Abstract
Stress produces significant alterations in sleep that appear to vary with the type, intensity and duration of the stressor. Brief manual restraint may be stressful in rodents, but is often required for experimental procedures. We examined the effects of brief manual restraint on sleep and its possible influence on sleep induced after footshock and after the opportunity to explore a neutral enclosure. Sleep was recorded during non-interrupted baseline and during 8-h light and 12-h dark periods after three sessions of 5-min manual restraint (M1-3), after 30-min in neutral enclosure alone (NE) or with previous manual restraint (mNE), and after 20 footshocks presented over the course of 30-min alone (FS) or with previous manual restraint (mFS). Compared to baseline, M1-3 increased total sleep and NREM during both light and dark periods, and significantly increased dark period REM. Both NE and mNE increased dark-period total sleep, NREM and REM; however, mNE also increased light-period total sleep and NREM, but not REM. FS and mFS increased total sleep, NREM and REM during the dark period and total sleep and NREM during light period. FS also significantly decreased light-period REM whereas mFS did not. M1, mNE and mFS significantly increased EEG delta power during NREM, but M2-3, NE and FS alone did not. The results revealed that manual restraint can increase sleep and EEG delta power and that increases in sleep may persist across repeated sessions whereas the magnitude of EEG delta power may vary across sessions. In addition, prior manual restraint may significantly alter the changes in sleep and EEG induced by footshock and by the opportunity to explore a neutral enclosure. The results suggest that mild stressors may interact in their effects on sleep.
Keywords: Manual restraint, Sleep, Activity, EEG power, Footshock, Stress
1. Introduction
Stress can have significant effects on sleep that vary with the type, intensity and duration of the stressor. Stress initially produces arousal [5] though many acute stressors (e.g., restraint [3,9,11,13,15], novelty [23,25,27], cage change [25,28], ether exposure [2,16], water maze [24] and footshock [20,24]) have been associated with subsequent increases in rapid eye movement sleep (REM) and/or non-REM (NREM) that occur at various latencies after the stressor is removed. However, inescapable footshock and cued [17,19] and contextual [22,30] reminders of inescapable footshock may induce more persistent reductions in subsequent REM than other stressors suggesting that this paradigm may be a unique model for exploring the anatomical basis mediating disrupted sleep induced by stress.
Experiments often require brief manual procedures to restrain animals for manipulations such as local drug infusions. Interestingly, in rats, relatively shorter duration restraint may have greater impact on sleep than longer duration restraint [11]. For example, compared to non-interrupted baseline, 30- and 60-min restraint was followed by 32% and 25% increases in NREM, respectively, whereas 120- or 240-min restraint did not significantly change subsequent NREM [11]. This suggests that brief manual restraint may produce significant alterations in subsequent sleep. Repeated handling is a standard experimental procedure used as a control procedure in attempts to reduce the potential effects of handling on experimental variables. However, many of the handling procedures used for laboratory animals result in a stress response that does not appear to readily habituate [1] and the true effectiveness of repeated handling as a control in sleep studies is largely unknown.
A significant issue that has received minimal attention is the possibility that even mild stressors may interact in their effects on sleep. Forced wakefulness and restraint produce enhancements in subsequent sleep, and given together produce greater enhancements than either alone [8]. This suggests that treatments with similar directional changes may produce additive effects in subsequent sleep. To our knowledge, the effect on sleep produced by stressors with potentially opposite influences or different time courses has not been examined. Therefore, in this work, we examined sleep and EEG power after repeated once daily, five min sessions of manual restraint. Afterwards, we examined sleep and EEG power after sessions of five min manual restraint followed by footshock or inescapable footshock alone, which may produce relatively long initial reductions in REM [18,19,22,30]. We also examined sleep and EEG power after manual restraint followed by exposure to a neutral enclosure and after the neutral enclosure alone. A neutral enclosure also is sometime used as a handling control for footshock stress [30], and can produce an initial arousal followed by significant increases in subsequent sleep that may be related to novelty and exploration [25,27,28,30]. This allowed us to examine the effects of repeated sessions of brief manual restraint as well as how this type of handling procedure influenced stressors that produced different types of changes in sleep.
2. Results
2.1. Sleep and Activity
Compared to uninterrupted baseline, all experimental treatments significantly (p < 0.05, paired t-test) decreased first h total sleep, NREM and REM, and significantly increased first h activity counts. Therefore, to better represent the changes occurring after the first h of recording, we compared the sleep and activity data for 7-h (second to eighth h) of the light period and the entire 12-h dark period. Bar graphs for these data as well as hourly plots for each condition are presented for M1-3 (Fig. 1), NE and mNE (Fig. 2), FS and mFS (Fig. 3) and activity (Fig. 4), and the corresponding F- and p- values for the comparisons are presented in Table 1.
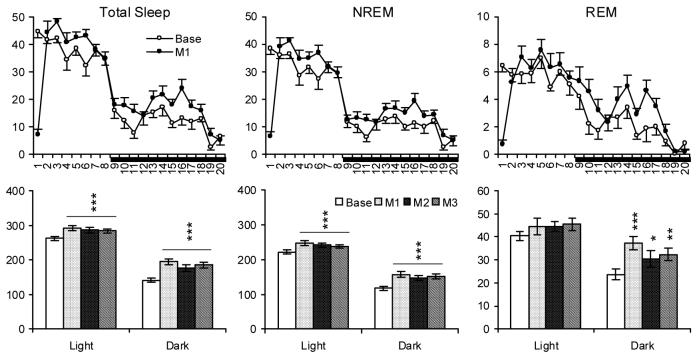
Fig. 1.
Total sleep, NREM and REM (min) plotted hourly (Upper Panels) and for totals during the 7-h light (from second to eighth h) and 12-h dark periods (Lower Panels) in time-matched baseline (Base) and after M1-3 (The effects for M1-3 were similar across days, and hourly data for M2-3 are not shown to simply the plots). Horizontal bar under the X-axis indicates the dark period. Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, ** < 0.01, *** p < 0.001, compared to Base).
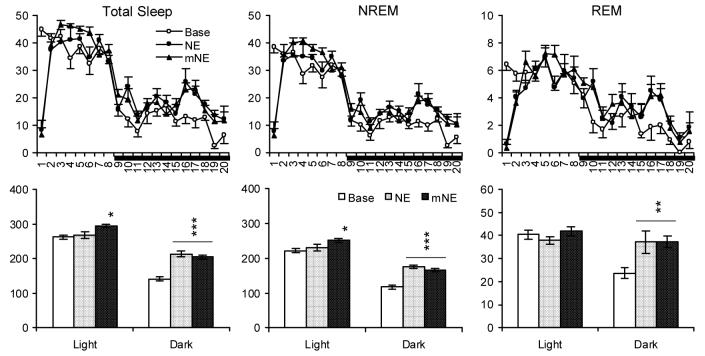
Fig. 2.
Total sleep, NREM and REM (min) plotted hourly (Upper Panels) and for totals during the 7-h light (from second to eighth h) and 12-h dark periods (Lower Panels) in time-matched baseline (Base) and after NE and mNE. Horizontal bar under the X-axis indicates the dark period. Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, ** < 0.01, *** p < 0.001, compared to Base).
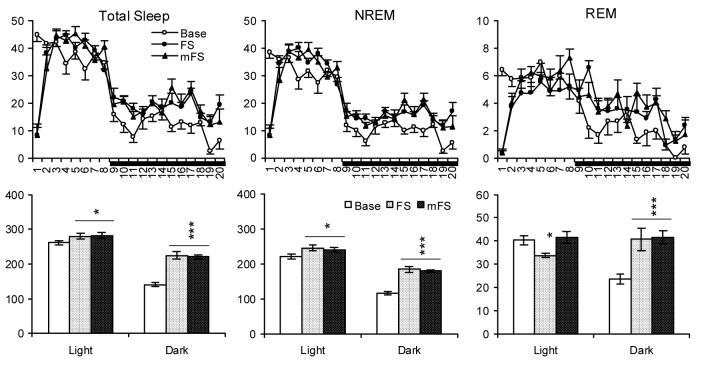
Fig. 3.
Total sleep, NREM and REM (min) plotted hourly (Upper Panels) and for totals during the 7-h light (from second to eighth h) and 12-h dark periods (Lower Panels) in time-matched baseline (Base) and after FS and mFS. Horizontal bar under the X-axis indicates the dark period. Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, *** p < 0.001, compared to Base).
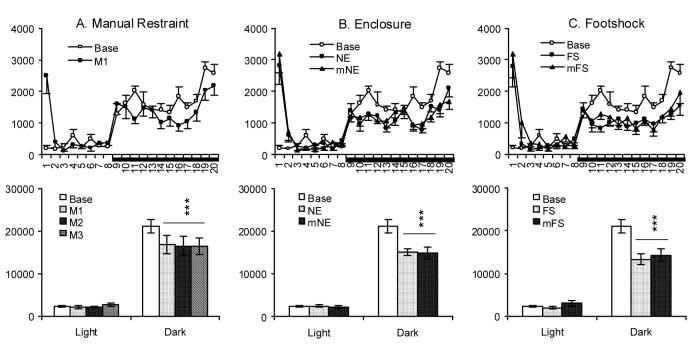
Fig. 4.
Activity (number of TTL pulses) plotted hourly (Upper Panels) and for totals during the 7-h light (from second to eighth h) and 12-h dark periods (Lower Panels) in time-matched baseline (Base) and after M1-3 (A, M1 only for hourly plots), NE and mNE (B), FS and mFS (C). Horizontal bar under the X-axis indicates the dark period. Values are means ± SEM. Comparisons were conducted with Tukey test (*** p < 0.001, compared to Base).
Table 1.
| TST | NREM | REM | ||||
|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | |
| Fig 1 | 24.24 | 24.54 | 20.44 | 19.25 | NS | 11.61 |
| F(3, 21) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| Fig 2 | 6.1 | 52.1 | 5.92 | 79.93 | NS | 11.9 |
| F(2, 13) | 0.014 | 0.0001 | 0.015 | 0.0001 | 0.001 | |
| Fig 3 | 4.99 | 58.94 | 6.47 | 77.84 | 6.54 | 17.66 |
| F(2, 13) | 0.023 | 0.0001 | 0.0001 | 0.0001 | 0.009 | 0.0002 |
| Fig 4 | A, F(3, 21) | B, F(2, 13) | C, F(2, 13) | |||
| Light | Dark | Light | Dark | Light | Dark | |
| NS | 12.64 | NS | 21.15 | NS | 65.14 | |
| <0.0001 | 0.0001 | 0.0001 | ||||
2.1.1. Manual Restraint
Light period sleep after brief manual restraint was characterized by increases in total sleep and NREM, but no significant alteration in REM, compared to baseline. Dark period sleep showed increases in total sleep, NREM and REM compared to baseline. Light period activity did not significantly differ from baseline; however dark period activity was decreased. Similar changes in sleep and activity were observed on each recording day (M1-3), and there was no significant difference across the manual restraint days when the absolute amounts of sleep were considered. However, considered as a percentage of baseline amounts, there were decreases from M1 to M3 in total sleep (M1: 11.7%, M2: 9.5%, M3: 8.4%; M1 vs. M3, p < 0.04, Tukey test) and NREM (M1: 12.0%, M2: 9.3%, M3: 7.6%; M1 vs. M3, p < 0.05, Tukey test).
2.1.2. Novel Enclosure
Experience with NE alone did not significantly alter light period sleep compared to baseline; however, dark period total sleep, NREM and REM were increased. By comparison, NE that was preceded by manual restraint was followed by significant increases in light period total sleep and NREM, but not in REM. Alterations in dark period sleep were similar to those observed with NE alone with significant increases in total sleep, NREM and REM. Both NE alone and mNE produced decreases in dark period activity with no observable alterations in light period activity.
2.1.3. Footshock
Footshock alone was followed by increases in light period total sleep and NREM and decreases in REM. In the dark period, total sleep, NREM and REM were increased relative to baseline. Footshock preceded by manual restraint produced similar changes in each recording period, except that the decrease in light period REM was not observed. Activity was decreased in the dark period after FS and mFS, but did not differ from baseline during the light period.
2.1.4. Dark Period Changes in Sleep
All of the treatments except NE alone significantly increased light period NREM, whereas none increased light period REM. In contrast, all were followed by increases in dark period NREM and REM. To determine the relative amounts of increases in dark period NREM and REM, we looked at the increases as percentages of increase relative to baseline and compared the amounts across conditions using paired t tests. The relative increases after manual restraint were higher for REM than for NREM after M1 (57.0% vs. 34.7%, p < .05) but did not differ after M2 (28.3% vs. 24.8%) and M3 (36.3% vs. 30.0%). The relative increase was greater in REM than in NREM for NE (36.9% vs. 28.9%, p < 0.05) but did not differ for mNE (32.1% vs. 29.3%). The relative increase was higher for REM than for NREM after both FS (71.7% vs. 57.4%, p < .05) and mFS (74.7% vs. 54.9%, < 0.05).
Comparisons across conditions indicated that the relative amount of change in dark period total sleep differed significantly (p < .05, Tukey tests) across treatments with less change after M1-3 (25-38%), intermediate change for mNE and NE (45-51%) and greater changes for mFS and FS (57-60%). Dark period activity showed parallel changes with lower reductions after M1-3 (20-22%), intermediate reductions after mNE and NE (28.9-29.3%) and greater reductions for mFS and FS (32-37%); however, the differences were only significant (p < .05, Tukey tests) for comparisons between M1-3 and mFS and between M1-3 and FS.
2.2. Spectral EEG power
We conducted spectral analyses of the EEG in four hour bins over the entire recording period for each condition. Figs. 5-7 presents plots of relative EEG power in 0.5 Hz bins from 0.5 to 20 Hz calculated for the first 4-h period for M1-3 (Fig. 5), NE and mNE (Fig. 6) and FS and mFS (Fig. 7) and time-matched baseline for each behavioral state. These plots guided the analysis across bins for selected frequency bands (0.5-5 Hz [delta] for NREM, 5.5-10 Hz [theta] for REM and 4.5-9 Hz [theta] for wakefulness).
Fig. 5.
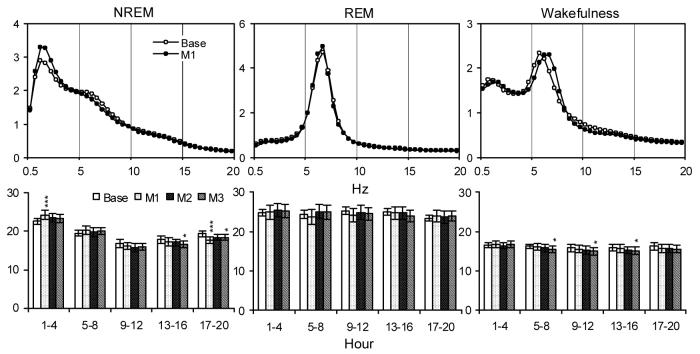
Relative EEG power (%) plotted for each 0.5 Hz bin from 0.5-20 Hz calculated during the first 4-h records (Upper Panels) in time matched baseline and after M1 (M2-3 are not shown for clarity), and totals calculated for selected frequency bands (0.5-5 Hz for NREM, 5.5-10 Hz for REM and 4.5-9 Hz for wakefulness) over five 4-h blocks in time matched baseline and after M1-3 (Lower Panels). Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, *** p < 0.001, compared to Base).
Fig. 7.
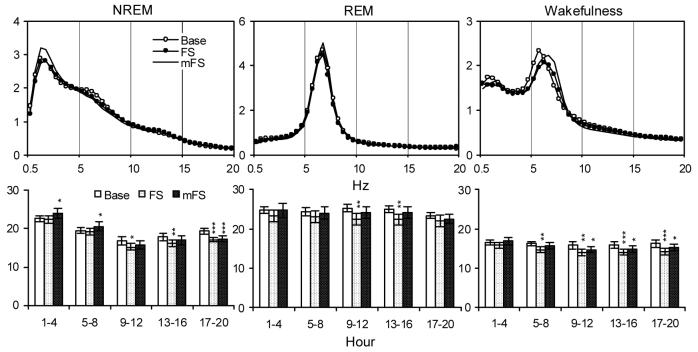
Relative EEG power (%) plotted for each 0.5 Hz bin from 0.5-20 Hz calculated during the first 4-h records (Upper Panels) and totals calculated in selected frequency bands (0.5-5 Hz for NREM, 5.5-10 Hz for REM and 4.5-9 Hz for wakefulness) over five 4-h blocks in time matched baseline and after FS and mFS (Lower Panels). Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, ** < 0.01, *** p < 0.001, compared to Base).
Fig. 6.
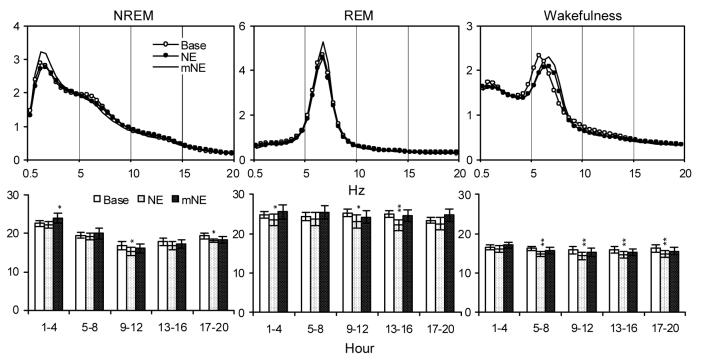
Relative EEG power (%) plotted for each 0.5 Hz bin from 0.5-20 Hz calculated during the first 4-h records (Upper Panels) and totals calculated in selected frequency bands (0.5-5 Hz for NREM, 5.5-10 Hz for REM and 4.5-9 Hz for wakefulness) over five 4-h blocks in time matched baseline and after NE and mNE (Lower Panels). Values are means ± SEM. Comparisons were conducted with Tukey test (* p < 0.05, ** p < 0.01, compared to Base).
2.2.1. Manual Restraint
The overall ANOVA for NREM delta power showed a significant interaction (treatment condition X time) for manual restraint [F(12, 84) = 6.77, p < 0.0001]. Post hoc tests found that NREM delta power after M1 was increased during the first 4-h period and decreased in the fifth 4-h period. Significant decreases in NREM delta were also seen after M3 in the fourth and fifth 4-h periods.
Manual restraint also produced significant alterations in theta power during wakefulness. This was characterized by a significant interaction in the ANOVA [F(12, 84) = 2.59, p < 0.006]. Post hoc analyses revealed that M1 and M2 did not significantly affect theta whereas after M3 theta power was significantly reduced during the second to fourth 4-h periods during wakefulness. There were no significant alterations in REM theta power in any of the analyses conducted for manual restraint.
2.2.2. Novel Enclosure
Significant alterations in NREM delta power were found in the analyses for NE. The ANOVA showed a significant treatment by time interaction [F(8, 56) = 4.45, p < 0.0003]. NREM delta was increased during the first 4-hour period after mNE, and decreased after NE during the third and fifth 4-h periods.
The overall ANOVAs for NE also showed significant interactions in the analyses of theta power during REM [F(8, 56) = 3.97, p < 0.0009] and wakefulness [F(8, 56) = 4.43, p < 0.0003]. NE alone produced either significant reductions, or trends toward reductions, in theta power during REM and wakefulness in all of the four h bins that were examined. By comparison, REM and waking theta power did not differ from baseline in any of the 4-h periods after mNE.
2.2.3. Footshock
Significant alterations in NREM delta power were also found in the analyses for FS as indicated by a significant interaction in the ANOVA [F(8, 56) = 10.15, p < 0.0001]. After mFS, NREM delta power was increased during the first and second 4-h period, and decreased during the fifth 4-h period. After FS alone, decreases in NREM delta power were seen during the third to fifth 4-h period.
The overall ANOVAs for FS showed significant interactions for the analysis of theta power during REM [FS, F(8, 56) = 4.28, p < 0.035] and wakefulness [F(8, 56) = 3.08, p < 0.006]. FS alone produced significant reductions or trends towards reductions in REM theta power, whereas mFS did not. However, waking theta power after FS was significantly reduced in the last four of five 4-h periods, and waking theta power after mFS was significantly reduced in the last three of five 4-h periods.
3. Discussion
The results revealed that brief manual restraint significantly alters subsequent sleep as indicated by changes in total sleep, NREM, REM and EEG power. Repeated manual restraint appears to reduce the extent of the changes it produces in sleep and EEG power. However, even after repeated sessions, brief manual restraint can alter the effects on sleep and EEG power produced by subsequent stressors.
Manual restraint (M1-3) significantly reduced NREM and REM during the first h of recording. This result is in accordance with findings that NREM and REM are reduced in this rat strain during the first h after open field [25], cage change [25] and novel enclosure [30] and suggests that brief manual restraint produces initial decreases in NREM and REM similar to those seen after other mild stressors [25]. Interestingly, the initial decrease in sleep is not often addressed in experiments examining the effects of restraint stress on sleep in rats [3,9,11,15], but it has been reported in mice [10,13].
The magnitude and duration of the initial decreases in sleep associated with stress appears to be affected by stressor intensity [11,17]. For instance, in Wistar rats, reductions in NREM and REM are mainly limited to the first h after mild stressors [25] whereas decreases in REM after inescapable footshock or conditioned reminders of inescapable footshock, which are putatively more stressful, can persist over several h [30].
Apart from decreases during the first h after manual restraint, increases in NREM and REM were observed during both light and dark periods, though the increases in light period REM did not reach significance. Increases in sleep were greater during the dark period than during the light period, and relatively greater in REM than in NREM during the dark period. These characteristics are similar to the alterations in sleep observed after experimental restraint [3,9,11,15] and other mild stressors that we have examined [25,27]. However, the amount of the increases in sleep, in particular REM, appear to be less than that induced by 30-, 60- and 120-min of experimental restraint [11], but more similar to that we observed after mild stressors in this strain [25]. In particular, the patterns of the initial decreases and subsequent increases after manual restraint are more similar to that after mild stressors on sleep [25] than other more typical experimental restraint manipulations [3,9,11,15].
The magnitude of EEG slow wave activity during NREM (0.5-5 Hz, SWA) is considered an indicator of sleep intensity [6,31,32]. Forced wakefulness, restraint and social stress have been demonstrated to increase SWA in rats [8,12,14]. Similarly, brief manual restraint (M1) also initially increased SWA though this was followed by reduced SWA during the last portion of the dark period. This suggests that, although manual restraint increased NREM time for M1-3 during both light and dark periods, the enhancement in NREM time during the initial light period of M1 may have involved more intense sleep.
Over three sessions of repeated restraint, the amount of initial decreases in REM and NREM were at a similar level. The magnitude of subsequent increases in NREM and REM appeared to become smaller over subsequent sessions, however, the increases were still significant. This suggests that the effects of manual restraint on sleep amounts may persist at least over the limited number of sessions that we examined. By comparison, significantly increased SWA was seen only after M1, not after M2 and 3, suggesting that habituation over repeated sessions may be particularly important for EEG power analysis.
The enclosure and footshock procedures in this study were identical to those we used in a previous study involving a number of rat strains [30]. In that study, we found that enclosure alone, used as a control for handling associated with the shock chamber, did not significantly affect total amounts of light period sleep, whereas footshock decreased total light period REM in Wistar rats [30]. The present work essentially replicated those findings for the NE and FS alone treatments. However, manual restraint administered prior to NE (mNE) resulted in an increase in light period NREM that was not seen after NE alone. In addition, manual restraint administered prior to FS (mFS) prevented the decrease in light period REM seen with FS alone. The effects appeared to be limited to light period sleep as no differences were seen during the dark period between NE and mNE or between FS and mFS. This suggests that manual restraint significantly altered the initial changes in sleep induced by NE and FS. Brief manual restraint thus appears to enhance NREM and REM when it interacts with other stressors, an effect similar to that reported for longer duration restraint applied via other methods [8].
Interestingly, prior manual restraint also markedly influenced NREM SWA compared to that seen with NE or FS alone. For example, SWA was increased the first 4-h recording period after mNE and mFS, and the increase was also significant during the second 4-h period after mFS. However, significant alterations in SWA were not seen during these periods after NE and FS alone. This suggests that manual restraint may have an enhancing effect on SWA. The decreases in the magnitude of dark period SWA after NE, mNE (not significant), FS and mFS may be associated with increases in less intense sleeping time as mentioned above.
Increases in dark period sleep were accompanied by decreases in activity. EEG theta activity has been linked to exploratory activity [4,7,33]. Thus, the significant decreases in waking theta after NE, FS and mFS in a number of recording periods may have been related to the decrease in activity. This view is supported by our recent examinations of the relationships among total sleep, activity, and wakefulness EEG theta power [29]. We found that individual Wistar rats exhibiting more sleep and less activity showed significantly less theta power in their waking EEG compared to rats showing less sleep and more activity. Furthermore, the magnitude of theta power was significantly reduced during the waking episodes with no activity compared to those with activity [29].
Wistar rats have a markedly greater sleep diurnal ratio (Light/Dark, 2.8) compared to the other strains that we have examined (e.g., 1.6 in Sprague-Dawley and Lewis, 2.1 in Fischer 344) [25]. Greater percentages of sleep in the light period could be a factor in greater dark period NREM after manipulations that interrupt sleep in the light period. For example, Wistar rats have significant increases in dark period NREM after cage change and open field [25] or novel enclosure [30] experienced in the early light period whereas other strains that we examined did not [25,30].
The increase in dark period NREM in Wistar rats after a mild stressor may involve altered homeostatic regulation of sleep as well as the stress response. For instance, increases in dark period NREM were in the range of 50-57% after NE, FS and mFS (43% after mNE), but were 25% to 35% after M1-3. These differences could be associated with the differences in the amount of sleep disruption across manipulations rather than differences in types of stressors, as M1-3 were of less duration than the other treatment conditions. However, changes in dark period REM appear to be more strongly associated with the intensity of the stressors, though all seven treatments were followed by increases. For example, increases in dark period REM were 57%, 28%, 36% after M1-3, respectively (the drop may reflect adaptation), 55-57% after NE and mNE, and 72-75% after FS and mFS. Interestingly, in mice we previously found that a single footshock did not influence subsequent dark period NREM and REM whereas 15 shock trials selectively decreased REM without a subsequent period of enhanced REM [19]. The difference between rats and mice may be due to differences in perceived intensity of the shock. For example, both rats and mice received the same low intensity footshock that may have been experienced as more intense in the mice for a variety of reasons including simple differences in the thickness of the foot pads. However, together, these findings suggest that changes in dark period NREM are not greatly associated with differences in stressor type or intensity whereas changes in dark period REM appear to have a closer link to variations in stressors.
In summary, this study demonstrates that brief manual restraint significantly alters subsequent sleep and can influence the alterations in sleep produced by other stressors. Such influences may be particularly important to consider in studies requiring handling to administer pharmacological or other treatments prior to stressors.
4. Experimental Procedure
4.1. Subjects
Eight male Wistar rats (Harlan, Indianapolis, IN) were used in the study. The rats were approximately 90 days of age at the start of the experiment. They were singly housed and given ad libitum access to food and water upon arrival until the completion of the experiment. The same room was used for housing the rats and recording sleep. The room was kept on a 12:12 light - dark cycle with lights on from 0700 to 1900 h, and ambient temperature was maintained at 24.5±0.5 C.
4.2. Surgery
Each rat was implanted with a transmitter (DataSciences ETA10F20) for recording EEG and activity via telemetry as described previously [21]. The body of the transmitter was implanted subcutaneously off midline and posterior to the scapula and it was attached to the skin with three sutures for stabilization. Leads from the transmitter were led subcutaneously to the skull and the bare ends placed in contact with the dura through holes in the skull. The electrodes were anchored to the skull with screws and dental cement. All surgical procedures were performed stereotaxically under aseptic conditions. Surgical anesthesia was achieved with isoflurane (5% induction; 2% maintenance). Ibuprofen (15 mg/kg—2.35 ml Children's Motrin® in 500 ml water) was provided in the water bottles 24 to 48 h prior to surgery and for a minimum of 72 h after surgery to alleviate potential post-operative pain. The rats were allowed a minimum of 14 days to recover prior to beginning the experiment. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 04-006).
4.3. Experimental protocol and data collection
After recovery from surgery, a 48 h recording of baseline sleep was obtained. The rats were then entered into the experimental protocols described below. All manipulations took place during the fourth h after lights on. After each treatment, the rats were returned to their home cages and sleep was recorded during the remaining 8 h of the light period and for the following 12 h dark period. EEG and activity was recorded via telemetry using procedures similar to those we have employed in mice [26] and rats [21].
Experiment 1 – manual restraint (M): Three sessions of manual restraint (M1-3) were conducted with a one day interval between each session. For restraint, the body and limbs of the rat were wrapped with a piece of disposable surgical drape and then gently restrained by hand to limit movement for 5 min. The drape was changed every time.
Experiment 2 – Neutral Enclosure (NE): 5 days after experiment 1, the rats received manual restraint as described above after which they were placed in a Plexiglas® chamber of approximately the same size as the chambers used for the application of footshock and allowed to explore for 30 min (mNE) or they were placed in the enclosure without prior manual restraint (NE). Each rat was studied in each condition with a 3-day interval between sessions. Half of the rats received mNE first and half received NE first.
Experiment 3 – Footshock Stress (FS): 5 days later after experiment 2, the rats received M, as described above, followed by FS (mFS) or footshock alone (FS). There was a 3-day interval between sessions. Each rat received each treatment with half receiving mFS first and half receiving FS first. FS was conducted as previously for shock training session [30]. Individual rats were placed in shock chambers. The rats were allowed to freely explore for 5 min after which they were presented with 20 footshocks (0.2 mA, 0.5-s duration) at 1.0 min intervals over the course of 20 min. Five min after the last shock, the rats were returned to their home cages. The entire procedure was of 30 min duration.
4.4. Determination of behavioral states and analysis in EEG power
Trained observers visually scored the EEG and activity records in 10 s epochs to determine wakefulness, NREM, and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and irregularly spaced and clustered TTL pulses on the activity channel. NREM was scored based on the presence of spindles interspersed with slow waves or desynchronization EEG. During REM, the EEG was characterized by a regular, low-amplitude theta rhythm. The EEG power density data were sorted according to behavioral states. Total EEG power from 0.5 to 40.0 Hz for individual animals was normalized to 100 for each state.
4.5. Data analysis
The time spent (min) in NREM, REM and total sleep (NREM+REM) as well as activity counts were processed and plotted to reveal hourly patterns during time matched baseline and after each experimental manipulation. Afterwards, the data were processed to obtain totals during selected periods for each condition. The relative densities of EEG power during wakefulness, NREM and REM were calculated in 0.5 Hz bins from 0.5 to 20 Hz for first 4 h records after each manipulation and for same period of time matched baseline. Afterwards, the EEG power in selected frequency bands was analyzed during each 4-h period over the 20 h of recording.
All statistical analyses were conducted using SigmaStat software (SPSS, Inc.). One-way repeated measures of analysis of variance (ANOVA) procedures were used in the analyses of sleep measures for each condition. Two-way repeated measures ANOVAs across treatment condition and time were used to analyze the EEG power data. After significant ANOVAs, post hoc comparisons of means were conducted with Tukey tests. Paired t-test were used in comparisons across conditions in measurements considered as percentage changes relative to baseline.
Footnotes
Supported by NIH grant MH64827 and MH61716
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 2.Bodosi B, Obal F, Jr., Gardi J, Komlodi J, Fang J, Krueger JM. An ether stressor increases REM sleep in rats: possible role of prolactin. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1590–8. doi: 10.1152/ajpregu.2000.279.5.R1590. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet C, Lger L, Baubet V, Debilly G, Cespuglio R. Influence of a 1 h immobilization stress on sleep states and corticotropin-like intermediate lobe peptide (CLIP or ACTH18-39, Ph-ACTH18-39) brain contents in the rat. Brain Res. 1997;751:54–63. doi: 10.1016/s0006-8993(96)01390-x. [DOI] [PubMed] [Google Scholar]
- 4.Brown BB, Shryne JE. EEG theta activity and fast activity sleep in cats as related behavioral traits. Neuropsychologia. 1964;2:311–26. [Google Scholar]
- 5.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. Ann N Y Acad Sci. 1998;851:311–35. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 6.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 7.Franken P, Malafosse A, Tafti M. Biochemistry. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–37. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Garcia F, Beltran-Parrazal L, Jimenez-Anguiano A, Vega-Gonzalez A, Drucker-Colin R. Manipulations during forced wakefulness have differential impact on sleep architecture, EEG power spectrum, and Fos induction. Brain Res Bull. 1998;47:317–24. doi: 10.1016/s0361-9230(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez MM, Valatx JL. Effect of intracerebroventricular administration of alpha-helical CRH (9-41) on the sleep/waking cycle in rats under normal conditions or after subjection to an acute stressful stimulus. J Sleep Res. 1997;6:164–70. doi: 10.1046/j.1365-2869.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Yang L, Parris BS, Tang X, Sanford LD. The effect of restraint stress on sleep in mice: Strain comparison. Sleep (Abstract Supplement) 2004;27:A401. [Google Scholar]
- 11.Marinesco S, Bonnet C, Cespuglio R. Influence of stress duration on the sleep rebound induced by immobilization in the rat: a possible role for corticosterone. Neuroscience. 1999;92 doi: 10.1016/s0306-4522(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 12.Meerlo P, de Bruin EA, Strijkstra AM, Daan S. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol Behav. 2001;73:331–5. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 13.Meerlo P, Easton A, Bergmann BM, Turek FW. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R846–54. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]
- 14.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–4. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 15.Rampin C, Cespuglio R, Chastrette N, Jouvet M. Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett. 1991;126:113–8. doi: 10.1016/0304-3940(91)90532-x. [DOI] [PubMed] [Google Scholar]
- 16.Roky R, Obâal F, Jr., Valatx JL, Bredow S, Fang J, Pagano LP, Krueger JM. Prolactin and rapid eye movement sleep regulation. Sleep. 1995;18:536–42. [PubMed] [Google Scholar]
- 17.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 18.Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139:169–83. [PubMed] [Google Scholar]
- 19.Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 20.Sanford LD, Xiao J, Liu X, Yang L, Tang X. Influence of avoidance training (AT) and AT cues on sleep in C57BL/6J (B6) and BALB/cJ (C) mice. Sleep (Abstract Supplement) 2005;28:A6–7. [Google Scholar]
- 21.Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2005 doi: 10.1016/j.brainres.2006.02.020. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 23.Schiffelholz T, Aldenhoff JB. Novel object presentation affects sleep-wake behavior in rats. Neurosci Lett. 2002;328:41–4. doi: 10.1016/s0304-3940(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 24.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–45. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Liu X, Yang L, Sanford LD. Rat strain differences in sleep after acute mild stressors and short-term sleep loss. Behav Brain Res. 2005;160:60–71. doi: 10.1016/j.bbr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002;25:691–9. [PubMed] [Google Scholar]
- 27.Tang X, Xiao J, Liu X, Sanford LD. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–47. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–29. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Tang X, Yang L, Sanford LD. Individual variation in sleep and activity in rats. Behav Brain Res. doi: 10.1016/j.bbr.2007.02.022. Under Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 31.Tobler I, Borbely AA. Short light-dark cycles and paradoxical sleep in the rat: effect of strain difference and hypophysectomy. Behav Biol. 1978;23:395–8. doi: 10.1016/s0091-6773(78)91435-9. [DOI] [PubMed] [Google Scholar]
- 32.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 33.Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]