Abstract
Supine weight-bearing exercise within lower body negative pressure (LBNP) alleviates some of the skeletal deconditioning induced by simulated weightlessness in men. We examined the potential beneficial effect in women. Because dietary acid load affected the degree of bone resorption in men during bed rest, we also investigated this variable in women. Subjects were 7 pairs of female identical twins assigned at random to 2 groups, sedentary bed rest (control) or bed rest with supine treadmill exercise within LBNP. Dietary intake was controlled and monitored. Urinary calcium and markers of bone resorption were measured before bed rest (BR) and on BR days 5/6, 12/13, 19/20, and 26/27. Bone mineral content was assessed by dual-energy X-ray absorptiometry before and after bed rest. Data were analyzed by repeated measures two-way analysis of variance. Pearson correlation coefficients were used to define the relationships between diet and markers of bone metabolism, and to estimate heritability of markers. During bed rest, all markers of bone resorption and urinary calcium and phosphorus increased (P < 0.001); parathyroid hormone (P = 0.06), bone-specific alkaline phosphatase (P = 0.06), and 1,25-dihydroxyvitamin D (P = 0.09) tended to decrease. LBNP exercise tended to mitigate bone density loss. The ratio of dietary animal protein to potassium was positively correlated with urinary calcium excretion for all weeks of bed rest in the control group, but only during weeks 1 and 3 for the exercise group. Pre-bed rest data suggested that many markers of bone metabolism have strong genetic determinants. Treadmill exercise within LBNP had less of a protective effect on bone resorption during bed rest in women than previously-published results had shown for its effect in men, but the same trends were observed for both sexes. Dietary acid load of these female subjects was significantly correlated with calcium excretion but not with other bone resorption markers.
Keywords: Exercise, Nutrition, Osteoporosis, Bone turnover markers, Weightlessness
Introduction
Bone loss during space flight remains a critical issue for the health of astronauts during long-duration missions [22]. Data from Skylab and Mir missions show that astronauts continue to lose bone despite participation in vigorous in-flight exercise protocols [11, 26, 35, 41]. The in-flight exercise program on early International Space Station (ISS) flights was also not successful at mitigating weightlessness-induced bone loss [25], although exercise hardware availability in those missions was inconsistent.
The effectiveness of treadmill exercise in preventing bone loss may be compromised by the low loading that can be comfortably achieved using a harness and bungee system to pull the crew member toward the running surface. Mir crew members using treadmill exercise with bungee-cord restraints had maximum mechanical loads of only 60–70% of body weight [44]. A similar loading configuration has been used on the ISS with a resistive exercise device, and in ISS crew members losses of bone mineral density were similar to those on Mir [25]. It is generally accepted that higher-impact exercise is more effective at maintaining bone than lower-impact exercise [14, 39, 46]. If high reaction forces could be developed through a more comfortable loading system, then maintenance of greater bone mineral density may result.
Because space flight research opportunities are limited, with small sample sizes and few flights, much of the research for countermeasure development is completed using ground-based analogs. Bed rest is a well-accepted analog for many of the physiological effects of space flight, especially musculoskeletal changes [5, 12, 13, 27, 34, 47, 51].
In a recent study of male identical twins [28], we found that supine treadmill exercise within lower body negative pressure (LBNP) mitigated some of the muscle [9] and bone [36] deconditioning associated with microgravity simulated by head-down-tilt bed rest. Moreover, dietary patterns in these subjects had a noticeable effect on the degree of bone loss during bed rest [49]. A higher ratio of acid to base precursors in the diet (estimated as the ratio of dietary animal protein to dietary potassium) had a greater negative effect on bone and calcium metabolism during bed rest than during ambulatory conditions. These findings were corroborated in a similar 30-d bed rest study in which use of an amino acid and carbohydrate supplement was associated with increased bone resorption in subjects who did not perform any exercise [50]. The proposed mechanism for the negative effect of dietary acid loads on bone is related to evidence that bone acts as a reservoir of basic ions to neutralize an acid load [8, 18, 31, 32, 51].
The primary objective of the present study was to determine if the LBNP treadmill exercise protocol would mitigate bone loss in women to a degree similar to that observed in men [36]. A secondary objective was to determine if the effect of dietary acid load on bone metabolism that we found in men [49] was evident in women.
A benefit of the design of this study is the use of identical twins as subjects [42]. This makes it possible to match subjects within groups for age, genetic makeup, and many environmental exposures [42]. Thus, a tertiary objective of this study was to estimate heritability of markers of bone metabolism.
Materials and methods
We have previously published reports [9, 19, 28, 36, 49] of a study with male subjects in which we used procedures identical to those used for the study reported here. Although in this report we compare the data from female subjects with data from male subjects published earlier [36, 49], none of the data from female subjects, nor the heritability data for men or women, have been published previously.
A 30-d bed rest study with 6° head-down tilt was conducted at the University of California, San Diego (UCSD) in the General Clinical Research Center (GCRC). Subjects were ambulatory for 6 d before beginning bed rest, and they remained ambulatory in the GCRC for 3 d of recovery after the 30-d bed rest. The protocol was approved by the National Aeronautics and Space Administration (NASA) Johnson Space Center Committee for the Protection of Human Subjects and the UCSD Institutional Review Board. All subjects provided written informed consent before participating.
Design
Seven pairs of identical twin women (mean age 24.4 y ± 2.6, weight 56.5 ± 9 kg) participated. One sister of each twin pair was randomly assigned to a control group (CON), while the other sister was assigned to an exercise (EX) group. All subjects were not studied concurrently; typically 2 pairs of identical twins were housed in the GCRC at a given time.
Before and during the 30-d bed rest protocol, all subjects were restricted to food and fluid intakes provided by the UCSD GCRC.
Menstrual cycles were not controlled for during this study. The dates of the last menstrual cycle before bed rest were recorded for 4 sets of twins, and the cycles during bed rest were predicted from those dates.
LBNP exercise protocol
The EX subjects exercised in an LBNP chamber with a sealed flexible waist seal [9, 28, 36, 43]. On 6 d per week, during LBNP, the EX subjects exercised for 40 min (40–80% pre-bed rest peak oxygen consumption) and then rested for 5 min during LBNP. Previous works describe LBNP exercise equipment and procedures in more detail [43].
Sample collection and biochemical measurements
Fasting (8 h) blood samples were collected three times before bed rest, on bed rest days 5, 12, 19, 26, and 30 (last day), and on post-bed rest days 1 and 2. Blood was collected into standard tubes, and was processed and aliquoted per standard conditions. Urine was collected (24-h pools) for 3 consecutive days before bed rest, on bed rest days 5 and 6, 12 and 13, 19 and 20, and 26 and 27, and for the first 2 days after bed rest. All urine samples were refrigerated immediately after collection, and aliquots were prepared for individual analytes. Serum and urine aliquots were stored at −70°C until analysis after completion of the study. Biochemical markers of bone and calcium metabolism were measured as previously described [36, 38].
DEXA
Measurements of each subject’s regional bone densities were made using a total-body DEXA scan (Lunar DPX-IQ or Hologic Delphi W). Scanning of a set of twins was performed on the same days, before and after bed rest. Two different facilities were used, one at UCSD Thornton Hospital (Lunar DPX-IQ) and the other at the UCSD GCRC (Hologic Delphi W). The same device was used for all pre- and post-bed rest measurements of a given set of twins. The scans were carried out using standard clinical protocol under standard conditions, and body position was monitored to be sure it was the same before and after bed rest
Bone mineral density (BMD) results were obtained by DEXA for the total body and 12 specific skeletal subregions. In each scan, subregions of interest were defined by standard DEXA protocol. When the automated analysis of a subregion of interest was not consistent with standard protocols, the subregion was re-analyzed manually by a single technician. The precision of the DEXA scanners was maintained by regular calibration using a standardized phantom.
Diet and dietary assessments
All food was prepared and daily intakes recorded as previously described [49]. Subjects were placed on an isocaloric diet consisting of 15% protein, 55% carbohydrate, and 30% fat, with daily intake of 3500 mg sodium, 800 to 1200 mg calcium, and at least 25 g of dietary fiber (1 g fiber/100 kcal). Initially, energy expenditure was estimated using the Harris-Benedict equation [20] times an activity factor based on the individual’s reported activity level. Energy was provided at a level designed to maintain body weight, which was monitored daily, within 1 kg of pre-bed rest weight. When possible, individual diets were modified to meet the preferences of each subject while maintaining the nutrient requirements outlined above. An 8-d menu cycle was used during the study. Nutrient data for all foods were obtained using Nutritionist IV (First DataBank, The Hearst Corporation, San Bruno, CA, USA).
Statistical analyses
Markers of bone metabolism and mineral metabolism were analyzed using 2-way repeated measures analysis of variance (ANOVA), with time as the repeated factor. The Bonferroni t-test was used post hoc to determine main-effect differences between the bed rest or reambulation period and the pre-bed rest period, and to assess differences between treatment groups over time. Linear associations between the ratio of animal or vegetable protein to potassium intake and urinary calcium or collagen crosslinks were described with Pearson correlation coefficients.
Since menstrual cycles were not controlled for in this study, a second analysis was performed to determine whether serum estradiol concentration had an effect on the crosslinks measured in this study. A repeated measures analysis of covariance (ANCOVA) was performed using the SAS (Cary, NC) procedure MIXED with estradiol levels as the covariable for all dependent variables. Default options for the MIXED procedure were used except that the best Kronecker product covariance structure for each data set was implemented. For all dependent variables except helical peptide (HP), the “un@un covariance structure” was used and for HP, the “un@ar@ covariance structure” was used. Research questions were addressed via a priori contrasts on adjusted means for the “treatment by test day” interaction for each dependent variable. Pair-wise contrasts among adjusted means from the ANCOVA were determined at each time point.
Four subjects’ (2 sets of twins) samples (blood and urine samples from before bed rest and bed rest days 4 and 12) thawed in transit from UCSD to the Johnson Space Center in Houston, but statistical analyses were performed with these samples included in the data set unless there was reason to believe that thawing would have affected measurement of an analyte (for example, osteocalcin rapidly degrades at temperatures greater than 4°C [33]). Whenever the thawing of samples affected the results, the differences between statistical analyses of all samples and analyses of all minus the thawed samples are reported.
The broad-sense heritability factor (H2) is an estimation of the portion of the total variance of a given phenotype that is attributable to genetic variance [21]. Knowing H2 in a study such as this is beneficial, to determine if heritability can explain most of the variance between twins in markers of bone metabolism before bed rest. If certain markers are in fact heritable and therefore similar between twins (groups) before bed rest, then any changes in these markers during the study are more likely to be the result of the treatment during the study. Heritability of markers of bone metabolism was assessed by determining the degree of similarity between the identical twins for the average pre-bed rest data point. Pre-bed rest data from one set of female twins who did not complete the bed rest portion of the study were included in the heritability analysis (n = 8 twin sets). The degree of similarity was evaluated by determining the correlation coefficient. The heritability of markers of bone metabolism in men was determined by using raw data from a study that was published previously except for heritability data. Marker heritability in men and women was compared by using analysis of covariance to compare slopes. These statistical analyses were performed using SAS, SigmaStat (SPSS), and StatView (Abacus Concepts, Inc.); the level of significance was 0.05.
Results
Effects of bed rest
Subjects did not lose weight during bed rest (mean ± SD weights were 56.5 ± 9.3 and 57.0 ± 9.6 kg before and after the study, respectively). Daily nutrient intake increased similarly during bed rest in the CON and EX groups (CON: 1967 ± 136 and 2321 ± 462 kcal for pre-bed rest and bed rest, respectively; EX: 1910 ± 83 and 2301 ± 357 kcal for pre-bed rest and bed rest, respectively).
In both CON and EX groups, urinary calcium was elevated after the second week of bed rest (Table 1), and serum total calcium (Table 2) was significantly less on the 2nd day of recovery after bed rest than before bed rest.
Table 1.
Urinary markers of bone and calcium metabolism before, during, and after 30 days of bed rest
| Pre-BR | BR 5/6 | BR 12/13 | BR 19/20 | BR 26/27 | Post-BR | |
|---|---|---|---|---|---|---|
| Calcium (mmol/d) | ||||||
| CON | 3.95 ± 1.32 | 4.09 ± 1.31 | 5.38 ± 1.73* | 5.76 ± 1.81* | 6.64 ± 2.54* | 5.22 ± 2.83 |
| EX | 3.90 ± 1.81 | 4.34 ± 1.87 | 4.67 ± 1.89* | 4.41 ± 1.41* | 5.46 ± 1.95* | 3.67 ± 1.16 |
| n-telopeptide (nmol/d) | ||||||
| CON | 385 ± 112 | 424 ± 145 | 530 ± 190* | 552 ± 152* | 633 ± 178* | 495 ± 115* |
| EX | 337 ± 109 | 364 ± 108 | 503 ± 280* | 533 ± 275* | 498 ± 213* | 506 ± 274* |
| Pyridinium crosslinks (nmol/d) | ||||||
| CON | 198 ± 56 | 229 ± 55 | 269 ± 69* | 312 ± 71* | 364 ± 119* | 374 ± 130* |
| EX | 193 ± 70 | 199 ± 59 | 229 ± 54* | 265 ± 86* | 291 ± 95* | 263 ± 94* |
| Deoxypyridinoline (nmol/d) | ||||||
| CON | 50 ± 19 | 58 ± 22 | 75 ± 25 | 75 ± 26* | 97 ± 28* | 88 ± 27* |
| EX | 50 ± 20 | 52 ± 21 | 55 ± 14 | 68 ± 21* | 82 ± 29* | 77 ± 45* |
| Helical peptide (μg/d) | ||||||
| CON | 465 ± 183 | 595 ± 231 | 669 ± 232# | 755 ± 354* | 843 ± 339*# | 749 ± 267* |
| EX | 464 ± 300 | 426 ± 234 | 439 ± 162 | 508 ± 198* | 507 ± 224* | 481 ± 189* |
| Creatinine (mmol/d) | ||||||
| CON | 1105 ± 208 | 1127 ± 235 | 1203 ± 279 | 1189 ± 242 | 1315 ± 276** | 1294 ± 302** |
| EX | 1113 ± 137 | 1177 ± 266 | 1336 ± 282 | 1286 ± 149 | 1403 ± 326** | 1314 ± 286** |
| Phosphorus (mg/d) | ||||||
| CON | 491 ± 124 | 661 ± 145* | 686 ± 268 | 754 ± 206* | 830 ± 358* | 604 ± 207* |
| EX | 494 ± 173 | 646 ± 115* | 713 ± 267 | 688 ± 210* | 819 ± 281* | 585 ± 222* |
Subjects were identical twins assigned at random to sedentary bed rest (CON) or bed rest combined with lower body negative pressure treadmill exercise (EX). Data are means ± SD for 7 subjects per group. BR, bed rest. For each subject, Pre-BR represents an average of 3 consecutive-day data collections. Data for all other time points are the average of two 24-h urine collections. Significant differences from Pre-BR are denoted as
P < 0.001,
P < 0.01.
Significant differences between CON and EX groups, P < 0.05.
Table 2.
Serum markers of bone and calcium metabolism before, during, and after 30 days of bed rest
| Pre-BR | BR 4 | BR 12 | BR 19 | BR 26 | Post-BR 0 | Post-BR 1 | Post-BR 2 | |
|---|---|---|---|---|---|---|---|---|
| Calcium (mmol/L) | ||||||||
| CON | 2.34 ± 0.10 | 2.33 ± 0.12 | 2.35 ± 0.08 | 2.37 ± 0.13 | 2.37 ± 0.14 | 2.40 ± 0.13 | 2.34 ± 0.14 | 2.32 ± 0.15* |
| EX | 2.33 ± 0.12 | 2.31 ± 0.13 | 2.27 ± 0.10 | 2.29 ± 0.12 | 2.35 ± 0.13 | 2.35 ± 0.15 | 2.31 ± 0.13 | 2.28 ± 0.15* |
| 25(OH)-vitamin D (nmol/L) | ||||||||
| CON | 68 ± 41 | 68 ± 39 | 64 ± 34 | 65 ± 29 | 66 ± 30 | 61 ± 22 | 59 ± 20 | 65 ± 25 |
| EX | 57 ± 34 | 58 ± 30 | 52 ± 27 | 55 ± 28 | 55 ± 27 | 63 ± 31 | 60 ± 31 | 57 ± 28 |
| 1,25(OH)2-vitamin D (pmol/L) | ||||||||
| CON | 126 ± 46 | 119 ± 53 | 134 ± 42 | 110 ± 35 | 101 ± 32 | 107 ± 16 | 101 ± 44 | 117 ± 36 |
| EX | 128 ± 41 | 94 ± 23 | 110 ± 34 | 119 ± 37 | 111 ± 37 | 114 ± 24 | 101 ± 29 | 111 ± 41 |
| Parathyroid hormone (pg/mL) | ||||||||
| CON | 20.8 ± 4.0 | 18.9 ± 3.7 | 17.0 ± 2.7 | 17.7 ± 5.2 | 16.6 ± 5.1 | 15.8 ± 4.4 | 18.4 ± 4.9 | 19.9 ± 6.3 |
| EX | 20.5 ± 6.0 | 22.9 ± 3.9 | 24.0 ± 8.2 | 19.3 ± 6.4 | 18.1 ± 2.9 | 19.1 ± 4.1 | 17.7 ± 2.8 | 20.2 ± 5.7 |
| BSAP (μg/L) | ||||||||
| CON | 18.7 ± 5.7 | 19.5 ± 5.3 | 18.5 ± 4.9 | 17.3 ± 4.0 | 17.8 ± 4.2 | 18.9 ± 3.6 | 18.2 ± 4.0 | 16.4 ± 2.9 |
| EX | 16.9 ± 3.1 | 15.7 ± 3.2 | 16.1 ± 3.1 | 15.1 ± 2.8 | 16.4 ± 3.4 | 15.2 ± 3.3 | 16.7 ± 2.9 | 16.1 ± 3.0 |
| Alkaline phosphatase (U/L) | ||||||||
| CON | 47 ± 14 | 47 ± 14 | 49 ± 14 | 47 ± 13 | 49 ± 12 | 47 ± 14 | 47 ± 14 | 45 ± 13 |
| EX | 47 ± 12 | 43 ± 10 | 40 ± 8 | 40 ± 8 | 42 ± 9 | 43 ± 12 | 43 ± 9 | 42 ± 10 |
| Osteocalcin (ng/mL)** | ||||||||
| CON | 12.4 ± 2.2 | 13.0 ± 3.1 | 12.9 ± 3.2 | 13.1 ± 2.7 | 14.2 ± 3.0 | 13.9 ± 3.6 | 11.5 ± 2.2 | 11.9 ± 2.6 |
| EX | 10.3 ± 3.3 | 10.4 ± 4.0 | 10.0 ± 3.3 | 12.4 ± 2.3 | 13.3 ± 2.4 | 12.3 ± 2.0 | 11.7 ± 1.4 | 11.1 ± 1.2 |
Subjects were identical twins assigned at random to sedentary bed rest (CON) or bed rest combined with lower body negative pressure treadmill exercise (EX). BR, bed rest. For each subject, Pre-BR represents an average of 3 consecutive-day data collections. Data are means ± SD for 7 subjects per group, except for osteocalcin, for which n = 5.
P < 0.001, different from Pre-BR.
There was a significant effect of time for osteocalcin, but the Bonferroni t-test revealed no significant difference between any BR value and the Pre-BR value.
In both groups, urinary n-telopeptide (NTX) excretion was greater during weeks 2 through 4 of bed rest than before bed rest, and it continued to be elevated after bed rest (Table 1). When the 4 frozen/thawed samples were excluded from the data set, in both CON and EX subjects NTX was significantly elevated during weeks 2 through 4 of bed rest, but was not different after bed rest. Other markers of bone resorption also had increased excretion rates during bed rest (Table 1). In both groups, excretion rates of pyridinium (PYD), deoxypyridinoline (DPD), and HP were elevated after the second (PYD) or third (DPD and HP) week of bed rest and had not returned to pre-bed rest levels by the end of the ambulatory post-bed rest recovery period. When the 4 frozen/thawed samples were excluded from the data set, DPD was significantly elevated after the second week of bed rest, and did not return to pre-bed rest levels after bed rest. When the rate of HP excretion by the women in this study was compared to that by males in a study with an identical protocol, the female data had more inter-individual variability (Fig. 1) [36]. HP data were not available when the male data were published. We have previously reported good correlations between HP and NTX excretion [52]. In Fig. 2, excretion of bone resorption markers by the EX group is expressed as a percentage of their excretion by the CON group and shown with previously published male data from an identical protocol [36]. For all of the markers of bone resorption, Figure 2 illustrates differences between female and male data. The EX group was significantly different from the CON group for the males but not for the females.
Fig. 1.
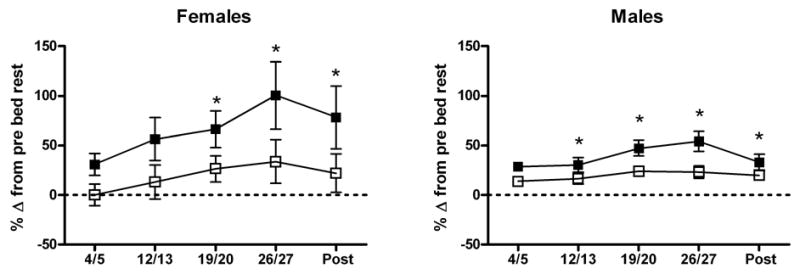
Urinary excretion of helical peptide, a bone resorption marker, during 30 d of head-down-tilt bed rest in sedentary (CON, ■) or exercise (EX, □) subjects. Data are expressed as percent change from pre-bed rest, calculated for each subject, with mean and SEM calculated for each group. Statistical analyses were performed on raw data. *Significant effect of bed rest, P < 0.001. Data for males are from an identical study performed with men as described previously [36].
Fig. 2.
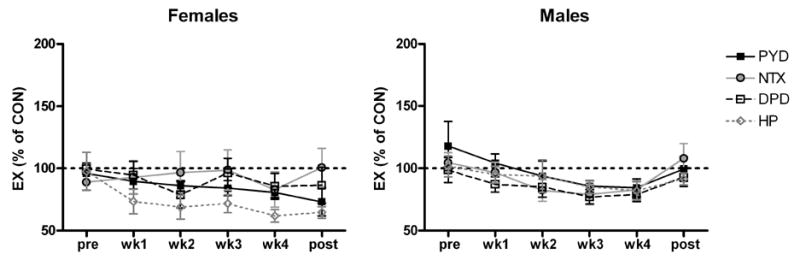
Urinary excretion of collagen crosslinks from the EX group expressed as a percentage of the crosslink in the CON group. Each EX twin was matched with their corresponding CON twin to determine the mean. Data are means ± SEM. Statistics were performed on raw data and are not presented here.
In both groups, urinary creatinine was significantly greater during the last week of bed rest and after bed rest than it was before bed rest (Table 1). However, when the two sets of samples that had thawed in transit were excluded from the data set, no significant differences in urinary creatinine occurred for the duration of the study.
For the subjects whose past menstrual cycles were recorded and used to predict their cycles during bed rest, the cycle was to start on average on bed rest day 9 (minimum day 3, maximum day 16). Within pairs of twins, cycles were an average of 4 days apart. In Fig. 3, serum estradiol concentration of the EX group is expressed as a percentage of the CON group to show that hormone levels of identical twins were not necessarily similar. The fluctuation in estradiol could explain some of the variability in crosslink excretion. When the crosslink data were analyzed with estradiol as a covariant, HP excretion was significantly greater in the CON group than in the EX group during weeks 2 (P < 0.05) and 4 (P < 0.01) during bed rest as well as after bed rest (P < 0.05).
Fig. 3.
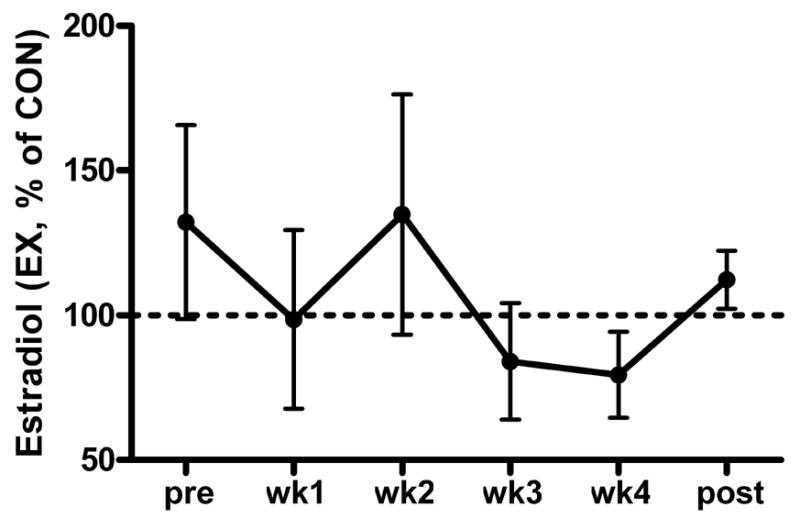
Serum estradiol from the EX subjects expressed as a percentage of serum estradiol of the corresponding CON twin. Data are means ± SEM.
Bone formation markers were not significantly changed during bed rest, although serum bone-specific alkaline phosphatase (BSAP) concentrations tended to decrease during bed rest (P = 0.06) (Table 2). For serum osteocalcin, the main ANOVA effect of time was statistically significant (P < 0.01), but post hoc comparisons showed that no time points were significantly greater than pre-bed rest in either group. During bed rest, reductions tended to occur in the active form of vitamin D, 1,25-dihydroxyvitamin D (P = 0.09), and parathyroid hormone (PTH) (P = 0.06).
Effects of exercise
Four of seven subjects exercised at least three sessions at 1.05 times one body weight (BW). The mean loading was 1.0 ± 0.1 BW.
Urinary calcium excretion in the EX group tended to be less than Ca excretion in the CON group (P = 0.05), but no differences between groups were found when comparisons were evaluated using the Bonferroni t-test (Table 1).
Urinary excretion of HP was significantly less in the EX group than in the CON group during weeks 2 and 4 of bed rest (Table 1).
Effects on bone mineral density
A significant interaction (P < 0.01) was found between treatment and time for the BMD in the femoral shaft region and total hip. Mean BMD in the femoral shaft and hip regions of EX twins after bed rest was not significantly different from their BMD before bed rest, but both femoral shaft and total hip BMD in the CON group were lower after bed rest (Table 3). BMD in other regions of the body showed no significant difference between the EX and CON groups.
Table 3.
Pre- to post-bed rest change in regional bone densities (DEXA)
| Region | Treatment | Pre-BR (g/cm2) | Post-BR (g/cm2) |
|---|---|---|---|
| Total body | CON | 1.100 ± 0.056 | 1.103 ± 0.047 |
| EX | 1.099 ± 0.079 | 1.098± 0.075 | |
| Legs | CON | 1.131 ± 0.069 | 1.126 ± 0.065 |
| EX | 1.129 ± 0.103 | 1.123 ± 0.098 | |
| Femoral neck | CON | 0.967 ± 0.123 | 0.966 ± 0.115 |
| EX | 0.956 ± 0.153 | 0.975 ± 0.159 | |
| Femoral shaft* | CON | 1.126 ± 0.063 | 1.103 ± 0.068 |
| EX | 1.108 ± 0.154 | 1.113 ± 0.153 | |
| Total hip* | CON | 0.976 ± 0.058 | 0.960 ± 0.061 |
| EX | 0.968 ± 0.122 | 0.971 ± 0.121 |
Subjects were identical twins assigned at random to sedentary bed rest (CON) or bed rest combined with lower body negative pressure treadmill exercise (EX). Data are means ± SD for 7 subjects per group.
Significant interaction (P < 0.01) between treatment and time, with bone density being lower after bed rest (BR) in the CON group but with no change in bone density after BR in the EX group.
Effect of diet
In the CON group, the ratio of dietary animal protein to dietary potassium was positively correlated with urinary calcium excretion during all weeks of bed rest (0.82, 0.95, 0.89, 0.84 for weeks 1–4 of bed rest, respectively, P <0.05). In this group, the slope of the regression line increased as the duration of bed rest increased (Fig. 4). Unlike what was observed in men [36], in both groups of women no significant associations were found between dietary animal protein:potassium and other markers of bone resorption.
Fig. 4.
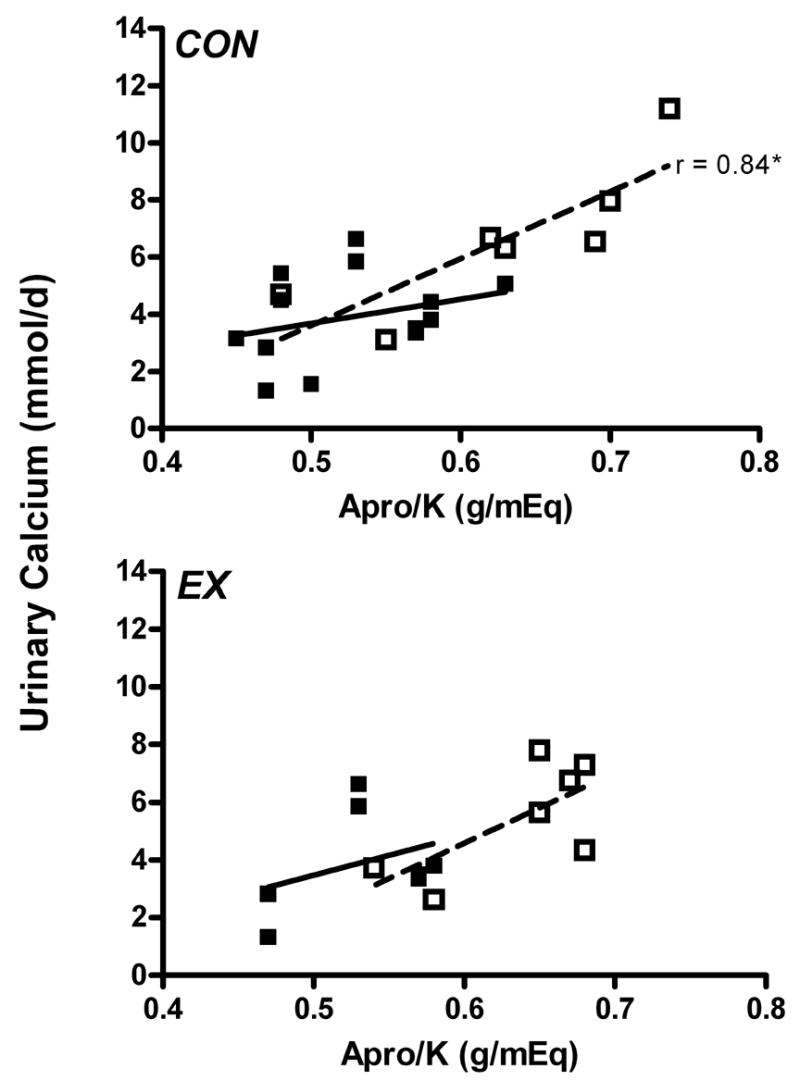
Correlation between urinary calcium excretion and the ratio of animal protein to potassium intake (Apro/K) before bed rest (■) and for bed rest week 4 (□). CON, control group; EX, exercise group. *P < 0.05.
Heritability of bone markers
Heritability estimates (H2) were determined for identical male twins who participated in a previous study [36] and for the women in the current study (Table 4, Fig. 5). All of the estimates were significant, except for PYD and BSAP in men. The only variable that had different slopes for the data from male and female subjects was DPD (Table 4).
Table 4.
Slopes and heritability coefficients for markers of bone metabolism in men and women
| Men | Women | |||
|---|---|---|---|---|
| Slope | H2 (%) | Slope | H2 (%) | |
| PTH | 1.31 ± 0.24 | 92* | 1.24 ± 0.32 | 85* |
| 25-OH vitamin D | 0.64 ± 0.18 | 82* | 0.67 ± 0.19 | 82* |
| 1,25-(OH)2 vitamin D | 0.66 ± 0.23 | 76* | 0.80 ± 0.16 | 90* |
| BSAP | 0.64 ± 0.28 | 68 | 0.39 ± 0.11 | 82* |
| Osteocalcin | 0.60 ± 0.21 | 77* | 0.65 ± 0.17 | 82* |
| DPD | 0.48 ± 0.11** | 88* | 1.03 ± 0.12 | 96* |
| NTX | 0.78 ± 0.11 | 94* | 0.75 ± 0.23 | 80* |
| PYD | 0.46 ± 0.34 | 49 | 1.06 ± 0.23 | 88* |
| Helical peptide | 0.77 ± 0.11 | 95* | 1.15 ± 0.52 | 78* |
| Urinary calcium | 0.96 ± 0.09 | 97* | 1.23 ± 0.25 | 89* |
Subjects were identical twins assigned at random to sedentary bed rest (CON) or bed rest combined with lower body negative pressure treadmill exercise (EX). Slope data are means ± SD for 8 subjects per group for all variables except osteocalcin, for which n = 6.
Heritability coefficients are statistically significant, P < 0.05.
Slopes for men and women are different, P < 0.05.
Fig. 5.
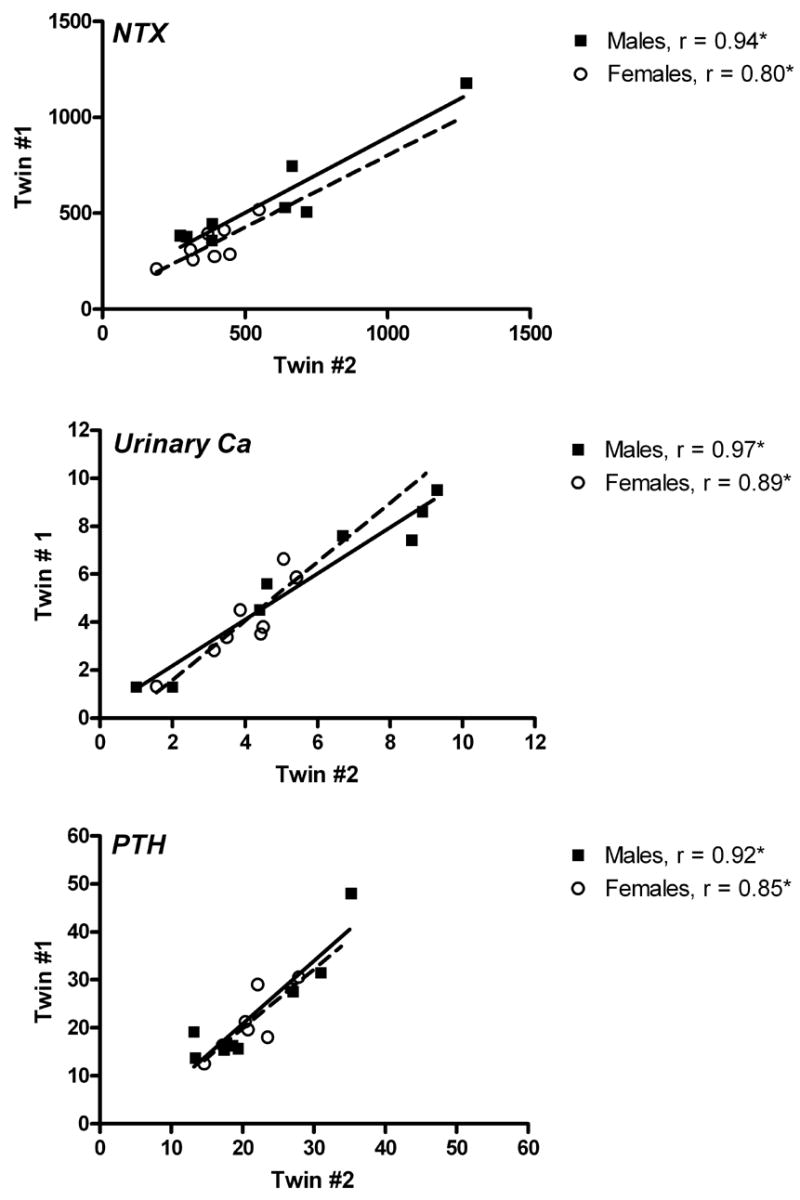
Correlation of parameters of pre-bed rest bone metabolism for male (solid line) and female (dashed line) twin pairs. Data are plotted as twin #1 vs twin #2 for urinary n-telopeptide excretion (NTX, nmol/d), urinary calcium excretion (mmol/d), and serum parathyroid hormone (PTH, pg/mL). Pearson correlation coefficients are reported. *P < 0.05.
Discussion
Supine treadmill exercise within LBNP effectively mitigated bone loss and changes in bone metabolism associated with simulated microgravity in men [9, 36], and while the results from women in the present study were not as consistent as those for men, similar trends existed. Femoral shaft and total hip BMD in the CON group was lower after bed rest than at baseline, but in the EX group there were no differences after bed rest. Urinary markers provide insight into the process of bone resorption, and they were consistent with the BMD data. A statistically significant difference between CON and EX groups was evident for only one measured crosslink, helical peptide. Variability was less among men than women, which contributed to gender differences in statistical significance. These trends between the CON and EX groups in women might have proven significant with a larger group size, but other explanations are also possible.
Different amounts of attenuation of bone resorption by exercise in male and female subjects might be related to their pre-bed rest fitness or tolerance of LBNP during the countermeasure. As a group, the male subjects were more fit and exercised at higher speeds than the women. Because of these factors, combined with a larger total body mass, the male subjects were likely to have experienced greater peak ground reaction forces, whether expressed in absolute terms or normalized to body mass [29]. Similarly, the male subjects exercised over a longer distance on the LBNP treadmill than the female subjects (men, 4.7 km/session; women, 4.3 km/session) and therefore may have had a greater total number of footfalls. In this setting, it would be impossible to determine which, if any, of these factors contributed most to greater protection against bone loss in the male subjects [36].
Other possible explanations for the disparate results between men and women are unrelated to the exercise. Data from several studies show that urinary bone resorption markers are more variable in women than men [6, 45]. Much of the variability is likely to be related to cyclic fluctuations of female sex hormones. Biochemical markers of bone resorption are clearly influenced by fluctuations in these hormones [10, 23, 48]. In one study, PYD varied as much as 50% from 3 days after ovulation to day 3 of the follicular period of the same menstrual cycle [48]. Because menstrual cycles were not controlled for in the present study, this variability with sex hormone fluctuations (Fig. 4) could explain why effects of exercise within LBNP were less obvious in women.
The effect of dietary patterns, specifically protein intake, on bone has been examined in many studies. The concept of evaluating the ratio of protein and potassium is based on a calculation to estimate net endogenous noncarbonic acid production [15]. Diet can influence endogenous acid production because food contains acid and base precursors (compounds that yield acid or base according to their metabolism post absorption) [32, 51]. An acidic metabolic environment has been theorized to stimulate bone resorption. Bone is a large reservoir for ions that can neutralize acid loads, and a small chronic alteration of acid-base homeostasis can induce noticeable changes in bone metabolism [2–4, 18]. Our previous results from bed rest studies in men [49, 50] led us to expect that the ratio of dietary animal protein to potassium would be positively associated with urinary calcium and markers of bone resorption in women. Although we did see a significant positive relationship with urinary calcium excretion during bed rest in both groups of women, the ratio had no similar relationship to markers of bone resorption. The reason for the difference between genders is unclear, but during bed rest, LBNP exercise or sex hormones may be more influential factors than diet, or may obscure its effect by increasing variability.
Collagen crosslink data are typically normalized to creatinine. This study provides evidence for why this should not be done: creatinine excretion significantly increased at the end of the study, thereby confounding the normalized crosslink results. We, and others, have previously shown that the approach of collecting 24-h pools, or even better, multiple 24-h pools significantly reduces the variability of collagen crosslinks [6, 17, 30, 37].
The use of identical twins in this study offered the advantage of matching treatment and control groups with respect to age, genetics, and environment to some degree. Because identical twins have identical genetics, we were able to estimate the heritability of traits related to bone and calcium metabolism. Further research using both monozygotic and dizygotic twins could more precisely determine which traits are attributable solely to genetic factors instead of a combination of genetic and environmental factors. Nonetheless, these data indicate that serum PTH and urinary excretion of calcium, NTX, DPD, and HP are predominantly heritable traits. Thus, the changes observed in these markers during bed rest are more clearly related to bed rest itself and/or the LBNP exercise. The heritability of these markers of bone resorption also provides a biochemical explanation for previously documented heritability of bone health [1, 7, 16, 24, 40]. The relatively high between-subject variability in bone markers is frequently criticized as limiting their utility, but our data suggest that the variability is likely related to genetics, and that studies to determine the causes of variability may reveal more about bone metabolism than expected.
Studies with flight analogs such as bed rest are critical for understanding and counteracting the negative effects of space flight. The constraints and limits on space flight research make analog studies essential to initial development of measures to counter bone loss associated with microgravity. Our present results document the difficulties in expanding findings to both sexes, and give reasons to evaluate gender differences in physiology further. They suggest that treadmill exercise within LBNP may be effective at mitigating bone loss associated with disuse in women, although a larger sample size would provide more powerful statistical analyses. These data, together with the male data previously published [36], provide preliminary evidence that LBNP exercise offers an effective countermeasure to mitigate bone loss associated with weightlessness. Further studies need to be performed to determine the ideal exercise time and intensity. Furthermore, studies need to be done to determine if confounding factors must be considered when designing countermeasures for bone loss associated with disuse in women.
Acknowledgments
We thank the staff of the USCD GCRC for their assistance in the conduct of this study, and especially Eva Brzezinski, M.S., R.D., for support of the dietary control and nutrient intake assessments. We also thank the identical twin pairs for their time and willingness to participate in this study. We also thank Eli Groppo, Debbie O’Leary, R. Scott Meyer, Dr. Micheal Zeigler, Kunihiko Tanaka, and Rachel Vanderlinden for their efforts on this project. The efforts of the staff of the NASA Johnson Space Center Nutritional Biochemistry Laboratory in the biological sample analyses and data management are appreciated. We thank Dick Calkins for advice and assistance with the statistical analyses, and Jane Krauhs for editing the manuscript.
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- BR
bed rest
- BSAP
bone-specific alkaline phosphatase
- BW
body weight
- DEXA
dual-energy X-ray absorptiometry
- DPD
deoxypyridinoline
- ELISA
enzyme-linked immunosorbent assay
- GCRC
General Clinical Research Center
- HP
helical peptide
- ISS
International Space Station
- LBNP
lower body negative pressure
- NASA
National Aeronautics and Space Administration
- NTX
n-telopeptide
- PTH
parathyroid hormone
- PYD
pyridinium
- RIA
radioimmunoassay
- UCSD
University of California, San Diego
Footnotes
All authors have no conflicts of interest.
Sources of support: This study was funded by NASA through grant NAG 9-1425 and by NIH through grant GCRC M01-RR00827 to UCSD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arden NK, Baker J, Hogg C, Baan K, Spector TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res. 1996;11:530–4. doi: 10.1002/jbmr.5650110414. [DOI] [PubMed] [Google Scholar]
- 2.Arnett T. Regulation of bone cell function by acid-base balance. Proc Nutr Soc. 2003;62:511–20. doi: 10.1079/pns2003268. [DOI] [PubMed] [Google Scholar]
- 3.Arnett TR, Dempster DW. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 1986;119:119–24. doi: 10.1210/endo-119-1-119. [DOI] [PubMed] [Google Scholar]
- 4.Arnett TR, Spowage M. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone. 1996;18:277–9. doi: 10.1016/8756-3282(95)00486-6. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Borderie D, Roux C, Toussaint B, Dougados M, Ekindjian OG, Cherruau B. Variability in urinary excretion of bone resorption markers: limitations of a single determination in clinical practice. Clin Biochem. 2001;34:571–7. doi: 10.1016/s0009-9120(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 7.Brown LB, Streeten EA, Shuldiner AR, Almasy LA, Peyser PA, Mitchell BD. Assessment of sex-specific genetic and environmental effects on bone mineral density. Genet Epidemiol. 2004;27:153–61. doi: 10.1002/gepi.20009. [DOI] [PubMed] [Google Scholar]
- 8.Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40:238–44. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- 9.Cao P, Kimura S, Macias BR, Ueno T, Watenpaugh DE, Hargens AR. Exercise within lower body negative pressure partially counteracts lumbar spine deconditioning associated with 28-day bed rest. J Appl Physiol. 2005;99:39–44. doi: 10.1152/japplphysiol.01400.2004. [DOI] [PubMed] [Google Scholar]
- 10.Chiu KM, Ju J, Mayes D, Bacchetti P, Weitz S, Arnaud CD. Changes in bone resorption during the menstrual cycle. J Bone Miner Res. 1999;14:609–15. doi: 10.1359/jbmr.1999.14.4.609. [DOI] [PubMed] [Google Scholar]
- 11.Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, Alexandre C. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–51. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson C, Hulley S, Vogel J, Hattner R, Bayers J, McMillan D. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19:1071–84. doi: 10.1016/0026-0495(70)90032-6. [DOI] [PubMed] [Google Scholar]
- 13.Dudley GA, Gollnick PD, Convertino VA, Buchanan P. Changes of muscle function and size with bedrest. Physiologist. 1989;32:S65–6. [PubMed] [Google Scholar]
- 14.Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA. Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 2005 doi: 10.1007/s00198-005-1938-9. [DOI] [PubMed] [Google Scholar]
- 15.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–83. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 16.Garnero P, Arden NK, Griffiths G, Delmas PD, Spector TD. Genetic influence on bone turnover in postmenopausal twins. J Clin Endocrinol Metab. 1996;81:140–6. doi: 10.1210/jcem.81.1.8550741. [DOI] [PubMed] [Google Scholar]
- 17.Ginty F, Flynn A, Cashman K. Inter and intra-individual variations in urinary excretion of pyridinium crosslinks of collagen in healthy young adults. Eur J Clin Nutr. 1998;52:71–3. doi: 10.1038/sj.ejcn.1600502. [DOI] [PubMed] [Google Scholar]
- 18.Green J, Kleeman CR. The role of bone in the regulation of systemic acid-base balance. Contrib Nephrol. 1991;91:61–76. doi: 10.1159/000420160. [DOI] [PubMed] [Google Scholar]
- 19.Hargens AR, Watenpaugh D, Lee SMC, Boda W, Smith SM, Macias B, Groppo E, Schneider S, O’Leary D, Meyer RS, Kawai Y. Physiologic countermeasures for long-duration space flight: review of treadmill exercise within lower body negative pressure. Journal of Adaption Medicine. 2004;7:2–6. [Google Scholar]
- 20.Harris JA, Benedict FG. A biometric study of basal metabolism in man, Publ 279. Washington, DC: Carnegie Institute of Washington; 1919. [Google Scholar]
- 21.Hartle DL, Jones EW. Genetics. Boston, MA: Jones Bartlett Publishers, Inc; 1998. [Google Scholar]
- 22.Holick MF. Microgravity-induced bone loss - will it limit human space exploration? Lancet. 2000;355:1569–70. doi: 10.1016/S0140-6736(80)02208-8. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss CE, Brommage R. Changes in bone turnover during the menstrual cycle in cynomolgus monkeys. Calcif Tissue Int. 2000;66:224–8. doi: 10.1007/s002230010044. [DOI] [PubMed] [Google Scholar]
- 24.Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16:371–8. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 25.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 26.LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. Bone mineral and lean tissue loss after long duration space flight. Journal of Musculoskeletal and Neuron Interaction. 2000;1:157–60. [PubMed] [Google Scholar]
- 27.LeBlanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–50. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 28.Macias BR, Groppo ER, Eastlack RK, Watenpaugh DE, Lee SM, Schneider SM, Boda WL, Smith SM, Cutuk A, Pedowitz RA, Meyer RS, Hargens AR. Space exercise and Earth benefits. Curr Pharm Biotechnol. 2005;6:305–17. doi: 10.2174/1389201054553653. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson J, Thorstensson A. Ground reaction forces at different speeds of human walking and running. Acta Physiol Scand. 1989;136:217–27. doi: 10.1111/j.1748-1716.1989.tb08655.x. [DOI] [PubMed] [Google Scholar]
- 30.Popp-Snijders C, Lips P, Netelenbos JC. Intra-individual variation in bone resorption markers in urine. Ann Clin Biochem. 1996;33 (Pt 4):347–8. doi: 10.1177/000456329603300411. [DOI] [PubMed] [Google Scholar]
- 31.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–61. doi: 10.1093/ajcn/59.6.1356. [DOI] [PubMed] [Google Scholar]
- 32.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–7. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 33.Seibel MJ, Woitge HW. Basic principles and clinical applications of biochemical markers of bone metabolism: biochemical and technical aspects. Journal of Clinical Densitometry. 1999;2:299–321. doi: 10.1385/jcd:2:3:299. [DOI] [PubMed] [Google Scholar]
- 34.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol. 1999;277(1 Pt 2):R1–10. doi: 10.1152/ajpregu.1999.277.1.r1. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Davis-Street JE, Fesperman JV, Calkins DS, Bawa M, Macias BR, Meyer RS, Hargens AR. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J Bone Miner Res. 2003;18:2223–30. doi: 10.1359/jbmr.2003.18.12.2223. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Dillon EL, DeKerlegand DE, Davis-Street JE. Variability of collagen crosslinks: impact of sample collection period. Calcif Tissue Int. 2004;74:336–41. doi: 10.1007/s00223-003-0149-7. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. Nutritional status assessment of International Space Station crew members. J Nutr. 2005;135:437–43. doi: 10.1093/jn/135.3.437. [DOI] [PubMed] [Google Scholar]
- 39.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep. 2005;3:46–51. doi: 10.1007/s11914-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 40.Tokita A, Kelly PJ, Nguyen TV, Qi JC, Morrison NA, Risteli L, Risteli J, Sambrook PN, Eisman JA. Genetic influences on type I collagen synthesis and degradation: further evidence for genetic regulation of bone turnover. J Clin Endocrinol Metab. 1994;78:1461–6. doi: 10.1210/jcem.78.6.8200950. [DOI] [PubMed] [Google Scholar]
- 41.Vico L, Collet P, Guignandon A, Lafage-Proust M-H, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–11. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 42.Wark JD, Nowson C. Influence of nutrition on bone health: The twin model approach. In: New SA, Bonjour JP, editors. Nutritional Aspects of Bone Health. Cambridge, UK: The Royal Society of Chemistry; 2003. [Google Scholar]
- 43.Watenpaugh DE, Ballard RE, Schneider SM, Lee SM, Ertl AC, William JM, Boda WL, Hutchinson KJ, Hargens AR. Supine lower body negative pressure exercise during bed rest maintains upright exercise capacity. J Appl Physiol. 2000;89:218–27. doi: 10.1152/jappl.2000.89.1.218. [DOI] [PubMed] [Google Scholar]
- 44.Whalen R. Musculoskeletal adaptation to mechanical forces on Earth and in space. Physiologist. 1993;36 (Suppl 1):S127–30. [PubMed] [Google Scholar]
- 45.Woitge HW, Scheidt-Nave C, Kissling C, Leidig-Bruckner G, Meyer K, Grauer A, Scharla SH, Ziegler R, Seibel MJ. Seasonal variation of biochemical indexes of bone turnover: results of a population-based study. J Clin Endocrinol Metab. 1998;83:68–75. doi: 10.1210/jcem.83.1.4522. [DOI] [PubMed] [Google Scholar]
- 46.Yung PS, Lai YM, Tung PY, Tsui HT, Wong CK, Hung VW, Qin L. Effects of weight bearing and non-weight bearing exercises on bone properties using calcaneal quantitative ultrasound. Br J Sports Med. 2005;39:547–51. doi: 10.1136/bjsm.2004.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 48.Zittermann A, Schwarz I, Scheld K, Sudhop T, Berthold HK, von Bergmann K, van der Ven H, Stehle P. Physiologic fluctuations of serum estradiol levels influence biochemical markers of bone resorption in young women. J Clin Endocrinol Metab. 2000;85:95–101. doi: 10.1210/jcem.85.1.6250. [DOI] [PubMed] [Google Scholar]
- 49.Zwart SR, Hargens AR, Smith SM. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr. 2004;80:1058–65. doi: 10.1093/ajcn/80.4.1058. [DOI] [PubMed] [Google Scholar]
- 50.Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM. Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol. 2005;99:134–40. [Google Scholar]
- 51.Zwart SR, Smith SM. The impact of space flight on the human skeletal system and potential nutritional countermeasures. Int SportMed Journal. 2005;6:199–214. [Google Scholar]
- 52.Zwart SR, Dekerlegand DE, Davis-Street JE, Smith SM. Assessment of urinary N-telopeptide: Point-of-care testing, sample types, and relationship to urinary helical peptide excretion. Clin Chim Acta. 2006;372:65–9. doi: 10.1016/j.cca.2006.03.013. [DOI] [PubMed] [Google Scholar]


