Abstract
In Chinese medicine, ginseng (Panax ginseng C.A. Meyer) has long been used as a general tonic or an adaptogen to promote longevity and enhance bodily functions. It has also been claimed to be effective in combating stress, fatigue, oxidants, cancer and diabetes mellitus. Most of the pharmacological actions of ginseng are attributed to one type of its constituents, namely the ginsenosides. In this review, we focus on the recent advances in the study of ginsenosides on angiogenesis which is related to many pathological conditions including tumor progression and cardiovascular dysfunctions.
Angiogenesis in the human body is regulated by two sets of counteracting factors, angiogenic stimulators and inhibitors. The 'Yin and Yang' action of ginseng on angiomodulation was paralleled by the experimental data showing angiogenesis was indeed related to the compositional ratio between ginsenosides Rg1 and Rb1. Rg1 was later found to stimulate angiogenesis through augmenting the production of nitric oxide (NO) and vascular endothelial growth factor (VEGF). Mechanistic studies revealed that such responses were mediated through the PI3K→Akt pathway. By means of DNA microarray, a group of genes related to cell adhesion, migration and cytoskeleton were found to be up-regulated in endothelial cells. These gene products may interact in a hierarchical cascade pattern to modulate cell architectural dynamics which is concomitant to the observed phenomena in angiogenesis. By contrast, the anti-tumor and anti-angiogenic effects of ginsenosides (e.g. Rg3 and Rh2) have been demonstrated in various models of tumor and endothelial cells, indicating that ginsenosides with opposing activities are present in ginseng. Ginsenosides and Panax ginseng extracts have been shown to exert protective effects on vascular dysfunctions, such as hypertension, atherosclerotic disorders and ischemic injury. Recent work has demonstrates the target molecules of ginsenosides to be a group of nuclear steroid hormone receptors. These lines of evidence support that the interaction between ginsenosides and various nuclear steroid hormone receptors may explain the diverse pharmacological activities of ginseng. These findings may also lead to development of more efficacious ginseng-derived therapeutics for angiogenesis-related diseases.
Panax ginseng
Background
Ginseng, Panax ginseng C.A. Meyer, a precious Chinese traditional medicinal herb, has been known clinically used in China for thousands of years. The genus name 'Panax' was derived from Greek. 'Pan' means 'all' and 'axos' means 'cure'. Literally 'Panax' can be translated as 'cure-all' or panacea. The herbal root is named ginseng because it is shaped like a man. Actually the term 'ginseng' represents two Chinese ideograms: 'gin' (pronounced ren) refers to 'man' and 'seng' (pronounced shen) refers to 'essence' It is believed to embody man's three mythical essences – body, mind and spirit. Thus it is also referred to as the lord or king of herbs [1]. Its medicinal efficacy was first documented in Shengnong Bencao Jing and it was later summarized by Li Shizhen in Bencao Gangmu and Zhongyao Zhi (Chinese Materia Medica) by People's Health Publishing House, Beijing, published in 1596 and 1959 respectively [1,2]. In the 18th century, the effectiveness of ginseng was recognized in the West, and subsequently, a large number of investigations were conducted on its botany, chemistry, pharmacology and therapeutic applications [3-7]. Ginseng has been used as a general tonic or adaptogen for promoting longevity especially in the Far East, especially China, Korea and Japan [8]. Ginseng is now one of the most popular herbal medicines used nutraceutically with an annual sale of over USD 200 million.
Ginseng is a deciduous perennial plant that belongs to the Araliaceae family. Currently, twelve species have been identified in the genus Panax (Table 1) [9]. Among them, Panax ginseng C. A. Meyer (Araliaceae), cultivated in China, Korea, Japan, Russia, and the US, P. quinquefolium L (American ginseng), grown in southern Canada and the US and P. notoginseng, cultivated in Yunnan and Guangxi provinces in China, represent the three most extensively investigated species. The pharmacological and therapeutic effects of ginseng have been demonstrated to affect the central nervous system (CNS), cardiovascular system, endocrine secretion, immune function, metabolism, biomodulating action, anti-stress, and anti-aging [5]. Recently, there have been controversies concerning the usefulness of ginseng in cancer therapy. Most studies claimed that the pharmacological effects of ginseng are attributed to its bioactive constituents such as ginsenosides, saponins, phytosterols, peptides, polysaccharides, fatty acids, polyacetylenes, vitamins and minerals [10]. In this review, we focus on the recent advances in the studies of ginsenosides on the modulation of angiogenesis (i.e. formation of blood vessels) which is a common denominator of many diseases, such as tumor and cardiovascular disorders (e.g. atherosclerosis).
Table 1.
Species of Ginseng belonging to the genus Panax.
| Scientific name | Common name (English name) | Geographical distribution |
| Panax ginseng C.A. Meyer | Asian ginseng Orient Ginseng Chinese Ginseng Korean Ginseng Red Ginseng White Ginseng Tartary Ginseng Tartary Root Kirin Ginseng |
Korea Japan China Russia Germany |
| Panax japonicus C.A. Meyer | Japanese Ginseng ChiKu |
East, Middle-south, South and South Yunnan Japan |
| Panax bipinnatifidus Seem | Feather-leaf bamboo ginseng Feather-leaved bamboo ginseng |
Hubei, China Nepal Eastern Himalayas |
| Panax notoginseng (Burkill) F.H. Chen | Sanchi Ginseng Tienchi Ginseng Sanchu Ginseng Notoginseng San-qi ginseng Tien-qi ginseng Yunnan ginseng |
Yunnan, Guangxi Guangdong, China |
| Panax omeiensis J. Wen | Omei ginseng | Sichuan, China Nepal Eastern Himalayas |
| Panax pseudoginseng Wallich | Himalayan Ginseng | South Tibet Nepal Eastern Himalayas |
| Panax quinquefolius L. | American Ginseng Occidental Ginseng Canadian Ginseng |
Northeast, North, East China Southern Canada America (from Maine to Minnesota, south to Florida and west to Oklahoma) |
| Panax stipuleanatus H.T. Tsai and K.M. Feng | Pingpien ginseng Baisanqi Tusangi Yesangi Zhujie qi |
Southern Yunnan, China |
| Panax trifolius L. | Dwarf ginseng Groundnut |
Ohio Pennsylvania Nova Scotia to Wisconsin and further south |
| Panax wangianus S.C. Sun | Narrow-leaved pseudoginseng | Sichuan, China |
| Panax vietnamensis Ha et Grushv. | Bamboo ginseng Vietnamese Ginseng |
|
| Panax zingiberensis C.Y. Wu and K.M. Feng | Ginger ginseng Ginger-like Pseudo-ginseng |
Yunnan, China |
[References: 5, 8, 9, MULTILINGUAL MULTISCRIPT PLANT NAME DATABASE-http://www.plantnames.unimelb.edu.au/Sorting/Panax.html#bipinnatifidus and Ginseng: A Concise Handbook. Edited by James A, Duke. Reference Publications, Inc. 1989. Michigan, USA]
Ginsenosides of ginseng
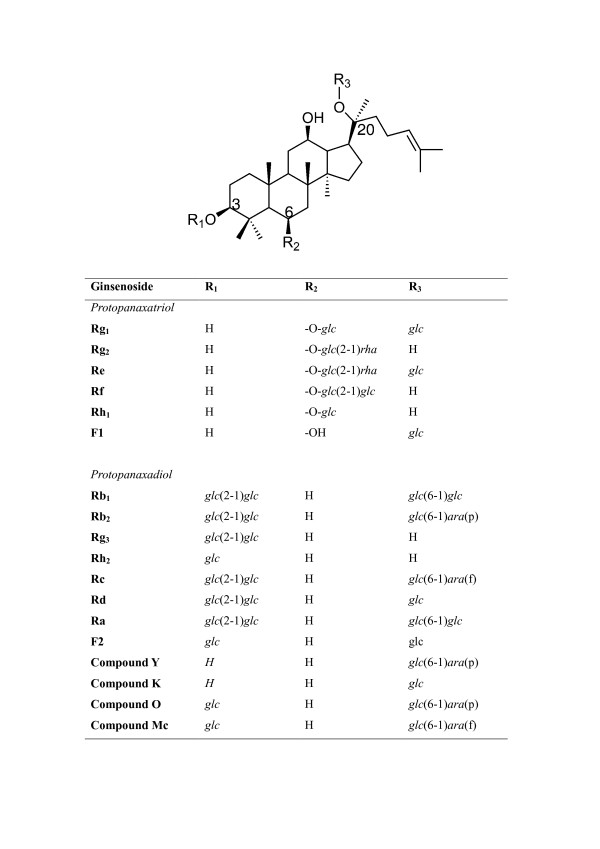
The most prominent constituent of ginseng is a saponin glycoside known as ginsenosides (Rx) (Figure 1). Recent research indicates that most of the pharmacological effects of ginseng are attributed to ginsenosides [11]. In general, the contents of ginsenosides vary widely ranging from 2 to 20% depending on the species, age and part of ginseng, and even vary with the preservation or extraction method [11-13]. More than 30 ginsenosides have been isolated, and characterized from various Panax species [14,15]. In terms of their chemical structural characteristics, ginsenosides can be classified into three major categories, namely protopanaxadiols (PPD) (e.g. Rb1, Rb2, Rc, Rd, Rg3, Rh2), protopanaxatriols (PPT) (e.g. Re, Rf, Rg1, Rg2, Rh1) and the oleanolic acid derivatives. Ginsenosides have a steroid-like skeleton consisting of four trans-rings, with modifications from each other depending the type (e.g. glucose, maltose and fructose), number of sugar moieties and the sites of attachment of the hydroxyl group (e.g. C-3, C-6, or C-20) (Figure 1). Ginsenosides are amphipathic in nature. The hydroxyl (-OH) group of ginsenosides allows both interactions between the polar head of the membrane phospholipids and the β-OH group of cholesterol, while the hydrophobic steroid backbone can interact with the hydrophobic side chains of fatty acids and cholesterol. Indeed, these physiochemical interactions are greatly determined by the numbers and sites of polar hydroxyl groups on each ginsenoside. Moreover, ginsenosides have been shown to interact with numerous membrane proteins such as ion channels, transporters and receptors, which leads to a broad range of physiological activities [16].
Figure 1.
The chemical structure of ginsenosides [6]. glc = glucosyl (C6H11O6-); rha = rhamnosyl (C6H11O5-); ara = arabinosyl (C5H9O5-); p = pyran; f = furan.
Angiogenesis
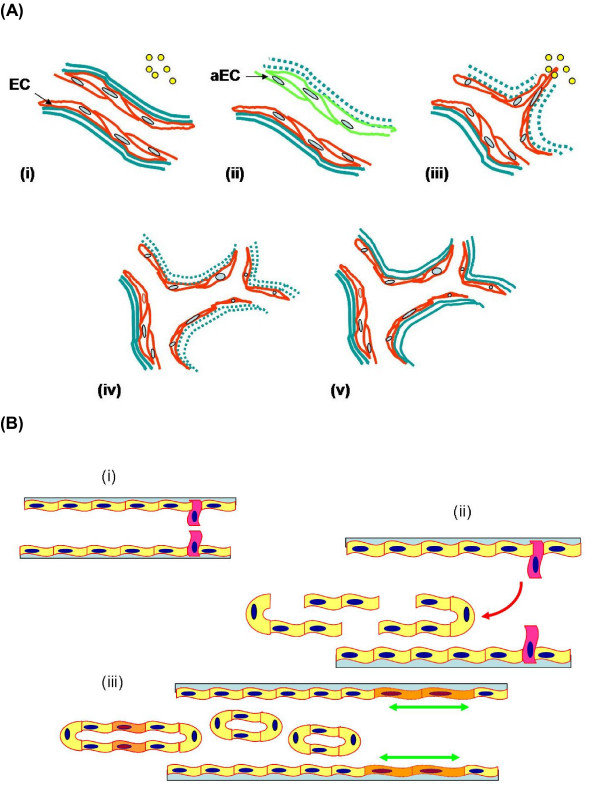
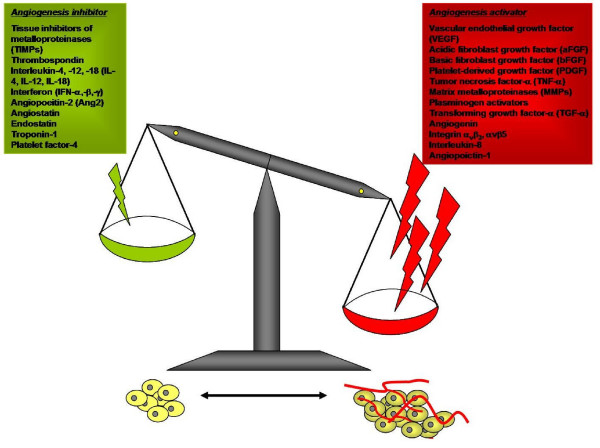
The term 'angiogenesis', first used by Hertig in 1935, refers to the formation of new blood vessels in the placenta [17]. Angiogenesis is a complex multi-step process which comprises activation, chemotactic invasion and migration, morphological alteration, proliferation, and capillary tube formation of endothelial cells (ECs) from pre-existing blood vessels (Figure 2). In this process, ECs have been shown to express all the information necessary to construct a vascular network. Under normal physiological conditions, most vasculature is quiescent, with only 0.01% of the ECs undergoing active cell division; thus angiogenesis is a relatively rare event that specifically occurs for a short and defined time period [18,19]. In addition, it is also tightly controlled by a relative balance of two groups of counteracting factors, namely angiogenic stimulators and inhibitors [20] (Figure 3).
Figure 2.
The process of angiogenesis. A) Sprouting angiogenesis: formation of blood vessels is a multi-step process, which includes (i) reception of angiogenic signals (yellow spot) from the surrounding by endothelial cells (EC); (ii) retraction of pericytes from the abluminal surface of capillary and secretion of protease from activated endothelial cells (aEC) and proteolytic degradation of extracellular membrane (green dash-line); (iii) chemotactic migration of EC under the induction of angiogenic stimulators; (iv) proliferation of EC and formation of lumen/canalisation by fusion of formed vessels with formation of tight junctions; (v) recruitment of pericytes and deposition of new basement membrane and initiation of blood flow. B) Non-sprouting angiogenesis – intussusceptive microvascular growth: it is initiated by (i) protrusion of opposing capillary walls towards the lumen; (ii) perforation of the EC bilayer and formation of many transcapillaries with interstitial core (red arrow); (iii) formation of the vascular tree from intussusceptive pillar formation and pillar fusion and elongation of capillaries (green arrows).
Figure 3.
The balance hypothesis of the 'angiogenic switch'. Angiogenesis is tightly controlled by the balance of two sets of counteracting factors – angiogenic activators and inhibitors. The stability of 'angiogenic switch' determines the time of initiation of the subsequent angiogenic process. When there are more angiogenic stimulators than angiogenic inhibitors, as in the case of solid tumors, normal wound healing or female endometrial repair, the 'angiogenic switch' will be turned on and angiogenesis will proceed. Furthermore, during the process, each step is strictly mediated by the balance of different types of angiogenic stimulators or inhibitors. In some pathological cases, the 'angiogenic switch' remains in the 'ON' mode which leads to 'non-stop' formation of new blood vessels and ultimately many physiological disorders and diseases.
Sprouting and intussuceptive (non-sprouting) modes of angiogenesis
Sprouting angiogenesis consists of several consecutive steps with extensive interactions between soluble factors, extracellular matrix (ECM) and cells. As shown in Figure 2, during the early stage of angiogenesis, angiogenic factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) emanate from conditioned cells (e.g. stromal cells, endothelial cells, blood) or surrounding tissues (e.g. ECM) to stimulate ECs [19]. These activated ECs secrete various types of enzymes including matrix metalloproteinases (MMPs), plasminogen activators (PA), gelatinase, collagenases and urokinases which bring about the degradation of basement membrane and ECM surrounding the parental blood vessels. Subsequently, the ECs migrate towards the angiogenic factors with the help of surface adhesion molecules or integrin receptors such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and integrins αvβ3 [21,22]. Upon the induction of various angiogenic factors, ECs proliferate and align in a bipolar mode to form capillary sprouts. Finally, these ECs form a lumen and a capillary loop through which the blood can flow.
Intussusceptive microvascular growth, a novel mechanism of blood vessel formation and remodeling, occurs by internal division of the pre-existing capillary plexus without sprouting. It is initiated by protrusion of opposing capillary walls into the lumen where a contact is created between the ECs. Then the endothelial bilayer is perforated and numerous transcapillary tissue pillars with an interstitial core are formed. An extensive vascular tree is formed from the subsequent intussusceptive pillar formation and pillar fusions [23-25].
Molecular regulation of angiogenesis
Among the angiogenic regulators, VEGF and nitric oxide (NO) are the critical angiogenic stimulators. We have demonstrated that they are closely related to ginsenoside-mediated angiomodulation. Both VEGF and NO play a critical role in the regulation of physiological angiogenesis including embryogenesis, reproduction and wound healing. They have also been implicated in pathological angiogenesis associated with tumors or many cardiovascular disorders (such as atherosclerosis or ischemic injury). VEGF, which belongs to the VEGF family, is expressed in different tissues including the brain, liver, spleen and many cell types [26]. In vitro, VEGF not only acts as a survival factor and mitogen for ECs, but also induces the expression of many angiogenesis-related factors, including ICAM-1, VCAM-1, uPA, PAI-1, uPAR and MMPs. All these gene products are related to the increase of ECM degradation, migration and tube formation of ECs, whereas their effects on intussusception remain to be investigated [27-30]. The critical roles played by VEGF in embryonic vasculogenesis and angiogenesis have been demonstrated [31,32]. Inactivation of a single VEGF allele in mice resulted in embryonic lethality between days 11 and 12. The growth of VEGF+/- embryos was retarded and there were many developmental anomalies.
Endothelium-derived NO has been implicated in mediating a multiplicity of processes involved in angiogenesis. In fact, there are many angiogenic factors, such as VEGF, transforming growth factor β (TGF-β) and fibroblast growth factor (FGF), which can up-regulate the expression of endothelial NO synthase (eNOS), thereby inducing the release of NO [33-36]. VEGF- or bFGF-activated human umbilical vein endothelial cells (HUVEC) have been found to secrete NO on Matrigel substratum and subsequently form a tube-like structure, whereas such tube formating action is abolished by eNOS antagonist, Nω-nitro-L-arginine methylester (L-NAME) [37-39]. On the one hand, NO increases EC proliferation by acting as a survival factor and enhances migration by augmenting the expression of αvβ3 and matrix-ECs interaction through the up-regulation of uPA [40-44]. Moreover, its vasodilating activity is the most prominent effect shown in the modulation of cardiovascular physiology [45]. On the other hand, disruption of the eNOS pathway can result in an impairment of normal angiogenesis, enhancement of tumor pathogenesis, and cardiovascular disorders [46-49].
VEGF and NO are working closely with each other in the modulation of angiogenesis, whereas NO is both an upstream and a downstream mediator or an effector of VEGF-dependent angiogenesis. Upon binding to its receptors, VEGF initiates complicated signaling cascades resulting in NO production and subsequent activation of ECs and smooth muscle cells in vessels, keratinocytes and macrophages in wounds, and tumor cells. Furthermore, through the activation of the PI3K/Akt pathway, NO can increase VEGF synthesis in a positive feedback manner [46,50-54].
Angiogenesis in health and diseases
Normal physiology
In health, angiogenesis is under stringent control by an 'angiogenic switch' (Figure 3), and rarely occurs in adults except for embryogenesis, placentation, endometrial repair and wound healing [20,55]. In the latter case, formation of neovessels is necessary to sustain newly formed granulation tissues, in such a way that the ECs divide with a turnover rate of about 5 days, giving rise to a new microvascular network. During the menstrual cycle, however, angiogenesis occurs in corpora lutea and endometrium with rapid growth and regression [56-59]. Thus angiogenesis is normally in the quiescent state but is capable of both rapid activation and shutting down.
Pathophysiology
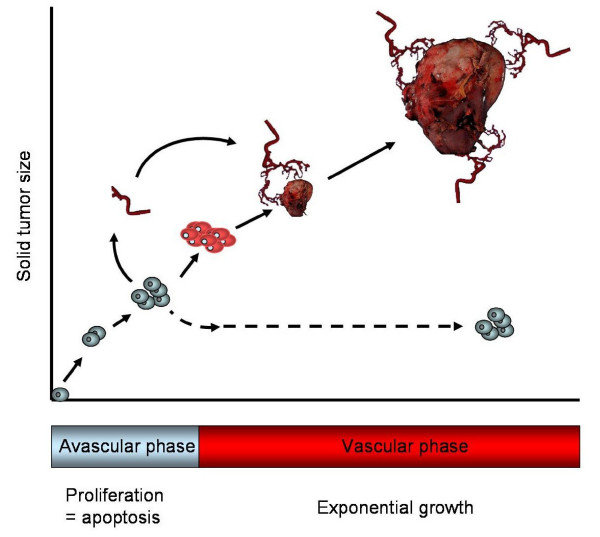
Excessive angiogenesis has been defined as a prominent pathological feature of many diseases such as tumor, rheumatoid arthritis, atherosclerosis, psoriasis and diabetic retinopathy [60-63] (Table 2). During the early stage of tumorigenesis, tumors are usually not angiogenic (Figure 4). Since oxygen can only diffuse to around 150–200 microns from capillaries, solid tumors can only grow to 1–2 mm3 autonomously at which stage they may exist for months or years without neovascularization (i.e. avascular phase). However, once tumor cells switch to the angiogenic phenotype (i.e. vascular phase), an extensive vascular network is constructed through sprouting or non-sprouting angiogenic mechanisms. The large amounts of angiogenic factors, mainly VEGF secreted by tumor cells or activated ECs, form a positive feedback on angiogenesis, leading to tumor growth and subsequent metastasis. [64,65]. A similar case has also been found in atherosclerosis, in which excessive angiogenesis enhances plaque growth in such a way to sustain perfusion, increase leukocyte exchange, deposition of proatherogenic plasma molecules and finally promote intraplague hemorrhage [66].
Table 2.
Angiogenic diseases
| Diseases/disorders | Organ/organ system |
| Atherosclerosis hypertension Vascular dementia Haemangioma Haemangioendothelioma Ischemic heart, limb |
Cardiovascular system |
| Diabetic retinopathy Ischemic retinopathy Neovascular glaucoma Cancer |
Ocular |
| Warts Kaposi's sarcoma Neoplasms Psoriasis Ulcer |
Skin |
| Dysfunctional uterine bleeding Endometriosis Neoplasms Placental insufficiency Cancer |
Reproductive system |
| Rheumatoid arthritis | Bones, joints |
| Ulcer Cancer Crohn's disease |
Digestive system |
Figure 4.
A fundamental step in tumorigenesis – angiogenesis. Angiogenesis is a critical step in the pathogenesis of solid tumors. Tumors remain in a dormant state (avascular phase) for a long time (up to several years), in which tumors keep their size within 1 – 2 mm3. When tumor progression starts, tumor cells secrete a large amount of angiogenic factors, mainly VEGF, to the surrounding tissues and blood capillaries. Once tumor angiogenesis is initiated, tumors enter a 'vascular phase' and become more aggressive. These newly formed blood vessels provide tumor cells with oxygen and nutrients for them to grow and for the initiation of metastasis.
An insufficient amount of angiogenic stimulators and/or an excess of angiogenic inhibitors can tip the balance resulting in inadequate or limited angiogenesis. Inadequate angiogenesis can also cause serious pathological outcomes such as stroke, Alzheimer disease, chronic wound, ulceration, ischemic coronary artery, critical limb ischemia, hypertension and hair loss [62,63]. In diseases such as ischemic coronary artery or limb ischemia, the functional blood flow is partially lost in certain organs or limbs. Poor blood circulation greatly prolongs the recovery period or even results in morbidity or death. A similar phenomenon has been found in the aberrant wound repair in diabetic and gastric ulcer [67,68].
Angiotherapy: angiogenesis as a therapeutic target
Since the discovery in the early 1970s that tumor progression is angiogenesis-dependent, the concept of 'angiotherapy' has been hypothesized as a therapeutic strategy [69]. Ideally, this strategy could be used in the case of insufficient angiogenesis (e.g. heart ischemia) through the stimulation of neovessel formation by introducing angiogenic stimulators. An over-burst of uncontrolled angiogenesis (e.g. tumors) could be treated using angiogenic inhibitors to shut down the formation of neovessels [70-72] (Table 2). After thirty years, this idea became a reality when the first-generation anti-angiogenic drugs called Avastin (bevacizumab) and Macugen (pegaptanib sodium) were approved by the Food and Drug Administration in the US in 2004 and 2005 respectively for clinical application in cancer patients [73].
In fact, a large number of anti-angiogenic agents (e.g. thalidomide, TNP-470, endostatin, and angiostatin) are being tested or undergoing preclinical or clinical trials either alone or in combination with conventional therapies [74-76]. Most of these drugs target the ECs rather than tumor cells, thereby affecting different stages of angiogenesis. Therefore, they are less likely to cause bone marrow suppression, gastrointestinal symptoms or hair loss than conventional tumor therapies.
Pharmacological actions of ginsenosides
Anti-tumor effects of ginsenosides
In Asia, ginseng has been used as a medicinal herb for a long time to treat various ailments such as malignant diseases. Experimental studies from the past two decades have shown that both the anti-tumor activities and therapeutic effects of ginseng are mainly attributed to ginsenosides which induce cell death and inhibit metastasis.
Induction of cell death
Several patterns of eukaryotic cell death, namely apoptosis, autophagy, paraptosis, mitotic catastrophe and necrosis, have been recognized [77-79]. The process of cell death can be either caspase-dependent or caspase-independent. It is also known that mitochondria play an important role in the initiation of apoptosis. Different ginsenosides induce apoptosis through various signaling cascades. Rh2 and compound K (the metabolites of Rb1) have been shown to induce apoptosis in prostate cancer cells, ovarian cancer cells, neuroblastoma cells and A549 lung adenocarcinoma cells by activating caspase-3 and -8 [80-83]. Kim et al. further demonstrated that such apoptotic activation can be Bcl-2, Bcl-xL or Bax independent [84-87]. In human neuroblastoma (SK-N-BE) and rat glioma models, Rh2-induced apoptotic cell death has been implicated via protein kinase C (PKC) and reactive oxygen-dependent mechanisms [88-90]. The apoptotsis – inducing activities of other ginsenosides such as Rb2, Rc, Rg3, RS4 and IH901 (20-O-(beta-D-glucopyranosyl)-20(S)-protopanaxadiol, an intestinal bacterial metabolite of ginseng formed from Rb1, Rb2 and Rc, have also been observed in different human tumor cell lines [87-89]. These findings suggest that induction of tumor cell apoptosis by ginsenosides may be one of the mechanisms in the elimination of tumor cells.
Anti-proliferation
Inhibition of cell cycle progression has also been implicated as a chemopreventive mechanism of ginsenosides. Rh2, Rg3, IH901 and PD have been shown to arrest the growth of human tumor cell lines such as A549 lung tumor cells, LNCaP and PC3 prostate carcinoma cells, U937 leukemia cells, SK-HEP-1 hepatoma cells and HeLa cells [80-83,91]. Mechanistically, Rh2 has been shown to arrest the cell cycle at the G1/G0 phase, and to prolong the S-phase in intestinal (Int-407 and Caco-2) cells and SK-HEP-1 hepatoma cells [92-94]. It also induces cell arrest by down-regulation of cyclin/Cdk complex kinase activity, inhibition of E2F release in MCF-7 breast carcinoma cells, or by modulation of three modules of MAP kinases in prostate carcinoma cells [81,95].
Active growth of tumor cells can also be attenuated by induction of terminal differentiation and this approach has been developed as one of the strategies in cancer treatment [96]. Previously, Zeng and Tu showed that Rh2-treated hepatocarcinoma (SMMC-7721) cells exhibited the morphological characteristics of mature cells. Results showed that Rh2 can reduce telomerase activity which affects transcriptional activity in cells and facilitates both cell differentiation and cell arrest [97,98]. Moreover, Rh2-induced cell differentiation has also been found in B16 melanoma cells and F9 teratocarcinoma cells [99,100]. After Rh2 treatment, B16 cells resembled epithelioid cells morphologically and the cells were arrested at the G1 phase. In vitro induction of differentiation of F9 cells was shown to involve the glucocorticoid receptor (GR). In the presence of a glucocorticoid antagonist (RU486), Rh1- or Rh2-treated F9 cells did not differentiate into endoderm-like cells. These hypotheses were further corroborated in the gel mobility shift assay, in which the glucocorticoid responsive element (GRE) was specifically detected, mainly in the nuclear extract of ginsenoside-treated F9 cells. Concomitantly the results were also recorded in the luciferase-reported gene assay.
Anti-invasion and metastasis
Cancer metastasis is a complex process involving angiogenesis and cell-cell interactions. Enzymes such as matrix metalloproteinases are known to play an important role in tumor invasion, metastasis as well as initiation of angiogenesis. Fujimoto et al. recently demonstrated that the invasiveness of the endometrial cancer cell lines HHUA and HEC-1-A was inhibited by treating the cells with ginsenoside Rb2 [101]. The inhibitory effect is due to suppression of MMP-2 expression. The association between down-regulation of MMP expression and reduction of invasiveness was also demonstrated in a highly metastatic HT1080 human fibroblast cell line. Purified ginseng components, PD and PT, down-regulated the expression of MMP-9 in HT1080 cells [102]. By contrast, the expression of MMP-2 was not affected by PD and PT. Whether the observed differential inhibition of MMP-2 and MMP-9 expression in these two groups of cells is cell type- or ginsenoside-dependent remains to be elucidated.
In addition, the anti-invasive effects of Rb2, 20(S)- and 20(R)-Rg3 have been further demonstrated by Shinkai et al. [103] and Mochizuki et al. [104]. Rg3 was found to significantly inhibit in vitro invasion of rat ascites hepatoma cells (MM1), B16FE7 melanoma cells, human lung carcinoma (OC10) and pancreatic adenocarcinoma (PSN-1) cells. Intravenous (10 μg/mouse) or oral (100–1000 μg/animal) administration of both Rg3 and Rb1 inhibited lung metastasis of B16-BL6 melanoma and colon 26-M3.1 carcinoma in mice [103-105]. Furthermore, Rg3 greatly reduced the volume or weight of tumors in xenotransplanted (e.g. Lewis lung carcinoma, human breast infiltrating duct carcinoma, human gastric tumor and B16-BL6 melanoma) or chemical-induced (e.g. hepatocellular carcinoma) tumor models [106-109].
Effects of ginsenosides on multi-drug resistance
The development of multi-drug resistance (MDR) is a major problem in cancer chemotherapy. Agents that can enhance the accumulation of chemotherapeutic drugs in tumor cells by targeting the MDR proteins is a novel approach to overcome this problem [110-112]. In an acute myelogenous leukemia cell model, PPT ginsenosides were found to exert a chemosensitizing effect on P-glycoprotein (Pgp)-mediated MDR leukemia cells by increasing intracellular accumulation of daunorubicin and doxorubicin [113]. The effect on the reversal of drug resistance was due to competitive binding of ginsenosides such as Rg3 with Pgp, thereby blocking drug efflux [114]. In other MDR leukemia cells such as daunomycin or vinblastine-resistant leukemia cell models, various ginsenosides including 20(S)-PPT, Rh2 and compound K were found to greatly enhance the cytotoxicity of anti-cancer drugs in a range of 2- to 46-fold through the blockage of drug efflux [115]. Moreover, other ginsenosides including Rc and Rd reduced the efflux pump activity in lymphoma cells [116]. Recently, we also demonstrated that ginsenosides could exert the chemosensitizing effect through direct interaction with breast cancer resistance protein (BCRP), but not Pgp in MCF-7 cells. Among the tested ginsenosides, Rh2, PPD and PPT have been found to increase the cytotoxicity of mitoxantrone (MX), a potent anti-tumor drug, to human breast carcinoma MCF/MX cells which overexpress BCRP. The potency order of ginsenosides to inhibit BCRP is PPD > Rh2 > PPT. Neither Rg1 nor Rh1 was observed to inhibit BCRP or Pgp, whereas Rg3 was shown to be mild inhibitors. Previously, the C-6 substitution was hypothesized to confer anti-tumor activity. These findings suggest that the C-6 substitution is also important to the BCRP-inhibitory activity of ginsenosides. In addition, the two glucose substitutions at C-3 position such as Rg3 nearly abrogate its effect to inhibit BCRP. Our findings indicated that the inhibition of MX efflux as mediated by BCRP and the enhanced uptake of MX is correlated to the attachment of the hydroxyl group and sugar substitution at different positions of the steroidal skeleton of ginsenosides [117].
A new class of anti-tumor drugs: ginsenosides Rg3 and Rh2
Both Rg3 and its metabolite form Rh2 have emerged in Mainland China and Taiwan as anti-cancer drugs in the form of capsules (e.g. 'Rg3 Shenyi Jiaonang' and 'GOOD LIFE ginsenoside Rh2 capsule'). Rg3 Shenyi Jiaonang suppresses tumor angiogenesis and prevents adhesion, invasion and metastasis of tumor cells [118]. Rh2 as an adjuvant agent was also tested in the nude mouse model with human ovarian cancer cells transplanted. In the presence of Rh2, cisplatin could significantly inhibit tumor growth in vivo and prolong survival time. Neither Rh2 nor cisplatin alone could inhibit tumor growth [119]. It was shown that chemotherapy supplemented with Rh2 is 60% more effective than chemotherapy alone. It could also mitigate the adverse effects of hair loss, anemia, nausea, vomit and poor appetite following chemotherapy or radiotherapy [120].
Both Rg3 and Rh2 are extracted from red ginseng which is processed by steaming and drying. During the process, the malonyl group at the C-6 is released and the glycosyl moiety at C-20 is partially detached to generate Rh1, Rh2 and Rg3 through deglycosylation similar to the deglycosylated product, compound K generated from the metabolic transformation of ginsenoside Rb1 by intestinal bacteria [121,122]. However, whether all of the ginsenosides generated by such post-treatment of white ginseng have similar anti-tumor effects are still not known.
Anti-angiogenic effects of ginsenosides
Various ginsenosides including Rg3, Rb2 and compound K demonstrated anti-angiogenic activity in different tumor models. Among them, Rg3 is the most extensively investigated. It exerts an anti-angiogenic action in different animal models when administered alone or in combination with other conventional chemicals. In those animal models which have been orthotopically implanted with different tumor cells such as human breast infiltrating duct carcinoma, ovarian carcinoma SKOV3 cells, B16-BL6 melanoma cells and colon 26-M3.1 carcinoma, Rg3 or Rb2 was found to inhibit angiogenesis in vivo. A significant reduction of intra-tumoral microvessel density (MVD), VEGF mRNA and VEGF protein levels in tumor tissues and sera of the tumor-bearing animals was observed [104-106,122]. Similar results were also reported when Rg3 was used in combination with a low dose of cyclophosphamide in bearing mice Lewis lung carcinoma [109]. These data indicate that one of the mechanisms of anti-metastatic effect of ginsenosides is probably related to suppression of tumor-induced angiogenesis [104].
In a recent study, we demonstrated that 20(R)-Rg3 (1–103 nM) was able to inhibit HUVEC proliferation, VEGF-induced chemoinvasion and tubulogenesis in vitro in a dose-dependent manner. In a Matrigel plug assay, 20(R)-Rg3 (600 nM) was able to reduce the hemoglobin content by 5-fold when compared with the positive control containing bFGF and heparin. The extent of microvascular sprouting was inhibited dose-dependently by 20(R)-Rg3 in the ex vivo organtypic cultures of rat aortic ring fragments (Figure 5). Basement membrane degrading enzymes, especially MMP-2 and -9, are secreted by activated ECs (or even tumor cells) throughout the angiogenic process. They have been demonstrated to play a critical role in cell invasion and migration, as well as angiogenesis. Our data further demonstrate that the anti-angiogenic effect of 20(R)-Rg3 was probably related to reduction of expression and activities of MMP-2 and -9 [124]. By using DNA microarray, 20(R)-Rg3 was found to inhibit the protein expression of angiogenic factors via several target genes (e.g. VEGF, bFGF and MMP-2) in both human lung adenocarcinoma cell line A549 and HUVEC304 cells [123]. Current research findings, indicating that some PD type ginsenosides (e.g. Rg3 and Rb2) exhibit potent anti-tumor and anti-angiogenic activities in vitro and in vivo, have far-reaching implications for future development of ginsenosides as an anti-tumor medicine.
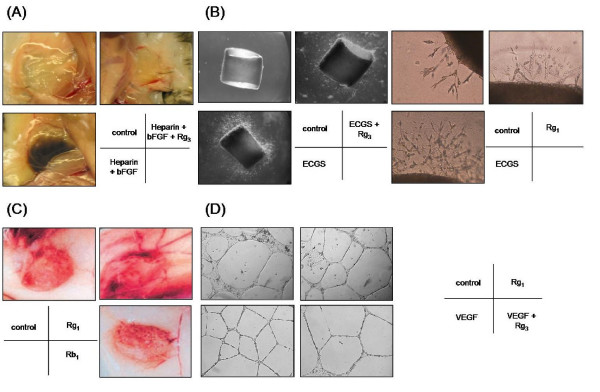
Figure 5.
Angiogenesis assays. Angiogenesis is a multi-step process, Different types of in vitro, in vivo or ex vivo bioassays have been designed to mimic the various steps of angiogenesis. (A) In vivo Matrigel Plug assay: liquid form Matrigel (500 μl) containing growth factor (e.g. bFGF) and/or ginsenoside is injected into the abdominal region of C57/BL mice subcutaneously. The Matrigel will solidify at 37°C and form a solid 'plug'. After 5 days of incubation, the mice are sacrificed and in vivo angiogenesis including endothelial cell invasion, migration and formation of neovessels can be examined [124]. (B) Ex vivo rat aortic ring sprouting assay: rat aortic fragments one millimeter in length are embedded in Matrigel and cultured in the presence of growth factors (e.g. endothelial cell growth supplements – ECGS) and/or ginsenosides. The extent of endothelial sprouting from the aortic fragment can clearly indicate the angiogenic properties of ginsenosides [124]. (C) In vivo sponge implantation assay: a sterile polyether polyurethane sponge (170 mm3) containing ginsenosides is inserted into the abdominal region of Balb/c mice. After incubation for 15 days, the animals are euthanized and neovascularization is examined as indicated by the growth of vessels in the granuloma tissue [129]. (D) In vitro tube formation assay: endothelial cells are seeded on the Matrigel and subsequently incubated in medium containing growth factors (e.g. VEGF) and/or ginsenosides. Endothelial cells will rearrange and alight into a tubular structure. Angiogenic properties of ginsenosides can be reflected from the number of tubes, branching points or tube area.
Angiogenic effects of ginsenosides
In contrast to the anti-angiogenic effects of ginsenosides such as Rb1 and Rg3, another group of ginsenosides, represented by the panaxatriols Rg1 and Re, have been found to be angiogenic. Rg1 increased HUVEC proliferation, migration and tube formation in a dose-dependent manner [125-128]. We further demonstrated its angiogenic effects using the in vivo Matrigel plug and ex vivo aortic ring sprouting models (Figure 5). Histological evaluation of the Matrigel implants indicated that functional neovessels were formed as induced by Rg1. The in vivo data collected seven days after the implantation of genipin-fixed porous acellular bovine pericardium (same as extracellular matrices), which was dip-coated with Re or Rg1, indicate that the density of neovessels and tissue hemoglobin content inside the matrices were significantly increased by both ginsenosides. Furthermore, vascularized neo-connective tissue fibrils were found to fill the pores in the matrices loaded with Re [125]. Similar angiogenic activities were also observed in the above assays when Rg1 was used [126]. Interestingly, ginsenoside-induced angiogenesis was found to be comparable to or even better than bFGF-induced angiogenesis in vivo. These data suggest that both ginsenosides are very potent angiogenic agents and may potentially be useful in therapeutic angiogenesis in tissue-engineering strategies
Ginseng contains two groups of ginsenosides with activity in the modulation of angiogenesis. These observed counteracting effects can be interpreted by the Yin/Yang theory in traditional Chinese medicine (TCM). In fact, we have, in our previous publications, demonstrated strong scientific evidence supporting the ancient Yin/Yang theory [129]. Administration of Rg1 or Rb1 alone was shown to exert counteracting effects in angiogenesis. Rg1 alone promoted functional neovascularization in the polymer scaffold in Matrigel implant model and the chemoinvasion of HUVEC. By contract, Rb1 exerted inhibition in both. Moreover, we found that ginseng extract reconstituted with a defined ratio of Rg1 and Rb1 could alter the angiogenic outcome. In an in vivo scaffold implant neovascularization model, administration of an extract with a higher concentration of Rg1 than Rb1 (Rg1:Rb1 = 5:2) resulted in the induction of significant angiogenesis in the implant compared with control implants. In contrast, overabundance of Rb1 (Rg1:Rb1 = 2:5) inhibited Rg1-induced neovascularization [130]. These counteracting actions manifested the logic of Yin/Yang theory of TCM, which advocates that everything has opposing Yin and Yang aspects, and these aspects are reciprocally regulated and inhibited by each other in such a way that a continuous state of dynamic balance is maintained. The contents of ginsenosides in P. ginseng, P. quinquefolius and P. notoginseng were then measured by an HPLC method. The data show that P. ginseng has a higher ratio of Rg1 to Rb1 (0.51 – 2.08), while P. quinquefolius has a lower ratio (0.07 – 1.4) [131,132]. These findings are also consistent with the TCM's attributes of 'hot' (i.e. stimulating) properties of P. ginseng and 'cool' (i.e. calming) properties of P. quinquefolius.
Functional genomics approach on the mechanistic study of ginsenoside
Functional genomics refers to the functions and interactions of genes towards a holistic view of the biological system in terms of gene expression and proteins. It focuses on the dynamics of gene transcription, translation and protein-protein interactions, instead of the static views of genomic information (e.g. gene sequences). It can be achieved by means of transcriptomics (differential expression of genes), proteomics (the study of total protein complement of a genome), phosphoproteomics and metabolomics studies (the study of the entire metabolic content of a cell or an organism). In general, the functional genomics approach is accompanied by high-throughput technology platforms such as the DNA microarray (i.e. DNA chip) technology and two-dimensional gel electrophoresis (2-DE) coupled with mass spectrometry for characterization of the abundant gene and protein products. The chip-based microarray allows a quantitative parallel assessment of gene expression and facilitates the study of complicated drug actions at the molecular level. This rapid increase in functional genomic-based drug studies enables an early and more accurate prediction and diagnosis of disease and disease progression. Understanding of the individual responses to drugs will have implications for their use and development by the pharmaceutical industry.
This approach was applied in the mechanistic study of ginsenoside Rg1 on HUVEC [127,133]. Our microarray data indicated that ginsenoside Rg1 could up-regulate a set of genes related to cell adhesion, migration and cytoskeleton such as RhoA, RhoB, IQ-motif-containing GTPase-activating protein 1 (IQGAP1), calmodulin (CALM2), Vav2 and laminin-α4 (LAMA4) in HUVEC. These proteins interact with one another in a hierarchical cascade pattern in modulating cell architectural dynamics. As we observed in the tube formation assay, HUVEC undergo rapid migration and morphological differentiation to form tubular structures by involving a dynamic assembly and disassembly of cytoskeletons. RhoA and RhoB, which belong to Rho family GTPases, have been found to regulate cell-cell adhesion and hence the motility through interfering with membrane ruffling, stress fiber formation and E-cadherin-mediated cell-cell adhesions [134-142]. The activities of GTPases are regulated by means of a molecular switch; cycling of the GDP-bound form (inactive state) and GTP-bound form (active state). As demonstrated by Bustelo et al., such transformation can be activated by guanine nucleotide exchange factors (Rho-GEFs) [143,144]. Vav2, one of Rho-GEFs, can increase the exchange of bound-GDP for a GTP molecule and translocate the Rho GTPases to the plasma membrane for interaction with its Rho effectors such as IQGAP1 [145]. Moreover, Vav2 can also affect cadherin-mediated cell-cell adhesion by binding with p120 catenin leading to formation of lamellipodia and membrane ruffling [146-148]. IQGAP1 acts as a negative regulator in E-cadherin-mediated cell-cell adhesion by interacting directly with β-catenin and the cytoplasmic domain of cadherin [149,150]. Over-expression of IQGAP1 is believed to compete with α-catenin for the same binding site on β-catenin and displace α-catenin from the β-catenin-α-catenin complex which disrupts the association of the complex with F-actin. Such cytomechanical modifications can subsequently cause the reduction of E-cadherin-mediated cell-cell adhesion that accounts for the changes in cell morphology and migration [151]. Moreover, laminins, which are the trimeric basement membrane glycoproteins, have been found to participate in the formation and differentiation of tubular structures of HUVEC in vitro and to provide a structural link between the ECM and the actin-based cytoskeletal system of cells [152-156]. Undoubtedly, microarray technology provides a high throughput platform for the elucidation of drug mechanism in study models.
On a different front, a proteomic study was carried out by Ma et al. to elucidate the cardiovascular protective role of Rg1 on ECs [133]. The data show that protein expression of MEKK-3, reticulocalbin, phosphoglycerate, 6-phosphogluconolactonase, zinc finger protein, NSAP1 protein, recombination-activating protein was increased, while that of eNOS, and mineralocorticoid receptor (MR) was decreased upon tumor necrosis factor-α (TNF-α) stimulation of HUVEC. However, Rg1 restored the expression of these proteins to the normal level. Western blotting and RT-PCR further validated that NO production in such TNF-α induced condition was correlated to the increase of eNOS mRNA and protein expression. They suggest that the increase of NO is related to the protective role of Rg1 on TNF-α stimulated HUVEC.
Ginsenosides and steroids
Steroid hormones and vascular homeostasis
A critical function of the vasculature is to provide a nutritional supply to tissues and organs. In general, proper vascular homeostasis is controlled by capillary permeability modulation, vasodilation and angiogenesis [157-159]. All these activities can govern the rate of material exchange, transportation and the site of distribution so as to meet the physiological needs of the human body. Steroid hormones, such as glucocorticoids, estrogens, progestagens, mineralocorticoids and androgens, play an important role in such vascular modulation at the molecular, cellular and even systematic levels [160-164].
Among the various steroid hormones, estrogen is the most promising example used to elaborate the maintaining and protective roles of steroid hormones on vascular homeostasis and cardiovascular disorders. The growing evidence implicated that estrogen reduces the risk of atherosclerosis by (1) decreasing the serum levels of both total and low-density-lipoprotein (LDL) cholesterol, while raising those of high-density-lipoprotein (HDL) cholesterol and triglycerides; (2) interfering with the inflammatory processes (e.g. monocyte adherence and trans-endothelial migration on the endothelium) in the vasculature; and (3) acting as an antioxidant [165-168]. Furthermore, estrogen is also a potent vasodilator, and hypotensive agents that can induce vascular relaxation by producing an increase of endothelium-derived vasodilators (e.g. NO). Mechanistic studies clearly indicate that while estrogen binds with its corresponding steroid hormone receptor – estrogen receptor (ERα, and ERβ), it may undergo either or both genomic and non-genomic actions inside the cells. Through the conventional pathway, activated-ER induces the gene transcription of eNOS, thereby increasing NO generation. Alternatively, through triggering the intracellular signaling (non-genomic) pathway, phosphorylated estrogen-bound ER (ligand-activated ER) has been found to induce rapid endothelial NO release via the PI3K-Akt-dependent pathway in HUVEC [169,170]. Various ginsenosides have been shown to exhibit an estrogen-like activity by acting as a functional ligand of ER [171-174].
Ginsenosides-mediated steroid-like activities
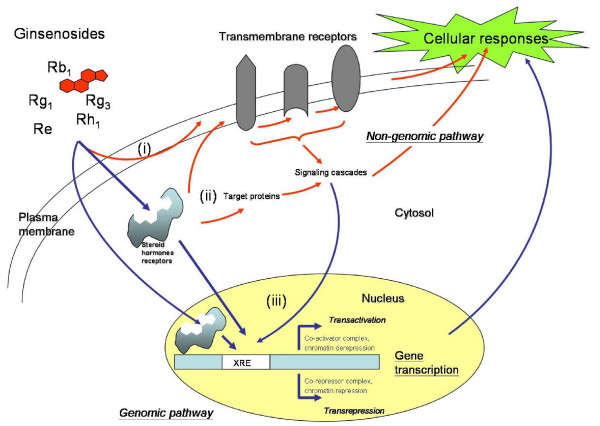
Ginsenosides could exhibit their actions through plasma membrane, cytosol, or even in the nucleus. They can initiate their mode of actions by binding to the membrane receptors (e.g. ATPase pump, ion transporters and channels, voltage gated channels and G-proteins) and subsequently activating the associated downstream signaling cascades. As described in the previous section, ginsenosides are amphipathic in nature, they can intercalate into the plasma membrane resulting in the alteration of membrane fluidity and trigger a series of cellular responses. Binding with the intracellular steroid hormone receptors including glucocorticoid receptor (GR), estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR) and mineralocorticoid receptor (MR), using their hydrophobic 'steroid-like' backbone is another alternative to trigger cellular responses. Like other steroids, ginsenosides can gain access to the nuclear receptors, where they regulate gene transcription by binding with the specific gene response elements to activate the so called 'genomic pathway' [175,176] (Figure 6).
Figure 6.
Schematic overview of ginsenosides-mediated genomic and non-genomic pathways. Ginsenosides possess a steroid-like skeleton composed of four trans-rings with different degrees of glyco-substitution. They are amphipathic in nature and can exhibit their actions at different cellular locations; such as the plasma membrane, cytosol and nucleus. Through the non-genomic pathway (indicated by red arrows), (i) they can initiate their actions by binding with the transmembrane receptors (e.g. ATPase pump, ion transporters and channels, voltage-gated channels and G-proteins) and subsequently activating the associated downstream signaling cascades. Moreover, they can intercalate into the plasma membrane resulting in an alteration of membrane fluidity and a trigger of a series of cellular responses. (ii) binding with steroid hormone receptors (SHRs) including glucocorticoid receptor (GR), estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR) and mineralocorticoid receptor (MR) present inside or outside the nucleus by using their hydrophobic backbone is another alternative to trigger downstream cellular responses. Those activated (phosphorylated) SHRs can activate the target molecules through a signaling cascade that brings about various cellular responses. (iii) the ligand-bound SHRs can translocate into the nucleus, where they regulate gene transcription by binding with the specific Response Elements (XRE). This is the so called 'genomic pathway' (indicated by blue arrows). Consequently, the altered gene products can affect the final cellular responses.
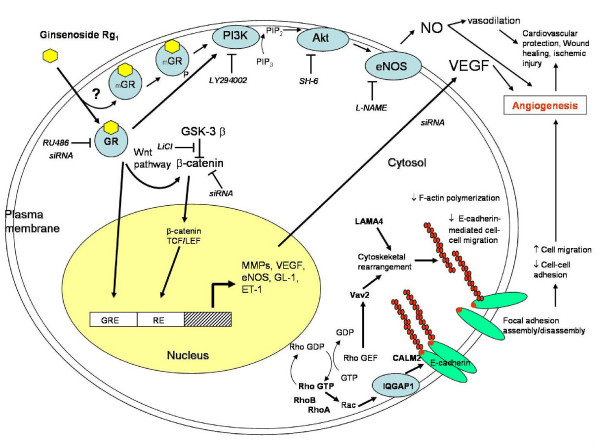
A previous study indeed demonstrated that Rg1 can trans-activate glucocorticoid response element (GRE)-luciferase activity by acting as a functional ligand of the GR [177]. Glucocorticoids (GCs) have been reported to activate the phosphatidylinositol-3 kinase (PI3K)/Akt pathway after binding with the GR [178]. Interestingly, our recent findings also indicate that Rg1 can act as GCs to increase the phosphorylation of GR, PI3K, Akt/PKB and eNOS, leading to an enhancement of NO production in HUVEC (Figure 7). Activation of eNOS was abolished by differential inhibition using RU486 (GR antagonist), LY294002 (PI3K inhibitor) and SH-6 (Akt inhibitor), indicating that Rg1 induced NO production from eNOS via the PI3K/Akt pathway. Moreover, knockout of GR by siRNA completely eliminated such reaction. Taken together, we concluded that such NO generation pathway was mediated through the activation of GR by ginsenoside Rg1 [179]. This nuclear receptor mediated response, which occurs within seconds or minutes upon stimulation and is independent of any transcriptional activity, is classified as the 'non-genomic' pathway [175,176] (Figure 6).
Figure 7.
Schematic overview of ginsenoside Rg1-mediated angiogenic action in HUVEC. Ginsenoside Rg1, which acts as a functional ligand of glucocorticoid receptor (GR) (either cytosol GR or membrane-bound GR-mGR), promotes angiogenesis through both non-genomic and genomic pathways. Through the non-genomic pathway, it increases nitric oxide (NO) production via the PI3K-Akt pathway: GR → phosphatidylinositol-3 kinase (PI3K)/Akt pathway → endothelial nitric oxide synthase (eNOS) pathway. Rg1 also increases vascular endothelial growth factor (VEGF) production through the GR → PI3K/Akt → GSK3β → β-catenin/TCF pathway. Gene expression profiling data indicated that Rg1 could increase the expression of a group of genes (e.g. Rho A, RhoB, IQGAP1, LAMA4, CALM2 and Vav2) which are related to cell-cell adhesion, migration and cytoskeletal remodeling.
In a later study, we discovered that the angiogenic Rg1 was able to enhance VEGF expression in ECs by increasing the level of β-catenin within the nucleus. We concluded that Rg1 induced increase of VEGF in HUVEC through activation of a PI3K/Akt → GSK3β→β-catenin/TCF pathway via GR [180]. In addition, the PI3K/Akt-mediated NO generation has been found in androgen-responsive LNCaP and estrogen-responsive MCF-7 cells by others [181]. Re was found to bind with steroid hormone receptors (including AR, ERα and PR). Lee et al., however, showed that ginsenoside Rh1 could only activate ER in the CV-1 cell model [173].
The estrogen-like activity of various ginsenosides including Rg1, Rh1 and Rb1 has been reported [170-172]. In fact, ginseng has been used in TCM for alleviation of the symptoms of menopause, which means ginseng, probably ginsenosides, can act as phytoestrogens to elicit an ER-mediated pathway for protecting the cardiovascular system. These recent research findings clearly indicate that ginsenosides may act as steroid hormones to activate various steroid hormone receptors to exert these pleiotropic responses. Although many mechanisms behind the physiological effects induced by ginsenosides are still not clear, their ability to interact with steroid hormone receptors may hold the key to finally elucidate these diverse actions of ginseng.
Conclusion
At present, ginseng is not only used as a therapeutic by traditional medical practitioners but is also as health supplements readily available in the commercial market. The diversity of highly desirable physiological effects of ginseng has intrigued scientists for years. In general, most of its pharmacological actions have been attributed to a group of triterpenoid saponins called ginsenosides. Although the modulatory effects of ginseng or probably ginsenosides have been extensively investigated, the actual molecular mechanisms remain largely unknown. Recently, it has been found that ginsenosides can act as functional ligands to activate different steroid hormone receptors. Through such mechanisms, ginseng can exert its effects on the human body by acting in a similar way as the steroid hormones. The interaction between ginsenosides and various nuclear steroid hormone receptors may explain the diverse activities of ginseng, which may eventually lead to further development of ginseng-derived therapeutics for angiogenesis-related diseases.
Abbreviations
2-DE: Two-dimensional gel electrophoresis
AR: Androgen receptor
BCRP: Breast cancer resistance protein
CALM2: Calmodulin
CNS: Central nervous system
ECM: Extracellular matrix
ECs: Endothelial cells
eNOS: Endothelial nitric oxide synthase
ER: Estrogen receptor
FGF: Fibroblast growth factor
GC: Glucocorticoids
GR: Glucocorticoid receptor
GRE: Glucocorticoid responsive element
HDL: High-density-lipoprotein
HUVEC: Human umbilical vein endothelial cells
ICAM-1: Intercellular adhesion molecule-1
IQGAP1: IQ-motif-containing GTPase-activating protein 1
LAMA4: Laminin-α4
LDL: Low-density-lipoprotein
L-NAME: Nω-nitro-L-arginine methylester
MDR: Multi-drug resistance
MMPs: Matrix metalloproteinases
MR: Mineralocorticoid receptor
MVD: Microvessel density
MX: Mitoxantrone
OH: Hydroxyl
PA: Plasminogen activators
PAI-1: Plasminogen activator inhibitor-1
Pgp: P-glycoprotein
PKC: Protein kinase C
PPD: Protopanaxadiols
PPT: Protopanaxatriols
PR: Progesterone receptor
RE Responsive element
Rho-GEFs: Guanine nucleotide exchange factors
Rx: Ginsenosides
TCM: Traditional Chinese medicine
TGF-β: Transforming growth factor β
TNF-α: Tumor necrosis factor α
uPA: Urokinase plasminogen activator
uPAR: Urokinase plasminogen activator receptor
VCAM-1: Vascular cell adhesion molecule-1
VEGF: Vascular endothelial growth factor
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
PYKY conceived and wrote the article and performed the experiments. NKM and TBN drafted the section of 'anti-tumor effects of ginsenosides'. YKC prepared all the chemical structures of ginsenosides. KWL and TPDF conducted the experiments. HWY and RNSW helped to compile the manuscript.
Acknowledgments
Acknowledgements
The present work was supported by earmarked research grant (HKBU 2171/03M and HKBU 2476/06M) from the Research Grant Council, Hong Kong SAR Government and a faculty research grant (FRG/04-05/II-36) from the Hong Kong Baptist University.
Contributor Information
Patrick Ying Kit Yue, Email: patrick@hkbu.edu.hk.
Nai Ki Mak, Email: nkmak@hkbu.edu.hk.
Yuen Kit Cheng, Email: ykcheng@hkbu.edu.hk.
Kar Wah Leung, Email: 03414604@hkbu.edu.hk.
Tzi Bun Ng, Email: b021770@mailserv.cuhk.edu.hk.
David Tai Ping Fan, Email: tpf1000@cam.ac.uk.
Hin Wing Yeung, Email: hwyeung@cmjournal.org.
Ricky Ngok Shun Wong, Email: rnswong@hkbu.edu.hk.
References
- Hu SY. A contribution of our knowledge of ginseng. Am J Chin Med. 1977;5:1–23. doi: 10.1142/s0192415x77000026. [DOI] [PubMed] [Google Scholar]
- Tao HC. Sheng-Nung-Pen-Tsao-Ching. Taipei, Taiwan: Chung-Hwa; 1955. [Google Scholar]
- Liu CX. Introduction on research of ginseng. Information of Traditional Chinese Medicine. 1975;2:9–11. [Google Scholar]
- Liu CX. Pharmacology and clinic of active principles of ginseng. Chinese Traditional Herbs and Drugs. 1975;7:57. [Google Scholar]
- Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- Jiang JW, Xiao QS. Handbook of Active Constituents of Medicinal Plants. Beijing: People's Health Publishers; 1985. pp. 503–516. [Google Scholar]
- Wang BX. Progress of pharmacological studies of ginseng. Yaoxue Xuebao. 1980;15:312–320. [PubMed] [Google Scholar]
- Yun TK. Brief introduction of Panax gingeng C.A. Meyer. J Korean Med Sci. 2001. pp. 3–5. [DOI] [PMC free article] [PubMed]
- Wen J, Zimmer EA. Phylogeny and biogeography of Panax L. (the ginseng genus, araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 1996;6:167–177. doi: 10.1006/mpev.1996.0069. [DOI] [PubMed] [Google Scholar]
- Lee FC. The Elixir of Life. Elizabeth, NJ: Hollyn International Corp; 1992. Facts about Ginseng. [Google Scholar]
- Huang KC. The Pharmacology of Chinese Herbs. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- Shoji J. Recent advances in the chemical studies on ginseng. In: Chang HM Yeung HW, Tso WW, Koo A, editor. Advances in Chinese Medicinal Materials Research. Singapore: World Scientific; 1985. [Google Scholar]
- Tanaka O, Kasai R, Morita T. Chemistry of ginseng and related plants: recent advances. Abstr Chin Med. 1986;1:130–152. [Google Scholar]
- Wang X, Sakuma T, Asafu-Adjaye E, Shiu GK. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhang C, Lu M, Sun F, Fu Y, Jin F. Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull (Tokyo) 2007;55:231–235. doi: 10.1248/cpb.55.231. [DOI] [PubMed] [Google Scholar]
- Banthorpe DV. Terpenoid. In: Mann J, editor. Natural Product. Essex: Longman Scientific and Technical; 1994. pp. 331–339. [Google Scholar]
- Hertig AT. Contrib. Embryol. 1935;25:37. [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Risau W. Mechanism of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factor. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Role of the matrix matelloproteinases and plasminogen activator-plasmin system in angiogenesis. Arterioscler Thrombovasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S, Haenni B, Burri PH. Implementation of intussusceptive microvascular growth in the chicken chorioallantoic membrane (CAM) Microvasc Res. 1997;53:33–52. doi: 10.1006/mvre.1996.1989. [DOI] [PubMed] [Google Scholar]
- Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res. 2000;86:286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–117. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- Marchio S, Primo L, Pagano M, Palestro G, Albini A, Veikkola T, Cascone I, Alitalo K, Bussolino F. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557–62. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem. 1995;270:9709–9716. doi: 10.1074/jbc.270.17.9709. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol. 1995;15:1255–1261. doi: 10.1161/01.atv.15.8.1255. [DOI] [PubMed] [Google Scholar]
- Wu HM, Yuan Y, McCarthy M, Granger HJ. Acidic and basic FGFs dilate arterioles of skeletal muscle through a NO-dependent mechanism. Am J Physiol. 1996;271:H1087–1093. doi: 10.1152/ajpheart.1996.271.3.H1087. [DOI] [PubMed] [Google Scholar]
- Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res. 1998;82:1007–1015. doi: 10.1161/01.res.82.9.1007. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:656–664. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- Ziche M, Parenti A, Ledda F, Dell'Era P, Granger HJ, Maggi CA, Presta M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ Res. 1997;80:845–852. doi: 10.1161/01.res.80.6.845. [DOI] [PubMed] [Google Scholar]
- Noiri E, Lee E, Testa J, Quigley J, Colflesh D, Keese CR, Giaever I, Goligorsky MS. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol. 1998;274:C236–244. doi: 10.1152/ajpcell.1998.274.1.C236. [DOI] [PubMed] [Google Scholar]
- Dulak J, Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659–666. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumor progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Dzau VJ. Derangements of the nitric oxide synthase pathway, L-arginine and cardiovascular diseases. Circulation. 1997;96:379–382. [PubMed] [Google Scholar]
- Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Luscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 1998;97:2494–2498. doi: 10.1161/01.cir.97.25.2494. [DOI] [PubMed] [Google Scholar]
- Frank S, Madlener M, Pfeilschifter J, Werner S. Induction of inducible nitric oxide synthase and its corresponding tetrahydrobiopterin-cofactor-synthesizing enzyme GTP-cyclohydrolase I during cutaneous wound repair. J Invest Dermatol. 1998;111:1058–1064. doi: 10.1046/j.1523-1747.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999;13:2002–2014. [PubMed] [Google Scholar]
- Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yla-Herttuala S, Martin JF. Cardiovascular gene therapy. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Feng X, Clark RAF. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B. Expression of vascular permeability factor (VEGF) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. VEGF mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF. Regulation and manipulation of angiogenesis in the primate corpus luteum. Reproduction. 2001;121:355–362. doi: 10.1530/rep.0.1210355. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Rogers AW. Human endometrial angiogenesis. Reproduction. 2001;121:181–186. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- Folkamn J. Tumor angiogenesis. Advance in Cancer Research. 1974;19:331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Walsh DA. Angiogenesis and arthritis. Rheumatology. 1999;38:103–112. doi: 10.1093/rheumatology/38.2.103. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Raghu K. Basement membranes: structure, assembly and role in tumor angiogenesis. Nat Med. 2003;3:442–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Yoshii S, Kaga S, Matsumoto M, Kugiyama K, Maulik N. Angiogenic strategy for human ischemic heart disease: brief overview. Mol Cell Biochem. 2004;264:143–149. doi: 10.1023/b:mcbi.0000044383.01785.05. [DOI] [PubMed] [Google Scholar]
- Aoki M, Morishita R. Therapeutic angiogenesis for ischemic diseases. Nippon Rinsho. 2006;64:762–768. [PubMed] [Google Scholar]
- Fan TP, Kohn EC, Eds . The New Angiotherapy. Totowa, NJ, USA: Human Press; 2002. [Google Scholar]
- Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Li C, Kumar S. Strategies for vascular targeting in tumors. Int J Cancer. 2002;100:123–130. doi: 10.1002/ijc.10462. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Specialization of tumor vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Pegaptanib: the first antiangiogenic agent approved for neovascular macular degeneration. Expert Opin Pharmacother. 2005;6:1421–1423. doi: 10.1517/14656566.6.8.1421. [DOI] [PubMed] [Google Scholar]
- Hori A, Ikeyama S, Sudo K. Suppression of cyclin D1 mRNA expression by the angiogenesis inhibitor TNP-470 (AGM-1470) in vascular endothelial cells. Biochem Biophys Res Commun. 1994;204:1067–1073. doi: 10.1006/bbrc.1994.2571. [DOI] [PubMed] [Google Scholar]
- Masiero L, Figg WD, Kohn EC. New anti-angiogenesis agents: review of the clinical experience with carboxyamido-triazole (CAI), thalidomide, TNP-470 and interleukin-12. Angiogenesis. 1997;1:23–35. doi: 10.1023/A:1018301031580. [DOI] [PubMed] [Google Scholar]
- Kohn EC. Endostatin and angiostatin: the next anti-angiogenesis generation. Angiogenesis. 1998;2:25–27. doi: 10.1023/a:1009046208807. [DOI] [PubMed] [Google Scholar]
- Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng CC, Yang SM, Huang CY, Chen JC, Chang WM, Hsu SL. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 2005;55:531–540. doi: 10.1007/s00280-004-0919-6. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–435. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- Liu K, Xu SX, Che CT. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000;67:1297–1306. doi: 10.1016/s0024-3205(00)00720-7. [DOI] [PubMed] [Google Scholar]
- Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, Kudoh K, Nagata I, Shinomiya N. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1989;89:733–740. doi: 10.1111/j.1349-7006.1998.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Jin SH, Lee YH, Kim SI, Park JD. Ginsenoside Rh2 induces apoptosis independently of Bcl-2, Bcl-xL, or Bax in C6Bu-1 cells. Arch Pharm Res. 1999;22:448–453. doi: 10.1007/BF02979151. [DOI] [PubMed] [Google Scholar]
- Kim ND, Kang SY, Kim MJ, Park JH, Schini-Kerth VB. The ginsenoside Rg3 evokes endothelium-independent relaxation in rat aortic rings: role of K+ channels. Eur J Pharmacol. 1999;367:51–57. doi: 10.1016/s0014-2999(98)00899-1. [DOI] [PubMed] [Google Scholar]
- Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur J Pharmacol. 1999;367:41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- Kim HE, Oh JH, Lee SK, Oh YJ. Ginsenoside RH-2 induces apoptotic cell death in rat C6 glioma via a reactive oxygen- and caspase-dependent but Bcl-X(L)-independent pathway. Life Sci. 1999;65:PL33–40. doi: 10.1016/s0024-3205(99)00252-0. [DOI] [PubMed] [Google Scholar]
- Oh SH, Lee BH. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicol Appl Pharmacol. 2004;194:221–229. doi: 10.1016/j.taap.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee YH, Park JH, Lee SK. Ginsenoside Rs4, a new type of ginseng saponin, concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepatoma SK-HEP-1 cells. Eur J Cancer. 1999;35:507–511. doi: 10.1016/s0959-8049(98)00415-8. [DOI] [PubMed] [Google Scholar]
- Li X, Guan YS, Zhou XP, Sun L, Liu Y, He Q, Fu L, Mao YQ. Anticarcinogenic effect of 20(R)-ginsenoside Rg3 on induced hepatocellular carcinoma in rats. Sichuan Daxue Xuebao Yixue Ban. 2005;36:217–220. [PubMed] [Google Scholar]
- Kang KA, Kim YW, Kim SU, Chae S, Koh YS, Kim HS, Choo MK, Kim DH, Hyun JW. G1 phase arrest of the cell cycle by a ginseng metabolite, compound K, in U937 human monocytic leukamia cells. Ach Pharm Res. 2005;28:685–690. doi: 10.1007/BF02969359. [DOI] [PubMed] [Google Scholar]
- Lee KY, Park JA, Chung E, Lee YH, Kim SI, Lee SK. Ginsenoside-Rh2 blocks the cell cycle of SK-HEP-1 cells at the G1/S boundary by selectively inducing the protein expression of p27kip1. Cancer Lett. 1996;110:193–200. doi: 10.1016/s0304-3835(96)04502-8. [DOI] [PubMed] [Google Scholar]
- Fujikawa-yamamoto K, Ota T, Odashima S, Abe H, Arichi S. Different response in cell cycle of tumor cells to ginsenoside Rh2. Cancer J. 1987;1:349–352. [Google Scholar]
- Popovich DG, Kitts DD. Ginsenosides 20(S)-protopanaxadiol and Rh2 reduce cell proliferation and increase sub-G1 cells in two cultured intestinal cell lines, Int-407 and Caco-2. Can J Physiol Pharmacol. 2004;82:183–190. doi: 10.1139/y04-001. [DOI] [PubMed] [Google Scholar]
- Oh M, Choi YH, Choi S, Chung H, Kim K, Kim SI, Kim DK, Kim ND. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–875. doi: 10.3892/ijo.14.5.869. [DOI] [PubMed] [Google Scholar]
- Leung KN, Mak NK, Fung MC. Cytokines in the differentiation therapy of leukemia: from laboratory investigations to clinical applications. Crit Rev Clin Lab Sci. 2005;42:473–514. doi: 10.1080/10408360500295154. [DOI] [PubMed] [Google Scholar]
- Zeng XL, Tu ZG. In vitro induction of differentiation by ginsenoside Rh2 in SMMC-7721 hepatocarcinoma cell line. Pharmacol Toxicol. 2003;93:275–283. doi: 10.1111/j.1600-0773.2003.pto930605.x. [DOI] [PubMed] [Google Scholar]
- Zeng XL, Tu ZG. Induction of differentiation by ginsenoside Rh2 in hepatocarcinoma cell SMMC-7721. Ai Zheng. 2004;23:879–884. [PubMed] [Google Scholar]
- Xia LJ, Han R. Differentiation of B16 melanoma cells induced by ginsenoside RH2. Yaoxue Xuebao. 1996;31:742–745. [PubMed] [Google Scholar]
- Lee YN, Lee HY, Chung HY, Kim SI, Lee SK, Park BC, Kim KW. In vitro induction of differentiation by ginsenoides in F9 teratocarcinoma cells. Eur J Cancer. 1996;32A:1420–1428. doi: 10.1016/0959-8049(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Aoki I, Toyoki H, Khatun S, Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. Eur J Gynaecol Oncol. 2001;22:339–341. [PubMed] [Google Scholar]
- Park MT, Cha HJ, Jeong JW, Kim SI, Chung HY, Kim ND, Kim OH, Kim KW. Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT1080 human fibrosarcoma cells. Mol Cells. 1999;9:476–483. [PubMed] [Google Scholar]
- Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, Kitagawa I. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res. 1996;87:357–362. doi: 10.1111/j.1349-7006.1996.tb00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao Y, Bai S, Shi Z, Zhang J. Effect of ginsenoside Rg3 on the progression of orthotopically xenotransplanted human breast cancer in nude mice and its mechanism. Sichuan Daxue Xuebao Yixue Ban. 2003;34:546–548. [PubMed] [Google Scholar]
- Tao H, Yao M, Zou S, Zhao D, Qiu H. Effect of angiogenesis inhibitor Rg3 on the growth and metastasis of gastric cancer in SCID mice. Zhonghua Waike Zazhi. 2002;40:606–608. [PubMed] [Google Scholar]
- Li X, Guan YS, Zhou XP, Sun L, Liu Y, He Q, Fu L, Mao YQ. Anticarcinogenic effect of 20(R)-ginsenoside Rg3 on induced hepatocellular carcinoma in rats. Sichuan Daxue Xuebao Yixue Ban. 2005;36:217–220. [PubMed] [Google Scholar]
- Kang XM, Zhang QY, Tong DD, Zhao W. Experimental study on anti-angiogenesis in mice with Lewis lung carcinoma by low-dose of cyclophosphamide combined with ginsenoside Rg3. Zhongguo Zhongxiyi Jiehe Zazhi. 2005;25:730–733. [PubMed] [Google Scholar]
- Mayer LD, Shabbits JA. The role for liposomal drug delivery in molecular and pharmacological strategies to overcome multidrug resistance. Cancer Metastasis Rev. 2001;20:87–93. doi: 10.1023/a:1013108524062. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Rabi T, Fonseca HB, Melnick SJ, Escalon EA. Novel plant triterpenoid drug amooranin overcomes multidrug resistance in human leukemia and colon carcinoma cell lines. Int J Cancer. 2003;105:784–789. doi: 10.1002/ijc.11180. [DOI] [PubMed] [Google Scholar]
- Szabo D, Keyzer H, Kaiser HE, Molnar J. Reversal of multidrug resistance of tumor cells. Anticancer Res. 2000;20:4261–4274. [PubMed] [Google Scholar]
- Choi CH, Kang G, Min YD. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003;69:235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3) Biochem Pharmacol. 2003;65:75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sung JH, Matsumiya S, Uchiyama M, Inouye Y, Kasai R, Yamasaki K. Reversal of daunomycin and vinblastine resistance in multidrug-resistant P388 leukemia in vitro through enhanced cytotoxicity by triterpenoids. Planta Med. 1995;61:409–413. doi: 10.1055/s-2006-958126. [DOI] [PubMed] [Google Scholar]
- Berek L, Szabo D, Petri IB, Shoyama Y, Lin YH, Molnar J. Effects of naturally occurring glucosides, solasodine glucosides, ginsenosides and parishin derivatives on multidrug resistance of lymphoma cells and leukocyte functions. In Vivo. 2001;15:151–156. [PubMed] [Google Scholar]
- Jin J, Shahi S, Kang HK, van Veen HW, Fan TP. Metabolites of ginsenosides as novel BCRP inhibitors. Biochem Biophys Res Commun. 2006;345:1308–1314. doi: 10.1016/j.bbrc.2006.04.152. [DOI] [PubMed] [Google Scholar]
- Wang X, Ling CO. Progress of the study on effective integredients from Chinese herbal medicine in anti-tumor metastasis. Zhonghua Wai Ke Za Zhi. 2002;40:606–608. [PubMed] [Google Scholar]
- Kikuchi Y, Sasa H, Kita T, Hirata J, Tode T, Nagata I. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside Rh2 and adjuvant effects to cisplatin in vivo. Anticancer Drugs. 1991;2:63–67. doi: 10.1097/00001813-199102000-00009. [DOI] [PubMed] [Google Scholar]
- Jia WW, Bu X, Philips D, Yan H, Liu G, Chen X, Bush JA, Li G. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy. Can J Physiol Pharmacol. 2004;82:431–437. doi: 10.1139/y04-049. [DOI] [PubMed] [Google Scholar]
- Dong A, Ye M, Guo H, Zheng J, Guo D. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett. 2003;25:339–344. doi: 10.1023/a:1022320824000. [DOI] [PubMed] [Google Scholar]
- Chi H, Ji GE. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett. 2005;27:765–771. doi: 10.1007/s10529-005-5632-y. [DOI] [PubMed] [Google Scholar]
- Chen MW, Ni L, Zhao XG, Niu XY. The inhibition of 20(R)-ginsenoside Rg3 on the expressions of angiogenesis factors proteins in human lung adenocarcinoma cell line A549 and HUVEC304 cell. Zhongguo Zhongyao Zazhi. 2005;30:357–360. [PubMed] [Google Scholar]
- Yue PY, Wong DY, Wu PK, Leung PY, Mak NK, Yeung HW, Liu L, Cai ZW, Jiang ZH, Fan TP, Wong RN. The angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Huang YC, Chen CT, Chen SC, Lai PH, Liang HC, Chang Y, Yu LC, Sung HW. A natural compound (ginsenoside Re) isolated from Panax ginseng as a novel angiogenic agent for tissue regeneration. Pharm Res. 2005;22:636–646. doi: 10.1007/s11095-005-2500-3. [DOI] [PubMed] [Google Scholar]
- Liang HC, Chen CT, Chang Y, Huang YC, Chen SC, Sung HW. Loading of a novel angiogenic agent, ginsenoside Rg1 in an acellular biological tissue for tissue regeneration. Tissue Eng. 2005;11:835–846. doi: 10.1089/ten.2005.11.835. [DOI] [PubMed] [Google Scholar]
- Yue PY, Wong DY, Ha WY, Fung MC, Mak NK, Yeung HW, Leung HW, Chan K, Liu L, Fan TP, Wong RN. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg(1) in vivo and in vitro. Angiogenesis. 2005;8:205–216. doi: 10.1007/s10456-005-9000-2. [DOI] [PubMed] [Google Scholar]
- Morisaki N, Watanabe S, Tezuka M, Zenibayashi M, Shiina R, Koyama N, Kanzaki T, Saito Y. Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. Br J Pharmacol. 1995;115:1188–1193. doi: 10.1111/j.1476-5381.1995.tb15023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- Cui JF. Identification and quantification of ginsenosides in various commercial ginseng preparations. Eur J Pharmacol Sci. 1995;3:77–85. [Google Scholar]
- Ma YC, Luo M, Mally L, Doucet M. Distribution and proportion of major ginsenosides and quality control of ginseng products. Chi J Med Chem. 1996;6:11–21. [Google Scholar]
- Ma ZC, Gao Y, Wang J, Zhang XM, Wang SQ. Proteomic analysis effects of ginsenoside Rg1 on human umbilical vein endothelial cells stimulated by tumor necrosis factor-alpha. Life Sci. 2006;79:175–181. doi: 10.1016/j.lfs.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPase Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. Commentary Cell adhesion amd Rho small GTPase. J Cell Sci. 1999;112:4491–4500. doi: 10.1242/jcs.112.24.4491. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. Rac p21 is involved in insulin-induced memebrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 samll GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Schmitz AAP, Govek EE, Bottner B, Aelst LV. Rho GTPases: signaling, migration and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regukation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Braga V. Epithelial cell shape: cadherins and small GTPases. Exp Cell Res. 2000;261:83–90. doi: 10.1006/excr.2000.5050. [DOI] [PubMed] [Google Scholar]
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1 and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphototyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Liu BP, Burridge K. Vav2 activates rac1, Cdc42 and RhoA downstream from growth factor receptor but not β1 integrins. Mol Cell Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelst LV, Schorey CD. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. P120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–579. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T. Role of IQGAP1, a target of small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Kaibuchi K. Cdc42, Rac1 and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun. 1999;262:1–6. doi: 10.1006/bbrc.1999.1122. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamurat T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1 a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Sephel GC, Weeks BSM, Martin GR, Kleinman HK, Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration and neurite outgrowth. J Biol Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- Grant DS, Tashiro K, Segui RB, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structure in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Nomizu M, Chung M, Delgado M, Kiuratomi Y, Yamada Y, Kleinman HK. Identification of laminin α1 and β1 chain peptides active for endothelial cell adhesion, tube formation and aortic sprouting. FASEB J. 1999;13:53–62. [PubMed] [Google Scholar]
- Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JCR. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2000;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–926. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Cornoldi A, Fini M. Effects of androgens on the cardiovascular system. J Endocrinol Invest. 2005;28:32–38. [PubMed] [Google Scholar]
- Critchley HO. Endometrial morphology and progestogens. Ernst Schering Res Found Workshop. 2005;52:55–88. doi: 10.1007/3-540-27147-3_4. [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Stier CT, Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev. 2002;10:97–107. doi: 10.1097/00045415-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. Nongenomic, ER-mediated activation of endothelial nitric oxide synthase: how does it work? What does it mean? Circ Res. 2000;87:956–960. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: possible mechanisms for gender differences in atherosclerosis. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- Bae EA, Shin JE, Kim DH. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab. 2004;89:3510–3515. doi: 10.1210/jc.2003-031823. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jin Y, Lim W, Ji S, Choi S, Jang S, Lee S. ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J Steroid Biochem Mol Biol. 2003;84:463–468. doi: 10.1016/s0960-0760(03)00067-0. [DOI] [PubMed] [Google Scholar]
- Chan RY, Chen WF, Dong A, Guo D, Wong MS. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab. 2002;87:3691–3695. doi: 10.1210/jcem.87.8.8717. [DOI] [PubMed] [Google Scholar]
- Tata JR. Signalling through nuclear receptors. Nat Rev Mol Cell Biol. 2002;3:702–710. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Dancey JE. Molecular targeting: PI3 kinase pathway. An Oncol. 2004;15:233–239. doi: 10.1093/annonc/mdh932. [DOI] [PubMed] [Google Scholar]
- Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RW. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta -catenin/TCF-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Bai CX, Kaihara A, Ozaki E, Kawano T, Nakaya Y, Awais M, Sato M, Umezawa Y, Kurokawa J. Ginsenoside Re, a Main Phytosterol of Panax Ginseng, Activates Cardiac Potassium Channels via a Non-Genomic Pathway of Sex Hormones. Mol Pharmacol. 2006;70:1916–1924. doi: 10.1124/mol.106.028134. [DOI] [PubMed] [Google Scholar]