Force data were submitted to a low pass digital filter to remove noise. Structural ductility was characterized as the ultimate strain (strain at ultimate load). The digital pictures of the trabeculae within the testing apparatus before and after testing were analyzed to identify any slipping of the specimen within the holders during the test. The thickness of the trabecula at the point of fracture was determined from digital images as the average of the thickness from the two sides of the broken specimen. After mechanical testing, the remaining portions of each trabecula between the grips (< 0.5 mg per specimen) were collected for collagen analysis.
Figure 2.
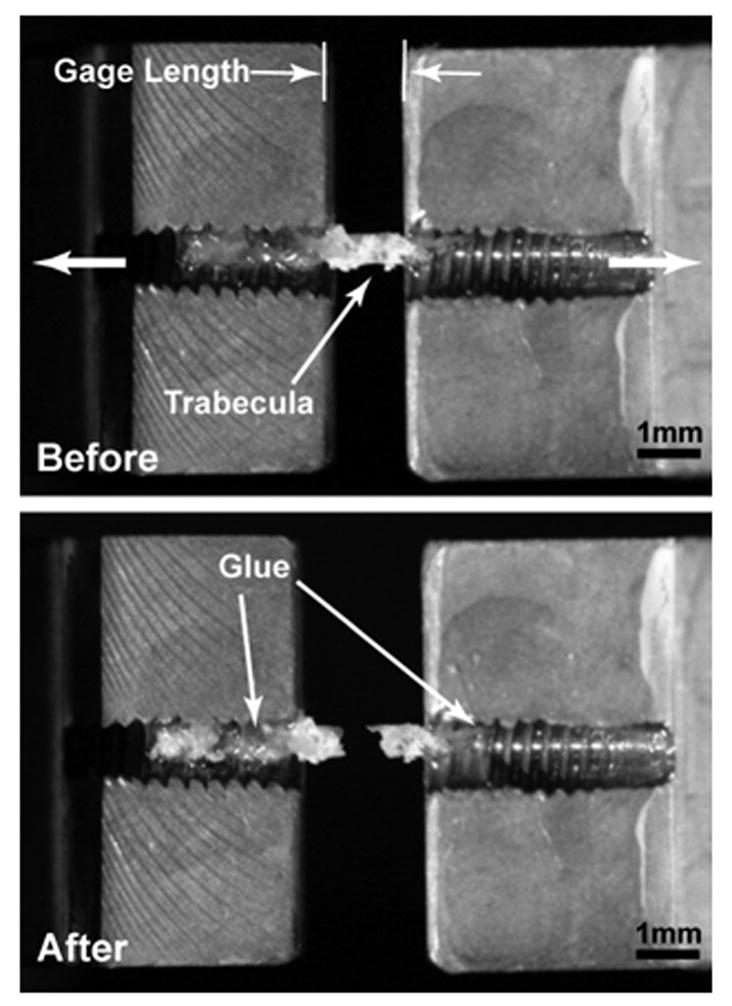
Digital photographs were taken of each trabecula within the holders a) before and b) after loading well beyond failure. The ultimate strain of this sample was 7.09%. Analysis of these images is used to determine the initial gage length, the thickness at the point of fracture and the length of trabecula embedded within each holder. If the amount of bone tissue within the holders before and after testing differed by more than 20% it was concluded that slipping had occurred at the interface and the test was discarded.
Collagen Cross-link Analysis
We measured the concentration of enzymatic cross-links hydroxylysyl-pyridinoline (HP, also referred to as pyridinoline) and lysyl-pyridinoline (LP, also referred to as deoxy-pyridinoline) (18,19,30). To characterize non-enzymatic cross-links we measured the concentration of pentosidine (31), a well characterized advanced glycation end product of the Maillard reaction, i.e. the spontaneous reaction of reducing sugars such as glucose with free amino groups of proteins (32). Although pentosidine typically accounts for only a small proportion of the Maillard end products, it is regarded as a marker for the accumulation of other non-enzymatic glycation products (30,33). All concentrations of cross-links were expressed relative to the amount of collagen.
Each specimen was hydrolyzed (108 C, 20–24 h) with 800 ml 6 M HCl in 5 ml teflon sealed glass tubes. The specimens were dried and redissolved in 800 ml water containing 10 mM pyridoxine (internal standard for the cross-links HP, LP, and pentosidine) and 2.4 mM homoarginine (internal standard for amino acids, Sigma, St. Louis, MO). HP an LP were calibrated against a standard from Metra Biosystems (Palo Alto, CA. USA); pentosidine was a gift from Prof. V.M. Monnier (Case Western Reserve University, Cleveland, OH, USA) and calibrated against a pentosidine standard from Prof. J.W. Baynes (University of South Carolina, Columbia, SC. USA). The dissolved hydrolysates were diluted 5-fold with 0.5% (v/v) heptafluorobutyric acid (Fluka, Buchs, Switzerland) in 10% acetonitrile for cross-link analysis; aliquots of the 5-fold diluted sample were diluted 50-fold with 0.1 M sodium borate buffer pH 8.0 for amino acid analysis. Derivatization of the amino acids with 9-fluorenylmethyl chloroformate (Fluka, Buchs, Switzerland) and reversed-phase high-performance liquid chromatography was performed on a Micropak ODS-80TM column (150 x 4.6 mm; Varian, Sunnyvale, USA) as described previously (30,34,35). The quantities of cross-links were expressed as the number of residues per collagen molecule, assuming 300 hydroxyproline residues per triple helix. This is a well-established procedure since hydroxyproline is a collagen-specific amino acid and the prolyl hydroxylation level in collagen is stable. To ensure precise collagen cross-link measurements, only samples having more than 10 μg total collagen mass were included.
A total of 360 individual trabeculae were tested in tension. A set of tests was discarded due to slipping at the holder-trabecula interface or low maximum loads that were not within our measurement capabilities (n = 42). Another 137 samples failed at the holder-trabecula interface and were also removed from consideration leaving a total of 221 successful mechanical tests (4–9 per donor). Of the original 360 samples, 180 samples were assayed for collagen analysis, 122 of which had sufficient collagen mass for analysis. A total of 76 samples (1–5 per donor, mean 2.38 ± 1.26, median 2.00) included data for both tensile ductility and collagen cross-linking. Mixed effects models were used to account for the different number of samples from each donor using the restricted maximum likelihood (REML) method. Because these mixed effects models found no significant donor effect on structural ductility or collagen status, linear regressions were used to identify relationships between mechanical properties, donor age and collagen status (JMP version 5.0, SAS Institute, Cary, NC, USA). However, because donor effects were apparent in trabecular thickness measures, the REML method was used in statistical models using specimen thickness.
Results
Trabeculae generally demonstrated large and highly variable values of ultimate strain. On average, the ultimate strain was 8.8 ± 3.7% (mean ± S.D.), ranged from 1.8 to 20.2% (Figure 3) and showed considerable intra-individual variation (Figure 4). The standard deviation of ultimate strain within donors ranged from 15–58% of the mean value and was typically 33–34%. Analysis of variance from the random effects portion of the model indicated that 73% of the variance in ultimate strain was within donors as opposed to between donors. As expected, thickness of the sample at the point of fracture was not correlated with ultimate strain (p = 0.36), but was significantly related to ultimate load (p <0.001). Ultimate strain was statistically similar in males (8.5 ± 3.6%) and females (9.1 ± 3.9%, p = 0.39). Intra-donor variance in ultimate strain did not depend on age (p = 0.22) or sex (p = 0.37). No relationship between donor age and ultimate strain was observed (p = 0.58, Figure 4).
Figure 3.
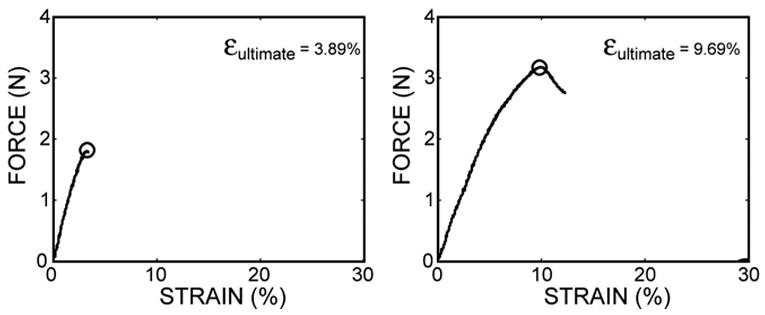
Typical force-strain curves for trabeculae tested in tension are shown. The ultimate strain (εultimate) is indicated. The sample on the right demonstrates the fact that fracture is not always associated with achievement of the ultimate load.
Figure 4.
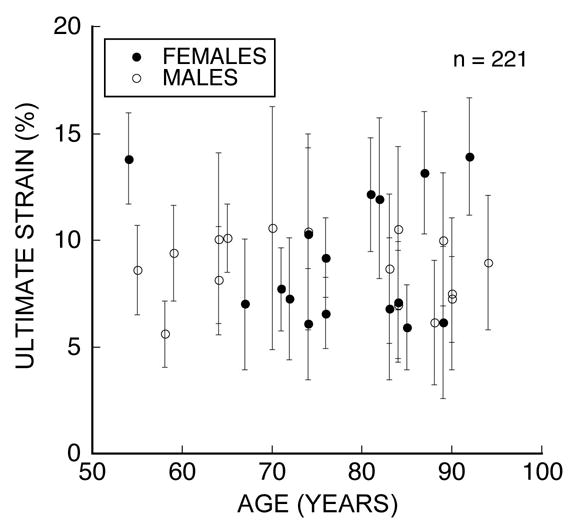
The average ultimate strain is shown relative to donor age in females and males. Error bars represent one standard deviation. No trends associated with age were identified.
Of the three types of collagen cross-links analyzed, only pentosidine was significantly related to the ultimate strain (Table 1). Greater pentosidine concentration was associated with lower ultimate strain (p = 0.01), but pentosidine accounted for only 9% of the observed variation (r2 = 0.09, Figure 5). HP and LP cross-links were not related to ultimate strain nor were combinations of the two (HP+LP, HP/LP). Variation in pentosidine across all specimens — as characterized by a six-fold difference between 10th and 90th percentiles — was about twice the variation of the other cross-links (Table 1), demonstrating the substantial variability. Pentosidine concentration in individual trabeculae did not depend on age (p=0.50) or sex (p = 0.08, Figure 6); HP and LP concentrations did decline with age (p < 0.01, Figure 6) but did not depend on sex (p = 0.67, 0.31 respectively).
Table 1.
The HP, LP and pentosidine concentrations are reported as median, 10th and 90th percentiles. The results of linear regressions between collagen cross-linking and ultimate strain are shown.
| HP (mol/mol collagen) | LP (mol/mol collagen) | Pentosidine (mmol/mol collagen) | Thickness at Fracture (mm) | |
|---|---|---|---|---|
| Median (10th,90th percentiles) | 0.37 (0.22, 0.76) | 0.17 (0.10, 0.30) | 2.25 (0.86, 5.18) | 0.47 (0.24, 0.77) |
| Regression with ultimate strain | p = 0.56 | p = 0.63 | p = 0.01 | p = 0.36 |
Figure 5.
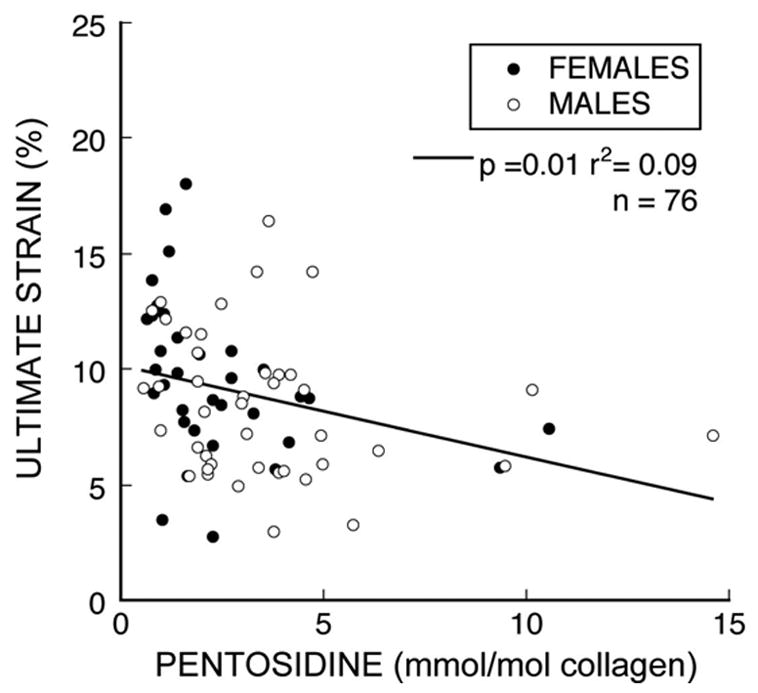
Increased pentosidine concentration was associated with reduced structural ductility, although only a small portion of the variance in ultimate strain was explained by pentosidine concentration.
Figure 6.
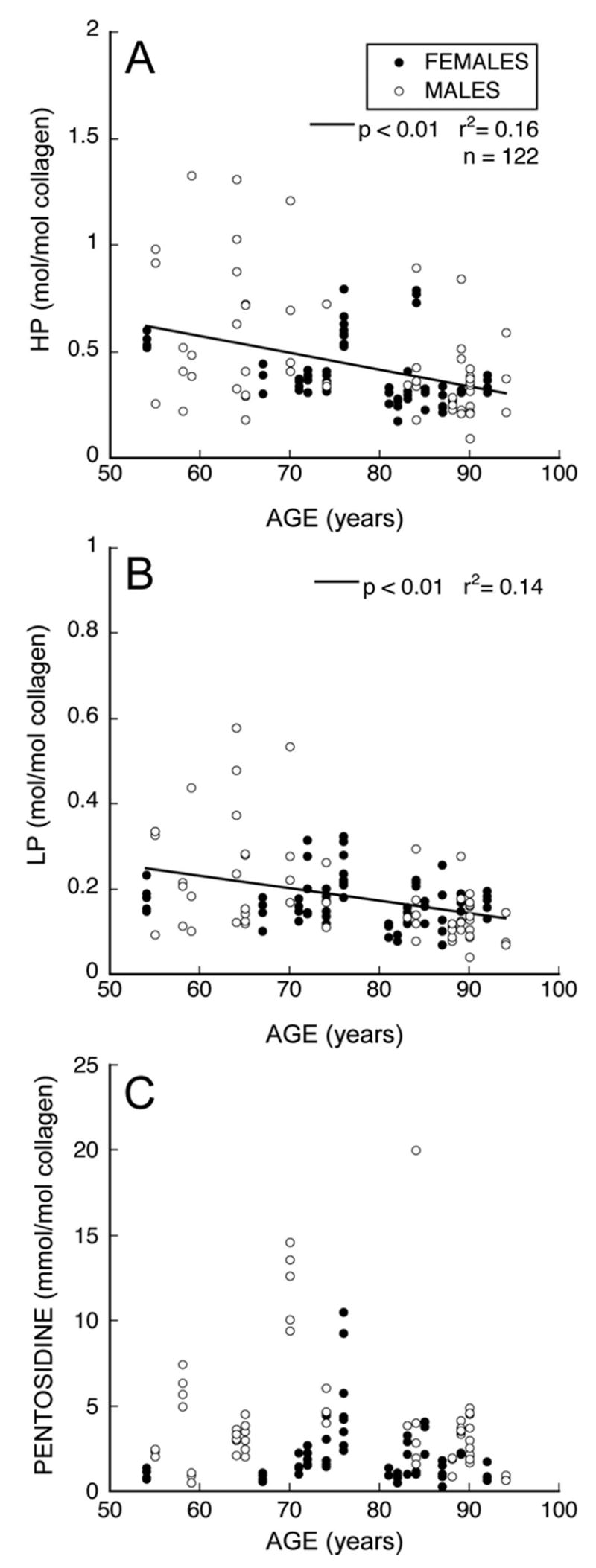
The distribution of measured collagen cross-links with age is shown for a) HP, b) LP and c) pentosidine. No significant relationship between donor age and pentosidine concentration was observed (n=122).
Discussion
In the current study we sought to determine whether pyridinium and non-enzymatic collagen cross-links are correlated with the structural ductility of individual trabeculae. We characterized structural ductility by the strain at maximum load-carrying capacity, and found that these strain measures varied substantially across and within donors. Pyridinium and pentosidine cross-links also varied substantially across trabeculae. Despite these large variations in both mechanical property and cross-link concentrations, only pentosidine showed a significant correlation with structural ductility, and even in this case the correlation was very weak. We conclude that the ductility of individual trabeculae varies tremendously, can be substantial, and is weakly influenced by non-enzymatic glycation.
A number of aspects of this study support the validity of these results. First, we used a tension testing protocol that avoids the complexities of trabecular buckling and loading artifacts that can occur in compression or bending tests. Tension tests are the norm in materials testing for characterization of ductility. Careful alignment of the specimens within the testing apparatus helped to reduce bending artifacts. By limiting our study to the evaluation of structural ductility we were able to gain insight into microfracture without making the results susceptible to errors caused by machining samples into a controlled shape or errors in the measurement of tissue elastic modulus or strength that are highly correlated with sample cross-section at the point of fracture (36). Ultimate strain, by definition, is not related to specimen cross-sectional area and, as expected, was independent of thickness at the point of fracture. Thickness at the point of fracture was, however, highly correlated with maximum load. Furthermore, methods in the protocol specifically targeted slipping at the trabecula-glue interface, a noted source of error in tension testing of individual trabeculae (36). Trabeculae were kept hydrated during the test, a condition known to influence ductility of trabeculae (37). Lastly, evaluation of collagen cross-linking was performed on the gage lengths of the specimens tested in tension, providing for direct evaluation of the effects of these collagen cross-links on the ductility of individual trabeculae.
There are limitations that should be realized when interpreting our results. First, uniaxial loading in tension is not a typical in vivo loading mode for individual trabeculae (38). However, about one third of the tissue that fails during compressive loading of cancellous bone is thought to fail by excessive tensile strain within the trabecular tissue (8,39), thus tensile properties may be important for determining apparent compressive or other properties of cancellous bone. The current study involved the removal of 27% of the tested samples due to failure within the sample holders. Such a large rate of test failure is not uncommon with samples of irregular shape. A much smaller percentage (12%) of the tests were discarded due to slipping at the holder-trabecula interface or extremely low ultimate loads. Due to the small size of the trabeculae, strain measurements were based on displacements of the testing device crosshead rather than on the trabeculae themselves. A detailed evaluation of the tension test using optical tracking on the surface of a subset of the specimens showed that the ductility measured by the substage apparatus was highly correlated with the maximum strain occurring on the surface of the trabecula, validating our use of cross-head displacement for characterization of ultimate strains within the trabecula (Appendix). Furthermore, the rate of applied load was greater than is typically used for mechanical testing of bone. However, as ultimate strain is insensitive to the rate of applied loading even in at rates of loading as high as 10%/second (40–42) the increased strain rate is not expected to greatly influence the results. Finally, due to the tiny amount of material available for assays from each specimen, the current study addressed only the three types of collagen cross-links (HP, LP, pentosidine) that have been associated with mechanical properties of human bone. Thus, while we found no appreciable effects of pyridinium and pentosidine collagen cross-links on ductility, it is conceivable that other types of collagen cross-links such as pyrrole cross-links could play a more prominent role (20). However, two recent studies failed to find correlations between pyrrolic cross-links and the mechanical properties of human cancellous bone (2,22).
Our findings regarding the structural ductility of individual trabeculae are quite different from measurements made in continuum level (5mm in smallest dimension) samples of cancellous bone. Previous work in our laboratory found the ultimate strain of vertebral cancellous bone in tension to be 1.59 ± 0.33% (mean ± S.D.) (43). The ultimate strain of femoral cortical bone in tension (a measure more directly related to material ductility rather than structural ductility) has been found to decline with age and has been shown to range from 2–4% (44,45). The ultimate strain in tension of individual trabeculae in the current study — about 8.8% on average — is much greater than ultimate strain values in cancellous bone or cortical bone. Our findings are therefore consistent with previous studies that found some human trabeculae and trabecular material to be capable of withstanding “large deformations” prior to fracture (29,37,46). The fact that structural ductility of individual trabeculae is typically four times greater than that of the cancellous bone structure as a whole suggests that significant bending of trabeculae occurs during compressive failure of cancellous bone, consistent with theoretical analyses (47).
Finite element analyses have suggested that if the fracture strain of individual trabeculae was commonly greater than 2%, microdamage (accumulation of damage within the tissue) rather than microfracture would be the primary mode of damage accumulation in cancellous bone (48). As the current study found that ultimate strain is typically much greater than 2%, it supports the assertion that microfracture is rare and provides a mechanical explanation for why there is such a low incidence of microfracture of vertically oriented trabeculae in cancellous bone, even after a substantial overload (6).
A key finding in this study was the significant relationship between pentosidine and structural ductility. The observed relationship is consistent with previous studies in untreated cortical bone samples from humans (14,49). Since pentosidine represents only a small portion of the intermolecular collagen cross-links in bone (18), it is possible that the observed relationship may be indicative of the effects of other NEG-mediated cross-links rather than that of pentosidine alone, although no other such NEG-mediated cross-links have yet been identified. Another possibility is that the observed relationship may be secondary to a correlation between degree of mineralization and NEG mediated cross-links caused by the age of the bone tissue (22). Degree of mineralization can have an important influence on bone ductility, and will be the subject of future studies. In the current study pentosidine concentration explained 9% of the variance in ultimate strain, a proportion of the variance similar to that found in uniformly shaped cortical bone sections (9%) (49). This suggests that factors such as the irregular morphology of the samples did not obfuscate the role of NEG-mediated cross-links in the current study. In contrast, Wang and colleagues found pentosidine to explain up to 35% of the variance in cortical bone toughness in bending (an alternate measure of ductility) (14). Differences in correlation coefficients between these two studies may be related to differences between ultimate strain and toughness measures or to the fact that the larger age range studied by Wang and colleagues (19–89 years of age v. 54–94 in the current study) may have made relationships more distinct. Just as important was the fact that pyridinium cross-links (HP, LP) were not correlated with structural ductility, a finding consistent with previous reports (11,14,49). We conclude that the ductility of individual trabeculae and probability of microfracture is not influenced by pyridinium cross-links but that NEG-mediated collagen cross-links can have a significant but minor effect, similar in magnitude to that found in cortical bone.
Acknowledgments
This work was supported by NIH grant AR41481. The authors thank David Forsyth and Tony Lobay for assistance with optical strain measurements and Brandon Laws and Jacky Wong for their assistance in sample preparation and data processing. Tissue samples were obtained from NDRI.
Appendix
Strain measurements were validated using optical measures of strain on the bone tissue surface. Individual trabeculae were dissected and treated with a biotin-avidin complex to bind 10 μm diameter microspheres throughout the bone surface. Treated trabeculae were tested in the manner described above but were not hydrated prior to testing to allow optical surface strain measurements. Tensile tests of 12 specimens were performed using a high-resolution digital video camera to record each test (ORCA 100, Hamamatsu Photonics K.K., Japan, 1.3 million pixels). Strain measurements were made based on the displacement of tracked features (microspheres and other recognizable features) using a Kanade-Lucas-Tomasi point tracker (50,51). Displacements were calculated assuming negligible out-of-plane motion of the features; a stereo camera setup was not necessary. The displacement of approximately 120 points was tracked in each test and interpolated to calculate strain throughout the gage length of the specimen. Results indicated that the ultimate strain measurements obtained from the crosshead displacements were strongly correlated with the 99th percentile of the surface strains, having a slope near 1 (Figure A1). These results demonstrate that the ultimate strain measured by the substage was a good indicator of the maximum surface strains that occur at fracture.
Figure A1.
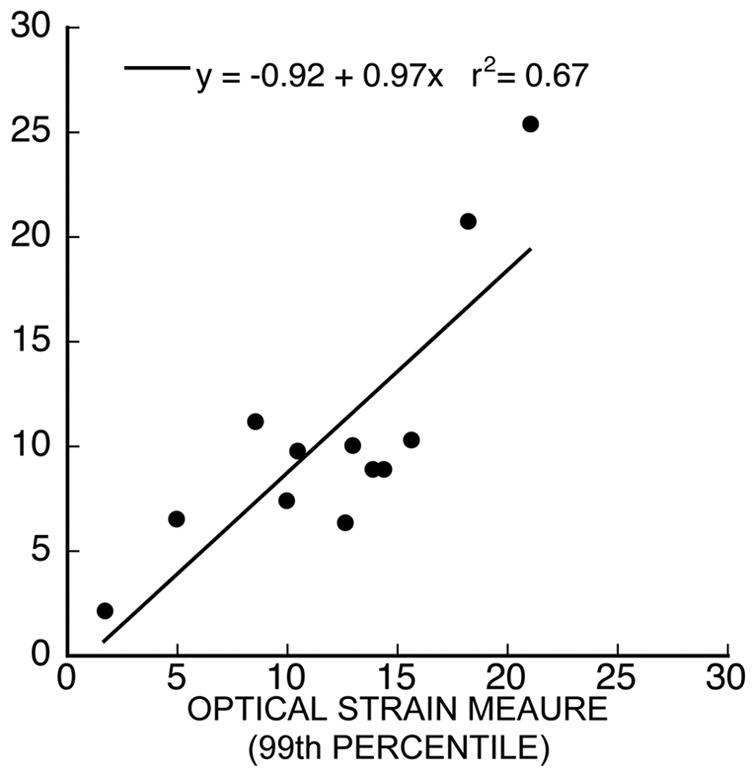
The 99th percentile of optical strain measurements was strongly correlated to fracture strain calculated using substage crosshead displacement with a slope near 1.0. The standard error of the regression was 3.86.
Footnotes
Ductility is a failure property that describes brittleness independent of strength; two structures may have the same strength, that is, they can both withstand the same magnitude of force, but one may easily fracture like chalk while another may be very ductile like rubber.
References
- 1.Burr DB. The contribution of the organic matrix to bone's material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 2.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002;17:1621–8. doi: 10.1359/jbmr.2002.17.9.1621. [DOI] [PubMed] [Google Scholar]
- 3.Guo XE, McMahon TA, Keaveny TM, Hayes WC, Gibson LJ. Finite element modeling of damage accumulation in trabecular bone under cyclic loading. Journal of Biomechanics. 1994;27:145–155. doi: 10.1016/0021-9290(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 4.Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcified Tissue International. 1984;36(Suppl 1):S123–128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- 5.Mosekilde L. Consequences of the remodelling process for vertebral trabecular bone structure: a scanning electron microscopy study (uncoupling of unloaded structures) Bone Miner. 1990;10:13–35. doi: 10.1016/0169-6009(90)90046-i. [DOI] [PubMed] [Google Scholar]
- 6.Fyhrie DP, Schaffler MB. Failure mechanisms in human vertebral cancellous bone. Bone. 1994;15:105–9. doi: 10.1016/8756-3282(94)90900-8. [DOI] [PubMed] [Google Scholar]
- 7.Fazzalari NL. Trabecular microfracture. Calcified Tissue International. 1993;53:S143–S147. doi: 10.1007/BF01673424. [DOI] [PubMed] [Google Scholar]
- 8.Cullinane DM, Einhorn TA. Biomechanics of bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. 2. Vol. 1. Academic Press; San Diego, CA, USA: 2002. pp. 17–32. [Google Scholar]
- 9.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:365S–371S. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 11.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. Journal of Biomedical Materials Research. 1999;45:108–16. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Zioupos P. Ageing human bone: factors affecting its biomechanical properties and the role of collagen. J Biomater Appl. 2001;15:187–229. doi: 10.1106/5JUJ-TFJ3-JVVA-3RJ0. [DOI] [PubMed] [Google Scholar]
- 13.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 15.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–4. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catanese JCI, Bank RA, TeKoppele JM, Keaveny TM. Increased cross-linking by non-enzymatic glycation reduces the ductility of bone and bone collagen. Proc Bioeng Conf. 1999;ASME BED-Vol. 42:267–68. [Google Scholar]
- 17.Lees S, Eyre DR, Barnard SM. BAPN dose dependence of mature crosslinking in bone matrix collagen of rabbit compact bone: corresponding variation of sonic velocity and equatorial diffraction spacing. Conn Tissue Res. 1990;24:95–105. doi: 10.3109/03008209009152426. [DOI] [PubMed] [Google Scholar]
- 18.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 19.Robins SP, Brady JD. Collagen cross-linking and metabolism. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Vol. 1. Academic Press; San Diego, CA, USA: 2002. pp. 211–223. [Google Scholar]
- 20.Knott L, Whitehead CC, Fleming RH, Bailey AJ. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem J. 1995;310:1045–51. doi: 10.1042/bj3101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banse X, Devogelaer JP, Lafosse A, Sims TJ, Grynpas M, Bailey AJ. Cross-link profile of bone collagen correlates with structural organization of trabeculae. Bone. 2002;31:70–6. doi: 10.1016/s8756-3282(02)00800-1. [DOI] [PubMed] [Google Scholar]
- 22.Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcified Tissue International. 1999;65:203–10. doi: 10.1007/s002239900683. [DOI] [PubMed] [Google Scholar]
- 23.Ryan SD, Williams JL. Tensile testing of rodlike trabeculae excised from bovine femoral bone. Journal of Biomechanics. 1989;22:351–5. doi: 10.1016/0021-9290(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 24.Mente PL, Lewis JL. Experimental method for the measurement of the elastic modulus of trabecular bone tissue. Journal of Orthopaedic Research. 1989;7:456–61. doi: 10.1002/jor.1100070320. [DOI] [PubMed] [Google Scholar]
- 25.Rho JY, Ashman RB, Turner CH. Young's modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. Journal of Biomechanics. 1993;26:111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- 26.Bini F, Marinozzi A, Marinozzi F, Patane F. Microtensile measurements of single trabeculae stiffness in human femur. Journal of Biomechanics. 2002;35:1515–1519. doi: 10.1016/s0021-9290(02)00182-3. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn JL, Goldstein SA, Choi K, London M, Feldkamp LA, Matthews LS. Comparison of the trabecular and cortical tissue moduli from human iliac crests. Journal of Orthopaedic Research. 1989;7:876–84. doi: 10.1002/jor.1100070614. [DOI] [PubMed] [Google Scholar]
- 28.Choi K, Kuhn JL, Ciarelli MJ, Goldstein SA. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. Journal of Biomechanics. 1990;23:1103–13. doi: 10.1016/0021-9290(90)90003-l. [DOI] [PubMed] [Google Scholar]
- 29.Choi K, Goldstein SA. A comparison of the fatigue behavior of human trabecular and cortical bone tissue. Journal of Biomechanics. 1992;25:1371–1381. doi: 10.1016/0021-9290(92)90051-2. [DOI] [PubMed] [Google Scholar]
- 30.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330:345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- 32.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mechanisms of Ageing and Development. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 33.Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 34.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 35.Bank RA, Jansen EJ, Beekman B, te Koppele JM. Amino acid analysis by reverse-phase high-performance liquid chromatography: improved derivatization and detection conditions with 9-fluorenylmethyl chloroformate. Anal Biochem. 1996;240:167–76. doi: 10.1006/abio.1996.0346. [DOI] [PubMed] [Google Scholar]
- 36.Lucchinetti E, Thomann D, Danuser G. Micromechanical testing of bone trabeculae - potentials and limitations. Journal of Materials Science. 2000;35:6057–6064. [Google Scholar]
- 37.Yeh OC, Tischner TT, Keaveny TM. Trans Orthop Res Soc. Vol. 26. San Francisco: 2001. Fracture strains of trabecular tissue exceed 20% in bending; p. 520. [Google Scholar]
- 38.Muller R, Gerber SC, Hayes WC. Micro-compression: a novel technique for the nondestructive assessment of local bone failure. Technol Health Care. 1998;6:433–44. [PubMed] [Google Scholar]
- 39.Morgan EF, Bayraktar HH, Yeh OC, Majumdar S, Burghardt A, Keaveny TM. Contribution of inter-site variations in architecture to trabecular bone apparent yield strains. J Biomech. 2004;37:1413–20. doi: 10.1016/j.jbiomech.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 40.McElhaney JH. Dynamic response of bone and muscle tissue. J Appl Physiol. 1966;21:1231–6. doi: 10.1152/jappl.1966.21.4.1231. [DOI] [PubMed] [Google Scholar]
- 41.Currey JD. The mechanical properties of bone. Clin Orthop. 1970;73:209–31. [PubMed] [Google Scholar]
- 42.Crowninshield RD, Pope MH. The response of compact bone in tension at various strain rates. Annals of Biomedical Engineering. 1974;2:217–225. [Google Scholar]
- 43.Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. Journal of Biomechanics. 1998;31:601–8. doi: 10.1016/s0021-9290(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 44.Burstein AH, Currey JD, Frankel VH, Reilly DT. The ultimate properties of bone tissue: the effects of yielding. Journal of Biomechanics. 1972;5:35–44. doi: 10.1016/0021-9290(72)90017-6. [DOI] [PubMed] [Google Scholar]
- 45.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. Journal of Bone and Joint Surgery American Volume. 1993;75:1193–205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Nazarian A, Muller R. Time-lapsed microstructural imaging of bone failure behavior. J Biomech. 2004;37:55–65. doi: 10.1016/s0021-9290(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 47.Gibson LJ. The mechanical behavior of cancellous bone. Journal of Biomechanics. 1985;18:317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 48.Yeh OC, Keaveny TM. The relative roles of microdamage and microfracture in the mechanical behavior of trabecular bone. Journal of Orthopaedic Research. 2001;19:1001–1007. doi: 10.1016/S0736-0266(01)00053-5. [DOI] [PubMed] [Google Scholar]
- 49.Keaveny TM, Morris GE, Wong EK, Yu M, Sakkee AN, Verzijl N, Bank RA. Collagen status and brittleness of human cortical bone in the elderly; 25th Annual Meeting of the American Society for Bone and Mineral Research; Minneapolis, MN, USA. 2003. p. M051. [Google Scholar]
- 50.Tomasi C, Kanade T. Carnegie Mellon University Technical Report. 1991. Detection and tracking of point features. [Google Scholar]
- 51.Birchfield S. Derivation of Kanade-Lucas-Tomasi Tracking Equation. Stanford, CA, USA: 1996. [Google Scholar]


