Abstract
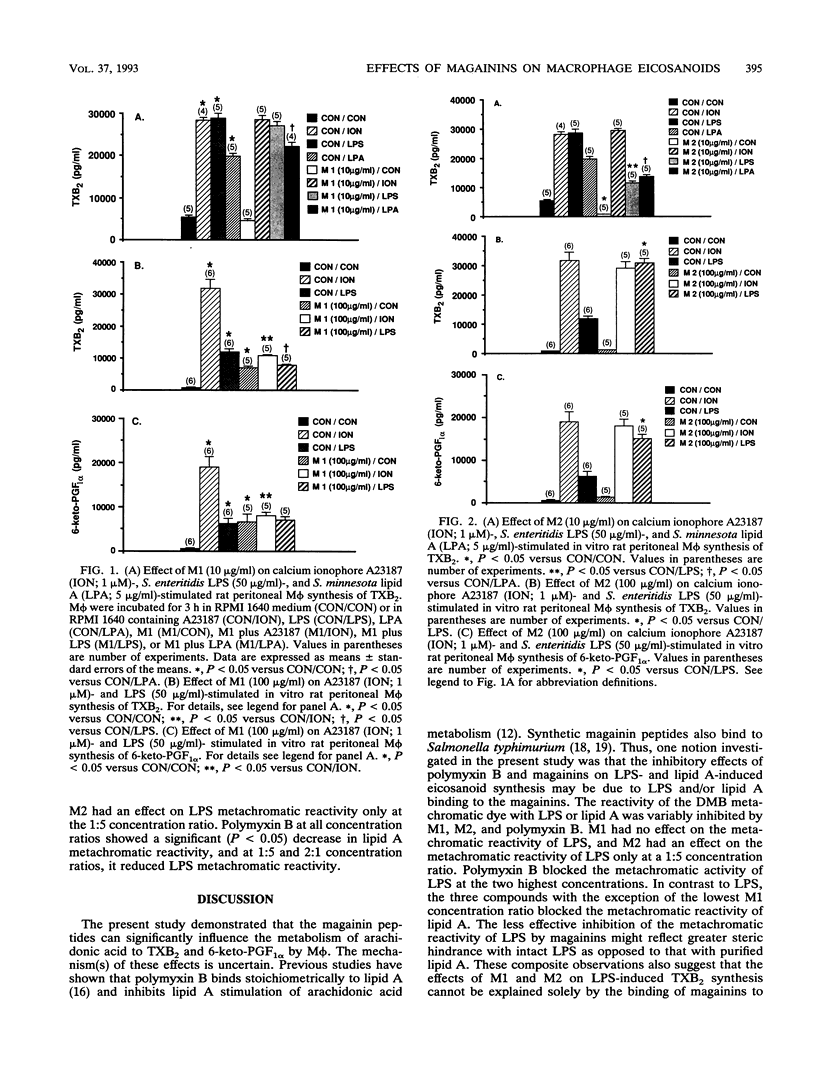
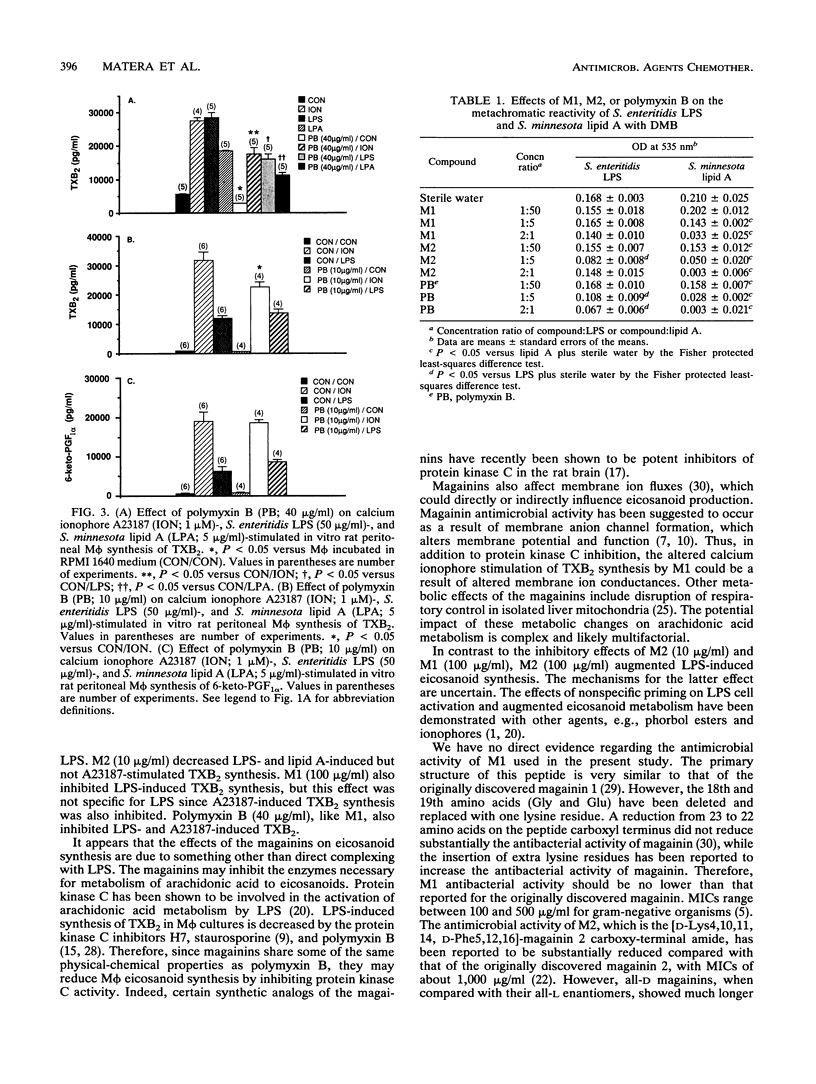
Magainins are novel polycationic peptides with broad-spectrum antimicrobial activity, including activity against gram-negative bacteria. Gram-negative bacteremia can elicit endotoxic shock that is associated with the increased formation of eicosanoids. Inhibition of eicosanoid synthesis has been shown to improve the outcome of experimental endotoxic shock. The aim of the present study was to test the in vitro effects of two magainin peptides, MSI-97 (M1) and MSI-98 (M2), on eicosanoid synthesis by rat peritoneal macrophages (M phi) stimulated by Salmonella enteritidis lipopolysaccharide (LPS; 50 micrograms/ml) and Salmonella minnesota lipid A (5 micrograms/ml) and to compare their effects on LPS reactivity with a metachromatic dye. M1 (100 micrograms/ml) significantly (P < 0.05) reduced LPS-stimulated synthesis of thromboxane B2 (TXB2), without changing 6-keto-prostaglandin F1 alpha in M phi. Similarly, M2 (10 micrograms/ml) significantly attenuated M phi synthesis of TXB2 stimulated by either LPS or lipid A. However, at a higher concentration (100 micrograms/ml), M2 but not M1 significantly augmented LPS-induced increases in TXB2 and 6-keto-prostaglandin F1 alpha. Polymyxin B (40 micrograms/ml) inhibited LPS production and lipid A-stimulated TXB2 production. M1 (100 micrograms/ml) and polymyxin B (10 and 40 micrograms/ml) also inhibited calcium ionophore A23187 (10 microM)-induced synthesis of TXB2. The lipid A moiety of LPS reacts with dimethylmethylene blue dye, providing a metachromatic assay of LPS. This metachromatic reaction with lipid A was significantly reduced by polymyxin B and M2 at all concentrations. M1 was effective only at the highest M1:lipid A concentration ratio (2:1). Thus, M1 and M2 share some similarities with polymyxin B in inhibiting lipid A reactivity with the dye, which suggests that these magainins may also bind to lipid A. However, M1 was devoid of any inhibitory effects on dye reactivity with S. enteritidis LPS and M2 was inhibitory at only one concentration ratio (1:5). In conclusion, the varied effects of the magainin peptides on LPS, lipid A, and M phi eicosanoid synthesis appear to depend on the type of peptide used and on its concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz B. A., Bevins C. L., Zasloff M. A. Magainins: a new family of membrane-active host defense peptides. Biochem Pharmacol. 1990 Feb 15;39(4):625–629. doi: 10.1016/0006-2952(90)90138-b. [DOI] [PubMed] [Google Scholar]
- Bessalle R., Haas H., Goria A., Shalit I., Fridkin M. Augmentation of the antibacterial activity of magainin by positive-charge chain extension. Antimicrob Agents Chemother. 1992 Feb;36(2):313–317. doi: 10.1128/aac.36.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Knapp D. R., Halushka P. V. Vasopressin stimulates thromboxane synthesis in the toad urinary bladder: effects of imidazole. J Pharmacol Exp Ther. 1979 Sep;210(3):344–348. [PubMed] [Google Scholar]
- Chen H. C., Brown J. H., Morell J. L., Huang C. M. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 1988 Aug 29;236(2):462–466. doi: 10.1016/0014-5793(88)80077-2. [DOI] [PubMed] [Google Scholar]
- Flynn P. M., Shenep J. L., Stokes D. C., Fairclough D., Hildner W. K. Polymyxin B moderates acidosis and hypotension in established, experimental gram-negative septicemia. J Infect Dis. 1987 Nov;156(5):706–712. doi: 10.1093/infdis/156.5.706. [DOI] [PubMed] [Google Scholar]
- Geisel J., Cook J. A., Coffee K. A., Wise W. C., Halushka P. V. Endotoxin-induced arachidonic acid metabolism requires de novo protein synthesis and protein kinase C activation. Biochim Biophys Acta. 1991 Aug 20;1085(1):15–20. doi: 10.1016/0005-2760(91)90226-8. [DOI] [PubMed] [Google Scholar]
- Juretić D., Chen H. C., Brown J. H., Morell J. L., Hendler R. W., Westerhoff H. V. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989 Jun 5;249(2):219–223. doi: 10.1016/0014-5793(89)80627-1. [DOI] [PubMed] [Google Scholar]
- Keler T., Nowotny A. Metachromatic assay for the quantitative determination of bacterial endotoxins. Anal Biochem. 1986 Jul;156(1):189–193. doi: 10.1016/0003-2697(86)90172-7. [DOI] [PubMed] [Google Scholar]
- Lüderitz T., Brandenburg K., Seydel U., Roth A., Galanos C., Rietschel E. T. Structural and physicochemical requirements of endotoxins for the activation of arachidonic acid metabolism in mouse peritoneal macrophages in vitro. Eur J Biochem. 1989 Jan 15;179(1):11–16. doi: 10.1111/j.1432-1033.1989.tb14514.x. [DOI] [PubMed] [Google Scholar]
- Matera G., Cook J. A., Hennigar R. A., Tempel G. E., Wise W. C., Oglesby T. D., Halushka P. V. Beneficial effects of a 5-lipoxygenase inhibitor in endotoxic shock in the rat. J Pharmacol Exp Ther. 1988 Oct;247(1):363–371. [PubMed] [Google Scholar]
- Matsuzaki K., Harada M., Handa T., Funakoshi S., Fujii N., Yajima H., Miyajima K. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim Biophys Acta. 1989 May 19;981(1):130–134. doi: 10.1016/0005-2736(89)90090-4. [DOI] [PubMed] [Google Scholar]
- Mazzei G. J., Katoh N., Kuo J. F. Polymyxin B is a more selective inhibitor for phospholipid-sensitive Ca2+-dependent protein kinase than for calmodulin-sensitive Ca2+-dependent protein kinase. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1129–1133. doi: 10.1016/0006-291x(82)91894-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Brown J. H., Morell J. L., Chen H. C., Huang K. P. Phosphorylation of magainin-2 by protein kinase C and inhibition of protein kinase C isozymes by a synthetic analogue of magainin-2-amide. FEBS Lett. 1990 Jul 2;267(1):135–138. doi: 10.1016/0014-5793(90)80307-5. [DOI] [PubMed] [Google Scholar]
- Rana F. R., Sultany C. M., Blazyk J. Interactions between Salmonella typhimurium lipopolysaccharide and the antimicrobial peptide, magainin 2 amide. FEBS Lett. 1990 Feb 26;261(2):464–467. doi: 10.1016/0014-5793(90)80616-q. [DOI] [PubMed] [Google Scholar]
- Rana F., Blazyk J. Outer membrane structure in smooth and rough strains of Salmonella typhimurium and their susceptibility to the antimicrobial peptides, magainins and defensins. Prog Clin Biol Res. 1989;292:77–85. [PubMed] [Google Scholar]
- Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J Biol Chem. 1989 Jun 5;264(16):9118–9121. [PubMed] [Google Scholar]
- Shenep J. L., Mogan K. A. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984 Sep;150(3):380–388. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- Soravia E., Martini G., Zasloff M. Antimicrobial properties of peptides from Xenopus granular gland secretions. FEBS Lett. 1988 Feb 15;228(2):337–340. doi: 10.1016/0014-5793(88)80027-9. [DOI] [PubMed] [Google Scholar]
- Terry A. S., Poulter L., Williams D. H., Nutkins J. C., Giovannini M. G., Moore C. H., Gibson B. W. The cDNA sequence coding for prepro-PGS (prepro-magainins) and aspects of the processing of this prepro-polypeptide. J Biol Chem. 1988 Apr 25;263(12):5745–5751. [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff H. V., Juretić D., Hendler R. W., Zasloff M. Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6597–6601. doi: 10.1073/pnas.86.17.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- Wise W. C., Cook J. A., Halushka P. V., Knapp D. R. Protective effects of thromboxane synthetase inhibitors in rats in endotoxic shock. Circ Res. 1980 Jun;46(6):854–859. doi: 10.1161/01.res.46.6.854. [DOI] [PubMed] [Google Scholar]
- Wrenn R. W., Wooten M. W. Dual calcium-dependent protein phosphorylation systems in pancreas and their differential regulation by polymyxin B1. Life Sci. 1984 Jul 16;35(3):267–276. doi: 10.1016/0024-3205(84)90110-3. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Martin B., Chen H. C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc Natl Acad Sci U S A. 1988 Feb;85(3):910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]


