Abstract
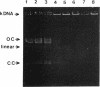
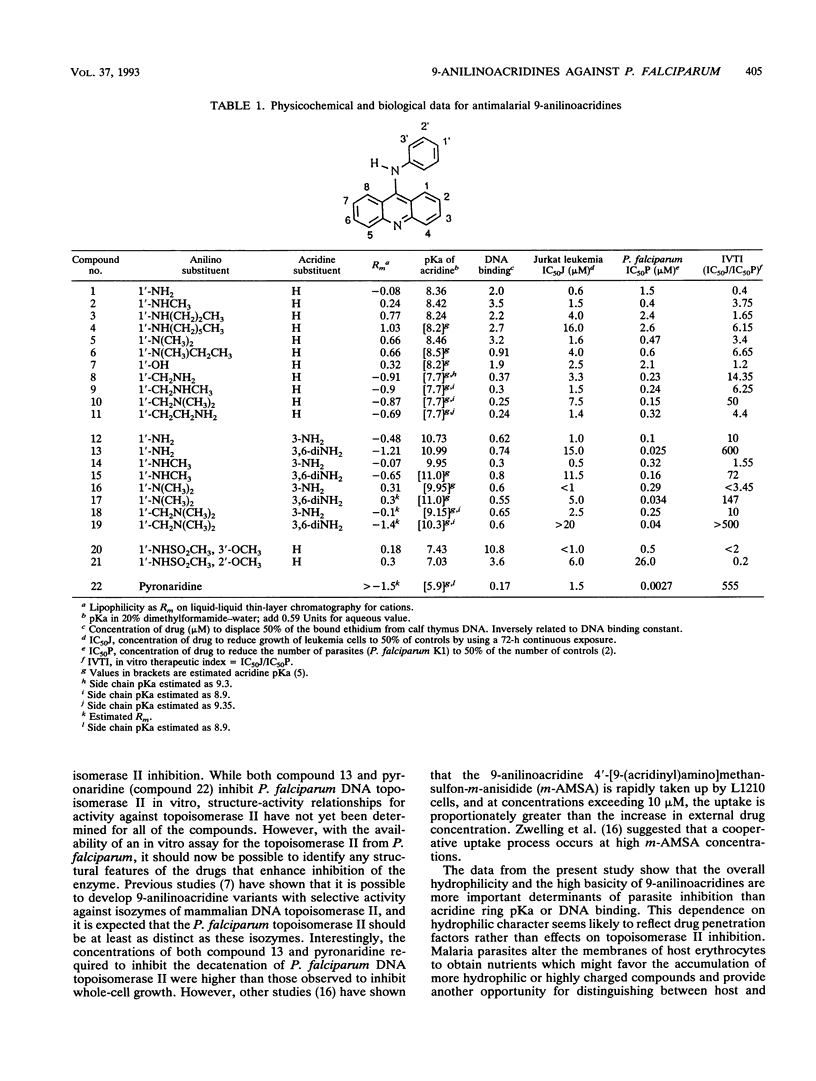
An in vitro investigation of the structure-activity profiles for a range of 9-anilinoacridines on drug-resistant Plasmodium falciparum is reported. C-3, 6-diamino substitution, low lipophilicity, and high pKa values substantially increased the activities of the 9-anilinoacridines tested. There appeared to be no correlation between DNA binding and antimalarial activity. 3,6-Diamino-1'-amino-9-anilinoacridine (compound 13) was the most active compound tested; it had a 50% inhibitory concentration of 25 nM. In vitro mammalian cell growth assays showed compound 13 to be one of the least cytotoxic 9-anilinoacridines (50% inhibitory concentration, 15 microM). Both compound 13 and the antimalarial drug pyronaridine inhibited the decatenation activity of P. falciparum DNA topoisomerase II at concentrations of 10 and 11 microM, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain B. F., Atwell G. J., Denny W. A. Potential antitumor agents. 17. 9-Anilino-10-methylacridinium salts. J Med Chem. 1976 Jun;19(6):772–777. doi: 10.1021/jm00228a007. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Seelye R. N., Atwell G. J. Potential antitumor agents. 14. Acridylmethanesulfonanilides. J Med Chem. 1974 Sep;17(9):922–930. doi: 10.1021/jm00255a003. [DOI] [PubMed] [Google Scholar]
- Chang C., Lin-Hua T., Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg. 1992 Jan-Feb;86(1):7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Denny W. A., Cain B. F., Atwell G. J., Hansch C., Panthananickal A., Leo A. Potential antitumor agents. 36. Quantitative relationships between experimental antitumor activity, toxicity, and structure for the general class of 9-anilinoacridine antitumor agents. J Med Chem. 1982 Mar;25(3):276–315. doi: 10.1021/jm00345a015. [DOI] [PubMed] [Google Scholar]
- Figgitt D., Denny W., Chavalitshewinkoon P., Wilairat P., Ralph R. In vitro study of anticancer acridines as potential antitrypanosomal and antimalarial agents. Antimicrob Agents Chemother. 1992 Aug;36(8):1644–1647. doi: 10.1128/aac.36.8.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay G. J., Baguley B. C., Snow K., Judd W. Multiple patterns of resistance of human leukemia cell sublines to amsacrine analogues. J Natl Cancer Inst. 1990 Apr 18;82(8):662–667. doi: 10.1093/jnci/82.8.662. [DOI] [PubMed] [Google Scholar]
- Fu S., Xiao S. H. Pyronaridine: A new antimalarial drug. Parasitol Today. 1991 Nov;7(11):310–313. doi: 10.1016/0169-4758(91)90267-r. [DOI] [PubMed] [Google Scholar]
- Riou J. F., Gabillot M., Philippe M., Schrevel J., Riou G. Purification and characterization of Plasmodium berghei DNA topoisomerases I and II: drug action, inhibition of decatenation and relaxation, and stimulation of DNA cleavage. Biochemistry. 1986 Apr 8;25(7):1471–1479. doi: 10.1021/bi00355a001. [DOI] [PubMed] [Google Scholar]
- Robbie M. A., Palmer B. D., Denny W. A., Wilson W. R. The fate of N1'-methanesulphonyl-N4'-(9-acridinyl)-3'-methoxy-2',5'-cyclohexadiene- 1',4'-diimine (m-AQDI), the primary oxidative metabolite of amsacrine, in transformed Chinese hamster fibroblasts. Biochem Pharmacol. 1990 May 1;39(9):1411–1421. doi: 10.1016/0006-2952(90)90422-h. [DOI] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Steffen R., Behrens R. H. Travellers' malaria. Parasitol Today. 1992 Feb;8(2):61–66. doi: 10.1016/0169-4758(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Liu L. F. Identification of DNA topoisomerase II as an intracellular target of antitumor epipodophyllotoxins in simian virus 40-infected monkey cells. Cancer Res. 1985 Nov;45(11 Pt 2):5872–5876. [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Michaels S., Kohn K. W. Cooperative sequestration of m-AMSA in L1210 cells. Biochem Pharmacol. 1982 Oct 15;31(20):3269–3277. doi: 10.1016/0006-2952(82)90561-5. [DOI] [PubMed] [Google Scholar]