Abstract
Glial cell line-derived neurotrophic factor (GDNF), neurturin (NTN), and their receptors, GDNF family receptor α-1 (GFRα-1) and GDNF family receptor α-2 (GFRα-2), are critically important for kidney and nervous system development. However, their role in skin biology, specifically in hair growth control, is as yet unknown. We have studied expression and function of GDNF, neurturin, GFRα-1, and GFRα-2 in murine skin during the cyclic transformation of the hair follicle (HF) from its resting state (telogen) to active growth (anagen) and then through regression (catagen) back to telogen. GDNF protein and GFRα-1 messenger RNA are prominently expressed in telogen skin, which lacks NTN and GFRα-2 transcripts. Early anagen development is accompanied by a significant decline in the skin content of GDNF protein and GFRα-1 transcripts. During the anagen-catagen transition, GDNF, GFRα-1, NTN, and GFRα-2 transcripts reach maximal levels. Compared with wild-type controls, GFRα-1 (+/−) and GFRα-2 (−/−) knockout mice show a significantly accelerated catagen development. Furthermore, GDNF or NTN administration significantly retards HF regression in organ-cultured mouse skin. This suggests important, previously unrecognized roles for GDNF/GFRα-1 and NTN/GFRα-2 signaling in skin biology, specifically in the control of apoptosis-driven HF involution, and raises the possibility that GFRα-1/GFRα-2 agonists/antagonists might become exploitable for the treatment of hair growth disorders that are related to abnormalities in catagen development.
The hair follicle (HF) represents an ectodermal-mesenchymal interaction unit that displays unique, life-long, cyclic transformations between stages of rapid growth and production of a pigmented hair shaft (anagen), apoptosis-driven regression (catagen), and relative resting (telogen). Every phase in the hair cycle is characterized by defined, tightly choreographed programs of tissue proliferation, differentiation, and apoptosis, that are controlled by the local balance of numerous growth-stimulatory and -inhibitory signals. 1-5 Among these growth-modulatory factors, several members of the transforming growth factorβ/bone morphogenetic protein (TGFβ/BMP) superfamily are recognized to play an important role, notably during HF morphogenesis and regression. 6-12 Therefore, it is interesting to explore which role other, more recently discovered members of this superfamily may play in the biology of hair growth.
Glial cell line-derived neurotrophic factor (GDNF), neurturin (NTN), artemin, and persephin, which share about 40% of amino acid sequence homology, are recently identified, distant members of the TGFβ superfamily, which are grouped together in the GDNF family of growth factors. 13-16 The biological activity of GDNF and NTN is mediated by their correspondingly high affinity receptors, GDNF family receptor α-1 (GFRα-1) and GDNF family receptor α-2 (GFRα-2), respectively, both sharing an intracellular-signaling component, the protein tyrosine kinase c-ret. 17-22 GDNF also shows lower-affinity interactions with GFRα-2, and NTN is able to bind with low affinity to GFRα-1. 20-23
Analyses of the distribution of GDNF, NTN, GFRα-1, and GFRα-2 messenger RNAs (mRNAs) during embryogenesis have identified wide expression patterns of these molecules outside of the nervous system, most notably under conditions of prominent epithelial-mesenchymal interactions, such as during kidney, gut, lung, and tooth development. 24-33 Indeed, the suggested role of GDNF in kidney organogenesis was confirmed by mice with null mutations of GDNF, GFRα-1, or c-ret genes, all of which are characterized by severe defects in kidney morphogenesis and in the developing of the enteric nervous system. 34-39 During kidney development, GDNF is secreted by the metanephric mesenchyme and increases cell proliferation, motility, and migration into the adjacent renal epithelium, thus playing a critical role in the outgrowth and branching of epithelial structures. 40,41
It was recently shown by gene targeting that NTN and GFRα-2 are essential for the development of postganglionic parasympathetic neurons, innervating lacrimal and salivary glands, and small intestine. 42,43 Although NTN- or GFRα-2-deficient mice are viable and fertile, there are no systematic studies available on the development in these mice of those organs that require epithelial-mesenchymal interactions during the development of tooth, lung, or kidney. This encourages exploration of the role of GDNF/GFRα-1- and NTN/GFRα-2-mediated signaling in the epithelial-mesenchymal interactions that underlie HF development and cycling. 3,5,11
The GDNF gene is transcribed in embryonic skin, 24,25 where GDNF mRNA was detected in both epithelial and mesenchymal components of vibrissae HF. 26,27 In addition, expression of NTN, GFRα-1, and GFRα-2 mRNA was found in the developing vibrissa follicles. 29,30,33 Whereas the involvement of other members of the TGFβ superfamily, like TGFβ-1 through 3, BMP-2, and BMP-4, in the control of HF development and cycling is now well appreciated, 9,11,12,44,45 the roles of GDNF and NTN in these processes remain to be defined.
Because the telogen-anagen transformation of each hair cycle recapitulates several aspects of HF morphogenesis, 1,5 and because the control of HF cycling, especially of the anagen-catagen transition, is of paramount clinical importance for the pathogenesis and management of hair growth disorders, 2,46 we have focused on exploring the role of GDNF, NTN, GFRα-1, and GFRα-2 in HF cycling. GDNF, NTN, GFRα-1, GFRα-2, and c-ret expression were characterized during all stages of HF cycling in normal adolescent C57BL/6 mice, using enzyme-linked immunosorbent assay (ELISA), semiquantitative reverse transcription–polymerase chain reaction (RT-PCR), in situ hybridization (ISH), and immunohistochemistry. In addition, HF cycling was compared by quantitative histomorphometry among heterozygous GFRα-1 knockout (+/−), homozygous GFRα-2 knockout (−/−), and the corresponding age-matched wild-type mice. 39,43 Finally, the functional effects of GDNF and NTN on HF cycling in vitro were assessed, using established skin organ culture techniques. Taken together, these studies provide evidence that GDNF and NTN modulate both anagen and catagen development, and that GFRα-1/GFRα-2 signaling serves as an important mechanism for driving HF cycling in adolescent skin.
Materials and Methods
Animal Models and Tissue Collection
C57BL/6 mice were purchased from Charles River Breeding Laboratories (Sulzfeld, Germany) and housed in community cages at the animal facilities of the Charité (Virchow Campus, Berlin, Germany). GFRα-1 knockout (+/−) and GFRα-2 knockout (−/−) mice were housed at the animal facilities of the Washington University School of Medicine (St. Louis, MO) and University of Helsinki (Helsinki, Finland). All mice were fed water and mouse chow ad libitum and were kept under 12-hour light/dark cycles.
GFRα-1 and GFRα-2 knockout mice were generated using conventional gene-targeting techniques as described previously, and genotyping of mutant animals was performed using PCR protocols for the mutated alleles. 39,43 Homozygous GFRα-1 knockout (−/−) mice die shortly after birth due to the absence of the kidney, whereas heterozygous GFRα-1 knockout (+/−) and homozygous GFRα-2 knockout (−/−) mice are viable, fertile, and display no obvious, macroscopically visible hair growth abnormalities. 39,43
Active hair growth (anagen) was induced by depilation of the back skin of 6- to 9-week-old C57BL/6 female mice in the telogen phase of the hair cycle as described previously. 47 In C57BL/6 mice, all key hair cycle stages 48 were studied, using at least five mice per time point: telogen (untreated skin), anagen II-VI (3–12 days after anagen induction by depilation [p.d.]), and catagen (17–19 days p.d.). 9,47
For the analysis of spontaneous HF cycling in infantile GFRα-1 knockout (+/−), GFRα-2 knockout (−/−), and the corresponding wild-type mice, skin was harvested 17 days after birth (P17), and four to five mice of every strain were studied (note that around P17 murine back skin HF enter synchronously into HF cycling by induction of their first catagen phase). 3,49,50 In all experiments, the neck region of the back skin was harvested parallel to the vertebral line and was embedded, using a special technique for obtaining longitudinal cryosections through the HF from a defined site. 51
Determination of Skin GDNF Protein Content by ELISA
For protein extraction, full-thickness samples of C57BL/6 mouse back skin, dissected at the level of the subcutis, including the panniculus carnosus muscle at distinct stages of the induced hair cycle, 47 were pulverized in liquid nitrogen. 10,52 Per 100 mg of skin, 0.5 ml of lysis buffer (50 mmol/L Tris/HCl, pH: 8.0, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L phenylmethylsulfonyl fluoride, 5 mmol/L iodacetamid, 10 mg/ml aprotinin, 0.2% sodium dodecyl sulfate, 1% nonidet, 1% Triton X-100) was added, and then samples were lysed in an ultrasonic bath for 10 minutes. After 1 hour of shaking at 4°C, the mixture was sonicated again in the ultrasonic bath for 10 minutes. The solution was centrifuged for 30 minutes at 14,000 × g and 4°C, and the supernatants were frozen and stored at −80°C. Quantification of the GDNF protein was performed in three independent experiments using a commercially available ELISA-kit by the manufacturer’s instructions (Promega, Madison, WI). Then data were pooled, means and standard errors of the mean (SEM) were calculated, and Student’s t- and analysis-of-variance (ANOVA) tests were used for statistical analyses.
RT-PCR
Semiquantitative RT-PCR analysis of GFRα-1, GFRα-2, and constitutively expressed β-actin was performed as previously described. 10,52 Total RNA was isolated from full-thickness back skin samples (homogenized in liquid nitrogen), using a single-step guanidine thiocyanate-phenol-chloroform method with RNAzol B (Biotech Laboratories Inc., Houston, TX). Skin samples, including the subcutaneous skeletal (panniculus carnosus) muscle layer complementary DNA, were synthesized by reverse transcription of 3 μg total RNA, using a complementary DNA synthesis kit (Invitrogen, San Diego, CA). The following sets of oligonucleotide primers were used: β-actin, 5′-TGG AAT CCT GTG GCA TCC ATG AAA C and 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′; GFRα-1, 5′-GC GGG CAA GGA AAC CAA CT and 5′-CAT AGG AGC ACA CAG GGA CG; 53 GFRα-2, 5′-TAT TGG AGC ATC CAT CTG GG−3′ and 5′-AGC AGT TGG GCT TCT CCT TTG−3′. 43 Amplification was performed using taq polymerase (GIBCO, Grand Island, NY) over 34 cycles, using an automated thermal cycler (Perkin Elmer Cetus). Each cycle consisted of the following steps: denaturation at 94°C (1 minute), annealing at 60°C (45 seconds), and extension at 72°C (45 seconds). PCR products were analyzed in three independent experiments by agarose gel electrophoresis and enzymatic digestion, using standard methods as described. 10 Staining was densitometrically assessed with a video scanner using Scan Pack 2.0 (Biometra, Göttingen, Germany). Then data were pooled, means and SEM calculated, and Student’s t- and ANOVA tests were used for statistical analyses.
ISH
ISH was carried out on mouse skin as described. 54,55 Eight-μm cryostat sections of paraformaldehyde-fixed skin were placed on RNase-free gelatin-coated slides and hybridized with a digoxigenin (Dig)-labeled synthetic riboprobe (labeled at the 3′-end with Dig-dUTP) that is complementary to bases 52–668 of the GDNF gene, to bases 349–936 of the NTN gene, to bases 294-1039 of the GFRα-1 gene, or to bases 37–400 of the GFRα-2 gene. 13,14,27,56 ISH was performed at 60°C for 17 hours with 160 μl of hybridization buffer, containing 50% formamide, 4× standard saline citrate, and 150 ng/ml of riboprobe. After hybridization, the slides were first washed in 2× standard saline citrate at 69°C for 1 hour, then in 0.1× standard saline citrate at 69°C for 1 hour. After washing, the slides were incubated with alkaline phosphatase-conjugated anti-digoxigenin antibody (3 hours, room temperature), and processed for reaction product development as described. 54 Incubation of sections with ribonuclease or corresponding sense probes was used as a negative control; cryosections of embryonic brain and kidney were used as positive controls. These controls confirmed the specificity and sensitivity of the ISH methodology.
Immunohistochemistry
Incubation of skin cryostat sections with rabbit antiserum against c-ret (dilution 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), followed by tetramethylrhodamine B isothiocyanate-conjugated F(ab)2 fragments of goat anti-rabbit immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, PA) was performed as described. 57-59 Incubation of skin sections without primary antiserum and cryostat sections of embryonic kidney were used as negative and positive controls, respectively. In addition, the preabsorption of primary antiserum against c-ret with 100 μg/ml of the corresponding antigenic peptide (37°C, 60 minutes) was used as a negative control. All sections were examined under a Zeiss Axioscope microscope and photodocumented with the help of a digital image analysis system (ISIS MetaSystem, Altlussheim, Germany).
Skin Organ Culture
Four-mm punch biopsies were prepared under sterile conditions from adolescent C57BL/6 mouse back skin at the late anagen VI to early catagen stage of the induced hair cycle (ie, day 17 after depilation 60 ), following previously described skin organ culture protocols 61,62 with some modifications. 55,63 Per experimental group, 8–10 randomized skin punches, derived from the back skin of three different mice, were placed (dermis down) on prehydrated gelatin sponges (Gelfoam, Upjohn Co., Kalamazoo, MI) in 35-mm Petri dishes, containing 5 ml of Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, 50 μg/ml l-glutamine, and antibiotic/antimycotic mixture (GIBCO, Grand Island, NY). After the addition of 0.5–50 ng/ml human recombinant GDNF (Promega, Madison, WI) or 0.5–50 ng/ml of NTN, organ-cultured skin was incubated at the air-liquid interphase for 48 hours at 37°C, in 5% CO2 and 100% humidity. At the end of the incubation, all skin fragments were washed repeatedly in phosphate-buffered saline buffer at 4°C and were quick-frozen for further processing of cryosections (see above), or they were fixed in 4% paraformaldehyde and embedded in paraffin for routine histology and histomorphometry.
Histomorphometry and Statistical Analysis
In adolescent skin, ISH or immunoreactive (IR) patterns were analyzed by studying at least 50 different HFs per mouse, and five mice were assessed per hair cycle stage. For each stage of HF cycling, the major staining patterns were recorded in previously prepared, computer-generated schematic representations of murine HF cycling, which allow a standardized, easily reproducible and systematic comparison of different follicular expression patterns. 9 For the precise identification of the defined stages of HF cycling, histochemical detection of endogenous alkaline phosphatase activity was used as described, because this allows visualizing the morphology of the dermal papilla (DP) as a useful morphological marker for staging HF cycling. 64
In the skin organ culture, the percentage of HF in different stages of catagen was assessed and calculated in 8–10 biopsies per group at a magnification of ×400 under a Zeiss Axioscope following accepted morphological criteria for classifying defined stages of catagen development 2,3,48,49,60,64 . A total of 120–150 HFs in 25–40 microscopic fields, derived from 8–10 biopsies per group, were analyzed and compared with that of a corresponding number of HFs from the vehicle control.
Also, the percentage of HFs in defined catagen stages was assessed in GFRα-1- knockout (+/−), GFRα-2- knockout (−/−) mice at P17, as well as in their corresponding age-matched wild-type littermates. During these days of postnatal development, the HF, after completion of morphogenesis, begins its life-long cycle of regression, resting and growth by spontaneous entry into the first catagen stage. 2,3,50 In normally cycling mice, this occurs around P16–17, and at P20–22 all HFs in the back skin reach telogen. All evaluations were performed by accepted morphological criteria of HF classification, 2,3,48,49,60 by two independent investigators using the blinded method. Only every 10th cryosection was used for analysis to exclude the repetitive evaluation of the same HF, and two to three cryosections were assessed from each animal. A total of 200–350 follicles in 50–60 microscopic fields, derived from four to five animals (approximately 50–60 follicles per animal) was analyzed and compared with that of a corresponding number of HFs from the appropriate, age-matched wild-type mice.
The distance between the epidermal stratum corneum and the subcutis/panniculus carnosus-muscle border was measured for the assessment of skin thickness in GFRα-1-knockout (+/−), GFRα-22-knockout (−/−), and the corresponding wild-type animals, as described before. 63 Skin thickness was analyzed in 50–60 microscopic fields, derived from four to five mutant animals, and was compared with that of a corresponding set of data from four to five age-matched wild-type mice. All data were pooled, and means and SEMs were calculated. Differences were judged as significant if P < 0.05, as determined by the independent Student’s t-test for unpaired samples.
Results
GDNF and NTN Expression in Normal Mouse Skin Are Hair Cycle-Dependent and Peak during Telogen and Late Anagen-Catagen
As an important phenomenological indicator for a possible involvement of GDNF- and NTN-related signaling in hair growth control, total GDNF protein content in the full-thickness adolescent mouse skin, as well as GDNF and NTN mRNA expression, were characterized by ELISA (Figure 1) ▶ and ISH during the induced, highly-synchronized murine hair cycle. The observed expression patterns of GDNF and NTN mRNA are documented by the representative examples and are schematically summarized in Figure 2 ▶ .
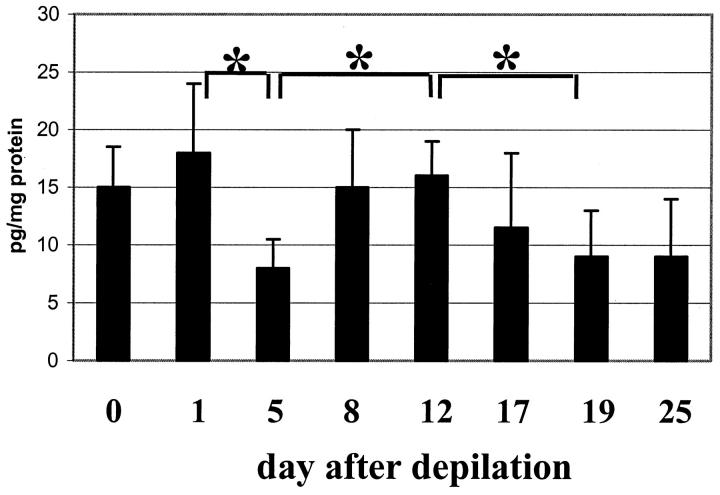
Figure 1.
GDNF protein content (by ELISA analysis) during the induced murine hair cycle. At distinct hair cycle stages (unmanipulated skin and day 25 p.d.: telogen, days 1–12 p.d.; anagen, days 17–19 p.d.; catagen), protein from full-thickness back skin (including the panniculus carnosus muscle) of C57BL/6 mice was extracted, and the content of GDNF antigen was assayed by ELISA in three independent experiments (means ± SEM of n = 5), *P < 0.05, Student’s t- and ANOVA tests. The x axis lists the days after anagen induction by depilation.
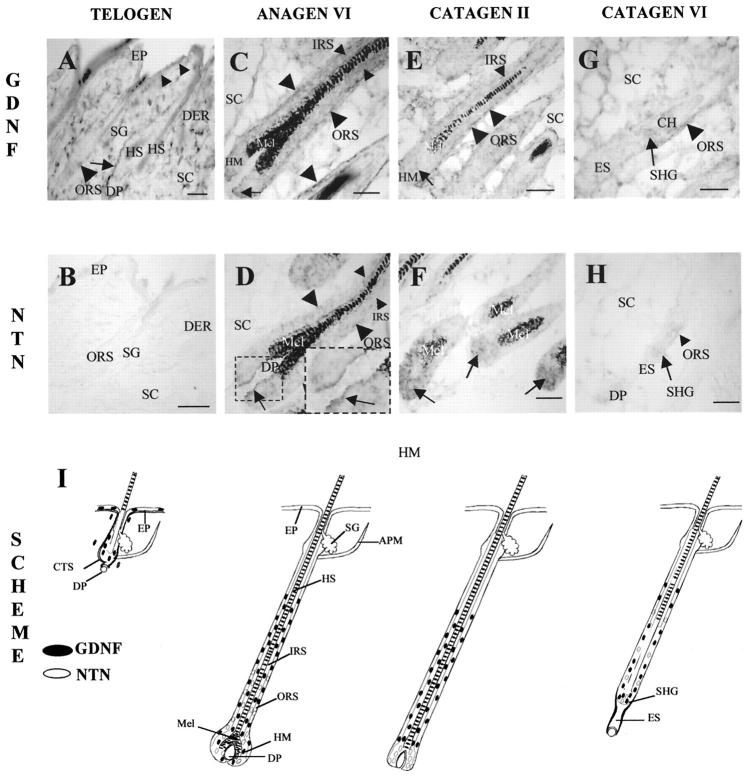
Figure 2.
Hair follicle-associated GDNF and NTN mRNA expression shows up-regulation during anagen-catagen transition. Cryostat sections (8 μm thickness) of adolescent C57BL/6 murine back skin in the defined stages of induced hair cycle (telogen, unmanipulated skin, day 0; anagen VI, 8–12 days after anagen induction by depilation; catagen II-VII, respectively, 17–19 days after anagen induction, 47,60 ) were processed for GDNF and NTN mRNA detection by in situ hybridization using corresponding Dig-labeled riboprobes 14,27 as described previously. 54,55 A–H: Representative examples of expression patterns. I: A schematic summary. A and B: Telogen expression patterns. A: GDNF mRNA in the connective tissue sheath around HFs (arrow), single basal KC in epidermis (small arrowheads), and single HF KC (large arrowhead). Note the prominent expression in single cells in the dermis. B: Absence of the NTN mRNA in the telogen skin. C and D: Anagen VI. C: Prominent GDNF mRNA expression in the central and proximal ORS (large arrowheads), IRS (small arrowheads), and in the hair matrix (arrow). D: NTN mRNA in the proximal ORS (large arrowheads), IRS (small arrowheads), and hair matrix (arrows). E and F: Catagen II. E: GDNF mRNA expression in the central and proximal ORS (large arrowheads), IRS (small arrowhead), and hair matrix (arrow). F: Prominent NTN mRNA expression in the hair matrix (arrows). G and H: Catagen VI. G: GDNF mRNA in the ORS (arrowhead) and in the secondary hair germ (arrow). H: Weak expression of NTN mRNA in the ORS (arrowhead) and secondary hair germ (arrow). I: Schematic representation of GDNF and NTN mRNAs during the adolescent murine hair cycle. Those cell populations with GDNF mRNA expression are depicted as black circles, whereas those with NTN mRNA are shown in gray. The different stages of hair cycle are indicated based on Chase (1954) with modifications. 2 The summary scheme was derived from analyzing >50 longitudinally sectioned follicles from the lower back of five harvested C57BL/6 mice per time point. DP, dermal papilla; EP, epidermis; ES, epithelial strand; IRS, inner root sheath; HF, hair follicle; HM, hair matrix; HS, hair shaft; Mel, melanin; ORS, outer root sheath; PCM, panniculus carnosus muscle; SC, subcutis; SHG, secondary hair germ. Scale bars: 100 μm.
In unmanipulated, full-thickness adolescent C57BL/6 murine back skin with all HF in telogen, constitutive steady-state levels of GDNF protein reached 15 pg/mg (Figure 1) ▶ . Prominent expression of GDNF mRNA was found in interfollicular mesenchyme and in the HF connective tissue sheath, whereas a relatively weak expression was seen in single basal epidermal keratinocytes (KCs) and in HF cells located above the DP (Figure 2, A and I) ▶ . Therefore, the steady-state levels of GDNF protein assessed by ELISA in full-thickness murine skin represent the product of intra- and extrafollicular-derived GDNF. In contrast, no expression of NTN mRNA was observed in telogen skin (Figure 2, B and I) ▶ .
Hair cycle induction and early stages of anagen development (anagen III–IV, day 5 after anagen induction) were accompanied by a significant decline (P < 0.05) of steady-state GDNF protein levels, compared with telogen and anagen I-II (Figure 1) ▶ . In anagen III-IV skin, no expression of GDNF mRNA was seen in the epidermis, whereas a decline of GDNF mRNA expression was apparent in dermal cells, and a relatively weak expression was found in the developing HF matrix (data not shown). Also, the appearance of NTN mRNA expression was found in single cells of the dermis, in the HF outer root sheath (ORS), and in the HF matrix adjacent to DP (not shown).
During further anagen development (anagen VI, days 8–12 after depilation), steady-state levels of GDNF protein significantly increased compared with early anagen skin (Figure 1) ▶ . GDNF mRNA expression was seen in the HF inner root sheath (IRS), ORS, and the hair matrix (Figure 2, C and I) ▶ . NTN mRNA was also found in the ORS and IRS, and a particularly strong NTN mRNA expression was seen in the hair matrix closely adjacent to the DP (Figure 2, D and I) ▶ .
During the subsequent spontaneous catagen development, levels of GDNF protein progressively declined again, compared with anagen VI levels (P < 0.05; Figure 1 ▶ ). GDNF mRNA was still present in the IRS and ORS, whereas a marked reduction of GDNF transcripts was found in the regressing HF matrix (Figure 2, E and I) ▶ . In contrast, NTN mRNA was prominently expressed in the matrix of catagen II HF (Figure 2, F and I) ▶ . It is interesting that catagen VI-VIII skin (day 19 after depilation) was characterized by relatively low, steady-state levels of GDNF protein (8–9 pg/mg protein, Figure 1 ▶ ). However, GDNF mRNA was still prominently expressed in the ORS and secondary germ (Figure 2, G and I) ▶ , whereas only weak NTN mRNA expression was seen in those compartments of the catagen VI HF (Figure 2, H and I) ▶ . During subsequent telogen development (day 25 p.d.), steady-state levels of GDNF protein were close to that of unmanipulated telogen skin (data not shown).
Taken together, these wave-like, hair cycle-associated patterns of GDNF- and NTN-expression changes in normal mouse skin suggest a differential involvement of GDNF/NTN signaling in the control of distinct hair cycle stages, particularly during the HF anagen-catagen transformation.
GFRα-1, GFRα-2, and c-ret Show Prominent Expression in the Hair Follicle Epithelium during Anagen-Catagen Transformation
To correlate the GDNF- and NTN- gene and protein expression in full-thickness skin with the expression patterns of their corresponding high-affinity receptors, GFRα-1 and GFRα-2, during different stages of HF cycling, semiquantitative RT-PCR, ISH, and immunohistochemistry for GFRα-1, GFRα-2, and c-ret were performed in the back skin of adolescent C57BL/6 mice. The observed expression patterns of GFRα-1/GFRα-2 mRNA and c-ret-immunoreactivity are documented by the representative examples and are schematically summarized in Figure 4 ▶ .
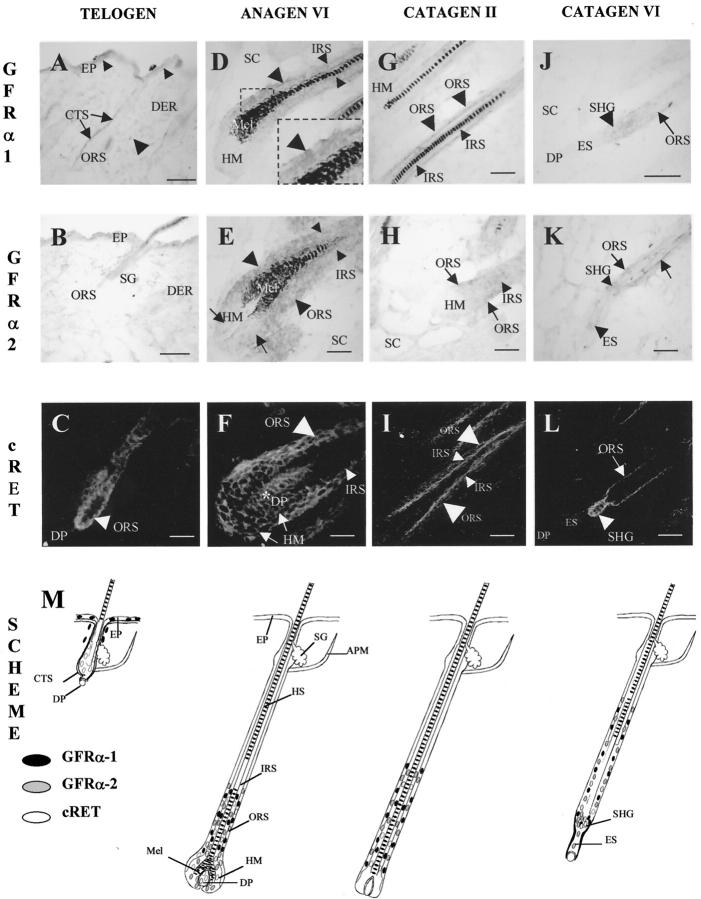
Figure 4.
HF-associated GFRα-1, GFRα-2 mRNAs and c-ret-immunoreactivity are increased during anagen-catagen transition. Cryostat sections (8 μm thickness) of adolescent C57BL/6 murine back skin in the defined stages of induced hair cycle (telogen, unmanipulated skin, day 0; anagen VI, 8–12 days after anagen induction by depilation; catagen II-VII, respectively, 17–19 days after anagen induction 47,60 ) were processed for GFRα-1, GFRα-2 mRNA detection by in situ hybridization and for c-ret-IR by immunohistochemistry. Representative examples of expression patterns are shown in A–L, and a schematic summary is given in M. A–C: Telogen. A: GFRα-1 mRNA in single cells of epidermis (small arrowheads) and dermis (large arrowhead), as well as in the HF connective tissue sheath (arrows). B: Absence of the GFRα-2 mRNA in the telogen skin. C: c-ret-IR in the HF ORS (arrowhead). D and F: Anagen VI. D: GFRα-1 mRNA in the proximal ORS (large arrowheads) and IRS (small arrowheads). E: Prominent expression of GFRα-2 mRNA in the proximal ORS (large arrowheads) and IRS (small arrowheads), and relatively weak expression in the hair matrix (arrows). F: c-ret-IR in the proximal ORS (large arrowhead), IRS (small arrowhead), hair matrix (arrows), and in dermal papilla (asterisk). G–I: Catagen II. G: GFRα-1 mRNA in the ORS (large arrowheads) and in the IRS (small arrowheads). H: Expression of GFRα-2 mRNA in the proximal ORS (arrow) and IRS (arrowhead). I: c-ret-IR in the IRS (small arrowheads) and in the ORS (large arrowheads). J–L: Catagen VI. J: GFRα-1 mRNA in the ORS (arrow) and in the secondary hair germ (arrowhead). K: expression of GFRα-2 mRNA in the ORS (arrows), secondary hair germ (small arrowhead), and single cells of epithelial strand (large arrowhead). L: c-ret-IR in ORS (arrow) and in the secondary hair germ (arrowhead). M: Schematic representation of GFRα-1, GFRα-2 mRNAs, and c-ret-IR during the adolescent murine hair cycle. Those cell populations with GFRα-1 mRNA expression are depicted as black circles, whereas those with GFRα-2 mRNA are shown in gray, and those with c-ret-IR are indicated as open circles. The different stages of hair cycle are indicated according to Chase (1954) with modifications. 2 The summary scheme was derived from analyzing >50 longitudinally sectioned follicles from the lower back of five harvested C57BL/6 mice per time point. DP, dermal papilla; EP, epidermis; ES, epithelial strand; IRS, inner root sheath; HF, hair follicle; HM, hair matrix; HS, hair shaft; Mel, melanin; ORS, outer root sheath; PCM, panniculus carnosus muscle; SC, subcutis; SHG, secondary hair germ. Scale bars: 100 μm.
By semiquantitative RT-PCR, we found that unmanipulated murine back skin with all HF in telogen was characterized by a prominent expression of GFRα-1 mRNA and by the absence of GFRα-2 transcripts (Figure 3) ▶ . Both GFRα-1 mRNA and c-ret-immunoreactivity were observed in the basal epidermal layer, GFRα-1 mRNA was also seen in the HF connective tissue sheath, whereas c-ret-IR was found in the HF ORS (Figure4, A, C, and M) ▶ . By ISH, no expression of GFRα-2 mRNA was found in telogen skin (Figure 4, B and M) ▶ .
Figure 3.
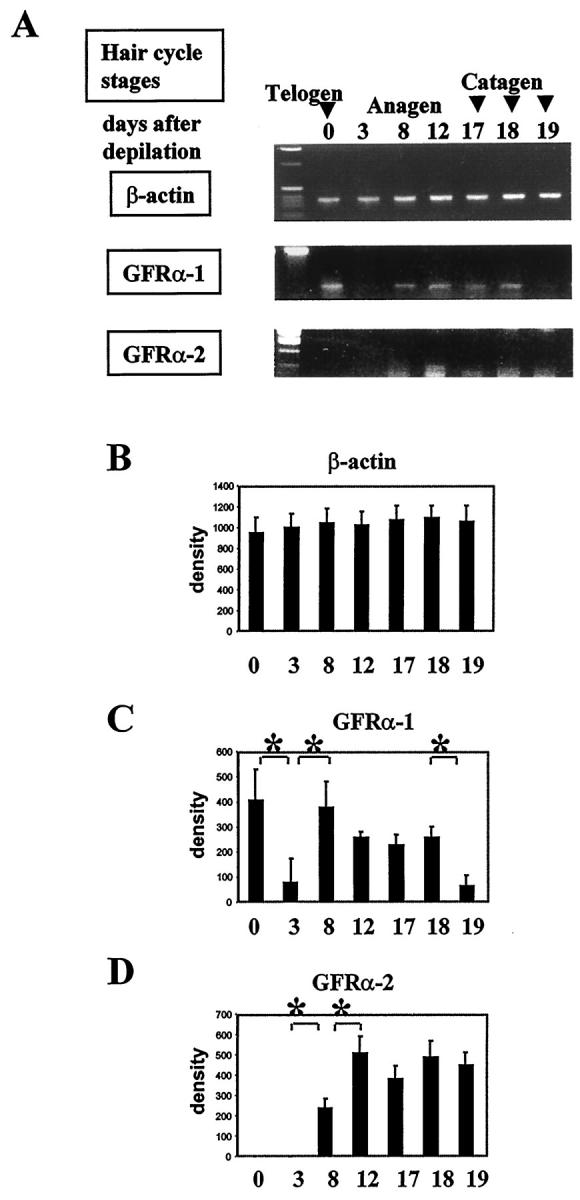
Semiquantitative RT-PCR of GFRα-1 and GFRα-2 mRNAs. Because it displays the highest degree of hair cycle synchrony, GFRα-1 and GFRα-2 expression was studied in full-thickness back skin from various stages of the depilation-induced hair cycle in adolescent C57BL/6 mice. At defined time points, total RNA was extracted from full-thickness back skin, including the panniculus carnosus muscle, was reverse-transcribed, and semiquantitative RT-PCR was performed using primers specific for β-actin, 10,55,63 GFRα-1, 53 and GFRα-2. 43 A: Representative gels from one of three experiments each are shown (days 0 and 25, telogen; days 1–12, anagen; days 17–19, catagen). B–D: Densitometric analysis of RT-PCR signals specific for β-actin (B), GFRα-1 (C), and GFRαGFRα-22 (D, means ± SD of n = 3); *P < 0.05, **P < 0.005, Student’s t- and ANOVA tests. The x axis in B–D lists the days after anagen induction by depilation (for corresponding hair cycle stages, see A).
In early anagen skin (anagen I-II, days 1–3 p.d.), a significant decrease of GFRα-1 and absence of GFRα-2 transcripts were found (Figure 3) ▶ . However, with further progression of anagen development (anagen VI, days 8–12 p.d.), GFRα-1 and GFRα-2 transcripts were significantly increased, compared with early anagen levels (Figure 3) ▶ . GFRα-1 and GFRα-2 mRNAs were prominently expressed in the proximal and central ORS and IRS, and, in addition, GFRα-2 mRNA was seen in lower amounts in the hair matrix (Figure 4, D, E, and M) ▶ . c-ret-immunoreactivity was homogeneously expressed in the proximal ORS, IRS, and hair matrix, as well as in the DP of anagen VI HF (Figure 4, F and M) ▶ .
Early steps of HF regression (catagen I-II, day 17 p.d.) were characterized by still prominent expression of GFRα-1 and GFRα-2 transcripts in skin (Figure 3) ▶ . By ISH, GFRα-1 and GFRα-2 mRNAs were expressed in the proximal ORS and IRS, whereas the hair matrix was negative for both markers (Figure 4, G, H, and M) ▶ . c-ret immunoreactivity was also found in the ORS and IRS of catagen II HF, whereas its expression in the hair matrix and in the DP was practically absent (Figure 4, I and M) ▶ .
During further catagen development (catagen VI, day 19 p.d.), GFRα-1 transcript was significantly decreased, compared with the anagen and early catagen levels, whereas the expression of GFRα-2 transcript was still prominent (Figure 3) ▶ . By ISH, only a weak GFRα-1 mRNA expression was seen in the ORS and in the secondary hair germ of catagen VI HF (Figure 4, J and M) ▶ . However, more prominent expression of GFRα-2 mRNA was found in the ORS, secondary hair germ, and the epithelial strand during catagen VI (Figure 4, K and M) ▶ . c-ret immunoreactivity was also seen in the proximal ORS and in secondary hair germ of the catagen VI HF (Figure 4, L and M) ▶ .
Taken together, these phenomenological data suggest that GFRα-1/GFRα-2-mediated signaling plays a role in the control of apoptosis-driven HF regression. This appeared to be in line with numerous reports that members of TGFβ superfamily are intimately involved, not only in the control of KC proliferation and differentiation, but also control KC cell death. 6,65-68 We, therefore, further explored the role of GDNF and NTN in HF anagen and catagen control in functional assays.
Acceleration of HF Regression in Heterozygous (+/−) GFRα-1- and Homozygous (−/−) GFRα-2 Knockout Mice
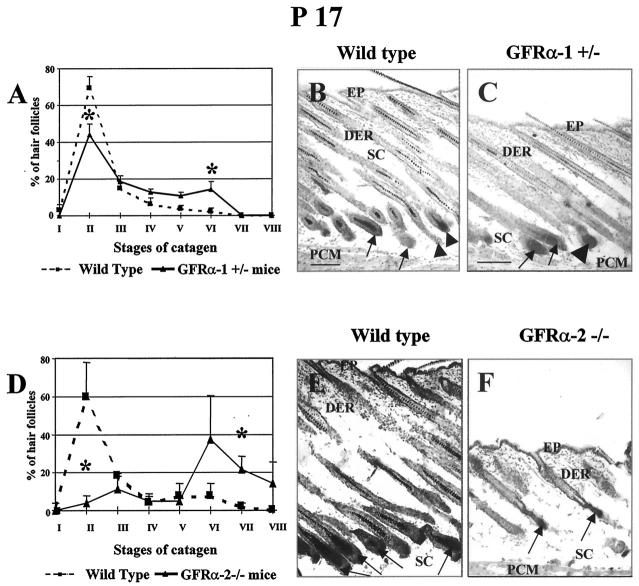
To further define the functional significance of the maximal levels of GFRα-1 and GFRα-2 transcripts during the HF transformation from anagen to catagen (Figure 3) ▶ and the prominent follicular expression patterns of GFRα-1/GFRα-2 mRNAs and c-ret protein during catagen (Figure 4) ▶ , the dynamics of spontaneous catagen development were examined in infantile heterozygous GFRα-1 (+/−) and homozygous GFRα-2 (−/−) knockout mice. 39,43
Compared with their age-matched wild-type controls, heterozygous GFRα-1 knockout (+/−) mice displayed a significant acceleration of their first spontaneous catagen development (Figure 5) ▶ . In contrast to wild-type skin, a significant decline (P < 0.05) in the percentage of catagen II HF and an increase of catagen V-VI HF were seen in GFRα-1 knockout (+/−) mice at P17 (Figure 5, A–C) ▶ , ie, a time-point when HFs in mouse back skin, after the completion of morphogenesis, begin their life-long cycle of regression, resting, and growth by spontaneous entry into the first catagen stage. 2,3,5,50 In addition, the skin thickness was substantially lower (P < 0.05; Figure 5 ▶ , Band C) in heterozygous GFRα-1 knockout (±) mice (521.5 ± 27.1 μm) at P17 compared with wild-type controls (618.8 ± 31.3 μm). Given that synchronized HF cycling in mice is associated with substantial fluctuations in skin thickness, especially with a significant reduction during catagen compared with anagen, 47,69,70 this provided further strong, indirect evidence that a partial deletion of GFRα-1 leads to catagen acceleration.
Figure 5.
Acceleration of catagen development in heterozygous (+/−) GFRα-1 and homozygous (−/−) GFRα-2 knockout mice. GFRα-1 and GFRα-2 knockout mice were generated using conventional gene-targeting techniques as described previously, and genotyping of mutant animals was performed using PCR protocols for the mutated alleles. 39,43 At P17, ie, a time-point when HFs in mouse back skin, after the completion of morphogenesis, begin their life-long cycle of regression, resting, and growth by spontaneous entry into the first catagen stage, 2,3,5,50 skin was harvested as described previously 51 and 4 to 5 mice of every strain were studied. For hair cycle staging, skin cryostat sections of mutant and corresponding age-matched wild-type mice were processed for detection of endogenous alkaline phosphatase activity, 64 and the percentage of HF in defined catagen stages was evaluated by well-defined morphological criteria. 49,60 A–C: GFRα-1 knockout (+/−) mice. A: In the skin of GFRα-1 knockout mice, HFs in early catagen (catagen II) were significantly reduced, and the percentage of follicles in later stages of catagen development (catagen V-VI) was significantly increased, compared with wild-type mice (asterisks indicate significant differences between identical stages of catagen development in wild-type versus GFRα-1 knockout mice, P < 0.05). B and C: At P17, wild-type mice show predominantly catagen II (B, arrows) and catagen III HF (B, arrowheads), characterized by the presence of elongated dermal papilla, yet surrounded by hair matrix KC. 49,60,74 GFRα-1 knockout mice display, together with catagen II (C, arrows), the appearance of catagen V HF, characterized by disappearance of the hair matrix and marked dermal papilla condensation (C, large arrowhead). In addition, the subcutis of GFRα-1 knockout mice was considerably thinner than that of wild-type skin (cf. B and C), which is highly suggestive of advanced catagen development. 69,70 D–F: GFRα-2 knockout (−/−) mice. D: In the skin of GFRα-2 knockout mice, HFs in early catagen (catagen II) were significantly reduced at P17, and the percentage of follicles in later stages of catagen development (catagen VI-VIII) was significantly increased, compared with wild-type mice (asterisks indicate significant differences between identical stages of catagen development in wild-type versus GFRα-2 knockout mice, P < 0.05). E and F: Predominance of late catagen HFs and dramatic reduction of skin thickness in GFRα-2 mutants (F, arrows), compared to wild-type mice, where the vast majority of HFs are still in the beginning of catagen (E, arrows). DER, dermis; EP, epidermis; PCM, panniculus carnosus muscle; SC, subcutis. Scale bars, 100 μm.
It is interesting that a more prominent acceleration of HF regression was seen in the homozygous GFRα-2 knockout mice, compared with the corresponding age-matched wild-type mice (Figure 5, D–F) ▶ . At P17, the vast majority of HFs in GFRα-2-null skin were already in late catagen, whereas most of the HFs in wild-type skin were still at the beginning of catagen (P < 0.05). Also, skin thickness in GFRα-2 mutants (349.6 ± 54.5) was dramatically reduced, compared with the wild-type controls (641.1 ± 72.9; P < 0.01), indicating a significantly advanced catagen progression under constitutive GFRα-2 deletion.
GDNF and NTN Induce a Retardation of Catagen Development in Organ Culture
Considering that the observed acceleration of catagen in GFRα-1 (+/−) and GFRα-2 (−/−) knockout mice might also be explained by the alterations of skin innervation in these mice, 71-73 GDNF and NTN proteins were added to the organ-cultured murine skin (ie, in the absence of functional skin nerves) with most HFs in the process of initiating the anagen VI-catagen transformation. For this purpose, biopsies were taken from normally cycling C57BL/6 mouse skin 17 days after anagen induction by depilation, 47 and were cultured for 48 hours in the presence or absence of 5 ng/ml GDNF or NTN.
Quantitative histomorphometry revealed that 5 ng/ml of GDNF or NTN indeed significantly retarded catagen de- velopment in situ. There was a significant increase in the percentage of HF in catagen I-II and a decrease of HF in catagen III-IV in those skin biopsies that had been cultured in the presence of 5 ng/ml GDNF or NTN (compared with vehicle controls, in which catagen III-IV HFs dominated, P < 0.05 - P < 0.001; Figure 6, A–C ▶ ). Similar changes were observed for 50 ng/ml of GDNF or NTN (data not shown). Therefore, GDNF and NTN can indeed retard catagen development.
Figure 6.
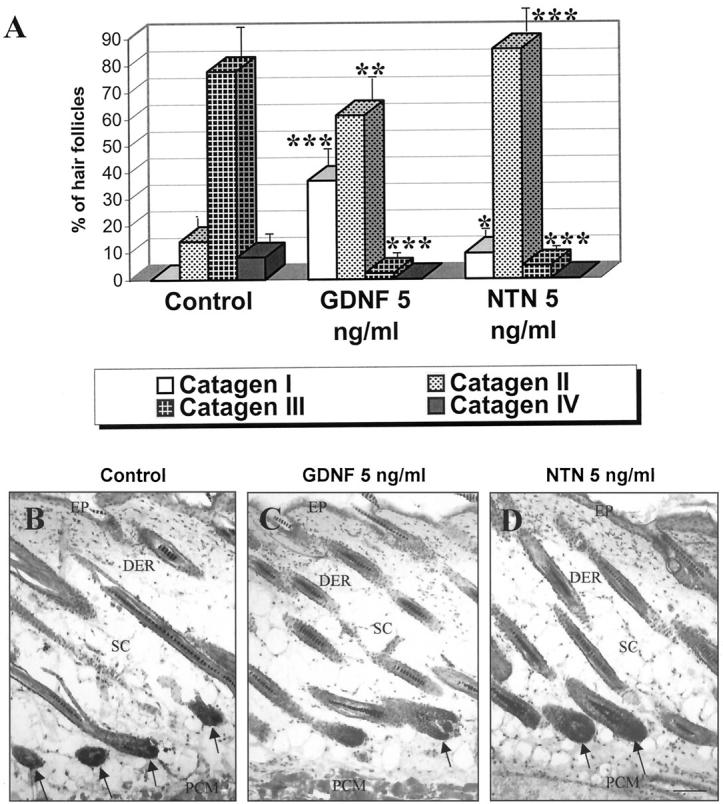
GDNF and neurturin retard catagen development in murine skin organ culture. Punch biopsies, taken from C57BL/6 mouse back skin at day 17 of the depilation-induced hair cycle (ie, with all HFs about to enter into the anagen VI-catagen transformation), were incubated during 48 hours in the presence of 5 ng/ml of GDNF or NTN, and percentage of HFs at the distinct catagen stages was evaluated. A: Compared with vehicle controls, a significant increase in the percentage of the catagen I-II and decline of catagen III-IV HF were seen in skin fragments incubated with 5 ng/ml of GDNF or NTN (mean ± SEM, n = 8–10 biopsies per group, Student’s t-test; asterisks, significant differences to the control, *P < 0.05, **P < 0.01, ***P < 0.001). B–D: Representative examples of skin biopsies treated either with vehicle control (B), GDNF (C), or NTN (D). Note the presence of catagen I HFs, characterized by the enlarged volume of proximal hair bulbs and dermal papilla (C, D, arrows) in skin biopsies after GDNF or NTN treatment. In contrast, control biopsy shows a predominance of catagen III HFs with shrunken hair bulbs and a reduced volume of the dermal papilla (B, arrows). Abbreviations: DER, dermis; EP, epidermis; PCM, panniculus carnosus muscle; SC, subcutis. Scale bars, 100 μm.
Discussion
This study provides the first evidence that GDNF, NTN, and their corresponding high-affinity receptors GFRα-1/GFRα-2 are expressed in adolescent mouse skin, that their expression changes significantly in a hair cycle-dependent manner, and that GDNF and NTN are func-tionally important for the HF anagen-catagen transition.
Our data show that GDNF, NTN, and GFRα-1/GFRα-2 receptors are differentially expressed in the unmanipulated telogen mouse skin. Substantial quantities of GDNF protein (15 pg/mg protein, measured by ELISA; Figure 1 ▶ ) are found in telogen skin, which is comparable to the skin content of TGFβ-1 protein. 10 GDNF mRNA is broadly distributed in adolescent mouse skin, and is expressed in both epithelial (basal KC of epidermis, single KC of the HF ORS, and hair germ) and mesenchymal cells (connective tissue sheath of the HF, cells in dermis; Figure 2 ▶ ), whereas the expression of GFRα-1 receptor appears to be restricted to the epithelial skin compartment (Figure 4) ▶ . However, no expressions of either NTN or GFRα-2 transcripts are seen in telogen skin by RT-PCR or by ISH (Figures 3 and 4) ▶ ▶ .
The steady-state levels of GDNF protein and GFRα-1/GFRα-2 gene transcription in full-thickness skin show synchronous hair cycle-dependent fluctuations, with minimal expression seen in early anagen and late catagen and maximal expression in telogen and late anagen-early catagen stages of the hair cycle (Figures 1 and 3) ▶ ▶ . Studying the roles for GDNF and NTN in the HF anagen-catagen transition, we show that, in striking contrast to TGFβ-1, which stimulates catagen development in human HF in vitro 7,8 and has transcript and protein levels that are maximally up-regulated during murine catagen development, 10 both GDNF and NTN inhibit spontaneous HF regression. We demonstrate that GDNF and NTN transcripts are expressed in the regressing follicular compartments (hair matrix, ORS, IRS) during early catagen (Figure 2) ▶ , whereas GFRα-1, GFRα-2, and c-ret are prominently expressed in the ORS, IRS, and in the secondary hair germ throughout the entire catagen (Figure 4) ▶ . It is most important that, during late anagen and catagen, GFRα-1 and GFRα-2 mRNAs are colocolized in the same follicular compartments (ORS, IRS, secondary hair germ) with their signal-transducing component c-ret (Figure 4) ▶ , which suggests that these receptors are likely to be functional.
The spatiotemporal expression patterns of these receptors and their signal-transducing component indicate that GFRα-1 and GFRα-2 are predominantly expressed in those HF compartments, which show only a very low degree of apoptosis during catagen. Together with our observations that constitutive complete or partial deletion of these receptors leads to an acceleration of catagen (Figure 5) ▶ , this suggests that neither of these receptors is involved in mediating apoptotic cell death during catagen. Rather, GFRα-1/GFRα-2 may promote cell survival or may mediate the release of anti-apoptotic signals during HF regression and serve as negative control mechanisms to prevent the appearance of massive, uncoordinated cell death.
Given that both GDNF and NTN retard catagen development in skin organ culture (Figure 6) ▶ , our data suggest that acceleration of catagen observed in GFRα-1/GFRα-2-deficient mice (Figure 3) ▶ is largely nerve-independent. Also, the prominent acceleration of catagen in neonatal GFRα-2-knockout mice associated with over 50% reduction of skin thickness cannot be explained only by malnutrition problems in these mice 43 because the gross weight of the mutants at P17 ± 1 (7.1 ± 0.7 g, n = 6) was only 5% less than that of their wild-type littermates (8.1 ± 0.7 g, n = 5).
Because catagen is an apoptosis-driven event of rapid organ involution, 2,74,75 GDNF and NTN, therefore, may also be capable of down-modulating apoptosis in selected KC populations. Although the exact mechanisms of the catagen-inhibitory action of GDNF and NTN remain to be dissected, GDNF may counterbalance KC apoptosis specifically in the regressing IRS and in the secondary hair germ, because both express GFRα-1, GFRα-2, and c-ret in catagen (Figure 4) ▶ . That GDNF has, in fact anti-apoptotic properties was recently demonstrated for dopaminergic neurons of the substantia nigra in vitro. 76
Alternatively, GDNF and NTN may also stimulate the terminal differentiation of GFRα-1+/GFRα-2+/c-ret+ secondary germ KC in the direct vicinity of the developing club hair (Figure 3, N and O ▶ ; Figure 4 ▶ ) because this cell population is involved in the formation of trichilemmal keratinization connecting club hair and ORS. 77,78
Taken together, our data demonstrate a previously unrecognized capacity of GDNF and NTN to operate as hair cycle modulators in adolescent mouse skin, namely to inhibit catagen development. This invites one to dissect the role of GFRα-1/GFRα-2/c-ret signal transduction pathways in the control of KC proliferation, differentiation, and/or apoptosis. Furthermore, pharmacological manipulation of these signaling pathways may become clinically exploitable for the treatment of hair growth disorders, the majority of which reflects problems of catagen control. 2,46
Acknowledgments
The excellent technical assistance of R. Pliet and E. Hagen is gratefully acknowledged. We are greatly indebted to Dr. J. Milbrandt and Dr. H. Enomoto for generously providing skin samples of GFRα-1 mutants.
Footnotes
Address reprint requests to Ralf Paus, M.D., Department of Dermatology, University Hospital Eppendorf, University of Hamburg, Martinstrasse 52, D-20246, Hamburg, Germany. E-mail: paus@uke.uni-hamburg.de.
Supported by grants from DFG (Pa 345/6-2 and 8-2) and Wella AG, Darmstadt, Germany (to R. P.).
References
- 1.Hardy MH: The secret life of the hair follicle. Trends Genet 1992, 8:55-61 [DOI] [PubMed] [Google Scholar]
- 2.Paus R: Control of the hair cycle and hair diseases as cycling disorders. Curr Opin Dermatol 1996, 3:248-258 [Google Scholar]
- 3.Stenn K, Parimoo S, Prouty S: Growth of the hair follicle: a cycling and regenerating biological system. Chuong C-M eds. Molecular Basis of Epithelial Appendages Morphogenesis. 1998, :pp. 111-156 R.G. Landes, Austin, TX, [Google Scholar]
- 4.Paus R: Principles of hair cycle control. J Dermatol 1998, 25:793-802 [DOI] [PubMed] [Google Scholar]
- 5.Paus R, Muller-Rover S, Botchkarev V: Chronobiology of the hair follicle: hunting the “hair cycle clock.” J Invest Dermatol 1999, 4:338–345 [DOI] [PubMed]
- 6.Sellheyer K, Bickenbach JR, Rothnagel JA, Bundman D, Longley MA, Krieg T, Roche NS, Roberts AB, Roop DR: Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc Natl Acad Sci USA 1993, 90:5237-5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philpott MP, Sanders D, Westgate GE, Kealey T: Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci 1994, 7(Suppl):S55-S72 [DOI] [PubMed] [Google Scholar]
- 8.Mori O, Hashisuka H, Sasai Y: Effects of transforming growth factor β1 in the hair cycle. J Dermatol 1996, 23:89-94 [DOI] [PubMed] [Google Scholar]
- 9.Paus R, Foitzik K, Welker P, Bulfone-Paus S, Eichmuller S: Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol 1997, 109:518-526 [DOI] [PubMed] [Google Scholar]
- 10.Welker P, Foitzik K, Bulfone-Paus S, Henz BM, Paus R: Hair cycle-dependent changes in the gene expression and protein content of transforming growth factor β1 and β3 in murine skin. Arch Derm Res 1997, 289:554-557 [DOI] [PubMed] [Google Scholar]
- 11.Philpott M, Paus R: Principles of hair follicle morphogenesis. Chuong CM eds. Molecular basis of epithelial appendage morphogenesis. 1998, :pp. 75-110 R.G. Landes Company, Austin, TX, [Google Scholar]
- 12.Botchkarev V, Botchkareva N, Roth W, Nakamura M, Chen L-H, Herzog W, Lindner G, McMahon J, Peters C, Lauster R, McMahon A, Paus R: Noggin is a mesenchymally-derived stimulator of hair follicle induction. Nat Cell Biol 1999, 1:158-164 [DOI] [PubMed] [Google Scholar]
- 13.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F: GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260:1130-1132 [DOI] [PubMed] [Google Scholar]
- 14.Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, Milbrandt J: Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 1996, 384:467-470 [DOI] [PubMed] [Google Scholar]
- 15.Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, H, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang L-C, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Henderson CE, Phillips HS, Johnson EM: Persephin, a novel neurotrophic factor related to GDNF, and neurturin. Neuron 1998, 20:245-253 [DOI] [PubMed] [Google Scholar]
- 16.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Milbrandt J: Artemin a novel member of GDNF ligand family, supports peripheral, and central neurons, and signals through the GFRalpha3-Ret receptor complex. Neuron 1998, 21:1291-1302 [DOI] [PubMed] [Google Scholar]
- 17.Durbec P, Marcos Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V: GDNF signalling through the Ret receptor tyrosine kinase. Nature 1996, 381:789–793 [DOI] [PubMed]
- 18.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupolas R, Louis JC, Hu S, Altrock BW, Fox GM: GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 1996, 85:1113-1124 [DOI] [PubMed] [Google Scholar]
- 19.Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar Grueso E, Pachnis V, Arumae U, Sariola H, Saarma M, Ibanez CF: Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 1996, 381:785–788 [DOI] [PubMed]
- 20.Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EM, Jr, Milbrandt J: TrnR2, a novel receptor that mediates neurturin, and GDNF signaling through Ret. Neuron 1997, 18:793-802 [DOI] [PubMed] [Google Scholar]
- 21.Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo JA, Devaux B, Poulsen K, Armanini M, Nozaki C, Asai N, Goddard A, Phillips H, Henderson CE, Takahashi M, Rosenthal A: A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature 1997, 387:717-721 [DOI] [PubMed] [Google Scholar]
- 22.Buj Bello A, Adu J, Pinon LG, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buchman VL, Davies AM: Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature 1997, 387:721–724 [DOI] [PubMed]
- 23.Airaksinen M, Titievsky A, Saarma M: GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci 1999, 13:313-325 [DOI] [PubMed] [Google Scholar]
- 24.Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF: Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol 1995, 130:137-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A: Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev 1996, 54:95-105 [DOI] [PubMed] [Google Scholar]
- 26.Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L: Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res 1996, 286:191-207 [DOI] [PubMed] [Google Scholar]
- 27.Suvanto P, Hiltunen JO, Arumae U, Moshnyakov M, Sariola H, Sainio K, Saarma M: Localization of glial cell line-derived neurotrophic factor (GDNF) mRNA in embryonic rat by in situ hybridization. Eur J Neurosci 1996, 8:816-822 [DOI] [PubMed] [Google Scholar]
- 28.Luukko K, Suvanto P, Saarma M, Thesleff I: Expression of GDNF and its receptor in developing tooth is developmentally regulated and suggests multiple roles in innervation and organogenesis. Dev Dyn 1997, 210:463-471 [DOI] [PubMed] [Google Scholar]
- 29.Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L: Neurturin and glial cell line-derived neurotrophic factor receptor-β (GDNFR-β), novel proteins related to GDNF and GDNFR-α with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J Neurosci 1997, 17:8506-8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luukko K, Saarma M, Thesleff I: Neurturin mRNA expression suggests roles in trigeminal innervation of the first branchial arch and in tooth formation. Dev Dyn 1998, 213:207-219 [DOI] [PubMed] [Google Scholar]
- 31.Nosrat CA, Fried K, Ebandal T, Olson L: NGF, BDNF, NT3, NT4, and GDNF in tooth development. Eur J Oral Sci 1998, 106(Suppl 1):94-99 [DOI] [PubMed] [Google Scholar]
- 32.Peters RJ, Osinski MA, Hongo JA, Bennett GL, Okragly AJ, HaakFrendscho M, Epstein ML: GDNF is abundant in the adult rat gut. J Auton Nerv Syst 1998, 70:115-122 [DOI] [PubMed] [Google Scholar]
- 33.Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R: Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci 1998, 18:4684-4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuchardt A, D’Agatti V, Larsson-Blomberg L, Constantini F, Pachnis V: Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor ret. Nature 1994, 367:380-383 [DOI] [PubMed] [Google Scholar]
- 35.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver Moore K, Rosenthal A: Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996, 382:76–79 [DOI] [PubMed]
- 36.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H: Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 1996, 382:73-76 [DOI] [PubMed] [Google Scholar]
- 37.Sanchez MP, Silos Santiago I, Frisen J, He B, Lira SA, Barbacid M: Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 1996, 382:70–73 [DOI] [PubMed]
- 38.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A: GFR α 1 is an essential receptor component for GDNF in the developing nervous system, and kidney. Neuron 1998, 21:53-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Milbrandt J: GFR α 1-deficient mice have deficits in the enteric nervous system, and kidneys. Neuron 1998, 21:317-324 [DOI] [PubMed] [Google Scholar]
- 40.Pepicelli CV, Kispert A, Rowitch DH, McMahon AP: GDNF induces branching and increases cell proliferation in the ureter of the mouse. Dev Biol 1997, 192:193-198 [DOI] [PubMed] [Google Scholar]
- 41.Tang MJ, Worley D, Sanicola M, Dressler GR: The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration, and chemoattraction of epithelial cells. J Cell Biol 1998, 142:1337-1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EMJ, Milbrandt J: Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 1999, 22:253-263 [DOI] [PubMed] [Google Scholar]
- 43.Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, Arumae U, Pasternack M, Saarma M, Airaksinen MS: Retarded growth and deficit in the enteric and parasympathetic nervous system in mice lacking GFRalpha2, a functional neurturin receptor. Neuron 1999, 22:243-252 [DOI] [PubMed] [Google Scholar]
- 44.Lyons KM, Pelton RW, Hogan BL: Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 1990, 109:833-844 [DOI] [PubMed] [Google Scholar]
- 45.Blessing M, Nanney LB, King LE, Jones CM, Hogan BL: Transgenic mice as a model to study the role of TGF-β-related molecules in hair follicles. Genes Dev 1993, 7:204-215 [DOI] [PubMed] [Google Scholar]
- 46.Paus R, Cotsarelis G: The biology of hair follicles. N Engl J Med 1999, 341:491-497 [DOI] [PubMed] [Google Scholar]
- 47.Paus R, Stenn KS, Link RE: Telogen skin contains an inhibitor of hair growth. Br J Dermatol 1990, 122:777-784 [DOI] [PubMed] [Google Scholar]
- 48.Chase HB: Growth of the hair. Physiol Rev 1954, 34:113-126 [DOI] [PubMed] [Google Scholar]
- 49.Straile WZ, Chase HB, Arsenault C: Growth and differentiation of hair follicles between activity and quiescence. J Exp Zool 1961, 148:205-222 [DOI] [PubMed] [Google Scholar]
- 50.Stenn KS, Combates NJ, Eilertsen KJ, Gordon JS, Pardinas JR, Parimoo S, Prouty SM: Hair follicle growth control. Dermatol Clin 1996, 14:543-557 [DOI] [PubMed] [Google Scholar]
- 51.Paus R, Hofmann U, Eichmüller S, Czarnetzki BM: Distribution and changing density of γ-delta T cells in murine skin during the induced hair cycle. Br J Dermatol 1994, 130:281-289 [DOI] [PubMed] [Google Scholar]
- 52.Pethö-Schramm A, Müller H-J, Paus R: FGF5, and the murine hair cycle. Arch Derm Res 1996, 288:264-266 [DOI] [PubMed] [Google Scholar]
- 53.Eng C, Myers SM, Kogon MD, Sanicola M, Hession C, Cate RL, Mulligan LM: Genomic structure and chromosomal localization of the human GDNFR-α gene. Oncogene 1998, 16:597-601 [DOI] [PubMed] [Google Scholar]
- 54.Panteleyev A, Paus R, Wanner R, Nürnberg W, Eichmüller S, Thiel R, Zhang J, Henz BM, Rosenbach T: Keratin 17 gene expression during the murine hair cycle. J Invest Dermatol 1997, 108:324-329 [DOI] [PubMed] [Google Scholar]
- 55.Botchkarev VA, Botchkareva NV, Welker P, Metz M, Lewin GR, Subramaniam A, Braun A, Lommatzsch M, Renz H, Paus R: A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. FASEB J 1999, 13:395-410 [DOI] [PubMed] [Google Scholar]
- 56.Suvanto P, Wartiovaara K, Lindahl M, Arumae U, Moshnyakov M, Horelli-Kuitunen N, Airaksinen M, Palotie A, Sariola H, Saarma M: Cloning, mRNA distribution, and chromosomal localization of the gene for glial cell line-derived neurotrophic factor receptor β, a homologue to GDNFR-α. Hum Mol Genet 1997, 6:1267-1273 [DOI] [PubMed] [Google Scholar]
- 57.Botchkarev VA, Eichmüller S, Johansson O, Paus R: Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol 1997, 386:379-395 [DOI] [PubMed] [Google Scholar]
- 58.Botchkarev VA, Eichmüller S, Peters EMJ, Pietsch P, Johansson O, Maurer M, Paus R: A simple fluorescent technique for co-visualization of mast cells and nerve fibers reveals selectivity and hair cycle-dependent changes in mast cell-nerve fiber contacts in skin. Arch Derm Res 1997, 289:292-302 [DOI] [PubMed] [Google Scholar]
- 59.Roloff B, Fechner K, Slominski A, Furkert J, Botchkarev VA, Bulfone-Paus S, Zipper J, Krause E, Paus, R: Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J 1998, 12:287–297 [DOI] [PubMed]
- 60.Paus R, Handjiski B, Czarnetzki BM, Eichmüller S: A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol 1994, 103:143-147 [DOI] [PubMed] [Google Scholar]
- 61.Li L, Paus R, Slominski A, Hoffman RM: Skin histoculture assay for studying the hair cycle. In Vitro Cell Dev Biol 1992, 28:695-698 [DOI] [PubMed] [Google Scholar]
- 62.Paus R, Lüftl M, Czarnetzki BM: Nerve growth factor modulates keratinocyte proliferation in murine skin organ culture. Br J Dermatol 1994, 130:174-180 [DOI] [PubMed] [Google Scholar]
- 63.Botchkarev VA, Welker P, Albers KM, Botchkareva NV, Metz M, Lewin GR, Bulfone-Paus S, Peters EMJ, Lindner G, Paus R: A new role for neurotrophin-3: involvement in the regulation of hair follicle regression (catagen). Am J Path 1998, 153:785-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Handjiski B, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R: Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol 1994, 131:303-310 [DOI] [PubMed] [Google Scholar]
- 65.Garlick JA, Taichman LB: Effect of TGF-β 1 on re-epithelialization of human keratinocytes in vitro: an organotypic model. J Invest Dermatol 1994, 103:554-559 [DOI] [PubMed] [Google Scholar]
- 66.Jiang CK, Tomic Canic M, Lucas DJ, Simon M, Blumenberg M: TGF β promotes the basal phenotype of epidermal keratinocytes: transcriptional induction of K5 and K14 keratin genes. Growth Factors 1995, 12:87–97 [DOI] [PubMed]
- 67.Benassi L, Ottani D, Fantini F, Marconi A, Chiodino C, Giannetti A, Pincelli C: 1,25-dihydroxyvitamin D3, transforming growth factor β1, calcium, and ultraviolet B radiation induce apoptosis in cultured human keratinocytes. J Invest Dermatol 1997, 109:276-282 [DOI] [PubMed] [Google Scholar]
- 68.Mitsui S, Ohuchi A, Hotta M, Tsuboi R, Ogawa H: Genes for a range of growth factors and cyclin-dependent kinase inhibitors are expressed by isolated human hair follicles. Br J Dermatol 1997, 137:693-698 [PubMed] [Google Scholar]
- 69.Hansen LS, Coggle JE, Wells J, Charles MW: The influence of the hair cycle on the thickness of mouse skin. Anat Rec 1984, 210:569-573 [DOI] [PubMed] [Google Scholar]
- 70.Maurer M, Handjiski B, Paus R: Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy induced alopecia. Am J Pathol 1997, 150:1433-1441 [PMC free article] [PubMed] [Google Scholar]
- 71.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD: IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 1997, 19:849-861 [DOI] [PubMed] [Google Scholar]
- 72.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW: Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science 1998, 279:1725-1729 [DOI] [PubMed] [Google Scholar]
- 73.Fundin B, Mikaels A, Westphal H, Ernfors P: A rapid and dynamic regulation of GDNF-family ligands and receptors correlate with the developmental dependency of cutaneous sensory innervation. Development 1999, 126:2597-2610 [DOI] [PubMed] [Google Scholar]
- 74.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R: Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol 1997, 151:1601-1617 [PMC free article] [PubMed] [Google Scholar]
- 75.Cotsarelis G: The hair follicle: dying for attention. Am J Pathol 1997, 151:1505-1509 [PMC free article] [PubMed] [Google Scholar]
- 76.Burke RE, Antonelli M, Sulzer D: Glial cell line-derived neurotrophic factor inhibits apoptotic death of postnatal substantia nigra dopamine neurons in primary culture. J Neurochem 1998, 71:517-525 [DOI] [PubMed] [Google Scholar]
- 77.Parakkal PF: Morphogenesis of the hair follicle during catagen. Z Zellforsch Mikrosk Anat 1970, 107:174-186 [DOI] [PubMed] [Google Scholar]
- 78.Pinkus H, Iwasaki T, Mishima Y: Outer root sheath keratinization in anagen and catagen of the mammalian hair follicle: a seventh distinct type of keratinization in the hair follicle: trichilemmal keratinization. J Anat 1981, 133:19-35 [PMC free article] [PubMed] [Google Scholar]