Abstract
The transformation of antenna to leg is a classical model for understanding segmental fate decisions in Drosophila. The spineless (ss) gene encodes a bHLH-PAS transcription factor that plays a key role in specifying the identity of distal antennal segments. In this report, we identify the antennal disc enhancer of ss, and then use enhancer-lacZ reporters to work out how ss antennal expression is regulated. The antennal determinants Distal-less (Dll) and homothorax (hth) are key activators of the antennal enhancer. Dll is required continuously and, when present at elevated levels, can activate the enhancer in regions devoid of hth expression. In contrast, homothorax (hth) is required only transiently both for activation of the enhancer and for specification of the aristal portion of the antenna. The antennal enhancer is repressed by cut, which determines its proximal limit of expression, and by ectopic Antennapedia (Antp). Repression by Antp is not mediated by hth, suggesting that ss may be a direct target of Antp. Finally, we show that ss+ is not a purely passive target of its regulators: ss+ partially represses hth in the third antennal segment and lies upstream of Dll in the development of the maxillary palp primordia.
Keywords: spineless, aristapedia, dioxin receptor, aryl hydrocarbon receptor, Distal-less, homothorax, antenna, Drosophila, cut, Antp
Introduction
How the identity of the antenna is specified in Drosophila was for many years a mystery. It was known that the Hox genes are not involved, since the anterior limit of their expression in the body lies just posterior to the antennal segment. In the last few years, it has become clear that at least three genes play key roles in specifying antennal identity: homothorax (hth) and Distal-less (Dll), which encode homeodomain proteins, and spineless (ss), which encodes a bHLH-PAS protein. The most important of these is hth. Mitotic recombination clones homozygous for hth− alleles can transform the entire antenna to a limb that is leg in identity, although not form (Casares and Mann 1998; 2001). Consistent with this transformation, hth is expressed throughout the antennal primordium in the first and second larval instars. However, during the late second or early third instar hth is repressed in the region of the antennal disc that gives rise to the arista, and down regulated in the next most proximal region, which gives rise to the third antennal segment (A3) (Fig. 1D). In leg discs, hth undergoes a more extreme distal repression, so that it comes to be expressed only in the most proximal segments (coxa and trochanter), where it serves to distinguish these segments from more distal ones (Abu-Shaar and Mann 1998; Wu and Cohen 1999). Hth functions as a heterodimer with the homeodomain protein Extradenticle (Exd), which is also required for antennal identity and normal proximo-distal subdivision of the leg (González-Crespo and Morata 1995; Rieckhof et al. 1997; Kurant et al. 1998; Pai et al. 1998).
Figure 1.
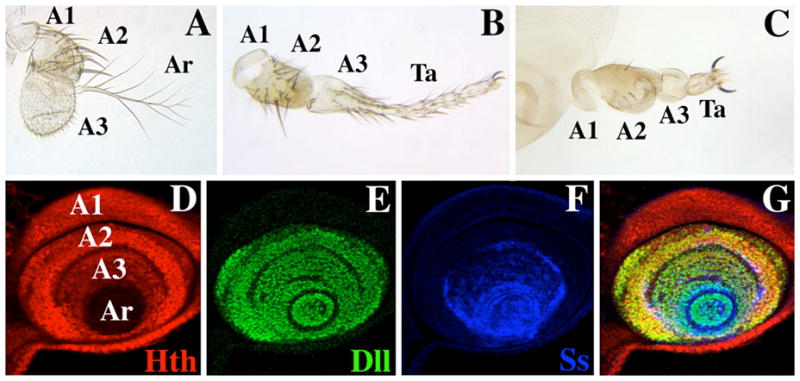
Antennae from wild-type and ss mutant adults, and expression of Hth, Dll, and the ss antennal reporter B6.9 in a mature antennal disc. A) A wild-type antenna. The first (A1), second (A2), and third (A3) antennal segments and the arista (Ar) are indicated. B) Antenna from an ssa-type mutant (ssD114.7/Df(3R)ssD114.4). Note that distal A3 and the arista are transformed to an almost complete set of tarsi (Ta). C) Antenna from a ss null mutant (ssD115.7/Df(3R)ssD114.4). Apart from a reduction in bristle size, A1 and A2 are basically normal; A3 is composed of naked cuticle, and the arista is reduced to a fifth tarsal segment (Ta) with claws. Panels D–G show a late third instar antennal disc triply labeled for Hth (red), Dll (green), and the ss antennal reporter B6.9 (blue). The primordia of A1, A2, A3, and Ar are indicated. Note that Hth (red) is present at a lower level in A3 than in A1 and A2, and is absent in the aristal region (D), Dll (green) is expressed in A2 and more distally (E), and the B6.9 ss antennal reporter (blue) is expressed in A3 and more distally (F). G) Merging of panels D–F.
The Dll gene is expressed in the distal portions of all of the ventral appendages, and loss-of-function alleles of Dll cause deletions of distal structures in these appendages. In the antenna, Dll is expressed in A2, A3, and the arista (Fig. 1E), and this entire expression domain is deleted in Dll− mutants (Cohen and Jürgens 1989). However, some weak alleles of Dll cause transformations of distal antennal structures toward leg, suggesting that Dll+ has a role in specifying distal antennal identity that is distinct from its requirement for distal limb development (Sunkel and Whittle 1987; Dong et al. 2000). Since Dll is expressed in the distal portions of all the ventral appendages, this function must depend upon interaction with some other factor that is differentially expressed in the legs and antennae. Dong et al. (2000) have suggested that this factor is Hth, and that the identity of A2, A3, and the arista is defined by the combined expression of hth and Dll. This idea is supported by the effects of hth− and Dll− alleles on the expression of antenna-specific genes, and by the finding that combined ectopic expression of Hth and Dll can cause transformations to antenna (Dong et al. 2000; 2002).
The ss gene is the Drosophila homolog of the mammalian Aryl Hydrocarbon receptor (AHR), also known as the dioxin receptor (for review see Schmidt and Bradfield 1996). In the antenna, ss is expressed continuously in A3 and more distal segments (Duncan et al. 1998) (Fig. 1F) and is required for these segments to develop with antennal identity (Struhl 1982; Burgess and Duncan 1990; Duncan et al. 1998). In ss− mutants, A3 develops with almost no specialization and consists of naked cuticle, while the region distal to A3 develops as a fifth tarsal segment with claws. Ectopic expression of ss can induce ectopic distal antennal structures, indicating that ss is an antennal determinant (Duncan et al. 1998). ss+ is also required for development of tarsal segments 1–4 in the legs, and is expressed transiently in a ring in the tarsal regions of the leg discs. Consistent with the model of Dong et al. (2000), ss expression in the antenna requires both Dll+ and hth+ (Duncan et al. 1998; this report). Thus, ss+ lies downstream of these genes, and likely executes many of their functions in specifying distal antennal identity.
In this report, we use lacZ reporters to identify the cis-regulatory elements responsible for driving ss expression in embryos and imaginal discs. We identify enhancers responsible for almost all aspects of ss expression. At least three distinct antennal enhancers are present; we focus on the enhancer responsible for expression in the larval antennal disc. We analyze the activity of this enhancer in clones homozygous for null alleles of other genes involved in limb development. We find that the antennal disc enhancer is positively regulated by Dll and hth; Dll is required continuously for activation, whereas hth is required only early in development. Clones expressing Dll ectopically can activate the enhancer in the absence of Hth expression, suggesting that Dll is its primary activator. The enhancer is repressed by cut, which defines the proximal limit of its activity. The antennal disc enhancer is also repressed by ectopic expression of Antennapedia (Antp). Repression by Antp can occur within clones induced long after the requirement for hth has passed, suggesting that ss is an independent target of Antp responsible for mediating transformations of distal antenna to leg. We also identify a ss enhancer that drives expression in a ring in the tarsal primordia of the legs and in the distal antenna. The latter expression likely accounts for the development of a full set of tarsal segments in the transformed antennae of ss mutants lacking only the antennal enhancer. Finally, we show that ss is not a purely passive target of its regulators; ss down regulates hth expression in A3 and lies upstream of Dll in development of the maxillary palps and perhaps also the bract cells of the legs.
Materials and Methods
Restriction fragments from ss λ clones (Duncan et al. 1998) were subcloned into the enhancer-tester vector pCaSpeR-hs43-βgal (Thummel and Pirrotta 1992) and germ-line transformants recovered by standard methods. Two independent insertions were recovered for each of the EX6.5 and EX4.3 fragments; three or more independent insertions were analyzed for the remaining fragments.
Dissection of B6.9 and EX6.5
The B6.9 subfragments E1.6, E1.9, E2.0, and EX1.9 were generated by EcoRI or EcoRI and XbaI digestion of λ clones from ss (Duncan et al. 1998). S4.9 was generated by digesting B6.9 with SpeI. Four subclones of E2.0, of 522, 542, 531, and 554 bp, were generated by PCR; these were cloned into pCR2.1 (Invitrogen), verified by sequencing, and then transferred to pCaSpeR-hs43-βgal. The EX6.5 fragment was subdivided by complete or partial digestion by PstI (see Fig. 2 in Supplementary Information).
Antibody staining
Primary antibodies used were mouse anti-Distal-less (Duncan et al. 1998), rabbit anti-Homothorax, mouse anti-Engrailed, mouse anti-Antennapedia and mouse anti-Dachshund (gifts of A. Salzberg, N. Patel, D. Brower and G. Mardon, respectively), mouse anti-β-galactosidase (Promega), rabbit anti-β-galactosidase (Cappel) and mouse anti-22C10 (Developmental Studies Hybridoma Bank). Secondary antibodies used were Cy3 Donkey anti-rabbit, Cy5 Donkey anti-rabbit and Cy3 Donkey anti-mouse (Jackson), FITC sheep anti-mouse and FITC goat anti-rabbit (Cappel). Antibody stainings were as described previously (Kankel et al. 2004), and all images were captured on a Leica confocal SP2 microscope.
X-Gal staining
Tissues were fixed in 0.5% glutaraldehyde in PBSTX (Phosphate Buffered Saline + 0.05% Triton X-100) and incubated in 10 mM NaH2PO4 pH 7.2, 150 mM NaCl, 3.3 mM K3[Fe(CN)6], 3.3 mM K4[Fe(CN)6], 0.1% Tween 20 and 0.1% X-Gal (all reagents from Sigma) for 20 minutes to overnight at room temperature.
Mitotic recombination
A single line of B6.9-lacZ (line 6; located in 3R) was used in all experiments. For 522-lacZ, lines 6A (chromosome 2) and 6B (chromosome 3) were used interchangeably, and for P732-lacZ, both lines 2A (X chromosome) and 6A (chromosome 3) were used. Because 522 is a weak reporter, its expression was always monitored in animals carrying two doses. To generate mitotic recombination clones, FRT40A, FRT42D, and FRT82B were used as appropriate. Clones were marked by expression of Ubi-GFP or, in a few cases, by expression of CD2. In all cases, hs-FLP122 was used to induce mitotic recombination. Crosses were made in glass vials, and cultures of desired age were heat shocked to induce FLP by immersion in a water bath at 37°C for 30 minutes. The following mutant alleles were used: ssD115.7 (Duncan et al. 1998), hth64-1 (provided by A. Salzberg), hthP2 (provided by R. Mann), DllSA1 (provided by E. Sanchez-Herrero), dacshund (dac)1 (Bloomington stock center) and aristaless (al)ex (provided by G. Campbell). All are null or strong loss-of-function alleles. A duplication of 60B-F (Duncan, unpublished), a region that includes Dll+, was used to test the dependence of reporter expression on Dll+ dosage. This duplication [Dp(2;2)D11] is located at the very tip of 2L.
Ectopic expression
Act5C>y+>GAL4 and Act5C>CD2>GAL4 were used interchangeably to produce ectopic expression clones. Excision of y+ or CD2 from these elements was induced using hs-FLP12. Clones were induced as described above, except the duration of the heat shock was 10 minutes. The UAS lines used included UAS-ss (line A1 in Duncan et al. 1998), UAS-hth (lines 15, 4, and 12; provided by H. Sun), UAS-Dll (provided by G. Morata), UAS-dac (line 21m5m4; provided by G. Mardon), UAS-Antp (provided by T. Kaufman), and UAS-al (line al6 al4; provided by T. Kojima). For combined Hth and Dll expression, the combinations UAS-hth4 UAS-Dll, UAS-hth12 UAS-Dll, and UAS-hth15; UAS-Dll were derived.
Results
The fragments tested for enhancer activity are shown in Fig. 2; together they cover all but the 3′ region of the gene. Transformants were examined for β-galactosidase expression in imaginal discs, the imaginal epidermis of the pupa, and embryos. In aggregate, the enhancers identified account for almost all aspects of ss expression (Duncan et al. 1998).
Figure 2.

Enhancer mapping within ss. The DNA scale is marked in kilobases. Above this scale are shown the ss transcribed region (exons are indicated as blue boxes; coding regions are dark blue, noncoding regions are light blue) and the locations of four ss mutants (A designates antennal transformations and B designates bristle reduction). The fragments tested for enhancer activity are indicated in red, and the expression patterns each drives are shown below for embryos (top), imaginal discs (middle) and for structures at the pupal stage (bottom). Embryonic expression is shown for only B6.9 and EX8.2. The expression patterns shown for EX6.5 are actually those of its P732 subfragment, which drives persistent, rather than transient, expression in the leg and antenna (see results). For all fragments, the embryonic and disc expression patterns shown correspond to patterns detected for the ss transcript (Duncan et al. 1998). Abbreviations: Ab (abdominal tergite), An (antenna), E (embryo), L (leg), P (maxillary palp), W (wing).
Our primary interest is in the expression of ss in the antenna. ss is expressed in the antennal segment of the embryo and in the distal portion of the antennal imaginal disc through larval and early pupal development. After disc eversion, ss transcripts are detected throughout the third antennal segment (A3) and the arista. Two fragments from ss contain antennal enhancers. The B6.9 fragment reproduces almost all aspects of ss antennal expression; it drives expression in the antennal segment of the embryo, and in the A3 and aristal regions of the antennal disc in the larval and pupal stages (Fig. 2). The EX8.2 fragment drives similar expression in the embryonic and pupal antenna, but does not drive expression in the larval antennal disc. The embryonic antennal expression of B6.9 and EX8.2 is seen from germ-band extension until the end of embryogenesis.
At pupal and late larval stages, ss is also expressed in the maxillary palp primordium, which lies just ventral and anterior to the antenna. Like A3, the palps are major olfactory organs of the fly. Both B6.9 and EX8.2 drive expression in the palp primordia in late larval and pupal stages (Fig. 2); B6.9 is expressed throughout the palp, whereas EX8.2 is expressed only in basiconic sensilla (not shown).
Transcripts from ss also accumulate in a ring in the tarsal regions of each of the legs (Duncan et al. 1998). This expression is transient, occurring at the late second and early third instars, and reflects the requirement for ss in the development of the tarsal regions of the legs. Tarsal ring expression is driven by the EX6.5 fragment, from the second intron of ss (Fig. 2). EX6.5 also drives a ring of expression in the antenna.
After pupariation, ss is expressed in association with most bristles, consistent with its requirement for normal bristle growth. Three bristle enhancers were identified. Two of these (contained within the E1.2 and X8.2 fragments) drive expression in most bristles of the fly (Fig. 2). A third bristle enhancer (located in the shared region of fragments EX6.5 and E5.0) drives expression in the taste bristles of the legs (Fig. 2), proboscis, and wing. ss is also expressed in the peripheral nervous system (PNS) of the embryo. The regulation of ss in the PNS is complex and involves redundant enhancers. For additional information on the ss bristle and PNS enhancers see Fig. 1 in Supplementary Data.
We focused our attention on the B6.9 fragment, as it drives antenna-specific expression through much of development. To define further the antennal enhancer(s) carried by B6.9, we divided the fragment into four subclones: E1.6, E1.9, E2.0, and EX1.9 (see Fig. 3). Only E2.0 drives expression in imaginal discs. In the antenna, E2.0 drives expression in a pattern identical to that of B6.9. However, E2.0 also drives expression in leg discs. Here, the pattern is similar to that of Dll, consisting of a large central patch and a proximal ring.
Figure 3.
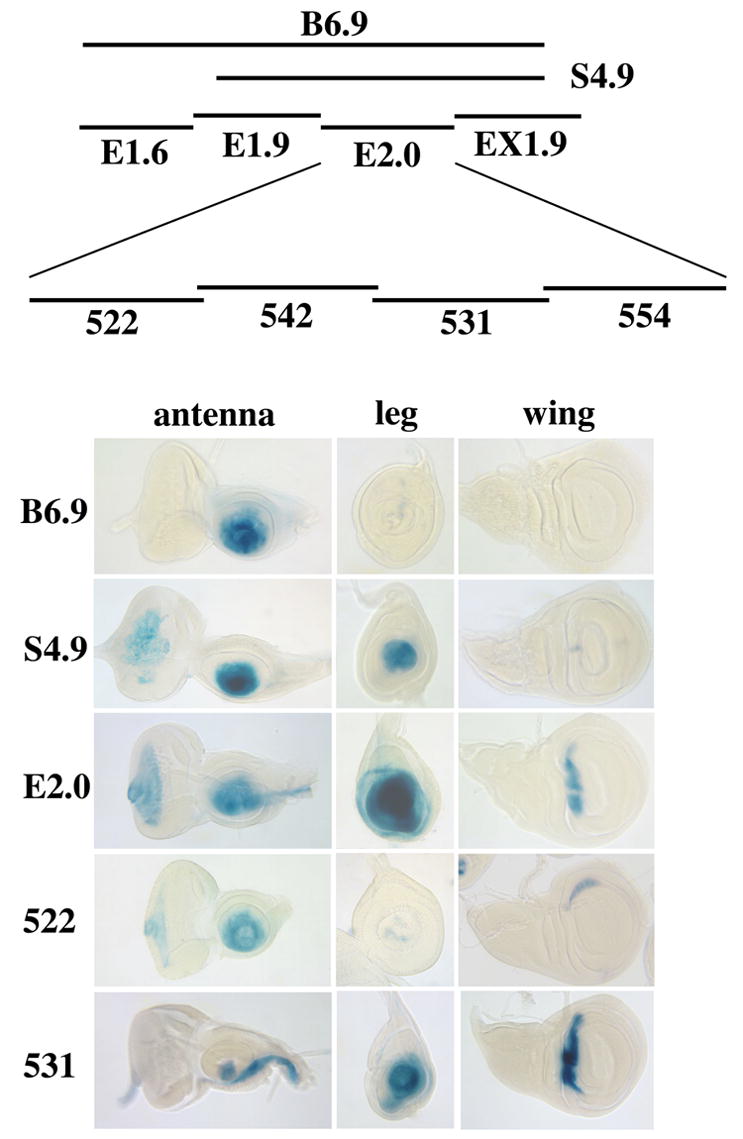
Dissection of the B6.9 fragment. Top: Summary of fragments tested using lacZ reporters. Bottom: X-Gal stained eye-antennal, leg, and wing discs showing the expression patterns driven by each fragment. B6.9 drives expression in the distal antenna, but not in leg or wing discs. In contrast, the S4.9 and E2.0 subfragments drive expression strongly in both antennal and leg discs. E2.0 also drives expression in a ventral stripe in the antennal disc and in the dorsal hinge region of the wing disc. S4.9 shows very weak expression in this same region of the wing disc. The leg and antennal disc expression of E2.0 prove to be largely separable, with the 522 and 531 fragments driving expression primarily in the distal antenna and leg, respectively. The separation is not complete, however, with 522 driving weak mottled expression in the distal leg, and 531 driving expression in the aristal region and in a ventral stripe in the antennal disc. 522 and 531 also drive expression in wing discs in the lateral and dorsal hinge regions, respectively. The E1.6, E1.9, EX1.9, 542 and 554 fragments do not drive expression in antennal or leg discs.
We further subdivided the E2.0 fragment into four subfragments of 522, 542, 531, and 554 bp (Fig. 3). To our surprise, we found that antennal and leg expression are largely separable: one fragment (522) drives expression primarily in the distal portion of the antennal disc, whereas a second (531) drives expression primarily in the distal leg. Antennal expression of 522 is similar to that of E2.0 and B6.9, with the exception that 522 usually shows weakened expression in the most central (aristal) region of mature discs. 522 also drives very weak, mottled expression in leg discs, which is restricted to the most distal region. In addition to its prominent leg expression, the 531 fragment drives expression in the most central region of the antennal disc and in a stripe in the region of the future palp and rostral membrane. The remaining two subfragments (542 and 554) do not drive expression in imaginal discs.
To determine which portion of the B6.9 fragment is responsible for restricting expression to the antenna, we examined an additional fragment, S4.9 (see Fig. 3). This fragment drives expression in both the antenna and leg, suggesting that the portion of B6.9 not contained within S4.9 represses expression in the leg. Significantly, B6.9 causes strong suppression of the mini-white reporter gene when homozygous, whereas S4.9 does not. The E1.6 subfragment, derived from the region deleted to produce S4.9, causes similar repression. These observations suggest that E1.6 contains a Polycomb response element (PRE) (for review see Kassis 2002). PREs are the sites of action of the Polycomb group (PcG) proteins, which function to maintain a transcriptionally silent state (for review see Ringrose and Paro 2004). The presence of a PRE within E1.6 suggests that the antennal specificity of B6.9 may be maintained by PcG-mediated repression. Homozygotes for some insertions of the EX8.2 fragment, which drives antennal expression in the embryo and pupa, also show repression of mini-white, suggesting the presence of at least one additional PRE in ss.
As described above, B6.9 drives expression in the antennal segment of the embryo as well as in the antennal disc. This embryonic antennal expression is due to a distinct enhancer located within the E1.9 subfragment. E1.9 drives expression in the antennal segment beginning at germ-band extension, and continuing until late in embryogenesis. Since E1.9 does not lie adjacent to EX8.2, which also drives expression in the antennal segment of the embryo, ss must have at least two embryonic antennal enhancers.
Regulation of antennal expression of ss by Dll and hth
To investigate how the ss antennal disc enhancer is regulated, we examined expression of the B6.9 and 522 antennal reporters within clones of cells homozygous for mutations in potential regulators. Our results indicate that Dll and hth play key roles in activating the ss antennal enhancer. Our results are consistent with the proposal that antennal identity is determined by the combined action of Dll and Hth (Dong et al. 2000). However, our results reveal strong context-dependence, indicating that other factors must also be involved.
Both B6.9 and 522 require Dll continuously for expression (Fig. 4A,E). The earliest Dll− clones studied were induced 2 days after egg laying (AEL). When examined one day later, these clones showed no or reduced expression of the reporters. Clones induced later in development behaved similarly. In contrast, hth is required only early in development for antennal expression of the reporters. For B6.9, hth− clones induced 0–2 days AEL showed autonomous loss of expression in the antenna (Fig. 4B). However, at later times B6.9 expression became partially or completely independent of hth. The requirement for hth is first lost in the aristal primordium; hth− clones induced 2–3 days AEL showed persistent B6.9 expression in this region but not more proximally (Fig. 4C). Clones induced after 3 days AEL showed normal B6.9 expression throughout the antenna (Fig. 4D). Because 522 drives weak and variable expression in the tarsal region of the leg, we focused our attention on hth− clones in A3 for this reporter. hth− clones induced from 0–2 days AEL usually showed an autonomous loss of 522 expression (Fig. 4F). Clones induced from 2–3 days AEL also lost 522 expression for the most part, but sectors within these clones usually showed persistent 522 expression (Fig. 4G). Most clones induced after 4 days AEL had no effect on 522 expression (Fig. 4H). The picture that emerges is that activation by Hth can be persistent for both reporters, but is more stable for B6.9 than for 522.
Figure 4.

Regulation of the B6.9 and 522 antennal reporters by Dll and hth. In all discs shown here, loss of GFP fluorescence (green) is used to mark clones, and β-galactosidase expression is shown in red. A, E: Dependence of B6.9 (A) and 522 (E) expression on Dll+. Expression of both reporters is lost within Dll− clones (arrowheads). This is true for clones induced throughout larval development. B–H: Control of B6.9 (B, C, D) and 522 (F, G, H) expression by hth. The images in B, C, and D are complicated by the fact that the B6.9 insertion studied is located in the same chromosome arm (3R) as hth. Consequently, the hth+/hth+ twin spot clones (recognized by strong green fluorescence) lack the B6.9 reporter altogether. B, F: hth− clones induced 1–2 days AEL. Such clones lose expression of B6.9 and 522 autonomously (arrowheads). C, G: hth− clones induced 2–3 days AEL. Such clones show persistent expression of B6.9 in the aristal region (arrow), but loss of expression more proximally (arrowhead in C). 522 expression is usually lost in such clones, although some persistence is seen (arrows in G). D, H: hth− clones induced 4–5 days AEL. B6.9 expression is unaffected (arrows in D), whereas 522 expression is lost in some clones (arrowhead in H) but retained in others (arrows in H). I–L: Effects on antennal identity of hth− clones induced at different times of development. I: A normal antenna heterozygous for the bristle markers (M(3)w Bsb) used to mark hth− clones. Note the reduction of the aristal (Ar) branches. J: An antenna containing hth− clones induced during the second day AEL [time corrected for the delay caused by heterozygosity for M(3)w]. Because the Minute technique was used to confer a growth advantage to clones, the antenna is probably entirely mutant. The entire antenna is transformed to leg, with the distal region developing as a set of tarsal segments (Ta) terminated by claws (Cl). K: An antenna containing hth− clones induced during the third day AEL. Note that partially formed tarsal segments are present, terminated by an arista (arrow). L: An antenna containing hth− clones induced during the fourth day AEL. Note that tarsal segments are absent, and that a complete arista including a basal cylinder (arrow) is present.
We used the apparent null allele hth64-1 (Kurant et al. 1998) in most experiments. However, we also tested hthP2, which has been used extensively by other groups as a null allele. We find that hthP2 is weaker than hth64-1 in its effects on B6.9 expression, with even very early hthP2 clones showing some expression of B6.9.
To determine when hth+ is required for normal antennal specification, we examined the cuticular phenotypes of hth− clones induced at progressively later times in development. We used the Minute technique to increase clone size, so that in most cases the antennae examined were largely or entirely mutant. Clones induced before about 2 days AEL (corrected for the slower development time of M(3)w heterozygotes) showed a complete transformation to leg (Fig. 4J). Clones induced in the third day were variable in phenotype, with many showing some aristal development (Fig. 4K). Clones induced in the fourth and fifth days showed normal development of the arista and basal cylinder (the segmented base of the arista), although more proximal segments continue to be transformed toward leg (Fig. 4L). These observations indicate that the aristal region becomes independent of hth sometime in the second or early third instar, roughly consistent with when the antennal enhancer becomes independent of hth. The restricted early requirement for hth in the distal antenna is consistent with the loss of hth expression here beginning in the second instar (Casares and Mann 1998).
To test further the roles of Dll and hth in activating ss, we examined the effects on the antennal reporters of clones expressing Dll and/or Hth ectopically. The results were not simple, and indicate that regulation of ss by Hth and Dll is strongly dependent on context. The results also suggest that Dll is the primary activator of the antennal enhancers. Below we describe the effects of ectopic expression clones in the antenna, leg, and wing discs.
1) Antennal discs
Clones expressing Dll frequently show ectopic expression of B6.9 and 522 when located in the region of the antennal disc ventral to the antenna, which includes the maxillary palp primordium (Fig. 5A,B). Activation of B6.9 and 522 is variable, with many Dll-expressing clones failing to activate the reporters. To determine whether endogenous hth expression is the critical determinant of this variation, we monitored Hth in Dll-expressing clones; activation of B6.9 and 522 by ectopic Dll is not correlated with the presence of Hth. Surprisingly, Hth-expressing clones can repress both B6.9 and 522 in the antenna (Fig. 5C,D). Thus, although Hth is required for the activation of these reporters, elevated levels of Hth represses them. Consistent with this observation, ss is normally expressed in regions where hth expression is reduced (A3) or absent (arista). Finally, clones expressing both Dll and Hth in the antennal disc behave much like clones expressing Dll alone (Fig. 5E,F).
Figure 5.
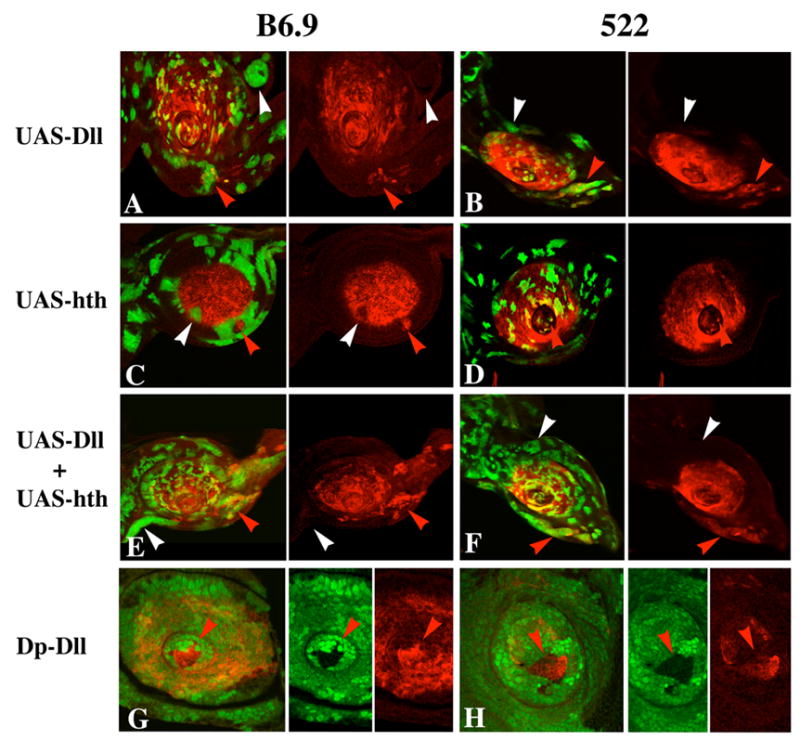
Effect of ectopic expression of Dll and Hth on expression of B6.9 and 522 in antennal discs. In all panels, clones are marked by GFP fluorescence (green) and β-galactosidase expression is in red. A, B: Dll-expressing clones induce expression of both B6.9 (A) and 522 (B) in the region ventral to the antenna (red arrowheads), but not elsewhere (white arrowheads). C, D: Hth-expressing clones often repress B6.9 and 522 proximally (white arrowheads), but upregulate 522 when located in the most distal (aristal) region (red arrowhead in D). Note that proximal Hth-expressing clones tend to follow the proximal limit of reporter expression (the A2–A3 boundary) for many cells (C). The disc shown in C is unusual in that a patch of β-galactosidase expressing cells is present proximal to the normal expression domain of the reporter (red arrowhead); whether this results from new induction of expression by the surrounding cells or from entrapment of originally distal cells is not clear. E, F: Clones expressing both Dll and Hth are very similar to clones expressing only Dll in their effects on B6.9 and 522 expression. G, H: Effects of varying Dll dosage on expression of 522 in antennal (G) and leg (H) discs. These discs contain clones carrying four doses of Dll+ (no green fluorescence) and two doses of Dll+ (bright green fluorescence), in a background of cells carrying three doses (light green fluorescence). Note the strong activation of 522 in the four-dose clones in both the antenna and leg (red arrowheads).
2) Leg discs
Dll-expressing clones in leg discs frequently activate B6.9 and 522, but only when located distally (Fig. 6A,C). This activation is not accompanied by expression of hth. Moreover, Dll-expressing clones in the most proximal region of the leg, where endogenous hth is expressed, do not activate the reporters. Clones expressing Hth often activate expression of the antennal reporters when located distally in the leg (Fig. 6E,G), as do clones expressing both Hth and Dll (Fig. 6I,K). Neither of the latter clone types activates reporter expression in the proximal leg.
Figure 6.
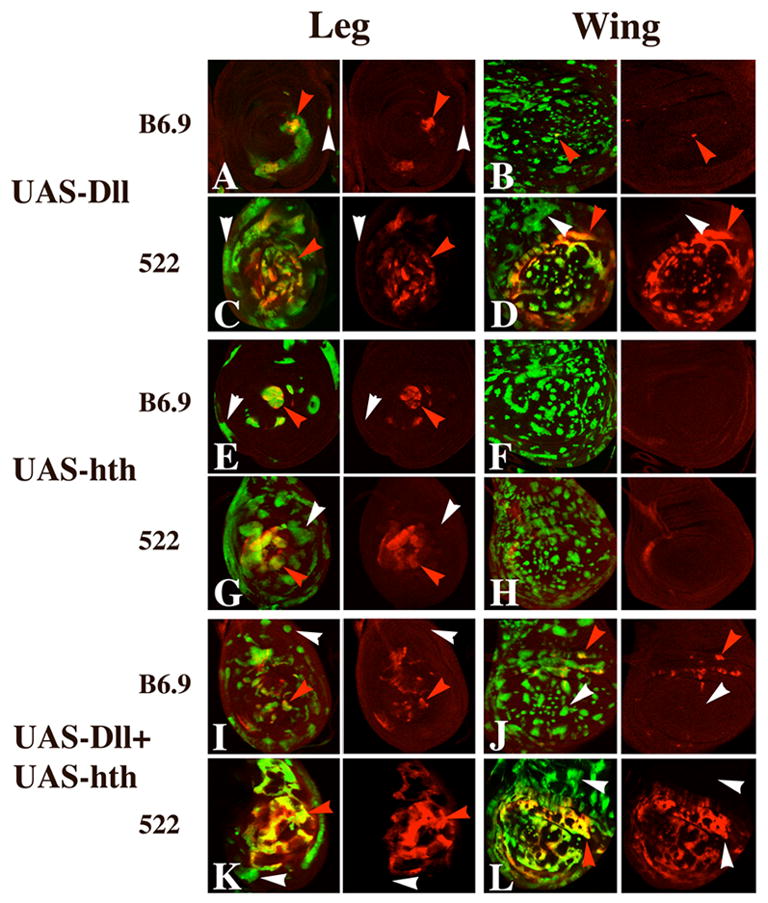
Effect of ectopic expression of Dll and Hth on expression of B6.9 and 522 in leg and wing discs. A–D: Dll-expressing clones activate 522 expression strongly in the distal leg (C) and in the wing blade (D) (red arrowheads), but not in more proximal regions of either disc (white arrowheads). Such clones can also activate B6.9 in the distal leg (red arrowhead in A) and, rarely, in the wing (red arrowhead in B). E–H: Hth-expressing clones also frequently activate both reporters in the distal leg (red arrowheads in E and G), but do not activate the reporters in the proximal leg (white arrowheads in E and G) or anywhere in the wing blade (F and H). I–L: Clones expressing both Dll and Hth behave much like clones expressing only Dll, with the exception that B6.9 is weakly activated in the wing hinge region in clones expressing both proteins (J).
The finding that Dll-expressing clones activate the antennal reporters in the distal leg is striking because Dll is normally expressed here anyway. Why should additional expression of Dll activate the antennal reporters? One possibility is that the level of Dll is critical. It could be that high levels of Dll can activate the antennal reporters in the absence of Hth, while lower levels of Dll also require Hth. Two lines of evidence support this idea. First, expression of both B6.9 and 522 in the antenna is reduced in Dll− heterozygotes (data not shown). Second, expression of the 522 reporter is increased in clones containing extra doses of Dll+. Using a duplication bearing Dll+ at the tip of 2L, we generated twin spots in which one clone has four doses of Dll+, and the other has two. As shown in Fig. 5H, the four dose clones show significant activation of 522 in the most distal region of leg discs, whereas two-dose clones show much weaker or no activation. Activation of 522 in the aristal primordium is also much stronger in the four-dose clones (Fig. 5G). Taken together, these observations suggest that Dll is the primary activator of the ss antennal disc enhancer.
3) Wing discs
The effects of Dll-expressing clones in the wing disc are strikingly dependent on position. The 522 reporter is activated strongly in the wing hinge region (which expresses hth endogenously) and in the pouch region (which does not express hth) (Fig 6D). B6.9 is activated only weakly in these locations (Fig. 6B). Neither reporter is activated in the notal region of the disc (which does express hth). Hth-expressing clones do not activate either reporter anywhere in the wing disc (Fig. 6F,H). Combined expression of Dll and Hth has effects that are very similar to expression of Dll alone (Fig. 6J,L).
In apparent contrast to our results, Dong et al. (2002) report that ss transcripts are induced along the compartment boundary of the wing pouch when Hth expression is driven here by dpp-GAL4. This induction was presented as evidence that ss is activated by the combined expression of Hth and Dll, despite the apparent induction of ss well away from the Dll-expressing wing margin. We find that the B6.9 reporter is not activated in the wing pouch in dpp-GAL4; UAS-hth animals (not shown) (522 has not been so tested). Therefore, it seems very likely that the ss transcripts induced in the wing pouch by dpp-GAL4/UAS-hth result from the activation of some other enhancer in ss. A likely candidate is the enhancer in the E5.0 fragment (see Fig. 2) that drives ss expression near the compartment boundary in the notal region of the wing disc. Transformation of wing pouch cells to a more proximal identity by ectopic Hth might be expected to activate this enhancer near the compartment boundary. Although one might argue that it does not matter which ss enhancer is activated by Hth in the wing blade, activation of the antennal enhancer implies transformation to antennal identity, whereas activation of the notal enhancer does not.
In summary, the effects of Dll and/or Hth-expressing clones in imaginal discs do not support a simple model of activation of B6.9 and 522 by combined Dll and Hth, as activation occurs within Dll-expressing clones that do or do not express hth, and many Dll-expressing clones that also express Hth do not activate the reporters.
We also asked whether Dll and hth are required for expression of the embryonic antennal enhancers of ss. In Dll− embryos, antennal expression of B6.9 is abolished, whereas expression of EX8.2 is unaffected (Fig. 7C). Consistent with these observations, B6.9 expression in the embryo is coincident with expression of Dll in the antennal segment (Fig. 7E), whereas expression driven by EX8.2 includes the Dll-expressing region as well as a few more anterior cells (Fig. 7J). Antennal expression of both B6.9 and EX8.2 is unaffected in hth− embryos (Fig. 7D,I).
Figure 7.
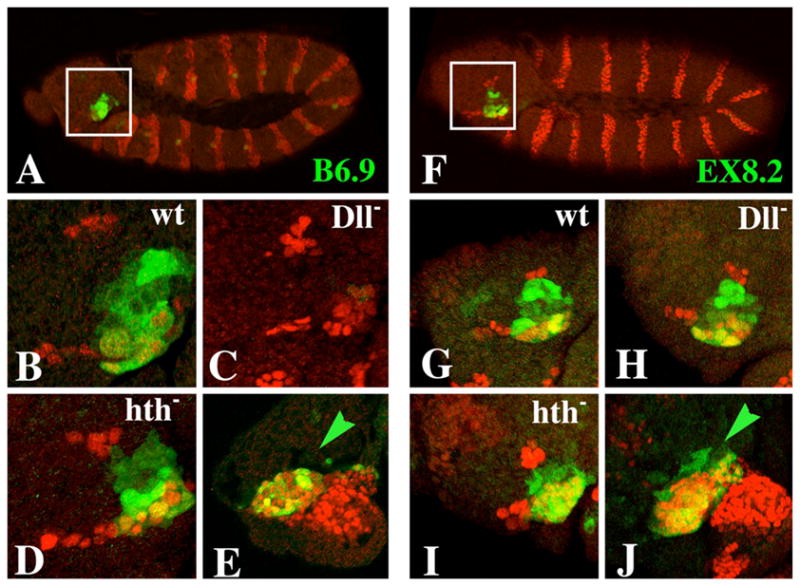
Expression of the B6.9 and EX8.2 ss antennal reporters in embryos. β-galactosidase is stained green in all panels. Engrailed (which marks the posterior of each body segment) is stained red in all panels except E and J, in which Dll is stained red. Embryos shown in panels E and J are at stage 13; all others are at stage 12. A–E: Expression of B6.9. A: B6.9 is expressed in the antennal segment (inset) and in a single lateral cell in each segment. B: A higher magnification of the inset region from another embryo. Antennal expression of B6.9 is lost in Dll− embryos (C), but is unaffected in hth− embryos (D). E: Expression of B6.9 in the antennal segment coincides precisely with that of Dll. F–J: Expression of EX8.2. Note that expression extends from one edge of the antennal segment to the other (F+G) (like expression of ss) and is unaffected in Dll− (H) and hth− (I) embryos. J: EX8.2 is expressed in all Dll-expressing cells in the antennal segment as well as a few more anterior cells (green arrow). B6.9 does not drive expression in these anterior cells (green arrow in E).
Regulation of hth and Dll by ss
Although our analysis shows that ss lies downstream of Dll and hth in the imaginal antenna, ss is not simply a passive target of these genes. In mature antennal discs, hth is expressed at a lower level in A3 than in A1 or A2. This reduction in A3 is due to repression by ss; hth expression in A3 is increased to A1 and A2 levels in ss− mutants or clones (Fig. 8D–F), while antennal expression of hth is strongly reduced within Ss-expressing clones (Fig. 8A–C). Reduced expression of hth in A3 is important for normal development, as ectopic expression of Hth causes deletion of A3 as well as aristal structures (Yao et al. 1999). Moreover, we find that Hth-expressing clones tend to be excluded from A3, with their borders often running along the A2–A3 boundary (Fig. 5C). Such exclusion is not seen for other clone types (e.g. clones expressing Dll), suggesting that partial repression of hth in A3 by ss generates a difference in cell affinities between the A2 and A3 regions.
Figure 8.
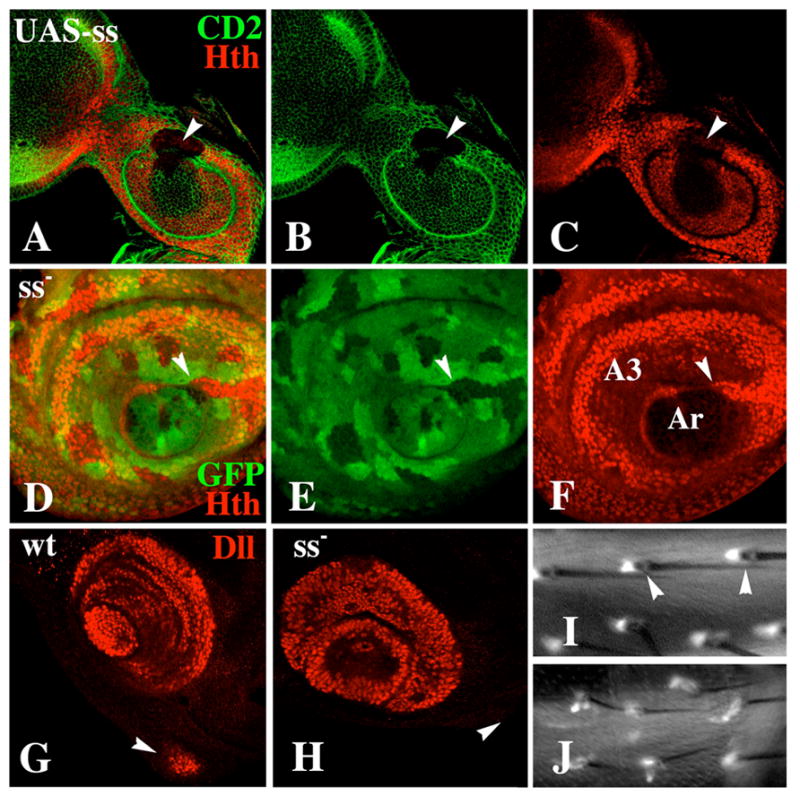
Effect of ss on expression of hth and Dll. A–C: A Ss-expressing clone (marked by loss of CD2) causes repression of hth in the antenna (arrowhead). D–F: A ss− clone causes increased expression of hth in A3 (arrowhead). G+H: Expression of Dll in wild-type and ss− antennal discs. In wild type, Dll expression in the maxillary palp primordium (arrowhead) is activated in the prepupa (G). In ss− mutants, Dll fails to be expressed in the palp primordium at this time (H). I+J: Expression of Dll-GAL4/UAS-GFP in ss+ (I) and ss− (J) adult femurs. (I) In wild type, Dll is expressed in a single cell associated with each bract (arrowheads). (J) In ss− mutants, bracts are absent from the femur, and Dll is expressed weakly in elongate cells that are associated with most bristles.
Although ss− clones have no effect on Dll expression in antennal discs, ectopic expression of Ss often causes ectopic activation of Dll (Duncan et al. 1998, Adachi-Yamada et al. 2005). We wondered whether this activation might reflect some process in which ss normally acts upstream of Dll, so we examined Dll expression in other contexts in ss− mutants. We were surprised to find that ss lies upstream of Dll in the development of the maxillary palps. In normal development, ss expression is first detected in the palp primordia in late larvae, while Dll expression is not detected until one to two hours after pupariation. In ss− mutants, Dll fails to be expressed at this time (Fig. 8G, H). Somewhat later a few cells do come to express Dll weakly in the palp regions, and severely truncated palps are produced in ss− animals.
ss may also lie upstream of Dll in the development of bracts, small pigmented outgrowths located proximally to most bristles in the legs. Dll is required for the development of bracts in the femur and tibia (Gorfinkiel et al. 1997; Campbell and Tomlinson 1998). Consistent with this requirement, in the adult femur Dll is expressed in single cells associated with each bract (Campbell and Tomlinson 1998). In the adult tibia, Dll is expressed in most or all cells, presumably including the bract-associated cells. We find that ss is required for bract formation in the femur (Fig. 8J), but not the tibia. In ss− legs, specific bristle-associated cells in the femur express Dll at what appears to be a reduced level, and these cells have dramatically altered morphology relative to the normal bract-associated cells (Fig. 8I, J). These observations leave the relationship between ss and Dll in bract development unresolved.
Regulation of antennal expression of ss by other genes
A third regulator of B6.9 and 522 is cut, which represses these reporters. In mature discs, cut is expressed in the first and second antennal segments (Johnston et al. 1998) in a pattern that is complementary to expression of B6.9 and 522 (Fig. 9A). Both reporters are expressed ectopically within cut− clones, and completely repressed within Cut-expressing clones (Figs 9B,C). These observations indicate that cut defines the proximal limit of ss expression in the antenna. Although cut is partially repressed within Ss-expressing clones (Fig. 9D) (see also Emerald et al. 2003), the distal limit of cut expression is not altered within ss− clones (not shown).
Figure 9.
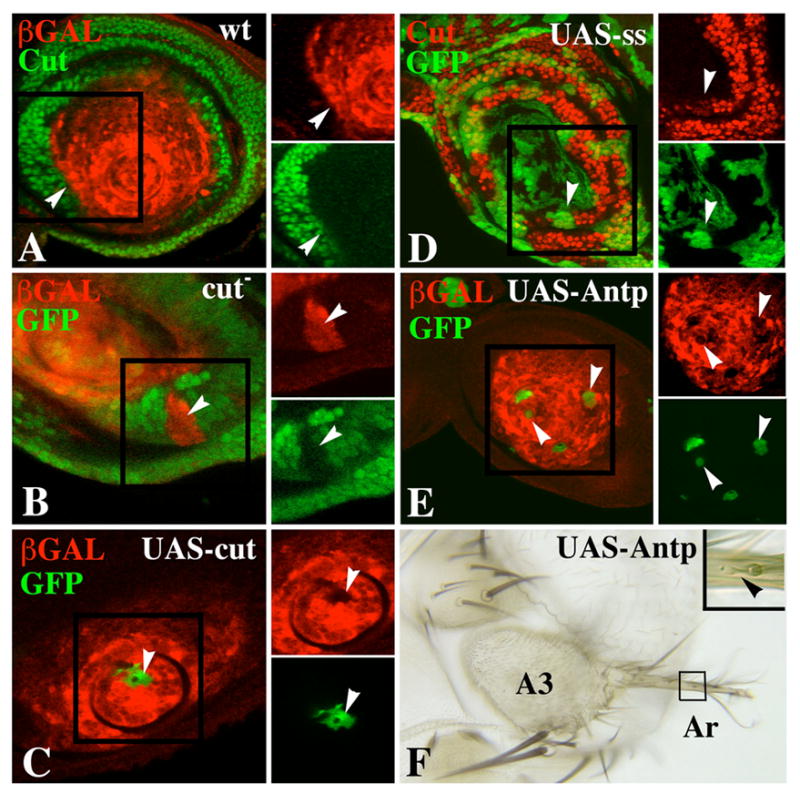
Mutual repression of ss and cut, and repression of the ss antennal reporters by ectopic Antp. A. Antennal disc double-labeled for Cut (green) and B6.9 expression (red) showing that the two expression patterns abut at the A2–A3 boundary (white arrowheads). B. A cut− clone (arrowhead) marked by loss of GFP fluorescence (green) shows ectopic expression of B6.9 (red). C. A clone expressing Cut ectopically (green) represses B6.9 expression (arrowheads). D. Clones expressing ss ectopically (green) partially repress cut expression in the proximal antenna (arrowheads). E. Clones expressing Antp ectopically (green) reduce B6.9 expression (arrowheads). These clones were induced 4–5 days AEL. F. Antenna containing Antp-expressing clone(s) induced 3–4 days AEL. The clone is marked by yellow. Note partial transformation of arista (Ar) to tarsus. Bristles on the partially transformed arista are bracted (arrowhead), indicating leg identity.
The B6.9 and 522 reporters are also negatively regulated by ss itself (data not shown). ss− clones up-regulate the 522 reporter within the distal antenna, but do not induce 522 expression outside of its normal domain of expression. Conversely, Ss-expressing clones weakly down-regulate both B6.9 and 522. Embryonic expression of the B6.9 and EX8.2 reporters in the antennal segment appears normal in ss− mutants.
It has long been known that ectopic expression of Antennapedia (Antp) or other Hox proteins in the antenna can cause a transformation of antenna to leg. This transformation has been attributed to the repression of hth (Casares and Mann 1998; Yao et al. 1999). Repression of hth early in development would be expected to lead secondarily to loss of ss expression, since Hth is required for ss activation. To determine whether Antp might act by other mechanisms to repress ss in the antenna, we examined expression of the antennal reporters within Antp-expressing clones induced at progressively later times of development. We found that Antp-expressing clones show autonomous repression of B6.9 and 522 regardless of the time of clone induction. Repression is seen even in small clones induced in the fourth day AEL (Fig. 9E). Since B6.9 and 522 become independent of hth much earlier, repression by Antp at these late times is clearly not mediated by hth, and may be direct. Consistent with their ability to repress ss, Antp-expressing clones induced late also cause transformations of the aristal and A3 regions to leg (Fig. 9F).
Finally, we examined the effects of dachshund (dac) (expressed in A3) and aristaless (al) (expressed in the aristal primordium) on B6.9 and 522 expression. dac− clones have no effect on expression of 522 or B6.9, whereas Dac-expressing clones weakly repress 522 and possibly also B6.9. al− and Al-expressing clones have no effect on the antennal reporters (data not shown).
The ss tarsal enhancer
To refine the mapping of the tarsal enhancer, we tested seven subclones of the EX6.5 fragment (see Fig. 2 in Supplementary Data). The results indicate that a core tarsal enhancer is located within a 732 bp PstI fragment (P732). P732 drives strong expression in a ring in the leg and antennal discs. Unlike EX6.5, for which tarsal β-galactosidase staining declines in the late larva, P732 drives strong expression even in mature leg and antennal discs. In everting leg discs, P732 is expressed from the distal half of the first tarsal segment through the fourth tarsal segment, which corresponds well to the domain that is deleted in ss− mutants. In the everted antenna, expression is seen in the basal cylinder and an adjacent portion of A3 (Fig. 2). We have tested several genes for their effect on P732 expression. Briefly, we find that Dll+ is required continuously for expression of P732 and that the proximal limit of P732 expression in the tarsus is defined by repression by dac.
Discussion
In this report, we use lacZ reporters to identify the enhancers responsible for most aspects of ss expression during embryonic and imaginal development. We find that antennal expression is driven by two large fragments from the ss 5′ region, B6.9 and EX8.2. Both of these fragments drive expression in the antennal segment of the embryo and in the distal portion of the pupal antenna. B6.9 is also expressed in the antennal disc through most or all larval development. Dissection of B6.9 allowed us to localize the larval antennal enhancer to a fragment of 522 bp. Our approach has been to use the B6.9 and 522 reporters as a proxy for ss expression in experiments to determine the effects of potential upstream regulators of ss. This strategy has its strengths and weaknesses, but has been made necessary by our inability to generate antisera against Ss. A major strength of the approach is that we are able to assess the effects of regulators on individual enhancers. It is likely that monitoring endogenous ss expression would give results that are less clear cut, as both the antennal and tarsal enhancers of ss are active within the antenna. A potential weakness is that our reporters may not faithfully reproduce the normal expression of ss. However, as far as we can tell, the antennal reporters reproduce ss expression very well. The expression of B6.9 and EX8.2 in the embryonic antennal segment and the pupal antenna corresponds very closely to that of endogenous ss. Expression of B6.9 and 522 in the larval antennal disc appears very similar or identical to that of ss+, and the transient requirement for hth+ in the activation of these reporters corresponds well to the transient requirement for hth+ in aristal specification. The tarsal enhancer P732 likely also reproduces the spatial pattern of ss+ expression, as its tarsal expression domain corresponds well to the region deleted in ss− mutants.
The results of our dissection of the B6.9 fragment were surprising. Removal of the left-hand 2 kb of B6.9 to produce S4.9 resulted in the loss of antennal specificity; S4.9 reporters are expressed in both antennal and leg discs (see Fig. 3). The E2.0 subfragment of S4.9 shows a similar expression pattern, and we have found that expression of this fragment in both leg and antennal discs is independent of Hth, but requires Dll continuously (data not shown). On further subdivision of the E2.0 fragment, we found that antennal and leg expression are separable; the 522 fragment is largely specific for the antenna, whereas the 531 fragment drives expression primarily in leg discs. To summarize, antennal specificity is present in B6.9, lost in S4.9 and E2.0, and regained in 522. How can we make sense of this? The region deleted from B6.9 to produce S4.9 clearly plays an important role in enforcing antennal specificity. Since this region contains a PRE (see below), one might suspect that it functions in larval stages to maintain repression of the enhancer outside of the antennal segment. However, that the E2.0 fragment has lost the requirement for Hth in both the antenna and leg (S4.9 has not been tested), suggests that the PRE-containing region might function in both locations. One possibility is that this region represses the enhancer in both antennal and leg discs. In the antenna this repression can be overcome by the combined action of Hth and Dll, while in the leg Dll alone is not sufficient for activation. When the PRE-containing region is deleted, repression is absent or reduced, so that Dll can activate the enhancer without assistance from Hth, and expression is seen in both antennal and leg discs. Why then is antennal specificity restored in the 522 subfragment? Perhaps this fragment is lacking a subset of Dll interaction sites so that it can no longer be activated by Dll alone, but requires combined activation by Hth and Dll. Although this model is consistent with many of our results, it does not provide a ready explanation for the leg specificity of the 531 fragment.
In addition to activation by combined Hth and Dll, the ss antennal disc enhancer is repressed by Cut and by ectopic Antp. Below we discuss each of these regulators separately. Regulatory interactions among ss, hth, Dll, and cut during normal antennal disc development are summarized in Fig. 10.
Figure 10.
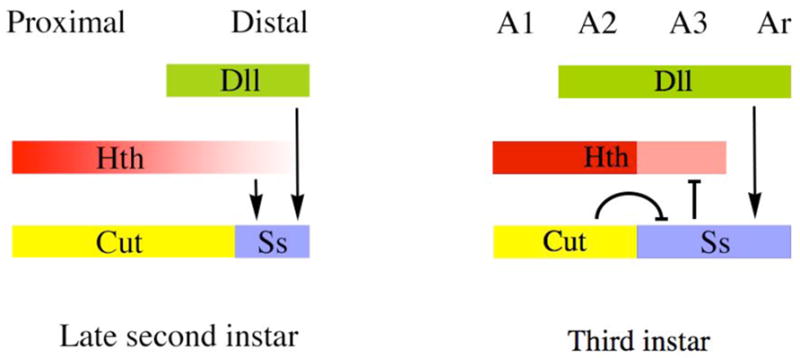
Summary of regulatory interactions among hth, Dll, cut, and ss. On the left are shown proximo-distal expression patterns in the late second instar, shortly after Dll and ss (B6.9) have become activated in the antennal disc and when hth expression is diminished distally. At this time, ss is activated by both hth and Dll. In the third instar (right), the ss antennal enhancer continues to be activated by Dll, but no longer requires hth for its expression. Instead, ss weakly represses hth, resulting in its down-regulation in A3. Repression by cut defines the proximal limit of ss expression during the third instar; regulatory interactions between cut and ss during the second instar have not been examined.
hth
We find that hth+ is required only transiently for activation of the B6.9 reporter. hth− clones induced in the embryo or first instar lose expression of B6.9 autonomously in both A3 and the aristal primordia. However, some time in the second instar expression of B6.9 becomes independent of hth. Consistent with this transient requirement, we show that hth+ is required only early in larval development for specification of the arista. hth− clones induced in the first and second instars show a transformation of the entire antenna to a leg-like appendage. However, clones induced after this time show normal aristal development. These temporal requirements are reflected in the expression pattern of hth: hth is expressed throughout the antennal primordium early in development, but in the second or early third instar is repressed in the central domain, which will produce the arista (Casares and Mann 1998).
The stable activation of B6.9 by Hth suggests that this fragment contains a “cellular memory module” (Maurange and Paro 2002; Cavalli and Paro 1998; 1999). The presence of a PRE within B6.9 is consistent with this idea. The ss locus binds Polycomb protein in salivary gland chromosomes (Zink and Paro 1989), and was recently shown to contain PREs by chromatin immunoprecipitation (Schwartz et al. 2006). In the latter work, ss PREs were localized to within the E1.6 subfragment of B6.9 as well as the EX8.2 fragment (Schwartz et al. 2006), both of which showed pairing dependent suppression in our work. PREs are generally thought of as functioning to stably repress genes. However, PREs can also be associated with activating elements to form memory modules that mediate stable activation. It seems likely that B6.9 contains such a module that responds to Hth. Like a memory module from the hedgehog gene (Maurange and Paro 2002), activity of the ss module is set sometime around the second instar. Surprisingly, we find that activation of the 522 reporter by Hth can also be persistent, although not as stable as for B6.9. The 522 fragment does not appear to contain a PRE, suggesting that Hth may directly recruit factors to the 522 element that cause semi-stable transcriptional activation.
We show that ss is not a completely passive target of hth; ss partially represses hth in antennal discs, which causes hth to be expressed at a lower level in A3 than in A2. This repression appears to be important for normal development, as ectopic expression of Hth can delete A3 (Yao et al. 1999). Moreover, we show that clones ectopically expressing Hth are largely blocked from entering A3 from the proximal (A2) side, suggesting that the different levels of Hth present in A2 and A3 cause a difference in cell affinities between these segments. Hth-expressing clones are similarly restricted to the two most proximal segments in leg discs, although here there is no endogenous expression of hth more distally (Wu and Cohen 1999).
Dll
In contrast to hth, Dll is required continuously for expression of both B6.9 and 522, as Dll− clones induced even very late in development lose expression of these reporters. This continuous requirement for Dll indicates that stable activation of the B6.9 memory module by Hth does not by itself commit the reporter to expression; rather, activation by Hth appears to render B6.9 open to interaction with Dll and perhaps other positive factors.
Three lines of evidence suggest that Dll is the primary activator of the ss antennal enhancer. First, we find that expression of B6.9 and 522 is sensitive to the dosage of Dll+. Expression of both reporters is reduced in animals carrying only one dose of Dll+, and for 522, expression is enhanced in clones having extra doses of Dll+. This dose sensitivity suggests that ss is a direct target of Dll. Second, we find that expression of both reporters is often induced within clones expressing ectopic Dll, even in the apparent absence of Hth expression. Such activation is seen in clones in the distal leg, wing, and elsewhere. Third, we find that the embryonic antennal enhancer carried by B6.9 is absolutely dependent upon Dll+, but independent of hth. Taken together, these observations suggest that Dll is a primary activator of the ss antennal enhancers. Hth may provide antennal specificity by boosting the level of activation by Dll in the antennal disc.
Surprisingly, we find that the regulatory relationship between ss and Dll is reversed in the maxillary palp. Here, ss is expressed prior to Dll and is required for the normal initiation of Dll expression. Although some Dll expression ultimately takes place in the palp primordium in ss− animals, this expression is weak and occurs in only a few cells. We have not worked out how ss is activated in the palp. However, it seems likely that dpp plays a role, as the 531 subfragment of B6.9 drives expression in a stripe in the region of the palp that roughly coincides with a stripe of dpp expression (Masucci et al. 1990). The positioning of ss upstream of Dll in the palp may explain why the region ventral to the antenna is so sensitive to ectopic expression of Ss. Strong activation of Dll here by ectopic Ss combined with endogenous expression of hth might be expected to cause frequent induction of ectopic antennae, as is observed (Duncan et al. 1998). Since ss is normally expressed in the palp, why should earlier ectopic Ss cause the palp primordium to develop as antenna? It seems likely that timing is key, but level of Ss expression could also be important.
The reciprocal regulatory roles of ss and Dll in the antenna and palp suggest a particularly close relationship between these genes. This relationship is reinforced by our finding that ss is required for the development of bracts in the femur, as is Dll (Campbell and Tomlinson 1998).
Our finding that Dll and Hth are both activators of the ss antennal reporters is consistent with the proposal that antennal identity is defined by the combined activity of these regulators (Dong et al. 2000). However, our results indicate that this model is an oversimplification. Examination of clones expressing Dll, Hth, or both proteins together revealed little correlation between activation of the B6.9 and 522 antennal reporters and combined expression of Dll and Hth. Strikingly, Dll-expressing clones often activate the reporters ectopically without any apparent concomitant expression of Hth, and clones expressing both proteins usually do not activate the reporters. These experiments also reveal strong context-dependence. Examples include the leg, where Dll-expressing clones can activate the reporters distally, but not proximally (where endogenous hth expression occurs) and the wing disc, where clones expressing Dll or both Dll and Hth activate the reporters in the wing pouch, but not at all in the notum. The level of expression of both proteins also appears to be key, as high levels of Dll can activate the reporters in the leg in the absence of Hth, and elevated levels of Hth can repress expression in the normal antennal domain. Previous results have shown that antennal structures can be induced by ectopic expression of Dll in the wing hinge region or proximal leg (which express hth endogenously), or by combined expression of Dll and Hth elsewhere (Casares and Mann 1998; Dong et al. 2000). While this is true, our results indicate highly variable effects in such ectopic expression experiments, and fail to detect the strongly synergistic activation of antennal identity by combined Hth and Dll implied by the model. Our results indicate that Dll is the primary activator of the ss antennal reporters, that Hth serves to promote this activity, and that activation by Dll and Hth is highly context dependent.
Consistent with direct control of the antennal reporters by Dll and Hth, two highly conserved regions within the 522 fragment contain apparent binding sites for Dll, Hth, and the Hth dimerization partner Extradenticle. The functional importance of these binding sites is currently being tested.
cut
We show that the proximal boundary of B6.9 and 522 expression is defined by repression by cut. This repression likely explains why ectopic Cut causes a transformation of arista to tarsus (Johnston et al. 1998). In prior work, cut has been shown to define the proximal expression limit of distal antenna (dan) and distal antenna related (danr) (Emerald et al. 2003); since ss lies upstream of these genes (Emerald et al. 2003; Suzanne et al. 2003), it seems very likely that their regulation by cut is indirect. The mechanism of action of Cut is not well understood, as only one direct target has been characterized in Drosophila (Valentine et al. 1998).
Antennapedia
We find that ectopic expression of Antp in the antenna represses the B6.9 and 522 reporters. This finding was expected, as it is well known that expression of Antp or other Hox genes in the antenna causes a transformation to leg. The conventional view is that this transformation results from the repression of hth by ectopic Hox proteins (Casares and Mann 1998; Yao et al. 1999). Repression of hth early in development would be expected to lead secondarily to loss of ss expression and loss of distal antennal identity. However, we find that clones expressing Antp repress the B6.9 and 522 reporters even when these clones are induced very late in development, long after the requirement for activation by hth has passed. Late repression of the antennal reporters by Antp must therefore occur independently of hth, and could be direct. One possibility, currently being tested, is that Antp might compete with Dll for binding to the 522 enhancer. Late repression of the ss antennal enhancer by Antp is consistent with the effects of Antp-expressing clones on antennal identity: such clones induced in the mid to late third instar cause transformations of distal antenna to leg.
In our work, we examined clones induced late that ectopically express Antp in a sustained fashion. In contrast, Gibson and Gehring (1988) studied the effects of pulses of Antp expression induced by one-hour heat shocks in a heat shock/Antp line. They found that transformations of arista to tarsus were induced by such pulses only when they are administered at the end of the second instar. Why do pulses of Antp at this time cause a stable, heritable transformation of the distal antenna? Our results suggest an explanation. The period sensitive to Antp pulses coincides roughly with when the ss antennal enhancer becomes independent of hth. This correlation suggests that pulses of Antp in the second instar cause heritable transformations by interfering with the stable activation of ss by Hth. Recently, Percival–Smith et al. (2005) reported that ectopic Antp does not repress hth in the antenna early in larval development. This observation suggests that Antp might act directly on the ss antennal enhancer to prevent its stable activation by Hth.
The regulation of ss by ectopic Antp suggests that Antp may normally play a significant role in repressing ss antennal enhancer activity in the legs. Although we have not yet tested this idea directly, it seems unlikely that Antp is primarily responsible for keeping the ss antennal enhancers inactive in the leg. Antp null clones do cause activation of the ss target gene dan (Emerald and Cohen 2004) in leg discs, implying ectopic activation of ss. However, this activation occurs only proximally, with the distal leg appearing to develop independently of Antp (Burgess and Duncan 1990, Casares and Mann 2001). Expression of Antp in the proximal leg may account for why Dll-expressing clones fail to activate B6.9 or 522 in this location. Ectopic activation of the ss antennal enhancers in the leg primordia of the embryo is not seen in an Antp null mutant (not shown).
Our studies suggest that antennal structures are specified in a combinatorial fashion by Hth, Dll, Ss, and probably other factors. In A3, all three proteins are required for normal antennal identity. In ss− antennae, hth continues to be expressed in A3 (although at elevated levels), as does Dll. Despite this continued expression of hth and Dll, A3 develops without antennal characteristics, and produces only naked cuticle. Thus, Hth and Dll are unable to specify A3 characters in the absence of Ss. Conversely, assuming that ss is stably activated in the antenna by Hth, as is B6.9, then hth− clones induced late would show persistent expression of both ss and Dll in A3. Such clones are transformed to leg, implying that Ss and Dll have no ability to direct A3 identity in the absence of Hth. Taken together, these observations suggest that Hth, Dll, and Ss must act together to specify A3 identity. This requirement for combined action accounts for why ectopic expression of Ss does not induce A3 tissue in the medial leg (Duncan et al. 1998), as hth is not normally expressed here. Our view of combinatorial control suggests that many A3-specific target enhancers might be identifiable in genome searches as regions that contain clustered binding sites for Hth, Dll, and Ss; tests of this prediction will be presented elsewhere.
In contrast to A3, the aristal primordium appears to be specified by ss and Dll acting together in the absence of hth expression. hth is expressed in the aristal region early in development, where it functions to establish ss expression, but it is soon repressed here. Therefore, for most of development, the arista is specified by Ss and Dll acting without input from Hth. Consistent with this picture, the arista adopts leg identity in ss null mutants, and ectopic expression of ss causes the distal tip of the leg to develop as arista (Duncan et al. 1998).
In ss− mutants the distal antenna is terminated by a single tarsal segment (the fifth). In contrast, in ss mutants that lack only antennal enhancer activity (e.g. the breakpoint mutations ssD114.3 and ssD114.7, see Fig. 2) the distal antenna develops with a near complete set of tarsal segments (see Fig. 1). This difference likely reflects the activity of the tarsal enhancer in the antenna. In support of this view, the ss tarsal enhancer drives expression in the segmented base of the arista (Fig. 2), a region known as the basal cylinder. This region transforms to tarsal segments 2–4 in Antp-induced transformations of antenna to leg (Postlethwait and Schneiderman 1971). However, the question arises as to why normal antennal expression of ss causes the proximal arista to develop as basal cylinder, whereas ss expression driven by the tarsal enhancer alone causes this same region to develop as tarsal segments. Likely the key difference is that expression driven by the tarsal enhancer is transient, whereas expression driven by the antennal enhancer is sustained. Perhaps transient expression of ss allows growth and subsegmentation to produce a full set of tarsal segments, whereas sustained expression inhibits growth, producing the basal cylinder. Consistent with this idea, sustained expression of ss driven by the GAL4 method can cause deletion of tarsi in the legs (unpublished observations). The levels of expression driven by the tarsal and antennal enhancers may also be important, as flies having only one dose of ss show a partial transformation of the basal cylinder to tarsus (Emmons et al. 1999). The ss tarsal enhancer drives weak expression in A3 as well as in the basal cylinder, likely accounting for the presence of some specialization of A3 in ss mutants lacking the antennal enhancers.
Our view that antennal identity is specified by the combined action of Hth, Dll, and Ss contradicts the now prevalent view that antennal identity is determined solely by hth (Casares and Mann 1998; 2001). The major evidence supporting the latter view is that early hth− clones transform the entire antenna to leg, and ectopic expression of Hth can induce ectopic antennal structures in the anal plates. In addition, Dll shows little antennal specificity, being expressed in the distal portions of all of the ventral appendages, and ss expression in the antenna is dependent upon hth+. Should hth be viewed as the antennal “selector” gene? hth does not seem to be a selector in the same sense as the Hox genes; it is expressed very broadly in the embryo and in other imaginal discs and plays no role in activating ss in the antennal segment of the embryo. Moreover, the ability of ectopic Hth to induce antennal structures is very limited: transformations of anal plate to distal antenna have been reported following ectopic expression of Hth or Meis1, a mammalian homolog (Casares and Mann 1998). However, Dong et al. (2000) were unable to reproduce this effect by ectopic expression of Hth, matching our own experience. That anal plates are susceptible to transformation at all is likely due to the fact that Dll and ss are coexpressed here in normal development (unpublished observations). A further dissimilarity is that hth acts only as an establishment regulator of ss in the antennal disc, unlike the continuous requirements usually seen for the Hox genes. Ultimately, assessment of the importance of hth will depend on whether its function in the antenna is conserved. The expression pattern of hth in the antenna does appear to be conserved in the milkweed bug Oncopeltus (Angelini and Kaufman 2004). However, localization of nuclear Exd, (a proxy for Hth expression) indicates that Hth is not differentially expressed in the antenna and leg of the cricket (Abzhanov and Kaufman 2000). Expression of hth in the crustacean Porcellio also appears to be identical in the second antenna and the legs (Abzhanov and Kaufman 2000). Characterization of hth, Dll, and ss expression and function in additional arthropods will be required to assess properly the importance of these genes in antennal specification.
Supplementary Material
Figure 1. Expression of ss-lacZ reporters in the embryonic peripheral nervous system (PNS) and in adult bristles. Neural cells marked by Mab 22C10 are stained in red, and β-galactosidase is stained in green. The E5.0 reporter (A, D) is expressed in most or all embryo PNS cells, whereas the EX6.5 (B, E) and X8.2 (C, F) reporters are expressed in subsets of the PNS cells. G-H: Expression of E1.2 and X8.2 in cells associated with abdominal tergite bristles. E1.2 drives lacZ expression in almost all bristles of the fly, exceptions being the bristles of the tarsal segments and the eye. ss mutants have little or no effect on the size of bristles in these regions. E1.2 labels the bristle, socket, and sheath cells associated with each bristle (G). X8.2 drives expression in all bristles except those of the eye, notum, and tarsal segments 3-5. X8.2 drives expression only in the socket cell (H).
Figure 2. Localization of the ss tarsal enhancer. The EX6.5 fragment was subdivided as indicated. Strong tarsal expression was driven by the P732 fragment and all larger fragments containing P732. As shown below, P732 drives expression in a ring in both antennal and leg discs. After disc eversion, expression in the leg is seen to include the distal portion of the first tarsal segment as well as tarsal segments 2 - 4. Expression in the antenna includes the basal cylinder and part of A3. The P732 and XP3003 fragments drive sustained lacZ expression in leg and antennal discs, whereas the RP4268 and RP5458 fragments show much weakened lacZ expression in late discs. Very weak tarsal expression is seen for some insertions of the P1190 and XP2271 reporters.
Acknowledgments
We are indebted to Danny Brower, Gerard Campbell, Graeme Mardon, Nipam Patel, and Adi Salzberg for antibodies, and the Bloomington Stock Center, Gerard Campbell, Thom Kaufman, Tetsuya Kojima, Graeme Mardon, Gines Morata, Adi Salzberg, Ernesto Sánchez-Herrero, and Henry Sun for Drosophila stocks. Thanks also to Joanna Mou for critical reading of the manuscript. This work was supported by NIH grant GM32318.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Kaufman TC. Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol. 2000;227:673–689. doi: 10.1006/dbio.2000.9904. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, Harumoto T, Sakurai K, Ueda R, Saigo K, O’Connor MB, Nakato H. Wing-to-leg homeosis by spineless causes apoptosis regulated by fish-lips, a novel leucine-rich repeat transmembrane protein. Molec Cell Biol. 2005;25:3140–3150. doi: 10.1128/MCB.25.8.3140-3150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol. 2004;271:306–321. doi: 10.1016/j.ydbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Burgess EA, Duncan I. Direct control of antennal identity by the spineless-aristapedia gene of Drosophila. Mol Gen Genet. 1990;221:347–352. doi: 10.1007/BF00259398. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–726. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. The ground state of the ventral appendage in Drosophila. Science. 2001;293:1477–1480. doi: 10.1126/science.1062542. [DOI] [PubMed] [Google Scholar]
- Cavalli G, Paro R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell. 1998;93:505–518. doi: 10.1016/s0092-8674(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Cavalli G, Paro R. Epigenetic inheritance of active chromatin after removal of the main transactivator. Science. 1999;286:955–958. doi: 10.1126/science.286.5441.955. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jürgens G. Proximal - distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. The EMBO Journal. 1989;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Jürgens G. Mediation of Drosophila head development by gap-like segmentation genes. Nature. 1990;346:482–485. doi: 10.1038/346482a0. [DOI] [PubMed] [Google Scholar]
- Dong PDS, Chu J, Panganiban G. Coexpression of the homeobox genes Distal-less and homothorax determines Drosophila antennal identity. Development. 2000;127:209–216. doi: 10.1242/dev.127.2.209. [DOI] [PubMed] [Google Scholar]
- Dong PDS, Dicks JS, Panganiban G. Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–1974. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes and Development. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerald BS, Curtiss J, Mlodzik M, Cohen SM. distal antenna and distal antenna related encode nuclear proteins containing pipsqueak motifs involved in antenna development in Drosophila. Development. 2003;130:1171–1180. doi: 10.1242/dev.00323. [DOI] [PubMed] [Google Scholar]
- Emerald BS, Cohen SM. Spatial and temporal regulation of the homeotic selector gene Antennapedia is required for the establishment of leg identity in Drosophila. Developmental Biology. 2004;267:462–472. doi: 10.1016/j.ydbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Emmons RB, Duncan D, Estes PA, Kiefel P, Mosher JT, Sonnenfeld M, Ward MP, Duncan I, Crews ST. The Spineless-Aristapedia and Tango bHLH-PAS proteins interact to control antennal and tarsal development in Drosophila. Development. 1999;126:3937–3945. doi: 10.1242/dev.126.17.3937. [DOI] [PubMed] [Google Scholar]
- Gibson G, Gehring WJ. Head and thoracic transformations caused by ectopic expression of Antennapedia during Drosophila development. Development. 1988;102:657–675. [Google Scholar]
- González-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes and Development. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Ostrow BD, Jasoni C, Blochlinger K. The homeobox gene cut interacts genetically with the homeotic genes proboscipedia and Antennapedia. Genetics. 1998;149:131–142. doi: 10.1093/genetics/149.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel MW, Duncan DM, Duncan I. A screen for genes that interact with the Drosophila pair-rule segmentation gene fushi tarazu. Genetics. 2004;168:161–180. doi: 10.1534/genetics.104.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis JA. Pairing-sensitive silencing, Polycomb group response elements, and transposon homing in Drosophila. Adv Genet. 2002;46:421–438. doi: 10.1016/s0065-2660(02)46015-4. [DOI] [PubMed] [Google Scholar]
- Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A. dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development. 1998;125:1037–1048. doi: 10.1242/dev.125.6.1037. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Miltenberger RJ, Hoffmann FM. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3′ cis-regulatory elements. Genes and Development. 1990;4:2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- Maurange C, Paro R. A cellular memory module conveys epigenetic inheritance of hedgehog expression during Drosophila wing imaginal disc development. Genes and Development. 2002;16:2672–2683. doi: 10.1101/gad.242702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH. the Homothorax homeoprotein activates the nuclear localization of another homeoprotein, Extradenticle, and suppresses eye development in Drosophila. Genes and Development. 1998;12:435–446. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival-Smith A, Teft WA, Barta JL. Tarsus determination in Drosophila melanogaster. Genome. 2005;48:712–721. doi: 10.1139/g05-021. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Schneiderman HA. Pattern formation and determination in the antenna of the homoeotic mutant Antennapedia of Drosophila melanogaster. Dev Biol. 1971;25:606–640. doi: 10.1016/0012-1606(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of Extradenticle requires Homothorax, which encodes an Extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Rehmsmeier M, Dura JM, Paro R. Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev. Cell. 2003;5:759–771. doi: 10.1016/s1534-5807(03)00337-x. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. AH receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Struhl G. spineless-aristapedia: a homeotic gene that does not control the development of specific compartments in Drosophila. Genetics. 1982;102:737–749. doi: 10.1093/genetics/102.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel CE, Whittle JRS. Brista: A gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Wilhelm Roux Arch Dev Biol. 1987;196:124–132. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- Suzanne M, Estella C, Calleja M, Sánchez-Herrero E. The hernandez and fernandez genes of Drosophila specify eye and antenna. Dev Biol. 2003;260:465–483. doi: 10.1016/s0012-1606(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Thummel C, Pirrotta V. New pCaSpeR P-element vectors. Drosophila Information Service. 1992;71:150. [Google Scholar]
- Valentine SA, Chen G, Shandala T, Fernandez J, Mische S, Saint R, Courey AJ. Dorsal-mediated repression requires the formation of a multiprotein repression complex at the ventral silencer. Molec Cell Biol. 1998;18:6584–6594. doi: 10.1128/mcb.18.11.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen SM. Proximodistal axis formation in the Drosophila leg: subdivision into proximal and distal domains by Homothorax and Distal-less. Development. 1999;126:109–117. doi: 10.1242/dev.126.1.109. [DOI] [PubMed] [Google Scholar]
- Yao L-C, Liaw G-J, Pai C-Y, Sun HS. A common mechanism for antenna-to-leg transformation in Drosophila: suppression of homothorax transcription by four HOM-C genes. Dev Biol. 1999;211:268–276. doi: 10.1006/dbio.1999.9309. [DOI] [PubMed] [Google Scholar]
- Zink B, Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989;337:468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Expression of ss-lacZ reporters in the embryonic peripheral nervous system (PNS) and in adult bristles. Neural cells marked by Mab 22C10 are stained in red, and β-galactosidase is stained in green. The E5.0 reporter (A, D) is expressed in most or all embryo PNS cells, whereas the EX6.5 (B, E) and X8.2 (C, F) reporters are expressed in subsets of the PNS cells. G-H: Expression of E1.2 and X8.2 in cells associated with abdominal tergite bristles. E1.2 drives lacZ expression in almost all bristles of the fly, exceptions being the bristles of the tarsal segments and the eye. ss mutants have little or no effect on the size of bristles in these regions. E1.2 labels the bristle, socket, and sheath cells associated with each bristle (G). X8.2 drives expression in all bristles except those of the eye, notum, and tarsal segments 3-5. X8.2 drives expression only in the socket cell (H).
Figure 2. Localization of the ss tarsal enhancer. The EX6.5 fragment was subdivided as indicated. Strong tarsal expression was driven by the P732 fragment and all larger fragments containing P732. As shown below, P732 drives expression in a ring in both antennal and leg discs. After disc eversion, expression in the leg is seen to include the distal portion of the first tarsal segment as well as tarsal segments 2 - 4. Expression in the antenna includes the basal cylinder and part of A3. The P732 and XP3003 fragments drive sustained lacZ expression in leg and antennal discs, whereas the RP4268 and RP5458 fragments show much weakened lacZ expression in late discs. Very weak tarsal expression is seen for some insertions of the P1190 and XP2271 reporters.


