Abstract
Interstitial cystitis (IC) is a debilitating disease that has been adversely affecting the quality of women’s lives for many years. The trigger in IC is not entirely known, and a role for the sensory nerves in its pathogenesis has been suggested. In addition to inflammation, increased mast cell numbers in the detrusor muscle have been reported in a subset of IC patients. Experimentally, several lines of evidence support a central role for substance P and neurokinin-1 (NK-1) receptors in cystitis. The availability of mice genetically deficient in neurokinin-1 receptor (NK-1R−/−) allows us to directly evaluate the importance of substance P in cystitis. An unexpected finding of this investigation is that NK-1R−/− mice present increased numbers of mast cells in the bladder when compared with wild-type control mice. Despite the increase in mast cell numbers, no concomitant inflammation was observed. In addition, bladder instillation of wild-type mice with a sensitizing antigen induces activation of mast cells and an acute inflammatory response characterized by plasma extravasation, edema, and migration of neutrophils. Antigen-sensitized NK-1R−/− mice also exhibit bladder mast cell degranulation in response to antigen challenge. However, NK-1R−/− mice are protected from inflammation, failing to present bladder inflammatory cell infiltrate or edema in response to antigen challenge. This work presents the first evidence of participation of NK-1 receptors in cystitis and a mandatory participation of these receptors on the chain of events linking mast cell degranulation and inflammation.
Interstitial cystitis (IC) is a chronic bladder inflammation affecting millions of women and causing severe pain, increased frequency of micturition, and even disability. 1 Clinical symptoms commonly associated with IC include increased urinary frequency and urgency, nocturia, suprapubic pressure, and pain that is generally relieved by voiding. 2 Major pathological findings in bladder biopsies from IC patients include an intense vascular component and increased numbers of mast cells and lymphocytes. 3,4 The intense tissue remodeling has implicated a role for neurotrophins 5 and mast cells 6-7 in IC concomitantly with increased urinary levels of histamine and tryptase. 5
Autoimmune and immune mechanisms have been implicated based on findings that at least a subset of IC patients present with allergy symptoms 8 and that immunoglobulin and complement deposits are present in affected bladders. 9 The immunological component is also supported by the findings of increased interleukin-6 levels in the urine of patients with IC 10 and evidence of urothelial cell activation. 11 Experimental animal models further support a possible role for an immunological component in cystitis. 12 Moreover, experiments in intact animals have indicated that antigen sensitization and challenge induce cystitis. 13,14 In this animal model, the inflammatory response is a complex cascade that involves disruption of the bladder permeability barrier. 15
The trigger in IC is not entirely known, and pain often appears out of proportion to standard laboratory and pathological evaluation, thus continually raising questions as to the role of the sensory nerves in its pathogenesis. 2 In fact, bladder biopsies from IC patients present increased density of neurokinin-1 (NK-1) receptors, 18 nerves, 19 and substance P-containing fibers. 20 Furthermore, the finding that sensory C fiber desensitization decreases urinary bladder hyperreflexia further supports a role for sensory peptides in this disorder. 16
Among the peptides released by C fibers, the tachykinins, substance P (SP) and NK-A, are known modulators of bladder inflammation. 17 SP is spontaneously released within the bladder wall, 21 activates NK receptors, and leads to increased vascular permeability 28 and expression of adhesion molecules. 29 The finding of increased NK-1 receptor density in experimental inflammation further supports a role for this receptor in cystitis. 22 In fact, NK-1 receptor antagonists reduce detrusor hyperreflexia caused by chemical 24 and bacterial 27 cystitis, decrease cyclophosphamide-induced inflammation, 25,26 and restore bladder endosomal fusion secondary to antigen-induced cystitis. 23
Products of mast cell degranulation activate sensory C fibers to release SP 7,17,30,31 and therefore induce inflammation. In this regard, recent experiments indicate that sensitized NK-1 receptor knockout (NK-1R−/−) mice do not mount an inflammatory response to antigen-complex stimulation. These results indicate a mandatory role for SP and its receptor in modulating the effects of mast cell degranulation. 32 Additional findings using NK-1R−/− mice indicate that SP plays a major role in other forms of inflammation, such as pancreatitis (34), Clostrium difficile toxin A-induced colitis, 33 and granuloma response of murine schistosomiasis. 35 However, little is known of how SP regulates other proinflammatory substances within the bladder wall.
The availability of NK-1R−/− mice allows us to directly evaluate the importance of NK-1 receptors in cystitis and to examine the mechanisms by which SP participates in the early events of inflammation.
Materials and Methods
Animals
NK-1R−/− (KO) and wild-type (WT) littermate control mice were generated by N. P. Gerard, and the colony at the University of Texas Medical Branch (UTMB) was genotyped as described previously. 32 Female mice weighing 20–30 g were used in this experiment, according to the approved animal protocol (UTMB, Animal Care & Use Committee protocol 98-05-033).
Sensitization Protocol
Groups of 32 mice (16 KO and 16 WT) were sensitized intraperitoneally (i.p.) with 1 μg of dinitrophenyl (DNP)4-human serum albumin (HSA) in 1 mg of alum on days 0, 7, 14, and 21. This protocol induces, in WT mice, sustained levels of immunoglobulin E (IgE) up to 56 days postsensitization. 36 One week after the last sensitization, cystitis was induced.
Induction of Cystitis
Sensitized WT and KO mice were anesthetized (ketamine, 40 mg/kg, and xylazine, 2.5 mg/kg i.p.), then transurethrally catheterized (24 Ga, 3/4 in; Angiocath, Becton Dickinson, Sandy, UT), and the urine was drained by applying slight digital pressure to the lower abdomen. The urinary bladders were instilled with either 150 μl of saline or DNP4-ovalbumin (-OVA; 1 μg/ml) infused at a slow rate to avoid trauma and vesicoureteral reflux. 37 To ensure consistent contact of substances with the bladder, infusion was repeated twice within a 30-minute interval. Twenty-four hours after instillation, mice were sacrificed with pentobarbital (20 mg/kg i.p.), and bladders were removed rapidly and fixed in buffered formalin for histology (n = 8 per group).
Alterations at Histological Level
The urinary bladder was evaluated for inflammatory cell infiltrates, mast cell numbers, and the presence of interstitial edema. A semiquantitative score with defined criteria of inflammation severity was used to evaluate cystitis. 38 A cross-section of bladder wall was fixed in formalin, dehydrated in graded alcohol and xylene, embedded in paraffin, and cut serially into four 5-μm sections (8 μm apart) to be stained with hematoxylin and eosin (H&E) and Giemsa. Histology slides were scanned with a CoolSNAP camera (RS Photometrics, Tucson, AZ) mounted on an Olympus microscope. Image analysis was performed with a MetaMorph Imaging System (Universal Imaging Corp., West Chester, PA). The severity of lesions in the urinary bladder was graded as follows: 1+, mild (infiltration of a low number of neutrophils in the lamina propria and little or no interstitial edema); 2+, moderate (infiltration of moderate numbers of neutrophils in the lamina propria and moderate interstitial edema); 3+, severe (diffuse infiltration of moderate to large numbers of neutrophils in the lamina propria and severe interstitial edema).
Identification of mast cells and quantification of their degree of degranulation was performed in Giemsa-stained sections because the presence of granules is the identifying characteristic for mast cells. Degrees of degranulation are presented as the percentage of mast cells per cross-section that exhibited degranulation.
Statistical Analysis
The statistical analysis of data was performed using Wilcoxon’s rank sum test. Results are expressed as mean ± SEM. The n values reported refer to the number of animals used for each experiment. In all cases, a value of P < 0.05 was considered indicative of significant difference. 39
Reagents
DNP was conjugated to OVA and HSA (Sigma Chemical Co., St. Louis, MO) as previously described. 40 Alum adjuvant was purchased from Intergen (Purchase, NY).
Results
Histological Severity of Antigen-Induced Cystitis
We first examined whether the bladder of sensitized mice developed inflammation secondary to antigen challenge. Bladders isolated from sensitized WT mice that were challenged with saline did not present any sign of inflammation or edema (Figure 1a) ▶ . WT sensitized mice challenged with antigen developed an inflammatory response characterized by vasodilation, edema, intense polymorphonuclear neutrophil (PMN) infiltration in the mucosa and submucosal layers (Figure 1b) ▶ , and activation of resident mast cells (Table 1) ▶ . This inflammatory response, which was observed 24 hours after antigen stimulation, had predominant characteristics of acute inflammation based on the strong vascular component, predominance of PMNs, and near absence of macrophages/monocytes (Figure 1b) ▶ . In contrast, histological quantification of the bladder sections indicated that NK-1R−/− mice had significant attenuation of congestion and edema of the mucosa, as well as reduced PMN infiltration in response to antigen challenge (Figure 1c ▶ ; Table 1 ▶ ). Histologically, antigen-challenged bladders of these mice appeared to be no different than those from sensitized WT mice exposed to saline (Figure 1a) ▶ .
Figure 1.
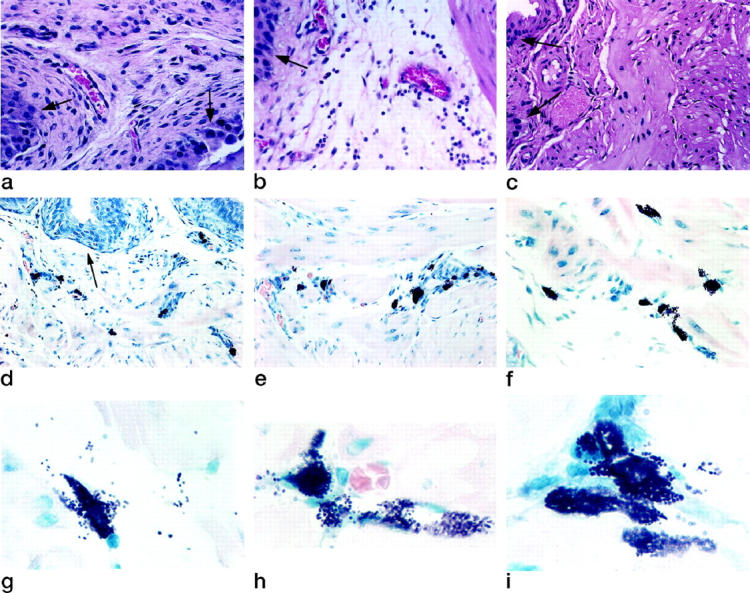
Histological evaluation of antigen-induced cystitis. a–c: Cell migration and edema. a: Sensitized WT mouse bladder 24 hours after intravesical instillation of saline, showing normal bladder submucosa architecture. b: Sensitized WT mouse bladder 24 hours after intravesical antigen challenge showing vasodilation, edema, and infiltration of cells in the submucosa. c: Sensitized NK-1R−/− mouse bladder 24 hours after intravesical antigen challenge showing lack of antigen-associated inflammatory changes. a–c were stained with hematoxylin and eosin. d–f: Mast cell distribution. Bladders were isolated from sensitized NK-1R−/− 24 hours after intravesical instillation of antigen. Mast cells were found in the suburothelial layer (d), around the blood vessels (e), and within the detrusor muscle (f). Results are representative of 16 NK-1R−/− mice. Original magnification, ×500. g–i: Mast cell activation. Bladders were isolated from sensitized NK-1R−/− 24 hours after intravesical instillation of antigen. Activated mast cells were found in the suburothelial layer (g) around the blood vessels (h) and within the detrusor muscle (j). Original magnification, ×1000. Identification of mast cells’ distribution and activation in d–i was performed in Giemsa-stained sections, because the presence of granules is the identifying characteristic for mast cells. a–i were analyzed by the investigator in a blinded fashion and quantification of mast cells, edema, and PMNs is presented in Table 1 ▶ . Results are representative of WT (n = 16) and NK-1R−/− mice (n = 16). Arrowsindicate urothelial cells.
Table 1.
Histological Severity of Antigen-Induced Cystitis Is Reduced in NK-1R−/− Mice Compared with WT Mice
| Mice | Histological severity of cystitis* | Mast cells† | |||
|---|---|---|---|---|---|
| Stimulus‡ | Edema | Neutrophil | Number | % Degranulation | |
| WT | Saline | 0.70 ± 0.14 | 0.18 ± 0.04 | 8.0 ± 0.7 | 4.4 ± 1.4 |
| WT | Antigen | 2.88 ± 0.06§ | 2.42 ± 0.11§ | 7.5 ± 0.4NS | 50.0 ± 6.0§ |
| NK-1R−/− | Saline | 0.52 ± 0.07 | 0.40 ± 0.12 | 39.0 ± 1.0¶ | 12.0 ± 1.3 |
| NK-1R−/− | Antigen | 0.60 ± 0.10NS | 0.42 ± 0.12NS | 33.0 ± 0.9§ | 60.0 ± 7.6§ |
*The histological severity of cystitis was graded by a score of 0–3.
†Number of mast cells per bladder cross-section.
‡DNP-HSA-sensitized mice were anesthetized and instilled either with saline or antigen (DNP-OVA). Inflammation was assessed after 24 hours as described in Materials and Methods (eight animals per group). Bladder tissue was fixed in formalin, and then paraffin embedded. Four serial sections (4 μm apart) were stained with H&E for histologic analysis in a blinded fashion. Additional sections were stained with Giemsa for evaluation of mast cell numbers. Values are means ± SEM. P values were obtained by using Wilcoxon’s rank sum test.
§P < 0.0001 versus the respective control (instilled with saline).
¶P < 0.0001 versus WT.
NSNonsignificant when compared with the respective control (instilled with saline).
Mast Cell Numbers and Activation
Morphological analysis was used to investigate the participation of the mast cell in cystitis. Urinary bladders isolated from sensitized NK-1R−/− mice that were exposed either to saline or antigen presented fourfold more mast cells than counterpart WT control mice. Increased numbers of mast cells were found in the subepithelial layers (Figure 1d) ▶ and within the detrusor smooth muscle around blood vessels (Figure 1e) ▶ and between muscle fibers (Figure 1f) ▶ . These results are summarized in Table 1 ▶ . In addition, antigen challenge of sensitized WT and NK-1R−/− mice induced activation of resident mast cells as indicated by intense degranulation (Figure 1, g–i ▶ ; Table 1 ▶ ). Despite the increased number of mast cells in the bladders of NK-1R−/− mice and the intense degranulation observed, there were no evident signs of edema or PMN infiltrate in response to antigen challenge (Figure 1, a–c) ▶ .
Discussion
Confirming our previous report with guinea pigs, 13,14 sensitized WT mice developed bladder inflammation in response to antigen challenge. In contrast, sensitized NK-1 R−/− mice did not develop bladder inflammation secondary to antigen stimulation. The reduction in the inflammatory response was not due to a decrease in peptide content or reduced mast cell numbers. Concerning peptide content, similar levels of SP were found in tissues isolated from NK-1R−/− and WT mice. 33,41
In regard to mast cell numbers, urinary bladders isolated from NK-1R−/− mice had fourfold more mast cells than those from WT control mice (Table 1) ▶ . In this context, we reported that products of mast cells stimulate sensory neurons and release SP. 17 In a positive feedback loop, an important aspect of SP action is its capacity to stimulate mast cells through activation of NK receptors 31 and to enhance mast cell degranulation secondary to other stimuli. 30 The capacity of these substances to stimulate each other’s release reciprocally suggests a nerve-mast cell axis 31 that may perpetuate cystitis, regardless of the initial stimulus. Our previous findings of a close physical contact between mast cells and sensory nerves containing SP in the urinary bladder further support this theory. 42 However, despite the increased numbers of mast cells, no other signs of inflammation were observed in the bladder of NK-1R−/− mice. This would indicate a mandatory participation of NK-1 receptors on the chain of events linking mast cell degranulation and inflammation. Therefore, we suggest that increased mast cell numbers without a concomitant participation of sensory peptides is not enough to induce bladder inflammation.
Another possible explanation for absence of inflammation in response to antigen stimulation is that NK-1R−/− mice are unable to mount an immune response. In this regard, it has been shown that splenocytes and granuloma cells in NK-1R−/− mice present an appreciable impairment in IgG2a and IgE expression. 35 However, NK-1R−/− mice also failed to respond with an inflammatory response when passively sensitized with an immunoglobulin, 32 indicating that the NK-1 receptor plays a major role downstream of immune-complex activation.
SP and its receptor are widely distributed and implicated in the regulation of a wide range of inflammatory pathways, such as activation of vascular endothelial cells, vascular permeability, plasma extravasation, 28 and expression of adhesion molecules. 29 The development and use of NK-1 receptor KO mice has demonstrated the central mechanistic role of this receptor in other forms of inflammation. 32-35 In the bladder, although the involvement of SP in cystitis has been suggested previously, 4,7,17,18,20 our results provide direct evidence for the importance of NK-1 receptors as an upstream regulator of an inflammatory cascade.
The results obtained with NK-1R−/− mice indicate that this receptor subtype is essential for induction of inflammation. Other pathways activated by SP appear to have minor contributions. These effects fall into two major categories. The first involves the effects of SP on NK-2 and NK-3 receptors. 24 Second, SP may also induce release of other mediators, such as bradykinin, which activates its own receptor. 21,30
Our data suggest that the NK-1 receptor is by far the most quantitatively important proximate mediator of this type of inflammatory response. The specific role of NK-2 and NK-3 receptors in inflammation needs to be reassessed. In addition, understanding receptor-independent pathways may be critical to refining any therapeutic strategy based on NK-1 receptor inhibition.
Our studies were performed 24 hours after antigen stimulation and, therefore, represent the early events of cystitis. However, they strongly indicate a mandatory role for SP and NK-1 receptors, mediating inflammation secondary to mast cell activation. Because both mast cells 7 and NK-1 receptors 18 are increased in bladder biopsies of IC patients, it is tempting to propose a role for sensory peptides in the pathogenesis of this disorder. Further studies, using a specific nonpeptide antagonist of NK-1 receptors, are necessary to investigate whether blockade of this receptor will reduce the inflammation and symptoms of IC.
Acknowledgments
The authors thank Mrs. Brenda Kenworthy for editing this manuscript.
Footnotes
Address reprint requests to Ricardo Saban, DVM, PhD, Assistant Professor, Department of Internal Medicine and Urology, University of Texas Medical Branch, 1108 The Strand, Room 219, Galveston, TX 77555-0632. E-mail: risaban@utmb.edu.
Supported by National Institutes of Health grants DK 55828–01 (to R. S.), DK51392 (to T. G. H.), and HL41587 (to N. P. G.).
References
- 1.Koziol JA, Clarck DC, Gittes RF: The natural history of interstitial cystitis: a survey of 374 patients. J Urol 1993, 149:465-469 [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L, Jr: The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol 1999, 161:553-557 [DOI] [PubMed] [Google Scholar]
- 3.Elbadawi A: Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 1997, 49:14-40 [DOI] [PubMed] [Google Scholar]
- 4.Granum RS, Theoharides TC: The role of mast cells in cystitis. Urol Clin N Am 1994, 21:41-50 [PubMed] [Google Scholar]
- 5.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M: Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 1999, 16:438-441 [PubMed] [Google Scholar]
- 6.Hofmeister MA, He F, Ratliff TL, Mahoney T, Becich MJ: Mast cells and nerve fibers in interstitial cystitis (IC): an algorithm for histologic diagnosis via quantitative image analysis and morphometry (QIAM). Urology 1997, 49:41-47 [DOI] [PubMed] [Google Scholar]
- 7.Theoharides TC, Pany X, Letourneau R, Sant GR: Interstitial cystitis: a neuroimmunoendocrine disorder. Ann NY Acad Sci 1998, 840:619-634 [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Murayama T, Mita H, Akiyama K, Taguchi H: Alternate occurrence of allergic disease and an unusual form of interstitial cystitis. Int J Urol 1998, 5:329-335 [DOI] [PubMed] [Google Scholar]
- 9.Lynes WL, Flynn SD, Shortliffe LD, Lemmers M, Zipser R, Roberts LJ, II, Stamey TA: Mast cell involvement in interstitial cystitis. J Urol 1987, 138:746-752 [DOI] [PubMed] [Google Scholar]
- 10.Erickson DR, Belchis DA, Dabbs DJ: Inflammatory cell types and clinical features of interstitial cystitis. J Urol 1997, 158:790-793 [DOI] [PubMed] [Google Scholar]
- 11.Liebert M, Wedemeyer G, Stein JA, Washington R, Jr, Faerber G, Flint A, Grossman HB: Evidence for urothelial cell activation in interstitial cystitis. J Urol 1993, 149:470-475 [DOI] [PubMed] [Google Scholar]
- 12.Bullock AD, Becich MJ, Klutze CG, Ratliff TL: Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. J Urol 1992, 148:1951-1956 [DOI] [PubMed] [Google Scholar]
- 13.Christensen MM, Keith I, Rhodes PR, Graziano FM, Madsen PO, Bruskewitz RC, Saban R: A guinea pig model for study of bladder mast cell function: histamine release and smooth muscle contraction. J Urol 1990, 144:1293-1300 [DOI] [PubMed] [Google Scholar]
- 14.Saban R, Christensen MM, Keith I, Graziano FM, Undem BJ, Aagaard J, Bjorling DE, Bruskewitz RC: Experimental model for the study of bladder mast cells degranulation and smooth muscle contraction. Sem Urol 1991, 9:88-101 [PubMed] [Google Scholar]
- 15.Lavelle JP, Apodaca G, Meyers SA, Ruiz WG, Ziedel ML: Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. Am J Physiol 1998, 274:F205-F214 [DOI] [PubMed] [Google Scholar]
- 16.Cruz F, Guimaraes M, Silva C, Reis M: Suppression of bladder hyperreflexia by intravesical resinferotoxin. Lancet 1997, 350:640-641 [DOI] [PubMed] [Google Scholar]
- 17.Bjorling DE, Saban MR, Saban R: Neurogenic inflammation of the guinea pig bladder. Med Inflamm 1994, 3:189-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchand JE, Sant GR, Kream RM: Increased expression of substance P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br J Urol 1998, 81:224-228 [DOI] [PubMed] [Google Scholar]
- 19.Hohenfellner M, Nunes L, Schmidt RA, Lampela A, Thuroff JW, Tanagho EA: Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol 1992, 147:587-591 [DOI] [PubMed] [Google Scholar]
- 20.Pang X, Marchand J, Sant GR, Theoharides TC: Increased number of SP-positive fibres in interstitial cystitis. Br J Urol 1995, 75:744-750 [DOI] [PubMed] [Google Scholar]
- 21.Saban R, Franz J, Bjorling DE: Spontaneous release of substance P (SP) and bradykinin (BK) by isolated guinea pig bladder. Br J Urol 1997, 79:516-524 [DOI] [PubMed] [Google Scholar]
- 22.Wang X-C, Saban R, Kaysen JH, Saban MR, Allen PL, Benes EN, Hammond TG: Nuclear factor kappa B mediates lipopolysaccharide-induced inflammation in the urinary bladder. J. Urol. In press [PubMed]
- 23.Hammond TG, Saban R, Bost KL, Harris HW Jr, Kaysen JH, Goda FO, Navar GL, Bjorling DE, Saban M, Zeidel ML: Inhibition of endosomal fusion during bladder inflammation is substance-P dependent. Am J Physiol. In press [DOI] [PubMed]
- 24.Lecci A, Giuliani S, Santicioli P, Maggi CA: Involvement of spinal tachykinin NK1 and NK2 receptors in detrusor hyperreflexia during chemical cystitis in anaesthetized rats. Eur J Pharmacol 1994, 259:129-135 [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliana S: Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol 1994, 111:1017-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfieri A, Gardner C: The NK1 antagonist GR203040 inhibits cyclophosphamide-induced damage in the rat, and ferret bladder. Gen Pharmacol 1997, 29:245-250 [DOI] [PubMed] [Google Scholar]
- 27.Lecci A, Tramontana M, Giuliana S, Criscuoli M, Maggi CA: Effect of tachykinin NK2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin in rats. J Urol 1998, 160:206-209 [PubMed] [Google Scholar]
- 28.Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N: The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med 1997, 3:904-907 [DOI] [PubMed] [Google Scholar]
- 29.Saban MR, Saban R, Bjorling DE, Haak-Frendscho M: The involvement of leukotrienes, TNFα, and the LFA1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J Leuk Biol 1997, 61:445-451 [DOI] [PubMed] [Google Scholar]
- 30.Saban R, Keith I, Bjorling DE: Neropeptide-mast cell interaction in interstitial cystitis. Sant GR eds. Interstitial Cystitis. 1997, :pp 53-65 Lippincott-Raven, Philadelphia [Google Scholar]
- 31.Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, Bienenstock J: Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol 1999, 163:2410-2415 [PubMed] [Google Scholar]
- 32.Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP: Neurogenic amplification of immune complex inflammation. Science 1996, 273:1722-1725 [DOI] [PubMed] [Google Scholar]
- 33.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP: Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 1998, 101:1547-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML: Role of Substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA 1998, 95:4760-4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum AM, Metwali A, Kim-Miller M, Li J, Qadir K, Elliott DE, Lu B, Fabry Z, Gerard N, Weinstock JV: The substance P receptor is necessary for a normal granulomatous response in murine schistomiasis mansoni. J Immunol 1999, 162:6080-6085 [PubMed] [Google Scholar]
- 36.Haak-Frendscho M, Saban R, Shields R, Jardieu P: Blockade of histamine release and tissue contraction by antibodies to IgE in an established IgE response. Immunology 1998, 94:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson DE, Russell RG: Animal models of urinary tract infection. Mobley, HLT Warren JW eds. Urinary Tract Infections: Molecular Pathogenesis and Clinical Management. 1996, :pp 377-403 American Society for Microbiology Press, Washington, DC, [Google Scholar]
- 38.Johnson DE, Lockatell CV, Russell RG, Hebel JR, Island MD, Stapleton A, Stamm WE, Warren JW: Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect Immun 1998, 66:3059-3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernard R: Hypothesis testing for correlation coefficients. Bernard R eds. Fundamentals of Biostatistics. 1990, :pp 443-455 PWS Publishers, Boston, [Google Scholar]
- 40.Little SR, Eisen AN: Preparation of immunogenic 2,4-dinitrophenyl and 2,4,6-trinitrophenyl proteins. Methods Immunol Immunochem 1967, 1:128-133 [Google Scholar]
- 41.Saban R, Nguyen N-B, Saban MR, Gerard N, Pasricha PJ: Nerve-mediated motility of ileal segments isolated from NK1 receptor knockout mice. Am J Physiol 1999, 277:G1173-G1179 [DOI] [PubMed] [Google Scholar]
- 42.Keith IM, Jin J, Saban R: Nerve-mast cell interaction in normal guinea pig urinary bladder. J Comp Neurosci 1995, 363:28-36 [DOI] [PubMed] [Google Scholar]


