Abstract
A subset of prolyl oligopeptidases, including dipeptidyl-peptidase IV (DPP IV or CD26, EC 3.4.14.5), specifically cleave off N-terminal dipeptides from substrates having proline or alanine in amino acid position 2. This enzyme activity has been implicated in the regulation of the biological activity of multiple hormones and chemokines, including the insulinotropic peptides glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Targeted inactivation of the CD26 gene yielded healthy mice that have normal blood glucose levels in the fasted state, but reduced glycemic excursion after a glucose challenge. Levels of glucose-stimulated circulating insulin and the intact insulinotropic form of GLP-1 are increased in CD26−/− mice. A pharmacological inhibitor of DPP IV enzymatic activity improved glucose tolerance in wild-type, but not in CD26−/−, mice. This inhibitor also improved glucose tolerance in GLP-1 receptor−/− mice, indicating that CD26 contributes to blood glucose regulation by controlling the activity of GLP-1 as well as additional substrates. These data reveal a critical role for CD26 in physiological glucose homeostasis, and establish it as a potential target for therapy in type II diabetes.
CD26 is a multifunctional glycoprotein expressed both as a soluble form in plasma and on the surface of several cell types, including epithelial and endothelial cells and lymphocytes (1). It has been involved in T cell costimulation, tumor suppression, and adenosine deaminase binding (2–4). In addition, CD26 is a serine protease (5) belonging to a subgroup of prolyl oligopeptidases, whose members specifically cleave off N-terminal dipeptides from proteins having proline or alanine in amino acid position 2 (6, 7). The biological activity of several hormones and chemokines can be abolished by CD26 in vitro (8). Thus, CD26 has been implicated in the regulation of immune, inflammatory, nervous, and endocrine functions (1). However, the physiological role of CD26 in these processes has not been established.
Among the substrates of dipeptidyl-peptidase IV (DPP IV; EC 3.4.14.5) enzyme activity are two hormones important for glucose regulation, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (9, 10). Whereas the intact forms of GLP-1-(7–36)-amide and GIP-(1–42) can enhance glucose-stimulated insulin secretion, this activity is abolished upon DPP IV-mediated removal of their N-terminal dipeptides in vitro (11). N-terminally truncated GLP-1 and GIP can be detected in the circulation, suggesting such cleavage may be a physiologically relevant mechanism of regulating their activity (10, 12). It remains unclear, however, precisely which enzymes are responsible for this processing of GLP-1 and GIP in vivo. Administration of chemical inhibitors of DPP IV activity leads to increased levels of GLP-1 in the circulation and causes enhanced insulin secretion and improved glucose tolerance in normal and diabetic animals (13–16). However, the precise molecular target of these pharmacological inhibitors has not been identified, in part because of the multiplicity of enzymes exhibiting DPP IV-like activity (17–21). The aim of this study was to clarify the physiological role of CD26 and determine its contribution to blood glucose control.
Materials and Methods
Generation of CD26−/− Mice.
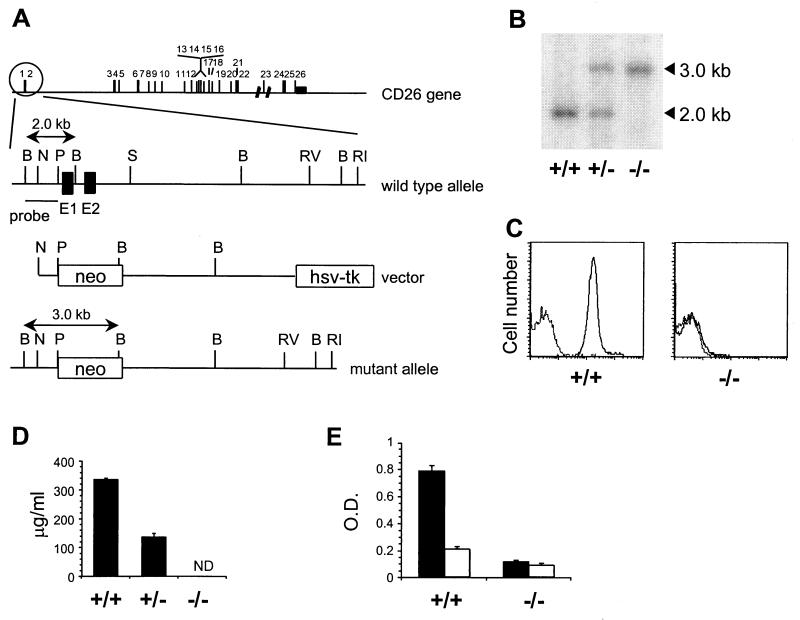
The CD26 targeting construct was generated by replacing a 2.3-kb PstI–SphI fragment in the 13.5-kb SalI–EcoRI CD26 genomic fragment (22) derived from the B10.A-derived λ clone 10 with a neo gene under control of the phosphoglycerate kinase 1 (PGK1) promoter. The construct, flanked by the herpes simplex virus TK gene, was linearized and electroporated into the C57BL/6-derived embryonic stem cell line B6III (23). Clones resistant to both G418 and ganciclovir were analyzed for single correct integration events by Southern blotting using a 0.7-kb BglII–NcoI fragment as a probe (Fig. 1A). Recombinant embryonic stem cells were injected into BALB/c blastocysts, resulting in 20 chimeric male mice. Six germ-line-transmitting animals, representing three independent targeting events, were bred with C57BL/6 females. Heterozygotes from each line were inbred, yielding homozygous CD26−/− mice on a C57BL/6 background.
Figure 1.
Generation of CD26-deficient mice. (A) Targeting strategy. Exons in the CD26 gene are numbered. The wild-type CD26 allele, targeting construct, and expected mutant allele are shown with exon 1 and 2 of CD26 as filled boxes and the neo and TK genes as open boxes. Restriction sites: B, BglII; N, NcoI; P, PstI; S, SphI; RI, EcoRI, RV, EcoRV. (B) Southern blot containing BglII-digested DNA from CD26 wild-type (+/+), heterozygous (+/−), and homozygous mutant (−/−) mice hybridized with a 0.7-kb BglII–PstI probe specific for regions upstream of the targeting construct. (C) Flow cytometric analysis of CD26 expression at the surface of thymocytes from CD26 wild-type (+/+) and homozygous mutant (−/−) mice stained with anti-CD26 mAb followed by FITC-labeled anti-Ig (solid line) or with FITC-anti-Ig alone (dotted line). (D) Soluble CD26 protein in serum from CD26 wild-type (+/+), heterozygous (+/−), and homozygous mutant (−/−) mice measured by ELISA. ND, not detectable. (E) DPP IV enzyme activity in plasma from CD26 wild-type (+/+) and homozygous mutant (−/−) mice (n = 7). The DPP IV substrate Gly-Pro-pNA (pNA, p-nitroanilide) was incubated in plasma alone (black bars) or plasma containing 10 μM valine-pyrrolidide (open bars).
Biochemistry.
Flow cytometric analysis of cell surface CD26 expression was done by staining thymocytes with the anti-CD26 mAb H194-112 (5) followed by FITC-conjugated anti-rat IgG. The levels of soluble CD26 protein in plasma were measured by ELISA using the anti-CD26 mAb H207-1082 and biotinylated anti-CD26 mAb H207-773 (24) as catching and detecting Ab, respectively, followed by addition of horseradish peroxidase-streptavidin and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate. Absorbance at 410 nm was measured at different time points. Plasma DPP IV activity was measured by incubating 20 μl of plasma with 100 μl of PBS containing 1 mM Gly-Pro-pNA (Bachem) for 60 min at 37°C, and the absorbance was determined at 410 nm (5, 24). For peptide stability measurements, 10 μl of 1 μg/μl solution of GLP-1-(7–36)-amide or GIP-(1–42) (both from Sigma) was incubated in 15 μl of EDTA-plasma from wild-type or CD26−/− mice at 37°C for 4 h. At the indicated time a 5-μl aliquot was withdrawn, trifluoroacetic acid was added to a final concentration of 20%, and the solution was briefly centrifuged to remove precipitated material. The supernatant was desalted on a Zip-Tip (Millipore) and 1 μl was used for analysis by matrix-assisted laser desorption ionization mass spectrometry. Sinapinic acid was used as matrix.
Metabolic Measurements.
Glucose tolerance tests were done on age- and sex-matched C57BL/6 wild-type, CD26−/−, or GLP-1R−/− mice fasted for 18 h. Glucose (2 g/kg) was given orally by gavage or intraperitoneally at time 0. Tail blood (5 μl) was collected at −30, 0, 30, 60, 120, and 180 min, and blood glucose concentration was determined on an EBIO Plus (Eppendorf). In some experiments, mice were given 5 ml/kg of a 4.12 mg/ml solution of valine-pyrrolidide (25) (obtained from L. B. Christiansen, Novo Nordisk) or the same volume of vehicle (water) as control, at time −30 min. As the volume of plasma required for insulin and GLP-1 measurements precluded sampling at multiple time points, a single blood sample was collected 15 min after an oral glucose challenge. A 200-μl sample of orbital blood was immediately mixed with 10.5 μl of 0.18 M EDTA/15,000 kallikrein inhibitor units/ml aprotinin/0.3 mM valine-pyrrolidide at 4°C and centrifuged 10 min at 10,000 × g at 4°C, and the plasma was collected on ice. Intact GLP-1 was measured by sandwich ELISA (detection range 0.2–50 pM) using a catching mAb (GLP1F5) specific for the C-terminal part of GLP-1 and a detecting mAb (mAb26.1) specific for GLP-1 having an intact N terminus. This assay will be described in detail elsewhere. Insulin levels were determined by using a kit (Linco) or by sandwich ELISA, using polyclonal antibodies GP114 and GP116, with rat insulin as standard (range 3 to 3000 pmol/liter) (26). All data are expressed as mean ± SEM. Statistical analysis was by two-tailed, unpaired Students t test, and differences are considered significant when P < 0.05.
Results
The single gene encoding both the transmembrane and the soluble form of CD26 (22) was inactivated by homologous recombination in embryonic stem cells. A targeting construct was designed to excise part of the promoter and exons 1 and 2, encoding the cytoplasmic and transmembrane domains of CD26 (Fig. 1A). Southern blot analysis of genomic DNA from wild-type, CD26+/−, and CD26−/− mice indicated that the mutant allele was correctly targeted (Fig. 1B). The analysis of heterozygous intercrosses showed that the mutant allele is transmitted with the expected Mendelian frequency. CD26−/− mice are fertile and appear healthy, although the body weight of male CD26−/− mice is slightly, but significantly, lower than wild-type controls (data not shown). To verify that the mutation of the CD26 gene led to a null phenotype, we measured CD26 expression on the surface of thymocytes. CD26−/− thymocytes completely lacked CD26 expression (Fig. 1C). Intermediate expression levels were observed with cells from heterozygous mice (data not shown). Because the catalytic domain resides in the C-terminal portion of CD26 it was important to exclude the possibility that a truncated soluble form of CD26 was still expressed from the mutant CD26 allele. The levels of CD26 protein in plasma were determined by ELISA measurements, showing that CD26 was absent from plasma derived from CD26−/− mice, and that CD26+/− mice expressed half as much CD26 as did wild-type mice (Fig. 1D). Despite the complete absence of CD26 protein, plasma from CD26−/− mice contained residual enzyme activity that degraded the prototypic DPP IV substrate, Gly-Pro-pNA, consistent with the existence of distinct enzymes displaying DPP IV-like proteolytic activity (Fig. 1E) (17–21).
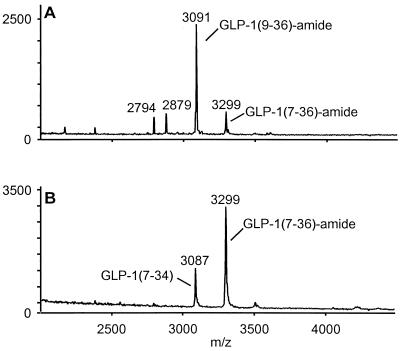
To evaluate the role of CD26 in the N-terminal processing of GLP-1, intact GLP-1-(7–36)-amide was incubated in plasma from CD26−/− mice or from wild-type controls and analyzed by mass spectrometry. After a 4-h incubation at 37°C in plasma from wild-type mice, a substantial proportion of GLP-1(7–36)-amide was N-terminally truncated to inactive GLP-1-(9–36)-amide (Fig. 2A). In contrast, incubation of intact GLP-1(7–36)-amide in plasma derived from CD26−/− mice resulted exclusively in C-terminal degradation, and possibly endo-degradation, with no detectable N-terminal truncation (Fig. 2B). Similar results were obtained after a 24-h incubation at 37°C (data not shown). Addition of an excess of valine-pyrrolidide, a pharmacological inhibitor of DPP-IV enzyme activity (25), to wild-type mouse plasma also prevented the accumulation of N-terminally truncated GLP-1-(9–36)-amide (data not shown). Taken together, these data indicate that CD26 is the major enzyme responsible for N-terminal processing of GLP-1 in serum.
Figure 2.
Lack of N-terminal degradation of GLP-1 in plasma from CD26−/− mice. Mass spectrometry analysis of GLP-1 degradation products after 4-h incubation of intact GLP-1-(7–36)-amide at 37°C in plasma from CD26 wild-type (A) or homozygous mutant (B) mice. The identity of peaks representing intact GLP-1-(7–36)-amide, N-terminally truncated GLP-1-(9–36)-amide, and C-terminally truncated GLP-1-(7–34) are indicated.
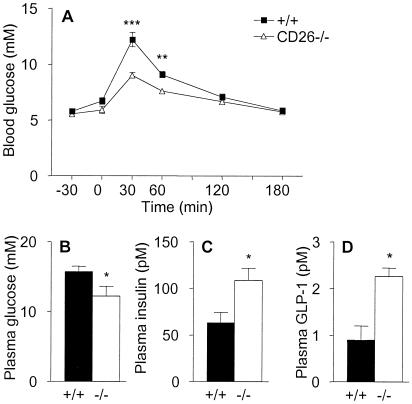
If CD26 mediates the majority of N-terminal processing of GLP-1 in vivo, increased levels of intact GLP-1 should accumulate in CD26−/− mice, leading to increased insulin secretion and enhanced glucose clearance. To test this possibility, oral glucose tolerance tests were done in wild-type and in CD26−/− mice. Blood glucose concentrations were significantly lower at 30 and 60 min after glucose challenge in CD26−/− mice compared with wild-type controls, indicating that lack of CD26 expression accelerates blood glucose clearance (Fig. 3A). The integrated glucose concentrations, calculated as area under the curves, were significantly lower (P < 0.05) in CD26-deficient compared with wild-type mice (data not shown). At 15 min after the oral glucose challenge, plasma glucose levels were already significantly lower in CD26−/− mice than in wild-type controls (Fig. 3B). Furthermore, glucose-stimulated insulin levels were significantly increased in the CD26−/− mice (Fig. 3C), in association with increased levels of intact, biologically active GLP-1 (Fig. 3D). In contrast, no significant differences were detected in the fasting levels of glucose, insulin, or GLP-1 in wild-type versus CD26−/− mice (data not shown), in agreement with the predominant incretin actions of GLP-1 (27, 28).
Figure 3.
Enhanced oral glucose tolerance and increased levels of insulin and GLP-1 in CD26−/− mice. (A) Blood glucose concentrations measured at various times before and after oral administration of glucose (given at time 0) to female wild-type or CD26−/− mice, as indicated. n = 7. Similar data were obtained in a repeat experiment and in two experiments on groups of male mice. (B–D) Levels of glucose (B), insulin (C), and intact GLP-1-(7–36) (D) in plasma from male wild-type (closed bars) and CD26−/− (open bars) mice taken 15 min after oral glucose challenge. n = 8. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Similar data were obtained in two additional experiments.
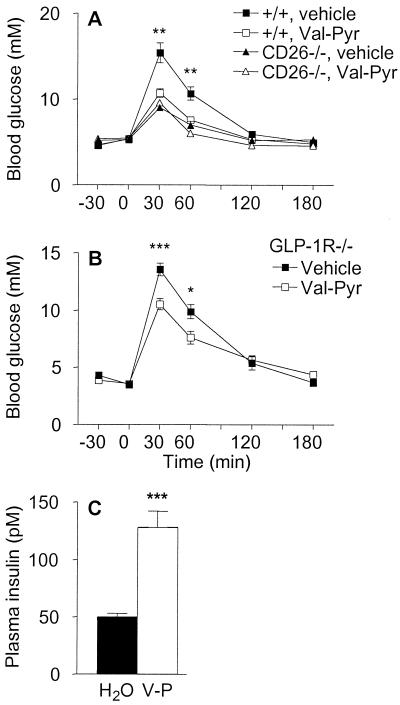
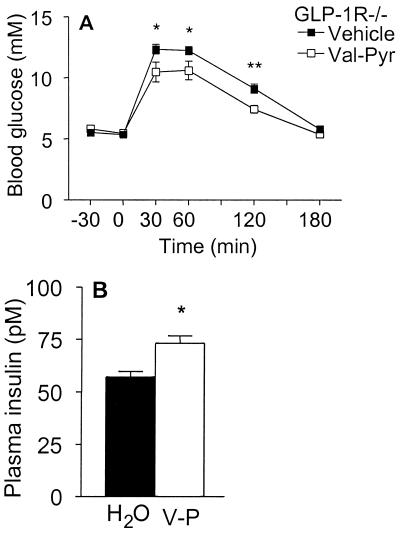
These results demonstrate that genetic inactivation of CD26 leads to reduced N-terminal degradation of GLP-1, increased levels of insulin, and improved glucose tolerance. To determine whether the improved glucose tolerance observed after inactivation of CD26 is mediated exclusively via a GLP-1-dependent signaling pathway, we evaluated the consequences of inhibiting the enzymatic activity of CD26 in GLP-1 receptor (GLP-1R)−/− mice. GLP-1R−/− mice completely lack GLP-1 signaling and are glucose intolerant because of lack of GLP-1 potentiation of insulin secretion (27). Inhibition of CD26 activity by using valine-pyrrolidide significantly improved glucose tolerance in wild-type mice (Fig. 4A). In contrast, valine-pyrrolidide had no effect on blood glucose levels in CD26−/− mice, demonstrating that this compound modulates glucose clearance specifically by suppressing the enzyme activity of CD26. Surprisingly, valine-pyrrolidide significantly reduced glycemic excursion even in GLP-1R−/− mice (Fig. 4B). This GLP-1-independent improvement in glucose tolerance was associated with enhanced insulin secretion (Fig. 4C).
Figure 4.
Specific pharmacological inhibition of CD26 enzymatic activity improves glucose tolerance, even in mice lacking GLP-1 signaling. (A) Female wild-type or CD26−/− mice were given vehicle (water) or 20.6 mg/kg valine-pyrrolidide (Val-Pyr) orally 30 min before oral glucose challenge. n = 8. Asterisks indicate level of significance of difference between wild-type mice given vehicle and valine-pyrrolidide. Similar data were obtained in another experiment with female mice and two experiments with male mice. Wild-type mice given valine-pyrrolidide exhibited significantly increased levels of insulin and intact GLP-1 at 15 min after the glucose challenge (data not shown). (B) Oral glucose tolerance test in male GLP-1R−/− mice given vehicle or 20.6 mg/kg valine-pyrrolidide 30 min before glucose challenge. n = 7–9. (C) Plasma insulin levels in GLP-1R−/− mice 15 min after oral glucose challenge. Mice were given vehicle (H2O) or valine-pyrrolidide (V-P) as indicated. n = 10–11. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
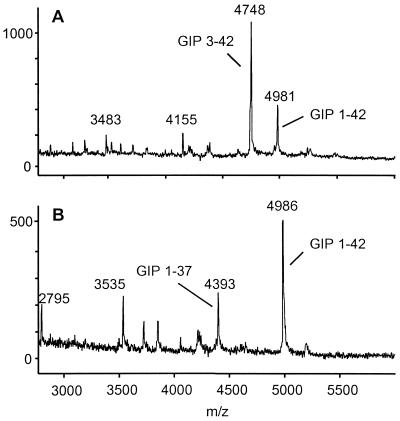
A possible explanation for the glucose-lowering effect of valine-pyrrolidide in GLP-1R−/− mice was that CD26 inhibition also resulted in decreased degradation of GIP. Mice lacking GIP receptors (GIPR−/−) are mildly glucose intolerant, indicating that GIP plays a role in normal blood glucose control (29). GIP can be inactivated by DPP IV activity in vitro (8). To assess the contribution of CD26 to GIP metabolism, intact GIP was incubated in plasma from wild-type or CD26−/− mice. Mass spectrometry analysis indicated that intact GIP was completely resistant to N-terminal cleavage when incubated at 37°C for 4 h in plasma from CD26−/− mice, whereas a large proportion of intact GIP was degraded by wild-type mouse plasma (Fig. 5). Thus, increased levels of intact GIP may contribute to the enhanced insulin secretion and glucose clearance in CD26-deficient mice and in wild-type or GLP-1R−/− mice treated with valine-pyrrolidide.
Figure 5.
CD26 is the major enzyme responsible for the N-terminal processing of GIP in serum. Mass spectrometry analysis of GIP degradation products after a 4-h incubation at 37°C in plasma from wild-type (A) or CD26−/− (B) mice. Peaks representing intact GIP-(1–42), N-terminally truncated GIP-(3–42), and C-terminally truncated GIP-(1–37) are indicated.
GLP-1 and GIP have generally been thought to act mainly as incretin hormones, potentiating glucose-stimulated insulin release after food intake. However, recent data revealed that GLP-1 also enhances glucose clearance independently of enteral glucose loading (11). To test whether the CD26-regulated pathway of glucose control involves additional substrates, besides GLP-1, that may contribute to glucose clearance in a nonincretin fashion, GLP-1R−/− mice were given water or valine-pyrrolidide and challenged with glucose intraperitoneally. Valine-pyrrolidide significantly increased the clearance rate of intraperitoneally administered glucose in GLP-1R−/− mice (Fig. 6A). Furthermore, circulating insulin levels were significantly increased in the GLP-1R−/− mice given valine-pyrrolidide compared with vehicle (Fig. 6B), suggesting that an insulin-dependent mechanism contributes to the accelerated clearance of i.p. glucose in animals given valine-pyrrolidide. These findings suggest that the CD26-mediated regulation of blood glucose is not dependent on a delay of gastric emptying, consistent with the lack of effect of another DPP IV inhibitor on paracetamol uptake (16).
Figure 6.
Inhibition of CD26 enzymatic activity accelerates clearance of glucose administered intraperitoneally in GLP-1R−/− mice. (A) Intraperitoneal glucose tolerance test in male GLP-1R−/− mice given vehicle (water) or valine-pyrrolidide at time −30 min and glucose at time 0. (B) Plasma insulin levels 15 min after glucose challenge in the animals shown in A, given vehicle (H2O) or valine-pyrrolidide (V-P), as indicated. n = 8–14. *, P < 0.05; **, P < 0.01.
Discussion
The results presented here demonstrate that the protease activity of CD26 plays an essential role in the physiological control of blood glucose. The precise mechanism whereby this occurs remains to be established, but most likely involves regulation of the activity of the insulinotropic hormones GLP-1 and GIP, and possibly other substrates that remain unidentified as yet. The function of such hormones includes the stimulation of insulin secretion upon food intake, facilitating glucose disposal from the blood. Our data indicate that CD26 plays a major role in controlling the rapid inactivation of these hormones, thereby helping to terminate the action of insulinotropic peptides once stimulation of insulin secretion from the β cell has been initiated.
Using CD26−/− mice, we showed that valine-pyrrolidide exerts all of its glucose-lowering effects by inhibiting CD26. Therefore, valine-pyrrolidide is a useful tool for dissecting how CD26 regulates glucose levels. As a first step in this direction, we show that although levels of intact GLP-1 are significantly increased in normal animals treated with valine-pyrrolidide, GLP-1 is actually not essential for CD26-mediated glucose regulation, since similar effects were seen in GLP-1R−/− mice treated with valine-pyrrolidide. We strongly suspect that GIP is involved in the non-GLP-1-mediated effect of valine-pyrrolidide. First, our data indicate that CD26 is responsible for the degradation of GIP in serum. Second, GLP-1R−/− mice exhibit increased GIP secretion and enhanced sensitivity to GIP actions, probably as an adaptation to the lack of GLP-1 signaling (30). Therefore, GLP-1R−/− mice may be abnormally hypersensitive to the increased levels of intact GIP that result from DPP IV inhibition. Enhanced GIP sensitivity may also explain why valine-pyrrolidide led to accelerated clearance of i.p. glucose loads in the GLP-1R−/− mice. Although analysis of results from GIPR−/− mice suggests that GIP does not normally play an essential role in clearance of i.p. glucose (29), it is possible that the increased GIP secretion and sensitivity of the GLP-1R−/− mice (30) enhances the effects of extending GIP half-life by CD26 inhibition, thereby revealing GIP actions that otherwise might not be obvious, possibly even including nonincretin-like actions. Alternatively, our data do not exclude the possibility that additional CD26 substrates may play a role in accelerating glucose clearance, possibly by both insulin-dependent and -independent mechanisms. These questions await the development and analysis of appropriate animal models.
In contrast to the phenotype of CD26−/− mice described here, rats with a spontaneous mutation in the CD26 gene exhibited normal glucose homeostasis (31). The reason for this difference in phenotype is unclear, but may be explained by the presence of significant residual circulating DPP IV-like enzyme activity in the rats (32).
Type II diabetes is a disease of epidemic proportions characterized by elevated blood glucose levels, in part because of relative insufficiency of insulin. Therapeutic agents that would accelerate glucose clearance by stimulating endogenous insulin secretion in a glucose-dependent manner, thereby avoiding hypoglycemic episodes, would represent an important advance in the treatment of this disease. Pharmacological inhibition of DPP IV enzyme activity improved glucose clearance in type II diabetic animals (13–16) and has been suggested as a promising strategy for the treatment of type II diabetes (33). However, for use in humans this strategy is complicated by the multiplicity of DPP IV-like enzymes that appear to have overlapping substrate and inhibitor specificities (17–21). Furthermore, the cleavage by these enzymes of a wide range of hormones and chemokines raise important concerns about the risk of side-effects. Our studies revealed that total lack of CD26 in mice is surprisingly tolerable, affecting primarily glucose metabolism. Mice with a targeted mutation in another DPP IV-like enzyme, fibroblast-activation protein, also were reported to develop without gross abnormalities (34). The data presented here indicate that specific pharmacological inhibition of CD26 is sufficient to improve glucose tolerance, establishing CD26 as a relevant, specific therapeutic target for the treatment of type II diabetes.
Acknowledgments
We thank E. Bentsen, M. Kristensen, A-G. Juul, G. Warcollier, and A. Loucif for technical assistance; L. B. Christiansen for valine-pyrrolidide; and E. Boel, K. Frederiksen, P. Golstein, B. Malissen, D. Mathis, and R. Unger for comments on the manuscript. This work was supported by institutional funding from the Institut National de la Santé et de la Recherche Médicale and the Centre National de la Recherche Scientifique and by grants from the Association pour la Recherche sur le Cancer, the Canadian Diabetes Association, and the Juvenile Diabetes Foundation International.
Abbreviations
- DPP
dipeptidyl-peptidase
- GLP-1
glucagon-like peptide 1
- GLP-1R
GLP-1 receptor
- GIP
glucose-dependent insulinotropic polypeptide
- pNA
p-nitroanilide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120069197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120069197
References
- 1.De Meester I, Korom S, Van Damme J, Scharpe S. Immunol Today. 1999;20:367–375. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto C, Schlossman S F. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 3.Wesley U V, Albino A P, Tiwari S, Houghton A N. J Exp Med. 1999;190:311–322. doi: 10.1084/jem.190.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kameoka J, Tanaka T, Nojima Y, Schlossman S F, Morimoto C. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 5.Marguet D, Bernard A M, Vivier I, Darmoul D, Naquet P, Pierres M. J Biol Chem. 1992;267:2200–2208. [PubMed] [Google Scholar]
- 6.Rawlings N D, Barrett A J. Methods Enzymol. 1994;244:19–61. doi: 10.1016/0076-6879(94)44004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott C A, McCaughan G W, Gorrell M D. FEBS Lett. 1999;458:278–284. doi: 10.1016/s0014-5793(99)01166-7. [DOI] [PubMed] [Google Scholar]
- 8.Mentlein R. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 9.Mentlein R, Gallwitz B, Schmidt W E. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 10.Kieffer T J, McIntosh C H, Pederson R A. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 11.Drucker D J. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 12.Deacon C F, Nauck M A, Toft-Nielsen M, Pridal L, Willms B, Holst J J. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 13.Deacon C F, Hughes T E, Holst J J. Diabetes. 1998;47:764–769. doi: 10.2337/diabetes.47.5.764. [DOI] [PubMed] [Google Scholar]
- 14.Pederson R A, White H A, Schlenzig D, Pauly R P, McIntosh C H, Demuth H U. Diabetes. 1998;47:1253–1258. doi: 10.2337/diab.47.8.1253. [DOI] [PubMed] [Google Scholar]
- 15.Pauly R P, Demuth H U, Rosche F, Schmidt J, White H A, Lynn F, McIntosh C H S, Pederson R A. Metab-Clin Exp. 1999;48:385–389. doi: 10.1016/s0026-0495(99)90090-2. [DOI] [PubMed] [Google Scholar]
- 16.Balkan B, Kwasnik L, Miserendino R, Holst J J, Li X. Diabetologia. 1999;42:1324–1331. doi: 10.1007/s001250051445. [DOI] [PubMed] [Google Scholar]
- 17.Jacotot E, Callebaut C, Blanco J, Krust B, Neubert K, Barth A, Hovanessian A G. Eur J Biochem. 1996;239:248–258. doi: 10.1111/j.1432-1033.1996.0248u.x. [DOI] [PubMed] [Google Scholar]
- 18.Shneider B L, Thevananther S, Moyer M S, Walters H C, Rinaldo P, Devarajan P, Sun A Q, Dawson P A, Ananthanarayanan M. J Biol Chem. 1997;272:31006–31015. doi: 10.1074/jbc.272.49.31006. [DOI] [PubMed] [Google Scholar]
- 19.Duke-Cohan J S, Gu J J, McLaughlin D F, Xu Y H, Freeman G J, Schlossman S F. Proc Natl Acad Sci USA. 1998;95:11336–11341. doi: 10.1073/pnas.95.19.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedermeyer J, Enenkel B, Park J E, Lenter M, Rettig W J, Damm K, Schnapp A. Eur J Biochem. 1998;254:650–654. doi: 10.1046/j.1432-1327.1998.2540650.x. [DOI] [PubMed] [Google Scholar]
- 21.Chiravuri M, Schmitz T, Yardley K, Underwood R, Dayal Y, Huber B T. J Immunol. 1999;163:3092–3099. [PubMed] [Google Scholar]
- 22.Bernard A M, Mattei M G, Pierres M, Marguet D. Biochemistry. 1994;33:15204–15214. doi: 10.1021/bi00254a032. [DOI] [PubMed] [Google Scholar]
- 23.Ledermann B, Burki K. Exp Cell Res. 1991;197:254–258. doi: 10.1016/0014-4827(91)90430-3. [DOI] [PubMed] [Google Scholar]
- 24.David F, Bernard A M, Pierres M, Marguet D. J Biol Chem. 1993;268:17247–17252. [PubMed] [Google Scholar]
- 25.Schon E, Born I, Demuth H U, Faust J, Neubert K, Steinmetzer T, Barth A, Ansorge S. Biol Chem Hoppe-Seyler. 1991;372:305–311. doi: 10.1515/bchm3.1991.372.1.305. [DOI] [PubMed] [Google Scholar]
- 26.Johansen T, Deckert M, Mandrup-Poulsen T, Malmlof K. J Endocrinol. 1999;162:87–93. doi: 10.1677/joe.0.1620087. [DOI] [PubMed] [Google Scholar]
- 27.Scrocchi L A, Brown T J, MacLusky N, Brubaker P L, Auerbach A B, Joyner A L, Drucker D J. Nat Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 28.Scrocchi L A, Marshall B A, Cook S M, Brubaker P L, Drucker D J. Diabetes. 1998;47:632–639. doi: 10.2337/diabetes.47.4.632. [DOI] [PubMed] [Google Scholar]
- 29.Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, et al. Proc Natl Acad Sci USA. 1999;96:14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pederson R A, Satkunarajah M, McIntosh C H S, Scrocchi L A, Flamez D, Schuit F, Drucker D J, Wheeler M B. Diabetes. 1998;47:1046–1052. doi: 10.2337/diabetes.47.7.1046. [DOI] [PubMed] [Google Scholar]
- 31.Pederson R A, Kieffer T J, Pauly R, Kofod H, Kwong J, McIntosh C H S. Metabolism. 1996;45:1335–1341. doi: 10.1016/s0026-0495(96)90112-2. [DOI] [PubMed] [Google Scholar]
- 32.Tavares W, Drucker D J, Brubaker P L. Am J Physiol Endocrinol Metab. 2000;278:E134–E139. doi: 10.1152/ajpendo.2000.278.1.E134. [DOI] [PubMed] [Google Scholar]
- 33.Holst J J, Deacon C F. Diabetes. 1998;47:1663–1670. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 34.Niedermeyer J, Kriz M, Hilberg F, Garin-Chesa P, Bamberger U, Lenter M C, Park J, Viertel B, Puschner H, Mauz M, et al. Mol Cell Biol. 2000;20:1089–1094. doi: 10.1128/mcb.20.3.1089-1094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]