Summary
Food allergy is a serious medical problem without definitive treatment at this time. Intense research focuses on severe peanut allergy. Recombinant peanut major allergens engineered to lose IgE binding capacity mixed with E coli showed great promise in a murine model of peanut anaphylaxis. Rectal vaccine containing E.coli expressing engineered recombinant major peanut allergens Ara h 1, 2, 3 is in preparation for first human clinical trials. Oral desensitization and sublingual immunotherapy with food extracts represent another approach that is being actively explored. Novel therapies must be carefully evaluated in respect to safety and long-lasting effect on oral food tolerance before being applied in clinical practice. Diversity of approaches and promising preliminary results bring hope for patients with food allergy.
Keywords: Food allergy, peanut allergy, immunotherapy, oral desensitization, sublingual immunotherapy, recombinant engineered allergens, recombinant engineered food proteins, food allergy therapy
Introduction
Food allergy is defined as an immune system-mediated adverse reaction to food. (1) Food allergies affect 2 – 4 % of adults and 6 – 8% of young children in the westernized societies. (2) An estimated 12 million of Americans, including 2 million school-age children suffer from food allergy. Food allergic reactions account for approximately 30,000 emergency room visits and severe food anaphylaxis is implicated in 150–200 deaths per year in the US. (3) Cow’s milk, egg white, soybean, wheat, peanut, tree nuts and fish are responsible for over 90% of food allergies in children. In adults, peanut, tree nuts, shellfish and fish are implicated as culprits in the majority of severe reactions, whereas the most common food allergens are fruits and vegetables that cause oral pruritus in persons with respiratory pollen allergy. (4–7) Majority of childhood food allergy to cow’s milk, egg white, soybean and wheat resolve with time whereas peanut, tree nut, fish and shellfish allergy tend to be a life-long condition. (8–10)
Diagnosis of food allergy relies on acquisition of detailed history regarding type of the food, amount ingested, timing and severity of symptoms; laboratory diagnostic tests for detection of food allergen-specific IgE antibody in the skin (skin prick test) or in serum (preferably ImmunoCAP); double-blind, placebo-controlled oral food challenge is the current gold standard for food allergy diagnosis. (11) (12)Feeding with suspected food under physician supervision (oral food challenge) is indicated in a subject without recent (past 6–12 months) history of convincing reactions, whose laboratory tests are below the high predictive value for clinical reactivity. (13;14) (Figure 1) Management of food allergy relies on dietary avoidance of the implicated foods and a prompt treatment of allergic reactions. Accidental food allergic reactions are very common; despite avoidance about 50% of peanut allergic children experienced at least one accidental reaction to peanut over 2 year period. (15)
Fig. 1.
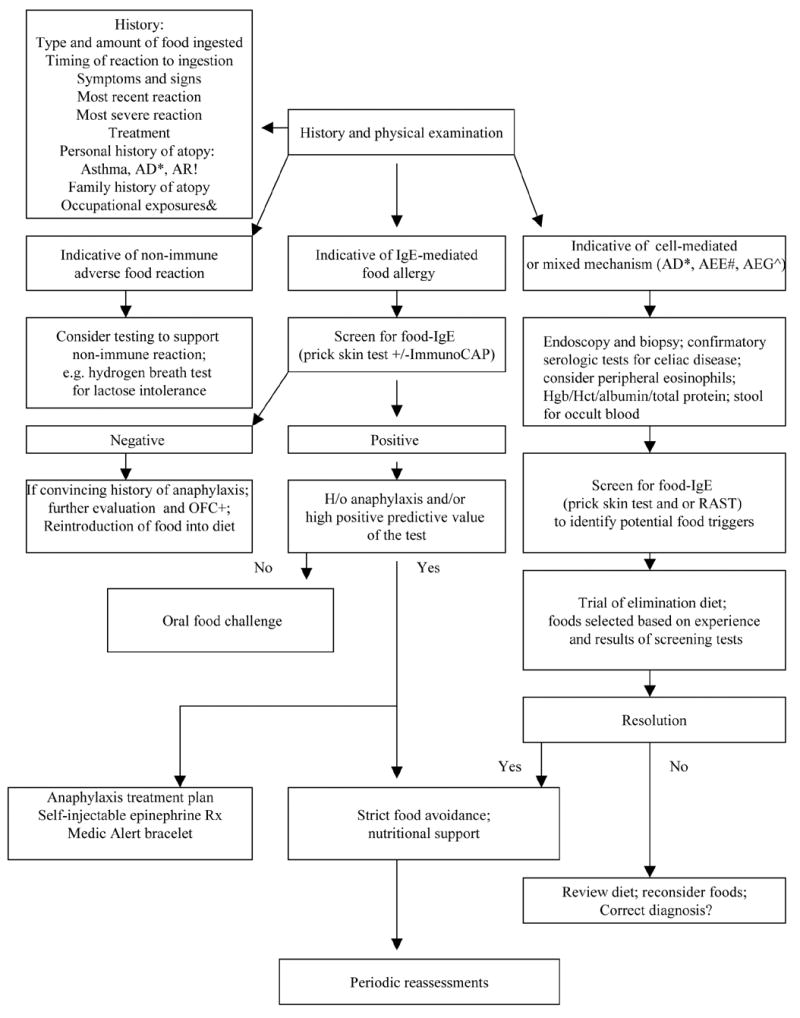
A scheme of current approach to diagnosis and management of food allergy & - Occupational exposures to inhaled food dust important in bakers and millers with asthma; AD*-atopic dermatitis; AR! – Allergic Rhinitis; AEE# – Allergic Eosinophilic Esophagitis; AEG^ – Allergic Eosinophilic Gastroenteritis; OFC+ – Oral Food Challenge
Recent epidemiologic data indicate that prevalence of peanut allergy doubled in the past 5–10 years in the US, UK, and Canada. (4;5;16;17) Currently peanut allergy is affecting about 1% of young children, a majority (80%) of whom will have life-long peanut allergy. (8) Peanut has been identified as a food allergen responsible for most of the severe and fatal food anaphylactic reactions in the US. (18) The reasons why peanut allergy prevalence is increasing are not known but considering rapid changes, environmental factors appear to play a more significant role. Current prevention guidelines that recommend delaying introduction of dietary peanut to children at risk for atopy until age 3 years have not been effective in decreasing the risk of peanut allergy. (19)
Considering the above factors, food allergy emerged as the important target for research on curative treatment and prevention, with a majority of efforts focusing on peanut, cow’s milk and egg allergy. This paper will review in detail the recent developments in the potential treatments for IgE-mediated food allergy utilizing native and engineered recombinant food proteins.
Immunotherapy with native food proteins
Subcutaneous peanut immunotherapy
Subcutaneous allergen immunotherapy is used as standard and effective treatment for allergic rhinitis, asthma and venom allergy. (20) Subcutaneous immunotherapy with peanut extract has been studied in a small number of subjects with peanut allergy. Subjects with confirmed peanut allergy were treated in a double-blind, placebo-controlled study with peanut immunotherapy or placebo. (21) Objective measures of efficacy included changes in symptom score during double-blind placebo-controlled food challenge (DBPCFC) to peanut and titrated end point prick skin tests (PST). Three subjects treated with peanut immunotherapy completed the study. These subjects displayed a 67% to 100% decrease in symptoms evaluated by DBPCFC and had a 2- to 5-log reduction in end point PST reactivity to peanut extract. One placebo-treated subject completed the study. This subject had essentially no change in DBPCFC symptom scores or PST sensitivity to peanut. Two other placebo-treated subjects underwent a second PST. These subjects had a 1- to 2-log increase in skin test sensitivity to peanut. All peanut-treated subjects were able to reach maintenance dose, and the rate of systemic reactions with rush IT was 13.3%. Due to a tragic pharmacy error, one control patient in the placebo group died of anaphylactic shock. This study provided preliminary data demonstrating the efficacy of injection therapy with peanut extract. In a follow up study, twelve patients with immediate hypersensitivity to ingestion of peanuts were recruited. (22) Six subjects were treated with injections of peanut extract: a maintenance level of tolerance was first achieved by a rush protocol, then maintained with weekly injections for at least 1 year. The other six were untreated control subjects. All patients underwent DBPCFC to peanut initially, after approximately 6 weeks, and after 1 year. All treated patients achieved the maintenance dose of 0.5 ml of 1:100 wt/vol peanut extract by the rush injection protocol. All experienced increased tolerance to DBPCFC and decreased sensitivity on titrated PST with peanut extract, whereas the threshold to oral peanut challenge and cutaneous reactivity to peanut extract were unchanged in the untreated control subjects. Systemic reactions were common in the treated group both during rush immunotherapy and with maintenance injections (39%). Only three patients remained tolerant of the full maintenance dose. The increased tolerance to oral peanut challenge was maintained in the three subjects who received full maintenance doses, but there was partial (n = 2) or complete (n = 1) loss of protection in the patients who required dose reduction because of systemic reactions. In addition, there was no long-term follow up data to evaluate the persistence of protection following discontinuation of peanut immunotherapy. Nevertheless, this pivotal clinical study demonstrated that food allergen could be successfully used to induce oral tolerance in food allergic subjects if safety was improved. The authors concluded that for future clinical applications, modified peanut extract of decreased allergenicity was needed.
Birch pollen immunotherapy for pollen-food allergy syndrome (oral allergy syndrome)
The phenomenon of cross-reactivity between birch tree pollen and allergens in raw apple is the basis for an approach that utilizes subcutaneous birch tree pollen immunotherapy to treat oral allergy to apple. Respiratory sensitization to pollen allergens (i.e., major birch allergen, Bet v 1) that cross-react with allergens in plant foods (i.e., major apple allergen, Mal d 1) results in oropharyngeal symptoms triggered by the ingestion of raw fruits and vegetables. (23) A prospective, non-randomized, non-blinded clinical trial of birch pollen subcutaneous immunotherapy in 49 adults with birch pollinosis and oral symptoms provoked by apple was recently reported. (24) Subjects received birch immunotherapy for 12, 24, or 36 months. Forty-one patients (84%) compared to no controls (0%, birch allergic adults who received no immunotherapy) reported a significant reduction (50–95%) or a total clearance (100%) of apple allergy symptoms after immunotherapy (P < 0. 001). Birch immunotherapy also induced a marked reduction in skin reactivity against fresh apple in 43 patients (88%). The effect of immunotherapy was inversely related with baseline skin reactivity. In contrast, baseline birch pollen-specific or apple-specific IgE antibodies levels did not correlate with immunotherapy effectiveness on apple allergy. No control subject reported a reduction in the severity of apple allergy or showed a decrease in skin reactivity at follow-up (P < 0.001). Immunotherapy with birch pollen extracts effectively reduced clinical apple sensitivity and skin reactivity in most cases after only 1 year of treatment. These effects were not paralleled by a similar reduction in apple-specific IgE. In a follow-up study, the duration of the effect of birch immunotherapy was evaluated in 30 birch pollen-allergic patients who experienced resolution of apple allergy and loss of skin test reactivity to fresh apple.(25) Symptoms and skin test reactivity was compared at the end of their immunotherapy course and 30 months after immunotherapy was stopped. Over 50% of patients were still able to tolerate eating apple at the 30-month follow-up visit, although the majority showed evidence of re-sensitization to apple by PST. Both studies were criticized for lack of randomized design and lack of an objective evaluation of the severity of apple allergy by DBPCFC. Despite limitations, these studies provided evidence that in a subset of birch allergic individuals with oral allergy to apple, birch pollen immunotherapy may provide a long-lasting improvement in apple allergy. Subsequent clinical trials, in which oral allergy to apple was diagnosed with DBPCFC, confirmed beneficial effect of birch IT on oral tolerance to apple in some patients. (26;27) Asero speculated that for some subjects with OAS, IT doses higher that typically needed to produce improvement in birch pollen rhinitis may be necessary to improve OAS. He also pointed out that most significant effects on OAS were observed in the studies that included adults mono-sensitized to birch tree pollen and not to other pollens.(28)
Oral desensitization in food allergy
Oral desensitization to food, or as proposed by Niggeman et al, specific oral tolerance induction (SOTI) is generating increasing interest as a potential approach to treatment of food allergy. (29;30) The rationale for using oral route is that oral ingestion of a food antigen results preferentially in the active non-response of the immune system towards that antigen. Animal studies suggest that high dose feeding of an antigen results in anergy or deletion of the antigen-specific T lymphocytes whereas intermittent feedings with small doses are more likely to result in the activation of the regulatory T cells and mediators. (31) Although it has been postulated that food allergy results from failure to develop or breech in oral tolerance, the direct evidence in human subjects has not been established.
In case of allergy to cow’s milk, peanut or egg, it has been assumed that the major route of sensitization is via gastrointestinal tract because these proteins are relatively resistant to proteolytic enzymes and low gastric pH. (2) In contrast, sensitization to proteins in raw fruits and vegetables that are highly cross-reactive with pollen allergens, predominantly occurs via respiratory tract and is primarily directed to inhaled pollen with subsequent reactivity to ingested cross-reactive food. (32) However, experimental murine models demonstrate that epicutanous sensitization to food allergens preferentially induces Th 2 responses and results in allergic responses to inhaled and ingested food proteins.(33–35) In mice, oral tolerance induction is highly dose dependent and differs for the allergenic proteins peanut and egg ovalbumin. (36) Tolerance to peanut requires a significantly higher oral dose than tolerance to ovalbumin. Low doses of peanut are more likely to induce oral sensitization and increased production of IL-4 and specific IgE upon challenge. Retrospective epidemiologic data from the United Kingdom collected as maternal recall and subject to recall bias, suggests that application of creams containing peanut oil is a risk factor for development of peanut allergy in infants, especially in the setting of atopic dermatitis and impaired skin barrier. (37) In addition, up to 50% of children with peanut and egg allergy experience their initial food allergic reaction to the first know ingestion of these foods, suggesting that prior exposures occurred either via unknown ingestion, such as resulting from trace amount cross-contamination from shared equipment, via transmission in breast milk, or potentially, via an alternative route, such as skin or respiratory tract. (38) Since about 90% of children with food allergy have atopic dermatitis and at least 40–50% of young children with persistent moderate to severe atopic dermatitis have food allergy, epicutaneous sensitization to the common food allergens via impaired skin barrier may represent an important underappreciated route and provide the rationale for trials of oral tolerance induction to these foods.
Current evidence in support of oral desensitization is limited to non-randomized clinical trials and case reports with little insight into the immunomodulatory effects of oral food desensitization. (Table 1) In addition, due to methodology issues, it is difficult to appreciate the actual effects of oral desensitization versus the natural resolution of food allergy to foods such as cow’s milk and egg that are typically outgrown by majority of children. Nevertheless, it appears that in most patients, tolerance to food is achieved and maintained for periods up to 6 months as long as the food is ingested regularly. (39;40) However, in some patients who reach maintenance dose, allergic symptoms re-develop if the food is not ingested on a regular basis, highlighting a concern whether oral desensitization is capable of inducing permanent tolerance. (29) In a subset, the full maintenance dose cannot be achieved due to allergic symptoms, but the patients benefit from increased threshold dose of food, are protected from reactions to trace amounts and benefit from increased safety, comfort and nutritional value, as long they continue to ingest the food on daily basis to guarantee maintenance of desensitization state. (40) The concept of oral immunomodulation and tolerance induction for therapy of food allergy is undeniably appealing, especially considering relatively low rate of serious adverse reactions and comfort of home administration in comparison to subcutaneous allergen immunotherapy. However, rigorous clinical trials are necessary to fully evaluate the role of oral desensitization in definitive treatment of food allergy. Finally, mechanistic studies are needed to understand the immunologic changes induced by oral desensitization.
TABLE 1.
Summary of current experience with oral food desensitization for IgE-mediated food allergy
| Study | Foods | Subjects | Starting dose | Time to maintenance | Success rate^ | Comments |
|---|---|---|---|---|---|---|
| Patriarca 1984 (76);
Open clinical trial |
CM (8)
Egg (8) Fish (2) Orange (1) |
N=19
Age: 5–55 years |
10 drops of CM in 10 ml of water; 4 drops/day
10 drops of beaten egg in 100 ml of water, 4 drops/day 10 ml of mixed fish commercial extract (eel, sardine, codfish, anchovy) in 90 ml of water, 4 drops/day Unspecified |
100 ml of undiluted CM/day in 104 days
120 drops of pure beaten egg/day in 90 days 200 g of cooked fish/day in 120 days 3 months |
5/8
6/8 2/2 1/1 |
Side effects in 11/19 patients: urticaria, pruritus, emesis, angioedema, abdominal pain, rhinitis, dyspnea;
Patients followed for 3-12 months; Subject with orange allergy reported resolution of allergy to plums and peaches. No insight into mechanism of desensitization. |
| Patriarca 1998 (77)
Clinical trial |
CM (6)
Egg (5) Fish (2) Apple (1) |
N=14
Age: 4–14 years |
Modification of previously published protocol (77) | Modification of previously published protocol (77) | 12/14 | All of the children who achieved maintenance continued to tolerate the foods at least 2–3 times per week for 3–6 years. 10/14 patients experienced side effect during treatment. |
| Bauer 1999 (78) | CM | N=1
Age 12 years |
1 ml/day of 0.01% milk diluted in water | Rush protocol: dose doubled every other hour; final dose 200 ml of undiluted milk achieved in 5 days | 1/1 | Patient tolerated CM daily for at least 6 month follow up. |
| Nucera 2000 (79) | CM | N=1
Age 6 years |
10 drops of milk in 10 ml of water; 4 drops per day | 100 ml of undiluted CM/day in 104 days | 1/1 | Child able to ingest CM and dairy products after 6 months. After 7 months: SPT to BLG, LLA, CS became negative;
IgE to milk proteins decreased significantly, whereas milk-IgG and IgA increased. PBMC stimulated with BLG produced significantly less IL-4 at 18 months than at baseline |
| Rueff 2001(80) | Celery | N=1
Age: 49 years |
0.1 ml of a commercial natural celery juice five times a day | 5 ml five times daily for 3 months | 1/1 | At 3 months patient tolerated 10 g of raw celery but developed flushing to 20 g; continued to ingest 25 ml of raw celery juice for 3 years |
| Patriarca 2003(39)
Clinical trial |
CM (29)
Egg (15) Fish (11) Orange (2); and other $ |
N=59
Age: 3–55 years |
Modification of previously published protocol (77) | Modification of previously published protocol (77) | 45/54 (83.3%) | 51% of patients experienced urticaria, emesis, diarrhea, or abdominal pain; In 9 patients (16.7%) protocol was stopped due to side effects; No differences between children and adults; SPT became negative after 18 months in 78%; Food- IgE decreased and food-IgG4 increased after 18 months |
| Meglio 2004(40) | CM | N=21
Age: 5–10 years |
1 drop of CM diluted 1:25 in water | 200 ml undiluted CM per day over 180 days | 15/21 (71.4%) | 3/21 reacted to minimal dose of diluted CM; 3/21 tolerated only 40–80 CM/day;
15/21 tolerated 200 ml CM/day for 6 months; Side effect rate 13/21; SPT to BLG and CS significantly decreased at 6 months(p<0.001), CM-IgE levels not significantly different |
| Rolinck- Werninghaus 2005 (29) | CM (1)
Egg (2) |
N=3
Age: 4–12 years |
0.0006 ml of CM per day
0.01 mg egg per day at home |
100 ml CM per day over
37 weeks, 2.5 g egg per day (1/2 egg) over 41–52 weeks |
3/3 | All patients had acute symptoms to food re-exposure following discontinuation of food for 2–14 days. The only study to re-challenge patients to food following a period of avoidance while on maintenance. |
| Patriarca 2006 (81) | Peanut | N=1
Age 38 years |
5.6 mg peanut per day on day | 40 g peanut/day over 7 days in the hospital | 1/1 | SPT to peanut became negative at 6 months, no significant changes in peanut-IgE (baseline 2.1, 6 months-1.5) or peanut-IgG |
| Pons 2006 (82) | Egg white | N=3 | Not specified | Rush immunotherapy on day 1 in the hospital, then build-up over several months | 3/3 | Percentage of egg-specific CD4+CD23high T cells increased starting between 3–6 months, in parallel with production of IL-10 by egg white- stimulated PBMC |
| Buchanan 2006 (83) | Peanut | N=7
Mean age 4.4 years |
Not specified | Rush phase and dose escalation in the hospital, maintenance at home | 7/7 at 6 months | During rush phase 4/7 required oral antihistamine. At 6 months there was mean 2.3-fold increase in peanut-IgG, and mean change in peanut-IgE of 0.9 fold. |
Symbols: Success rate defined as regular ingestion of the tested food for at least 6 months; $ One of each Apple, peach, lettuce, orange, beans and corn; Abbreviations: CM, cow’s milk; SPT, skin prick test; BLG, beta-lactoglobulin; ALA, alpha-lactalbumin; CS, casein; PBMC, peripheral blood mononuclear cells;
Sublingual immunotherapy with food extract
Another approach to food therapy is sublingual immunotherapy with food extracts. Recently, a randomized, double-blind, placebo-controlled trial of sublingual immunotherapy with commercial hazelnut extract for treatment of hazelnut allergy was conducted.(41) Adult subjects with hazelnut allergy confirmed by DBPCFC to hazelnut were randomly assigned into 2 treatment groups: hazelnut immunotherapy (12) or placebo (11). Patients kept the immunotherapy solution in the mouth for at least 3 minutes and then discharged it. All patients receiving hazelnut immunotherapy reached the planned maximum dose in 4 days according to a rash schedule under physician supervision in the hospital, followed by home administration of a daily maintenance dose of 5 drops of the most potent extract over 5 months (November-March). The maintenance daily dose contained 188.2 μg of Cor a 1 and 121.9 μg of Cor a 8, major hazelnut allergens. Systemic reactions were observed in 0.2% of the total doses administered, were limited to the rush build up phase and were treated with oral antihistamines. Mean threshold dose of ingested hazelnut for objective symptoms increased from 2.3 g to 11.6 g (P = 0.02; active group) versus 3.5 g to 4.1 g (NS; placebo) at follow-up evaluation. Almost 50% of patients who underwent active treatment reached the highest dose (20 g) of hazelnut during follow-up DBPCFC, compared to 9% in the placebo group. Levels of serum hazelnut-specific IgG4 antibody and total serum IL-10 increased only in the active group, but there were no differences in hazelnut-specific IgE antibody levels pre- and post-immunotherapy. These preliminary data are encouraging, especially in regard to safety but more studies are needed to determine optimal duration of immunotherapy and persistence of protective effect when immunotherapy is discontinued. In addition immunotherapy trials with different food allergens and in different patient populations (children versus adults, subjects with well-defined anaphylactic reactivity to foods) must be conducted before final assessment of the role of sublingual immunotherapy with food in treatment of food allergy.
Recombinant food proteins
In the past decade a tremendous progress was accomplished in the area of identification of the relevant food proteins. Major food allergens, such as those in cow’s milk, egg white, soybean, wheat, peanut, tree nuts, seeds, fish and shellfish were characterized in regard to their structure and function. Birch tree pollen-cross reactive proteins in foods of plant origin (fruits, vegetables, legumes, and tree nuts) responsible for oral allergy syndrome were extensively studied. (32) Severity of peanut allergy was correlated to the diversity of epitopes recognized on major peanut allergens, Ara h 1, Ara h 2, and Ara h 3 by B cells. (42–44) Cow’s milk, egg and peanut allergic subjects who lacked IgE antibodies against certain sequential epitopes of the major allergens were found to be more likely to achieve tolerance to these foods than subjects whose IgE antibodies were directed against those epitopes. (45–48)
Identification of the most relevant food allergens was followed by generation of recombinant proteins. (49) To date, a significant number of recombinant food allergens have been synthesized, including major peanut allergens (Ara h 1, 2, 3); major allergens from English walnut (Jug r 1), hazelnut (Cor a 1.04), and cashew (Ana o 1); tropomyosins from crab, lobster, shrimp and snail; parvalbumins from salmon, carp and codfish; lipid transfer proteins from carrot, bell pepper, tomato, wheat, and cherry; birch Bet v 1 cross-reactive major allergens from carrot, apple, and celery; soybean glycinin; and egg white ovomucoid. Most recombinant food proteins were evaluated in regard to their ability to bind specific IgE antibody from allergic patients’ sera and some were used for skin prick testing. (44;50;51) The availability of pure recombinant allergens should allow for customization of diagnostic tests and result in more precise testing for an individual food-allergic patient. It should also allow for improved safety of testing procedures by eliminating contaminations and undeclared allergens in wild-type allergen extracts. (52)
Animal models of food hypersensitivity
Due to ethical concerns about serious side effect of food immunotherapy, animal models of food allergy were crucial in evaluating efficacy and safety of experimental therapies. Li et al developed well-characterized murine models of IgE-mediated cow’s milk and peanut hypersensitivity that were sensitized by the oral route and developed anaphylaxis following oral feeding. (53;54) In a peanut model, C3H/HeJ mice were sensitized orally with freshly ground whole peanut and cholera toxin as adjuvant and challenged orally 3 and 5 weeks later with crude peanut extract. (54) Peanut-specific IgE was induced by oral peanut sensitization, and hypersensitivity reactions were provoked by feeding peanut to sensitized mice. The symptoms were similar to those seen in human subjects. (Fig. 2) Ara h 1- and Ara h 2-specific IgE antibodies were present in the sera of mice with peanut allergy. Furthermore, these Ara h 2-specific IgE antibodies bound the same Ara h 2 isoforms and major allergenic epitopes as antibodies in the sera of human subjects with peanut allergy. Splenocytes from mice with peanut allergy exhibited proliferative responses to Ara h 1 and Ara h 2. This murine model of peanut allergy mimics the clinical and immunologic characteristics of peanut allergy in human subjects and has been used subsequently in many studies
Fig. 2.
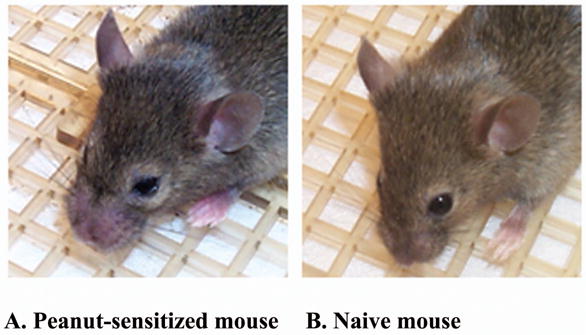
Murine model of peanut anaphylaxis.
A. Peanut allergic-mouse with snout, eye and paws edema, and pilar erecti following oral peanut challenge (score 3) B. Non-peanut allergic mouse asymptomatic on feeding with peanut (score 0).
C3H/HeJ mice were sensitized intragastrically (i.g) with PN and cholera toxin on days 0, 7 and 14. At week 3, mice were challenged with 10 mg crude peanut extract i.g. Symptoms were scored using a 0–5 point scale, with 0 for no signs of reaction, and 5 for death.
Reprinted from LI XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks E et al. Persistent protective effect of heat-killed E.coli producing “engineered” recombinant peanut proteins in a murine model of peanut allergy. (J Allergy Clin Immunol 2003; 112 (1):159–167, with permission from American Academy of Allergy, Asthma and Immunology).
Immunotherapeutic approaches based on recombinant food proteins
An early study found that the intramuscular immunization of naïve AKR/J (H-2K) and C3H/HeJ (H-2K) mice with plasmid DNA encoding Ara h 2 prior to intraperitoneal peanut sensitization had some protective effect in AKR/J mice, but induced anaphylactic reactions in peanut-sensitized C3H/HeJ mice following peanut challenge. (55) Li et al also found no reduction in peanut-IgE antibody levels and more severe anaphylactic symptoms following oral challenge in peanut allergic mice following treatment with pDNA-expressing Ara H 2. However, in another study, oral chitosan embedded Ara h 2 had a protective effect in preventing sensitization in AKR mice. (56) These data indicate that pDNA-based immunotherapy may not be effective in reversing established IgE-mediated hypersensitivity.
Synthetic immunostimulatory oligodeoxynucleotides containing unmethylated CpG motifs (ISS)-conjugated allergen administration was more effective than a mixture of antigen and ISS in the suppression of allergic airway responses probably due to the enhanced dendritic cell uptake of ISS-allergen. The concept of ISS potentiation of Th 1 responses to food allergens was explored Li et al who immunized C3H/HeJ mice intradermally with ISS-linked Ara h 2, or ISS-linked Amb a 1 as a control. (57) Four weeks following immunization, mice were intragastrically sensitized with peanut and challenged with Ara h 2 five weeks later. ISS-Ara h 2 treated mice did not develop symptoms and had significantly lower plasma histamine levels following oral challenge compared to ISS-Amb a 1-treated mice. Nguyen et al found that intradermal immunization with a mixture of ISS and β-gal, but not with ISS alone or β-gal alone, provided protection against fatal anaphylaxis induced by intraperitoneal β–gal sensitization and challenge that was associated with an increase in IgG2a/IFN-γ and a reduction in IgE/IL-4, and IL-5. (58) This effect was comparable to immunization with the pDNA-encoding β-gal. These results suggest that antigen-ISS immunization may have a prophylactic effect against allergy, however, the ability to reverse established food allergy remains to be determined.
Recombinant engineered food proteins
Immunotherapy with native peanut was found to have unacceptably high rate of adverse reactions to immunotherapy injections but the concept of utilizing immunomodulation with peanut protein to increase oral tolerance to peanut was confirmed. (21;22) Subsequent studies concentrated on generation of “hypoallergenic” recombinant peanut proteins that lost the ability to interact with IgE antibodies directed against native peanut but retained the ability to interact with T cells. Such engineered recombinant peanut proteins were expected not to activate mast cells and thus ensure improved safety profile.
In the initial study, peanut major allergen Ara h 1 was cloned and characterized as a member of the vicillin storage protein family. (59) RNA isolated from peanut species (Florunner) was used to construct an expression library for screening with serum IgE from patients with peanut allergy by western blot analysis. A large number of clones with intense binding to peanut-allergic patients’ sera were selected. When incubated with peanut allergic patients’ sera, 94% of patients that bound to wild-type Ara h 1 also bound to recombinant Ara h 1. Subsequently, two additional major peanut allergens were cloned and characterized: Ara h 2 and Ara h 3. (60–62) The IgE-binding epitopes of each of these allergens have been determined. Amino acids critical to each epitope were identified and site-directed mutagenesis of the allergen cDNA clones was used to produce engineered recombinant allergens. Engineered recombinant peanut allergens were then analyzed through immunobloting with peanut-allergic patients’ sera. (63) Fifty seven patients were tested for IgE binding to Ara h 1, 52 patients for Ara h 2, and 52 patients for Ara h 3. The range of IgE binding to engineered recombinant allergens was from 5.4–125% of wild-type Ara h 1, 0–99.8% of wild-type Ara h 2, and 19.3–141% of wild-type Ara h 3. Peripheral blood mononuclear cells (PBMC) from peanut-allergic subjects were incubated in the presence of engineered recombinant peanut allergens and proliferation was assessed by the incorporation of radioactive thymidine into the DNA of dividing cells. PBMCs from 12 peanut-allergic individuals were tested for each wild- type and engineered recombinant allergen. The average stimulation index produced by the engineered recombinant allergens in comparison to its wild-type counterpart was 72% for Ara h 1, 104% for Ara h 2, and 72% for Ara h 3. Subsequently, wild-type Ara h 2 and engineered recombinant Ara h 2 were compared in regard to their ability to interact with T cells, ability to bind IgE and ability to release mediators from passively sensitized RBL-2H3 cell line. Multiple T cell epitopes were identified on Ara h 2. Amino acid regions of Ara h 2, 11–35, 86–125, and 121–155 contained the majority of peptides that interact with T cells from peanut allergic patients. Wild-type and engineered recombinant Ara h 2- stimulated proliferation of T cells from peanut-allergic patients to similar levels. In contrast, engineered recombinant Ara h 2 had greatly reduced IgE-binding capacity and released significantly lower amounts of β-hexosaminidase, a marker of IgE mediated RBL-2H3 degranulation compared to the wild-type allergen. (64) These studies demonstrated that the engineered recombinant peanut allergens retained the ability to interact with T cells while they lost the ability to interact with IgE antibodies directed against native peanut proteins, supporting their use in trials of peanut immunotherapy.
Recombinant major apple allergen (rMal d 1) with 5 point mutations introduced by site-directed mutagenesis was recently evaluated in 14 adults with birch tree pollen allergy and apple allergy. (51) IgE reactivity to mutated rMal d 1 was two-fold lower than that of the wild-type molecule (95%CI: 1.7–2.4). RAST inhibition showed a 7.8 fold decrease in IgE-binding capacity (95%CI: 3.0–12.6). The biological activity of mutant rMal d 1 assessed by skin prick test and basophil histamine release was decreased 10–200-fold. In addition, DBPCFC in two subjects confirmed lower allergenicity of mutant rMal d 1 in comparison to wild-type rMal d 1 as measured by increased threshold dose inducing symptoms and maximum severity of reaction at the peak dose. This is the first study to demonstrate hypoallergenicity of engineered recombinant food allergen by an oral food challenge. These results are very encouraging but must be interpreted with great caution, as point mutations may result in formation of new allergenic epitopes and paradoxically increase allergenicity of mutated allergens for some patients. (63)
Engineered recombinant peanut immunotherapy
In vivo efficacy of the engineered recombinant peanut proteins was tested in the murine model of peanut anaphylaxis. (65) Mice were sensitized to whole peanut and then desensitized by intranasal administration of engineered recombinant Ara h 2 (three doses a week for 4 weeks). Desensitization with the engineered recombinant Ara h 2 protein suppressed synthesis of Ara h 2-IgE and resulted in significantly decreased symptoms on oral peanut challenge compared to a control group treated with wild-type Ara h 2.
Engineered recombinant peanut proteins mixed with bacterial adjuvants immunotherapy
Bacteria are potent stimulants of Th 1 immune responses and increase IFN-γ production. Heat-killed Listeria monocytogenes (HKLM) was shown to reverse established allergic airway hyperreactivity in mice. (66) In a dog model, a single subcutaneous treatment with a mixture of HKLM, milk and wheat significantly reduced immediate skin test reactions and prevented anaphylactic symptoms on oral food challenge. (67) Li et al tested the efficacy of a mixture of HKLM and modified peanut proteins (Ara h 1, Ara h2, and Ara h3). (68) Peanut-allergic C3H/HeJ mice were treated 10 weeks following sensitization with a mixture of the three major peanut allergens and HKLM (modified (m)Ara h 1–3 plus HKLM) administered subcutaneously. All mice in the sham-treated groups exhibited anaphylactic symptoms with a median symptom score of 3, whereas only 31% of mice in the mAra h 1–3 plus HKLM group developed mild anaphylaxis, with a low median symptom score of 0.5 (on a scale from 0 to 5). Changes in core body temperature, bronchial constriction, plasma histamine, and peanut-specific IgE levels were all significantly reduced. This protective effect was markedly more potent than in the mAra h 1–3 protein alone-treated group. HKLM alone did not have any protective effect. Reduced IL-5 and IL-13, and increased IFN-γ levels were observed only in peanut-stimulated splenocyte cultures from mAra h 1–3 plus HKLM-treated mice. These results showed that immunotherapy with modified peanut proteins and HKLM was effective for treating peanut allergy in this model, and might be a potential approach for treating peanut allergy.
The safety concerns about using potentially pathogenic bacteria in humans resulted in selecting a non-pathogenic strain of Escherichia coli as a bacterial adjuvant. In subsequent studies heat-killed E. coli (HKE) expressing modified peanut proteins administered subcutaneously was investigated and it was found that lower doses for desensitization were required using HKE. (69) However, considering potential complications resulting from subcutaneous route of administration in humans, further studies focused on the vaccine administered per rectum. Since non-pathogenic E. coli bacteria reside in the colon, it was assumed that rectal delivery would provide superior safety regarding possible infectious complications as well as severe adverse reactions. In addition, the rectal route is non-invasive and could be safely used in young children that would be most likely the target for this vaccine.
The long-term immunomodulatory effect of HKE producing mutated Ara h 1, 2, and 3 (HKE-MP123) administered rectally (pr) was investigated in a murine model of peanut anaphylaxis. (70) Peanut-allergic C3H/HeJ mice received 0.9 (low dose), 9 (medium dose), or 90 (high dose) μg HKE-MP123 pr, HKE-containing vector (HKE-V) alone, or vehicle alone (sham) weekly for 3 weeks. Mice were challenged 2 weeks later. Second and third challenges were performed at 4-week intervals. After the first challenge, all 3 HKE-MP123 and HKE-V-treated groups exhibited reduced symptom scores (P <0.01, 0.01, 0.05, 0.05, respectively) compared with the sham-treated group. Only the medium- and high-dose HKE-MP123-treated mice remained protected for up to 10 weeks after treatment accompanied by a significant reduction of plasma histamine levels compared with sham-treated mice (P <.05 and.01, respectively). (Fig. 3) IgE levels were significantly lower in all HKE-MP123-treated groups (P <0.001), being most reduced in the high-dose HKE-MP123-treated group at the time of each challenge. In vitro IL-4, IL-13, IL-5 and IL-10 production by peanut-stimulated splenocytes of high-dose HKE-MP123-treated mice were significantly decreased (P <0.01, 0.001, 0.001, and 0.001, respectively), and IFN-γ and TGF-β production were significantly increased (P <0.001 and 0.01, respectively) compared with sham-treated mice at the time of the last challenge. Treatment with rectal HKE-MP123 can induce long-term “down-regulation” of peanut hypersensitivity, which might be secondary to decreased antigen-specific Th 2 and increased Th 1 and T regulatory cytokine production. The rectal vaccine is currently being standardized in preparation for a phase I human clinical trial. The comparison of experience with native, recombinant, and engineered recombinant allergen immunotherapy for food allergy is presented in Table 2.
Fig. 3.
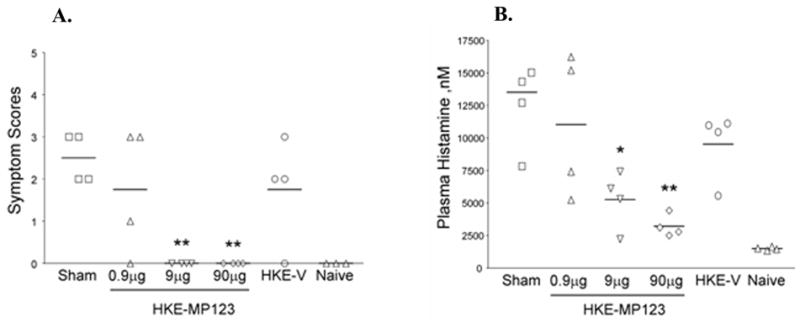
Persistent protection against peanut-induced anaphylactic reactions by HKE-MP 123. Mice were challenged at 10 weeks after the last HKE-MP 123 treatment. A. Anaphylactic symptom scores were determined 30 minutes after challenge. Each point indicates an individual mouse. Bars indicate the median of 4 mice in each group. B. Histamine plasma levels were measured in blood samples obtained 30 to 40 minutes after the challenge. Data are means +SEM for each group of 4 mice. *P<0.05, and **P<0.01 versus sham. (Re-printed with permission from Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol 2003; 112(1):159–167, with permission from American Academy of Allergy, Asthma and Immunology)
TABLE 2.
The comparison of native, recombinant, and engineered recombinant allergen immunotherapy (IT) for food allergy
| Therapy | Mechanism of Action | Effects | Comments |
|---|---|---|---|
| Conventional peanut IT | Altered T-cell responses, up- regulation of suppressor cells in allergen IT | Increased oral peanut tolerance | Subcutaneous injections of gradually increasing doses of allergen, unacceptably high rate of serious adverse events |
| Birch pollen IT for oral allergy to apple | Marked reduction in skin test to raw apple; IT inversely correlated with baseline skin test but not with serum apple or birch-IgE | Significant reduction or total resolution of oral allergy to raw
Golden Delicious apple in a subset of patients receiving IT for at least 12 months |
Clinical effect lasting for up to 30 months after discontinuation in >50% of patients |
| Oral desensitization | Presumed oral tolerance induction; decreased skin test reactivity, increased serum food-IgG/IgG4, no change in food-IgE, increased food-specific CD4+CD23high T cells, increased IL-10 on food antigen stimulation | Tolerance to regular servings of food in most subjects (70–80%) maintained as long as food ingested on regular basis for up to 6 months; in a subset increased threshold dose for clinical reactions | Up to 50% experience systemic side effects; some patients require uninterrupted ingestion of food to maintain desensitized tolerant state; rigorous clinical trials necessary to determine safety and efficacy |
| Sublingual IT with hazelnut extract | Presumed oral tolerance induction; increased serum hazelnut-IgG4 and total IL-10 level, no change in hazelnut-IgE | Increased oral hazelnut tolerance | Systemic reactions in only 0.2% of doses during build up phase, treated with oral antihistamines; rigorous clinical trials necessary to determine safety and efficacy |
| Plasmid DNA- based IT | Induces prolonged humoral and cellular responses due to CpG motifs in the DNA backbone | Protection against peanut anaphylaxis in sensitized AKR/J mice, but induction of anaphylaxis in C3H/HeJ (H-2K) mice; no effect on peanut-IgE antibody levels | Serious concerns regarding safety in view of strain- dependent effects in mice, concern for excessive Th 1 stimulation and autoimmunity |
| Immunostimulatory sequences (ISS- ODN) | Potent stimulation of Th 1 via activation of antigen- presenting cells, natural killer cells, and B cells; increased Th 1 cytokines | Protection against peanut sensitization in mice | Not shown to reverse established peanut allergy, concern for excessive Th 1 stimulation, and potential for autoimmunity |
| Engineered recombinant peanut IT | Binding to mast cells eliminated, T-cell responses comparable to native peanut allergens | Protection against peanut anaphylaxis in mice | Improved safety profile compared with conventional IT, requires identification of IgE binding sites |
| Heat-killed bacteria mixed with or expressing engineered recombinant peanut proteins | Potentiation of Th 1 and T- regulatory cytokine responses | Protection against peanut anaphylaxis in mice, lasting up to 10 weeks after treatment | Concern for toxicity of bacterial adjuvants, excessive Th 1 stimulation, and potential for autoimmunity; heat-killed E. coli expressing modified peanut allergens administered rectally viewed as the safest approach for future human studies |
Non- allergen-specific approaches to food allergy treatment
In addition to research focusing on immunotherapy with native and engineered recombinant food proteins, alternative approaches to food allergy therapy have been investigated, including monoclonal anti-IgE therapy, Chinese herbs, probiotics, and cytokine therapy. (71–75)
Footnotes
Funding support: K23AI059318 to A. Nowak-Wegrzyn
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117(2 Suppl MiniPrimer):S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2003;111(2 Suppl):S540–S547. doi: 10.1067/mai.2003.134. [DOI] [PubMed] [Google Scholar]
- 3.Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmested County: a population-based study. J Allergy Clin Immunol. 1999;104:452–456. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999;103(4):559–562. doi: 10.1016/s0091-6749(99)70224-1. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112(6):1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 6.Munoz-Furlong A, Sampson HA, Sicherer SH. Prevalence of self-reported seafood allergy in the US. J Allergy Clin Immunol. 2004;113:S100. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108(6):881–890. doi: 10.1067/mai.2001.118515. [DOI] [PubMed] [Google Scholar]
- 8.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107(2):367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 9.Wood RA. The natural history of food allergy. Pediatrics. 2003;111(6 Pt 3):1631–1637. [PubMed] [Google Scholar]
- 10.Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005;116(5):1087–1093. doi: 10.1016/j.jaci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117(2 Suppl MiniPrimer):S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 12.Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J Allergy Clin Immunol. 1988;82:986–997. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 13.Sampson HA, Ho DG. Realationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 14.Sampson HA. Utility of food-specific IgE concentrations in prediciting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 15.Bock SA, Atkins FM. The natural history of peanut allergy. J Allergy Clin Immunol. 1989;83(5):900–904. doi: 10.1016/0091-6749(89)90103-6. [DOI] [PubMed] [Google Scholar]
- 16.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: Data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110(5):784–789. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 17.Kagan RS, Joseph L, Dufresne C, Gray-Donald K, Turnbull E, Pierre YS, et al. Prevalence of peanut allergy in primary-school children in Montreal, Canada. J Allergy Clin Immunol. 2003;112(6):1223–1228. doi: 10.1016/j.jaci.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic Infant Formulas Pediatrics. 2000;106(2):346–349. [PubMed] [Google Scholar]
- 20.Nelson HS. Advances in upper airway diseases and allergen immunotherapy. J Allergy Clin Immunol. 2006;117(5):1047–1053. doi: 10.1016/j.jaci.2005.12.1306. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90(2):256–262. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 22.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99(6 Pt 1):744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 23.Valenta R, Kraft D. Type I allergic reactions to plant-derived food: A consequence of primary sensitization to pollen allergens. J Allergy Clin Immunology. 1996;97:895. doi: 10.1016/s0091-6749(96)80062-5. [DOI] [PubMed] [Google Scholar]
- 24.Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28:1368–1373. doi: 10.1046/j.1365-2222.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- 25.Asero R. How long does the effect of birch pollen injection SIT on apple allergy last? Allergy. 2003;58(5):435–438. doi: 10.1034/j.1398-9995.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 26.Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59(12):1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 27.Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34(5):761–769. doi: 10.1111/j.1365-2222.2004.1939.x. [DOI] [PubMed] [Google Scholar]
- 28.Asero R. Effects of birch pollen SIT on apple allergy: a matter of dosage? Allergy. 2004;59(12):1269–1271. doi: 10.1111/j.1398-9995.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 29.Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005;60(10):1320–1322. doi: 10.1111/j.1398-9995.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 30.Niggemann B, Staden U, Rolinck-Werninghaus C, Beyer K. Specific oral tolerance induction in food allergy. Allergy. 2006;61(7):808–811. doi: 10.1111/j.1398-9995.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 31.Ko J, Mayer L. Oral tolerance: lessons on treatment of food allergy. Eur J Gastroenterol Hepatol. 2005;17(12):1299–1303. doi: 10.1097/00042737-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 33.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101(8):1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh KY, Tsai CC, Wu CH, Lin RH. Epicutaneous exposure to protein antigen and food allergy. Clin Exp Allergy. 2003;33(8):1067–1075. doi: 10.1046/j.1365-2222.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 35.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35(6):757–766. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 36.Strid J, Thomson M, Hourihane J, Kimber I, Strobel S. A novel model of sensitization and oral tolerance to peanut protein. Immunology. 2004;113(3):293–303. doi: 10.1111/j.1365-2567.2004.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 38.Vadas P, Wai Y, Burks AW, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA. 2001;285(13):1746–1748. doi: 10.1001/jama.285.13.1746. [DOI] [PubMed] [Google Scholar]
- 39.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17(3):459–465. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 40.Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow's milk allergy. Allergy. 2004;59(9):980–987. doi: 10.1111/j.1398-9995.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 41.Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116(5):1073–1079. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116(4):893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 43.Lewis SA, Grimshaw KE, Warner JO, Hourihane JO. The promiscuity of immunoglobulin E binding to peanut allergens, as determined by Western blotting, correlates with the severity of clinical symptoms. Clin Exp Allergy. 2005;35(6):767–773. doi: 10.1111/j.1365-2222.2005.02252.x. [DOI] [PubMed] [Google Scholar]
- 44.Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy 1. J Allergy Clin Immunol. 2006;118(1):250–256. doi: 10.1016/j.jaci.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Beyer K, Ellman-Grunther L, Jarvinen KM, Wood RA, Hourihane J, Sampson HA. Measurement of peptide-specific IgE as an additional tool in identifying patients with clinical reactivity to peanuts. J Allergy Clin Immunol. 2003;112(1):202–207. doi: 10.1067/mai.2003.1621. [DOI] [PubMed] [Google Scholar]
- 46.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159(4):2026–2032. [PubMed] [Google Scholar]
- 47.Jarvinen KM, Chatchatee P, Bardina L, Beyer K, Sampson HA. IgE and IgG binding epitopes on alpha-lactalbumin and beta-lactoglobulin in cow's milk allergy. Int Arch Allergy Immunol. 2001;126(2):111–118. doi: 10.1159/000049501. [DOI] [PubMed] [Google Scholar]
- 48.Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow's milk allergy. J Allergy Clin Immunol. 2002;110(2):293–297. doi: 10.1067/mai.2002.126080. [DOI] [PubMed] [Google Scholar]
- 49.Valenta R, Vrtala S, Laffer S, Spitzauer S, Kraft D. Recombinant allergens. Allergy. 1998;53(6):552–561. doi: 10.1111/j.1398-9995.1998.tb03930.x. [DOI] [PubMed] [Google Scholar]
- 50.Reuter A, Lidholm J, Andersson K, Ostling J, Lundberg M, Scheurer S, et al. A critical assessment of allergen component-based in vitro diagnosis in cherry allergy across Europe. Clin Exp Allergy. 2006;36(6):815–823. doi: 10.1111/j.1365-2222.2006.2492.x. [DOI] [PubMed] [Google Scholar]
- 51.Bolhaar ST, Zuidmeer L, Ma Y, Ferreira F, Bruijnzeel-Koomen CA, Hoffmann-Sommergruber K, et al. A mutant of the major apple allergen, Mal d 1, demonstrating hypo-allergenicity in the target organ by double-blind placebo-controlled food challenge. Clin Exp Allergy. 2005;35(12):1638–1644. doi: 10.1111/j.1365-2222.2005.02390.x. [DOI] [PubMed] [Google Scholar]
- 52.Lidholm J, Ballmer-Weber BK, Mari A, Vieths S. Component-resolved diagnostics in food allergy. Curr Opin Allergy Clin Immunol. 2006;6(3):234–240. doi: 10.1097/01.all.0000225166.90768.d6. [DOI] [PubMed] [Google Scholar]
- 53.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103(2 Pt 1):206–214. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 54.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106(1 Pt 1):150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Huang CK, Schofield BH, Burks AW, Bannon GA, Kim KH, et al. Strain-dependent induction of allergic sensitization caused by peanut allergen DNA immunization in mice. J Immunol. 1999;162(5):3045–3052. [PubMed] [Google Scholar]
- 56.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava K, Li XM, Bannon GA, et al. Investigation of the use of ISS-linked Ara h2 for the treatment of peanut-induced allergy [Abstract] J Allergy Clin Immunol. 2001;107:S233. [Google Scholar]
- 58.Nguyen MD, Cinman N, Yen J, Horner AA. DNA-based vaccination for the treatment of food allergy. Allergy. 2001;56 (Suppl 67):127–130. doi: 10.1034/j.1398-9995.2001.00937.x. [DOI] [PubMed] [Google Scholar]
- 59.Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity 26. J Clin Invest. 1995;96(4):1715–1721. doi: 10.1172/JCI118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eigenmann PA, Burks AW, Bannon GA, Sampson HA. Identification of unique peanut and soy allergens in sera adsorbed with cross-reacting antibodies. J Allergy Clin Immunol. 1996;98(5 Pt 1):969–978. doi: 10.1016/s0091-6749(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 61.Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997;342(2):244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 62.Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, et al. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest. 1999;103(4):535–542. doi: 10.1172/JCI5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bannon GA, Cockrell G, Connaughton C, West CM, Helm R, Stanley JS, et al. Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy 9. Int Arch Allergy Immunol. 2001;124(1–3):70–72. doi: 10.1159/000053672. [DOI] [PubMed] [Google Scholar]
- 64.King N, Helm R, Stanley JS, Vieths S, Luttkopf D, Hatahet L, et al. Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res. 2005;49(10):963–971. doi: 10.1002/mnfr.200500073. [DOI] [PubMed] [Google Scholar]
- 65.Srivastava KD, Li XM, King N, et al. Immunotherapy with modified peanut allergens in a murine model of peanut allergy. J Allergy Clin Immunol. 2002;109:S287. [Google Scholar]
- 66.Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. Heat-killed Listeria monocytogene as an adjuvant converts established murine TH2-dominated immune responses into TH1-dominated responses. J Immunol. 1998;161:4146–4152. [PubMed] [Google Scholar]
- 67.Frick OL, Bachanan BB, Delval G, Umetsu DT. Allergen immunotherapy with heat-killed Listeria monocytogenes as adjuvant prevents allergeic reactions in highly sensitized dogs [Abstract] J Allergy Clin Immunol. 2001;107:S232. [Google Scholar]
- 68.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170(6):3289–3295. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 69.Stanley JS, Buzen F, Cockrell G, West M, Srivastava K, Sampson HA, et al. Immunotherapy for peanut allergy using modified allergens and a bacterial adjuvant. J Allergy Clin Immunol. 2002;109(1):S93. [Google Scholar]
- 70.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112(1):159–167. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 71.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348(11):986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy 1. J Allergy Clin Immunol. 2005;115(1):171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh KY, Hsu CI, Lin JY, Tsai CC, Lin RH. Oral administration of an edible-mushroom-derived protein inhibits the development of food-allergic reactions in mice. Clin Exp Allergy. 2003;33(11):1595–1602. doi: 10.1046/j.1365-2222.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 74.Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clin Immunol. 2001;101(2):220–228. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- 75.Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113(1):115–119. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 76.Patriarca C, Romano A, Venuti A, Schiavino D, Di RV, Nucera E, et al. Oral specific hyposensitization in the management of patients allergic to food 1. Allergol Immunopathol (Madr ) 1984;12(4):275–281. [PubMed] [Google Scholar]
- 77.Patriarca G, Schiavino D, Nucera E, Schinco G, Milani A, Gasbarrini GB. Food allergy in children: results of a standardized protocol for oral desensitization 1. Hepatogastroenterology. 1998;45(19):52–58. [PubMed] [Google Scholar]
- 78.Bauer A, Ekanayake MS, Wigger-Alberti W, Elsner P. Oral rush desensitization to milk. Allergy. 1999;54(8):894–895. doi: 10.1034/j.1398-9995.1999.00228.x. [DOI] [PubMed] [Google Scholar]
- 79.Nucera E, Schiavino D, D'Ambrosio C, Stabile A, Rumi C, Gasbarrini G, et al. Immunological aspects of oral desensitization in food allergy 4. Dig Dis Sci. 2000;45(3):637–641. doi: 10.1023/a:1005430231735. [DOI] [PubMed] [Google Scholar]
- 80.Rueff F, Eberlein-Konig B, Przybilla B. Oral hyposensitization with celery juice 13. Allergy. 2001;56(1):82–83. doi: 10.1034/j.1398-9995.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- 81.Patriarca G, Nucera E, Pollastrini E, De Pasquale T, Lombardo C, Buonomo A, et al. Oral rush desensitization in peanut allergy: a case report 12. Dig Dis Sci. 2006;51(3):471–473. doi: 10.1007/s10620-006-3157-4. [DOI] [PubMed] [Google Scholar]
- 82.Pons L, Buchanan AD, Steel PH, Staats HF, Burks AW. CD4+CD25 high T regulatory cells in egg-allergic children undergoing oral desensitization. Journal of Allergy and Clinical Immunology. 2006;117(2):S42. [Google Scholar]
- 83.Buchanan AD, Scurlock AM, Jone SM, Christie L, Althage KM, Steele PH, et al. Oral desensitization and induction of tolerance in peanut-allergic children. Journal of Allergy and Clinical Immunology. 2006;117(2):S327. [Google Scholar]


