Abstract
Diabetes of even short duration accelerates the death of capillary cells and neurons in the inner retina by a process consistent with apoptosis. We examined whether the process is accompanied by changes in the expression of endogenous regulators of apoptosis. In postmortem retinas of 18 diabetic donors (age 67 ± 6 years, diabetes duration 9 ± 4 years) the levels of pro-apoptotic Bax were slightly, but significantly, increased when compared with levels in 20 age-matched nondiabetic donors (P = 0.04). In both groups, Bax localized to vascular and neural cells of the inner retina. Neither pro-apoptotic Bcl-XS, nor pro-survival Bcl-XL appeared affected by diabetes. The levels of these molecules could not be accurately quantitated in lysates of retinal vessels because of variable degrees of glial contamination. However, studies in situ showed in several pericytes, the outer cells of retinal capillaries, intense Bax staining often in conjunction with DNA fragmentation. Bovine retinal pericytes exposed in vitro to high glucose levels for 5 weeks showed elevated levels of Bax (P = 0.03) and increased frequency of annexin V binding, indicative of early apoptosis. Hence, human diabetes selectively alters the expression of Bax in the retina and retinal vascular pericytes at the same time as it causes increased rates of apoptosis. The identical program induced by high glucose in vitro implicates hyperglycemia as a causative factor in vivo, and provides a model for establishing the role of Bax in the accelerated death of retinal cells induced by diabetes.
A salient feature of the process that leads to diabetic retinopathy is the loss of retinal vascular cells. Pericytes, the smooth muscle-like cells that envelope capillaries and may have contractile functions, 1 are lost early, leaving behind empty pockets of basement membrane that have been called “ghosts.” 2 Endothelial cells are lost as well, as demonstrated by the development of acellular, nonperfused capillaries, which herald retinal ischemia and neovascularization. 2,3 The degenerative changes appear to involve neural cells also, especially in the inner retina, with evidence of fragmentation and pyknosis of ganglion cells and cells of the inner nuclear layer. 4 We and others have noted recently that both vascular and neural cells of the diabetic retina undergo changes consistent with apoptosis. 5,6 Accelerated apoptosis was found to precede the characteristic diabetic vascular histopathology 5,6 and may therefore be key to the development of the microangiopathy and abnormal retinal function occurring in diabetes.
The molecular basis for the apoptogenic effects of diabetes in the retina is not yet identified. Proteins of the Bcl-2 family are well known inducers and integrators of survival and death signals 7 and may play a role in the cellular effects of diabetes. The pro-survival family members can inhibit apoptosis induced by a variety of cytotoxic insults, and the pro-apoptotic members mostly work by heterodimerizing with pro-survival proteins and antagonizing their effects, although the Bax group can also kill directly by damaging organelles. 7 Hence, the relative concentration of these proteins is an important determinant of their final impact on cell fate, and their regulation is transcriptional as well as posttranslational. 7 Decreased expression of the pro-survival molecule Bcl-X L lowers cellular tolerance to stress-induced apoptosis, 8 and down-regulation of Bcl-2 is a concomitant of the apoptosis of thyroid follicular cells in human Hashimoto’s thyroiditis. 9 Conversely, almost complete disappearance of pro-apoptotic Bax is a concomitant of human breast cancer. 10,11 Of immediate relevance to diabetes, increased expression of Bax mediates enhancement of apoptosis in the pre-implantation blastocyst in a mouse model of diabetic embryopathy. 12
In a previous study, we had observed that in the adult human retina Bcl-2 is expressed predominantly if not solely in Müller glial cells, and that its levels are not modified by diabetes. 13 We have now examined the effects of diabetes on another pro-survival member of the family, Bcl-XL, and two pro-apoptotic members, Bcl-XS and Bax. Having detected increased Bax levels in the diabetic retinas, we examined whether they can be induced by hyperglycemia and are associated with the death of retinal pericytes, a hallmark of diabetic retinopathy. 2
Materials and Methods
Eye Donors and Specimens
Human eyes were obtained from certified eye banks through the National Disease Research Interchange. Criteria for acceptance or rejection of specimens were described previously. 13,14 We studied eyes from a total of 18 diabetic (12 males and 6 females, age 67 ± 6 years, duration of diabetes 9 ± 4 years) and 20 nondiabetic donors (16 males and 4 females, age 65 ± 6 years). Duration of diabetes of less than 15 years was chosen to address mostly if not solely background, nonproliferative retinopathy. 15 The time elapsed from death to enucleation was 3.5 ± 2 hours for the diabetic donors and 3 ± 1 hour for the nondiabetic donors. Eyes were either shipped unfixed to the laboratory on wet ice, or one of the eyes was fixed in 10% buffered formalin by the eye banks. The time to fixation was 11 ± 4 hours and the time to processing of the unfixed eyes 31 ± 7 hours for both the diabetic and nondiabetic specimens. The unfixed retinas were isolated and separated from the pigmented epithelium, and processed for extraction of total protein or isolation of retinal vessels. The fixed retinas were processed for preparation of frozen retinal sections 13 or trypsin digests. 5
Isolation of the Retinal Vascular Network
To examine vascular cells in situ, trypsin digests were prepared from fixed human retinas as described. 5 To extract vascular proteins, unfixed retinas were cut in four quadrants and placed in distilled water (2 hours at 4°C) to permit osmotic lysis of the neural and glial elements. 16 The preparation was then transferred to a 60-mm petri dish containing 200 μg (580 U) DNase I Type II (Sigma Chemical Co., St. Louis, MO) in 5 ml distilled water. During the incubation, the DNase solution was gently and repeatedly pipetted on the tissue until the preparation looked transparent (approximately 10 minutes.). The preparation was transferred back to water and the inner limiting membrane was gently pulled away under the dissecting microscope using a tweezers and keeping the vascular network flat and untangled. If required, remaining neural and glial elements were eliminated by further gentle agitation in distilled water. After microscopic verification of its purity, the vascular network was homogenized in lysis buffer (see below). In some preparations, two of the quadrants were mounted on silane-coated slides and fixed in 10% buffered formalin for immunohistochemical studies. Residual neural contamination of the isolated vascular preparations was titrated by comparing in Western blots the level of neuron-specific enolase (NSE) in 5 μg of vascular lysate with that in 5, 0.5, 0.25, and 0.1 μg of lysate prepared from the contralateral whole retina. Glial contamination was similarly assessed by comparing the level of glial fibrillary acidic protein (GFAP) in Western blots of vascular and total retinal lysates, and by performing GFAP immunohistochemistry on the isolated fixed vessels.
Culture of Retinal Pericytes
Bovine retinal pericytes were isolated and cultured as previously described. 17 Briefly, bovine retinas were gently dissected and extensively washed with Dulbecco’s modified Eagle’s medium (DMEM) to eliminate residual pigment epithelium. Retinas were homogenized with one stroke of a hand-held homogenizer, and the homogenate was washed thoroughly with DMEM over an 88-μm mesh to dissociate the neural retina. The material remaining on the mesh was digested with 0.1% collagenase type 2 (Worthington Biochemical, Lakewood, NJ) for 1 hour at 37°C. The digested material was pipetted onto 88 μm mesh, and the small vessel fragments and single cells passing through the mesh were pelleted by centrifugation, washed 2× in DMEM, resuspended in complete medium, and plated in 100-mm culture dishes. Cultured retinal pericytes showed the characteristic variable size, stellate shape with multiple processes, and lack of confluency and of multiple layers. Endothelial cell contamination was tested with anti-von Willebrand factor antibodies and glial contamination with antibodies against GFAP. Cultures at first and subsequent passages were routinely found to be free of contaminants. Pericytes were grown in DMEM containing 5 mmol/L glucose and supplemented with 10% fetal calf serum, antibiotics, and antimycotics. All experiments were performed in second or third passage cells. Treatment with high glucose (25 mmol/L D-glucose added) was begun 5, 3, or 1 week before harvest; mannitol (25 mmol/L) was used as control for the effects of hypertonicity.
Western Blot Analysis
Retinas, isolated retinal microvessels, or cultured pericytes were homogenized in lysis buffer (30 mmol/L Tris-HCl, 10 mmol/L EGTA, 5 mmol/L EDTA, 1% Triton X-100, 250 mmol/L sucrose) containing 1 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, 15 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml pepstatin, and 1 mmol/L Na3VO4. The homogenate was sonicated three times for 2 seconds and centrifuged at 16,000 × g for 15 minutes at 4°C. The supernatant was collected and stored at −80°C. The protein concentration of each lysate was determined with the Bradford method 18 using bovine serum albumin (BSA) as the standard. The lysates were diluted 1:3 in Laemmli Sample Buffer (Bio-Rad, Hercules, CA) supplemented with 2-mercaptoethanol to a final concentration of 5%, and boiled for 5 minutes. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting, and visualization of immunoreactive bands were performed as described previously. 13 The primary antibodies and their concentrations were as follows: rabbit polyclonal anti-Bcl-X (Santa Cruz Biotechnology, Santa Cruz, CA) 0.5 μg/ml, mouse monoclonal anti-human NSE (Dako, Carpinteria, CA) 1 μg/ml, and rabbit polyclonal anti-GFAP (Dako) 0.1 μg/ml. Rabbit anti-human Bax antiserum 1712 11 and anti-Bcl-X antiserum 1695 19 were raised to synthetic peptides conjugated to ovalbumin. The antisera were thus pre-absorbed in 0.1 mol/L Tris buffer, pH 7.8, containing 0.1 mg/ml ovalbumin and 1% milk for 1 hour at 37°C before being applied to the membranes at a 1:1000 dilution.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To confirm that the single Bax species identified in the human retina could be designated as Bax α, we performed RT-PCR using primers encompassing intron 5, which is unspliced in Bax β. 20 The primers were designed based on the GenBank sequences of human Bax α and Bax β mRNAs (accession numbers L22473 and L22474, respectively). The forward primer TTG GAC TTC CTC CGG GAG CG corresponded to bp 421–440 of exon 5, and the reverse primer CAG TCC AAG GCA GCT GGG GC to bp 580–561 of exon 6. Bax α forms would yield a 180-bp fragment, whereas β forms would yield a 810-bp fragment. RT-PCR was performed as described previously 14 using 35 cycles of amplification. The specificity of the 180-bp PCR product was verified by digestion with BamHI and Bst O1, which yielded fragments of the expected size.
Immunohistochemistry
Formalin-fixed retinal sections and vascular preparations were rehydrated in phosphate buffered saline (PBS) for 20 minutes, blocked with 2% BSA in PBS for 10 minutes, and incubated overnight in a moist chamber with the primary antibody diluted in PBS containing 2% BSA and 0.5% Triton X-100. For Bax and Bcl-X immunohistochemistry, the antisera were pre-absorbed in 1 ml of 0.1 mol/L Tris buffer, pH 7.8, containing 0.1% ovalbumin, 2% BSA, 2% goat serum, and 0.1% human serum for 1 hour at 37°C; and the histological preparations were pre-blocked in 0.1 mol/L Tris containing 2% BSA, 2% goat serum, and 0.5% Triton X-100 for 1 hour at room temperature. The pre-absorbed antisera were used at a 1:1000 dilution; negative controls received the same dilution of nonimmune rabbit serum pre-absorbed as the primary antiserum. GFAP antibodies were used at 0.8 μg/ml; the negative controls received an equivalent concentration of nonimmune rabbit IgG. After extensive washes in PBS, the preparations were reacted with the appropriate secondary antibodies and processed for immunofluorescence or peroxidase immunohistochemistry. 14
Transferase-Mediated dUTP Nick End Labeling (TUNEL) Assay
To test whether intense Bax immunoreactivity in cells of human retinal vessels was associated with vascular cell apoptosis, retinal trypsin digests from three diabetic and four age-matched nondiabetic donors were tested with the terminal deoxynucleotidyl TUNEL assay (In situ Cell Death Detection Kit; Boehringer Mannheim Biochemicals, Indianapolis, IN) as described previously. 5 On completion of the TUNEL reaction, the preparations were reacted with the Bax antiserum as described above, and Bax immunoreactivity was detected with a Cy3-conjugated secondary antibody. The green fluorescence of TUNEL-positive nuclei with chromatin fragmentation and the red fluorescence of Bax were observed under a Zeiss fluorescence microscope.
Annexin V Binding
Annexin V binding was used to detect early stages of apoptosis in bovine retinal pericytes cultured in high glucose. Cells that had been exposed to high glucose for 1, 3, or 5 weeks and companion cells cultured in normal glucose medium were trypsinized and processed following the ApoAlert Annexin V protocol (Clontech Laboratories, Palo Alto, CA). At least 20,000 events were analyzed by flow cytometry using a single laser emitting excitation light at 488 nm.
Statistical Analysis
The data are summarized with the mean ± SD. Statistical analysis was performed with the unpaired t-test for results obtained in human specimens and analysis of variance for those obtained in cultured cells.
Results
Bax and Bcl-X in the Human Diabetic Retina
The yield of total retinal protein was identical in the diabetic and control group (9.2 ± 2 mg per retina). Bax immunoblots (Figure 1) ▶ showed a single band of 22 to 23 kd, confirmed to correspond to Bax α, the membrane form of Bax, 20 by RT-PCR analysis. The Bax α signal was often more pronounced in diabetic than in control specimens. In the group of 18 diabetic donors, retinal Bax levels were 40 ± 13 densitometric units/μg protein versus 32 ± 9 in control donors (P = 0.04). The interassay coefficient of variation for the six samples tested in more than two blots was 6 ± 5%, indicating excellent reproducibility. Bax levels did not correlate with age or diabetes duration, nor with the time elapsed from death to eye enucleation or processing.
Figure 1.
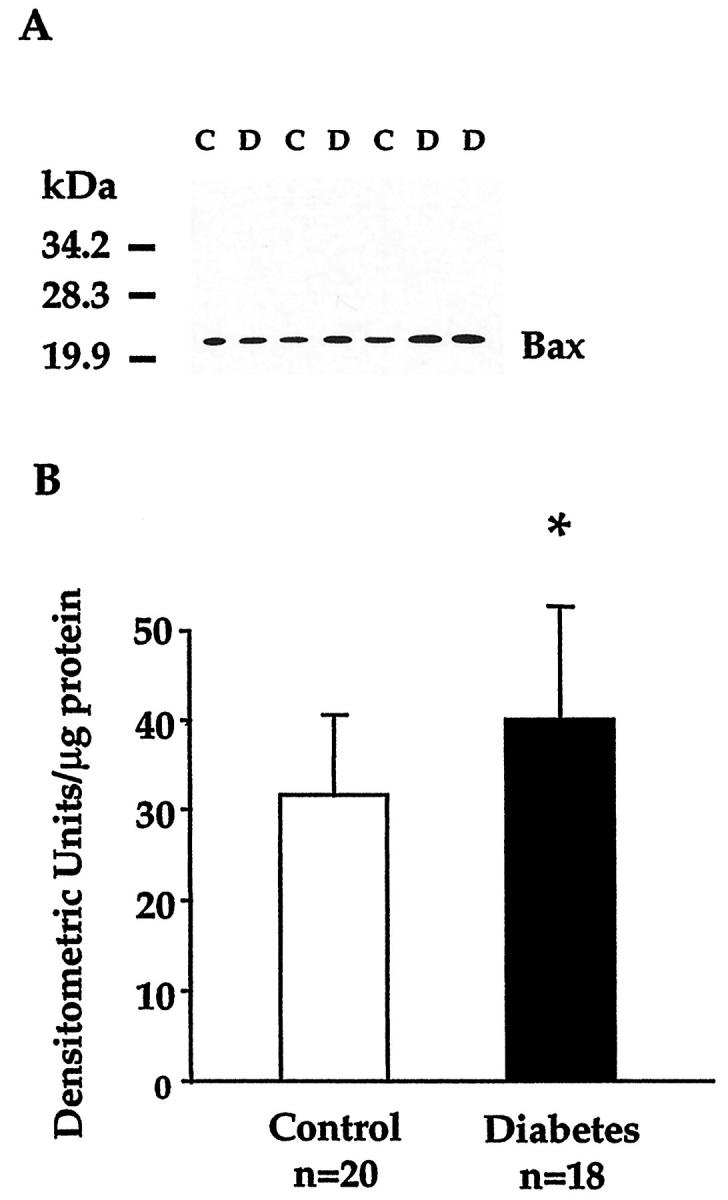
Immunoblot analysis of Bax in the human retina. A: Blot representative of the signals obtained in retinal lysates from control and diabetic donors. Retinal proteins (10 μg/lane) from three nondiabetic control (C) and four diabetic donors (D) matched for age and sex were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gels and immunoblotted with anti-human Bax antiserum. Molecular weight markers are indicated on the left. B: Densitometric analysis of retinal Bax levels. Bars present the mean ± SD of the intensity of Bax signals in the control and diabetic group. *P = 0.04 (unpaired t-test).
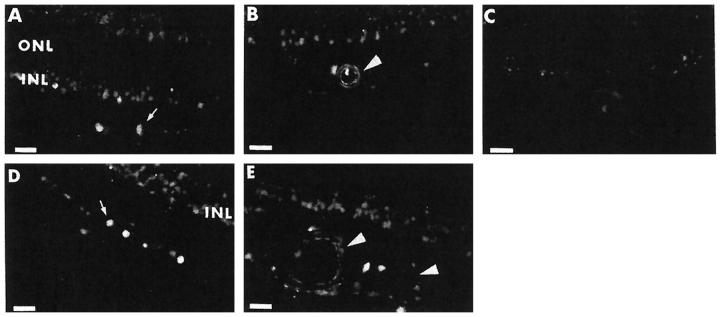
Bax immunoreactivity was almost exclusively present in the inner retina (Figure 2) ▶ , with specific staining detected in cells of the inner nuclear layer, ganglion cells (and, often, their nuclei), and the endothelial and medial layers of blood vessels. There was no evidence of glial staining. The distribution was similar in diabetic and control retinas, and differences in staining intensity between the two groups could not be confidently appreciated.
Figure 2.
Localization of Bax by immunofluorescence in the human retina. A and D: Bax immunoreactivity is present in ganglion cells (arrow) and inner nuclear layer (INL) in the retina of a 68-year-old male with an 11-year history of diabetes (A) and a 71-year-old male nondiabetic (D). The outer nuclear layer (ONL) is negative. B and E: Bax immunoreactivity in the endothelial lining and medial layer of a large vessel (arrowhead), as well as in cells of the inner nuclear layer in the retina of a 61-year-old female with a 7-year history of diabetes (B) and a 56-year-old male nondiabetic (E). C: Negative control obtained by using nonimmune rabbit serum on a retinal section from the donor shown in B. Scale bars, 22 μm.
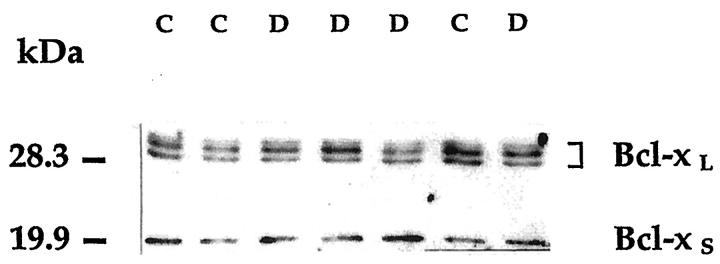
Western blot of retinal Bcl-X (Figure 3) ▶ yielded a doublet of proteins with an apparent molecular mass of 29 to 31 kd, and a smaller protein of ∼19 kd. The doublet corresponds to the long isoform of Bcl-X as reported in other tissues 19 and is thought to reflect initiation from a cryptic translation initiation codon in addition to the usual start site. 21 The 19-kd band corresponds to Bcl-XS. 19 Diabetes did not change retinal Bcl-X expression appreciably. Retinal levels of Bcl-XL were 72 ± 34 densitometric units/μg protein in the diabetic group and 62 ± 26 in the control group (P = 0.3), and the levels of Bcl-XS were 24 ± 18 and 19 ± 15, respectively (P = 0.3).
Figure 3.
Immunoblot analysis of Bcl-X in the human retina. The blot is representative of the signals obtained in retinal lysates from control and diabetic donors. Both Bcl-X isoforms are present in the retina. Symbols and procedures are as in the legend to Figure 1 ▶ ; immunoblotting was performed with the Santa Cruz Biotechnology anti-Bcl-X antibody.
A fully informative study of the topography of Bcl-X in the human retina would have required discrete identification of the long and short isoform in situ, but good reagents for this purpose are not currently available, in part because the domain missing in Bcl-XS is highly conserved within Bcl-2 family members. 19 Moreover, both antibodies used for Bcl-X detection showed in Western blot cross-reactivity with at least one unknown retinal protein in addition to Bcl-X. We reasoned, however, that we could trust negative findings. The Bcl-X antiserum reacted solely with vessel walls; the Bcl-X antibodies from Santa Cruz Biotechnology also showed minimal cytoplasmic staining of cells in the inner nuclear layer. The outer nuclear layer and glia did not show immunoreactivity.
Bax and Bcl-X in Human Retinal Microvessels
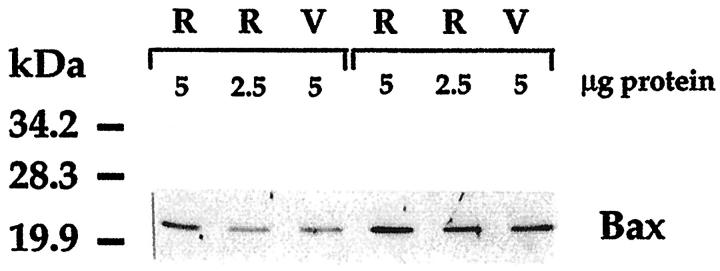
To obtain data on Bax and Bcl-X expression specific to microvessels, we isolated the vascular tree from unfixed retinas using hypotonic lysis. Microscopically, the vascular networks appeared free of contamination and in Western blots showed a greater than 20-fold enrichment in proteins such as occludin and endothelial nitric oxide synthase unique to endothelial cells (data not shown). Bax appeared as a single band in immunoblots of vessel lysates (Figure 4) ▶ , and the amount of Bax per microgram of protein was approximately half the amount measured in the whole retinal lysate prepared from the contralateral retina. Diabetic donors showing in the whole retina Bax levels greater than in nondiabetic controls tested in the same assay also showed Bax levels greater than controls in the microvessels of the contralateral retina (Figure 4) ▶ . Of the two Bcl-X isoforms, only Bcl-XL could be detected in up to 20 μg of vascular lysates.
Figure 4.
Representative immunoblot comparing Bax levels in human whole retina (R) and vessels (V) isolated from the contralateral retina by hypotonic lysis. In the specimens of both a 71-year-old nondiabetic (left three lanes) and a 71-year-old diabetic donor, Bax levels per microgram of protein are in vessels approximately half those in whole retina. The Bax signal, more intense in the whole retina of the diabetic donor, was also more intense in the vessels of his contralateral retina when compared with the respective specimens of the nondiabetic donor.
To assess whether the microvascular preparations were sufficiently pure to be used in quantitative studies, they were tested for neural (NSE immunoreactivity) and glial (GFAP immunoreactivity) contamination. Neural material was reproducibly found to account for approximately one-fortieth of the protein in vessel lysates. Because total retina NSE levels were superimposable in control and diabetic subjects, NSE could be used as a quantitative index of neural contamination. In contrast, the extent of glial contamination appeared highly variable in different vascular preparations, most likely reflecting residual glial processes enmeshed in the basement membrane common to microvessels and glia. The presence of GFAP-positive material surrounding variable lengths of the retinal capillaries was confirmed by immunohistochemistry. This, coupled with the facts that retinal GFAP levels are variable among individuals, and increased in diabetic subjects, 13 precluded the possibility of accurately quantitating glial contamination. Although Bax was not detected in retinal glia (Figure 2) ▶ , glial contamination rendered inaccurate the estimation of vascular protein, ie, the reference for quantitative comparison of Bax levels between diabetic and nondiabetic vessels.
Bax Expression and Pericyte Apoptosis in Situ
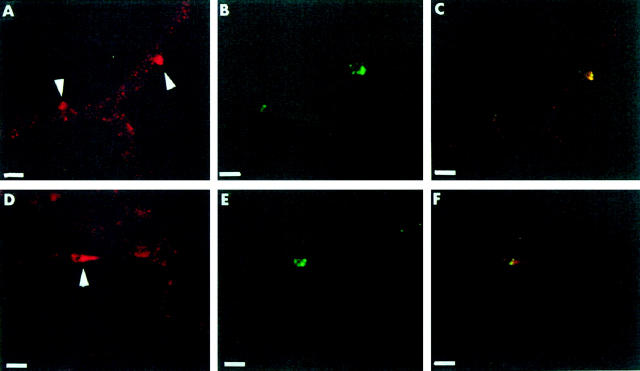
The pattern of Bax expression in retinal vessels could, however, be examined in situ. This approach offered the opportunity to also determine any relationship that focal increases in Bax expression may have with apoptotic events. Retinal trypsin digests from 3 diabetic and 4 nondiabetic donors were studied for Bax immunoreactivity after performing the TUNEL reaction. A faint granular staining was present throughout the capillary bed (Figure 5) ▶ , consistent with low basal expression of Bax in these vessels, and was not obviously different in diabetic and control specimens. In accordance with previous observations, TUNEL-positive vascular cells were more numerous in diabetic (5 ± 4 per one-sixth of retina) than in nondiabetic (1 ± 1) retinal vessels. Several TUNEL-positive pericyte nuclei in the diabetic vessels were surrounded by bright Bax staining (Figure 5) ▶ . The intense red (Bax) and green (TUNEL) fluorescence colocalized in the same area, but not within the same structures, suggesting increased cytoplasmic Bax expression in cells with evidence of apoptotic nuclear fragmentation. Only a few cells showed bright Bax staining as an isolated feature, whereas some TUNEL-positive cells did not show Bax staining. Of note, in a nondiabetic trypsin digest the only TUNEL-positive pericyte nucleus was surrounded by intense Bax immunoreactivity
Figure 5.
Colocalization of Bax- and TUNEL-positive pericytes in diabetic retinal vessels. A−C: In a trypsin digest from a 76-year-old male with a 10-year history of diabetes, intense Bax staining (A) is shown in two pericytes (arrowheads) using a secondary antibody conjugated to Cy3 (red). DNA fragmentation (B) detected with the TUNEL reaction (green) is present in the same pericytes, as confirmed by observation under a dual-pass fluorescein isothiocyanate/rhodamine filter set (C). D−F: In a trypsin digest from a 67-year-old male with a 7-year history of diabetes, Bax staining (D) is localized in a pericyte cytoplasm (arrowhead) surrounding a TUNEL-positive nucleus (E). Localization of the two signals to the same pericyte is shown in F. Scale bars, 13 μm.
Bax Expression and Apoptosis in Retinal Pericytes Cultured in High Glucose
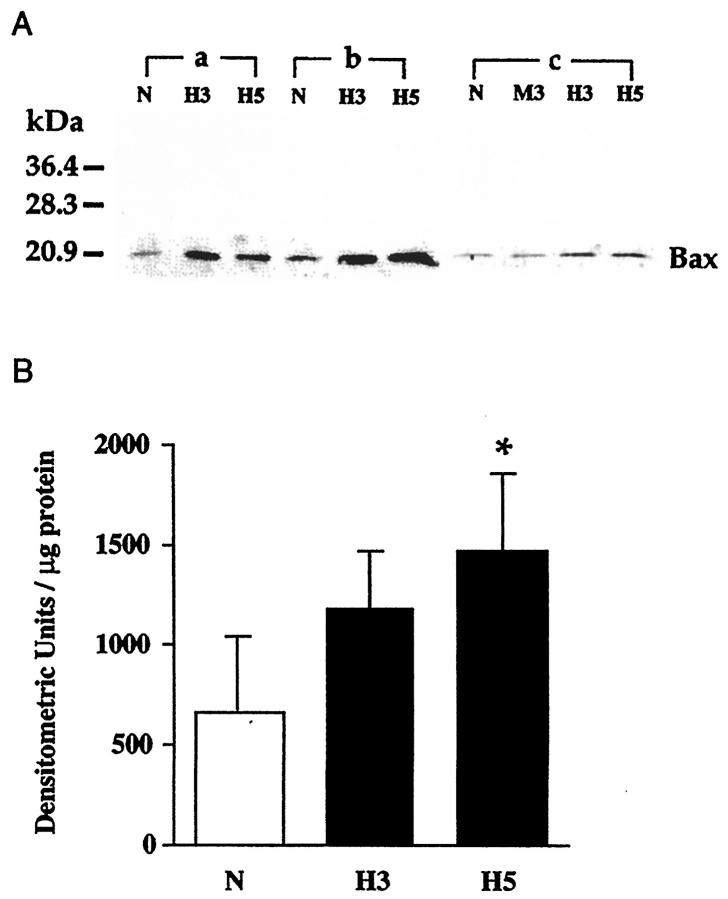
To begin investigating mechanisms for Bax overexpression in diabetic retinal cells, bovine retinal pericytes were cultured in the presence of high glucose for several weeks. One week exposure to high glucose did not appreciably alter Bax levels (118 ± 37% of control). After 3 weeks of high glucose treatment (Figure 6) ▶ , Bax levels had almost doubled (1186 ± 270 densitometric units/μg protein versus 658 ± 364 in control cultures), and were significantly greater than control values in pericytes exposed to high glucose for 5 weeks (1469 ± 388 densitometric units/μg protein, P = 0.03). Hypertonicity induced by 25 mmol/L mannitol had no effect. At the same time points, the levels of Bcl-XL were not changed by high glucose (94 ± 1, 102 ± 1, and 95 ± 12% of control, respectively), nor were the levels of Bcl-XS (87 ± 3, 100 ± 4, and 96 ± 2% of control).
Figure 6.
Immunoblot analysis of Bax in bovine retinal pericytes cultured in media containing 5 mmol/L glucose (N), 30 mmol/L glucose for 3 weeks (H3), 25 mmol/L mannitol + 5 mmol/L glucose for 3 weeks (M3), and 30 mmol/L glucose for 5 weeks (H5). A: Cell protein lysates (20 μg/lane) from three different isolates (a, b, and c) were electrophoresed and immunoblotted as described in the legend to Figure 1 ▶ . B: Densitometric analysis of Bax levels in retinal pericytes. Each bar represents the mean ± SD of the results obtained in the three different isolates each analyzed in two independent experiments. *P = 0.03 versus normal glucose (analysis of variance).
In the same cultures, the frequency of cells binding annexin V (an index of early stages of apoptosis) was unchanged after one week in high glucose medium, but progressively increased after 3 and 5 weeks’ exposure to high glucose (Table 1) ▶ .
Table 1.
Apoptosis in Cultures of Bovine Retinal Pericytes Exposed to High Glucose
| Annexin V-positive cells (% of total) | |||
|---|---|---|---|
| Normal glucose (5 mmol/L) | High glucose (30 mmol/L) | Mannitol (25 mmol/L) | |
| 1 week | 1.3 ± 0.4 | ||
| 3 weeks | 2.0 ± 0.6 | 1.0 ± 0.5 | |
| 5 weeks | 1.2 ± 0.7 | 3.3 ± 1.1 |
Values are means ± SD of the results obtained in three independent experiments, each performed in a different pericyte isolate. *P = 0.01 versus normal glucose.
Discussion
We have presented evidence that Bax, a pro-apoptotic member of the genetic program that governs apoptosis, is a target of diabetes in the retina and of high glucose in cultured retinal pericytes, and that its overexpression in retinal pericytes is associated with apoptosis.
These observations advance the notion that diabetes induces an apoptogenic environment in the retina. Our original studies had proposed apoptosis as the mode of death of retinal vascular cells in human and experimental diabetes on the basis of nuclear chromatin morphology coupled with TUNEL. 5 We sought supportive evidence and mechanistic insight by examining if diabetes alters the balance of endogenous regulators of apoptosis. Bcl-2, found almost exclusively in the retinal glia, was present at similar levels in diabetic and nondiabetic retinas. 13 We have now observed that expression of another pro-survival molecule, Bcl-XL, which in the postmortem human retina localized mostly to blood vessels, is also apparently unchanged by diabetes, as is the expression of its pro-apoptotic isoform, Bcl-XS. Bax immunostaining was limited to the inner retina, and was prominent in ganglion cells and vascular cells, the cell types that are known to undergo accelerated apoptosis in diabetes. 5,6 We found increased overall level of Bax in whole retinal lysates from diabetic donors indicating that in these retinas some cell populations, or some cells within given populations, were overexpressing Bax. This could not be readily appreciated in retinal sections immunostained for Bax owing to the limited quantitative capabilities of immunohistochemistry.
To examine if retinal vascular cells were among Bax overexpressing cells, we prepared isolated vascular networks. We discovered however, even in preparations that appeared pure microscopically, a sizable amount of glial contamination, which could not be accurately titrated and precluded Bax quantitation in vascular extracts. When we turned to analysis of the vessels in situ, we observed numerous focal increases in Bax staining localized around pericyte nuclei and often associated with TUNEL-positive fragmentation of the same nuclei. Similar colocalization 22 or at least temporal association 23,24 of Bax overexpression and TUNEL have been reported in cells of the central nervous system and the retina after experimental induction of transient ischemia or optic nerve lesions, which are well known stimuli of apoptotic death. Of course, the frequency of Bax- and TUNEL-positive cells was much greater in such acute experimental settings than in the diabetic retinal vessels, where our snapshots of apoptosis could only capture the few cells dying at that particular point in time within the chronic degenerative process. Of note, we did not encounter endothelial cells staining intensely for Bax in diabetic retinal vessels. This may reflect a different temporal relationship of Bax overexpression to apoptosis in endothelial cells and pericytes, or indicate that retinal endothelial cell death in diabetes occurs through mechanisms that do not involve Bax. The distribution of Bcl-2 family members as well as the regulation of apoptosis are often cell-specific within tissues. 19,25 These possibilities require further investigation using model systems.
The observation that high glucose increases Bax levels in cultured pericytes at the same time as it increases in these cells the rate of apoptosis establishes that a metabolic abnormality characteristic of diabetes is sufficient to up-regulate Bax expression and stimulate a death pathway in retinal cells. Such notion could not be confidently derived from the findings in diabetic retina and retinal vessels in situ, where Bax expression may have been influenced by functional or structural sequelae of the chronic diabetes state, in addition to the prevailing abnormal milieu. That hyperglycemia may at least contribute to altering Bax levels in the diabetic retina is also suggested by the fact that high glucose in vitro selectively up-regulated Bax without affecting the levels of the two Bcl-X isoforms, exactly as observed in the diabetic retina. A role for hyperglycemia in inducing apoptosis mediated by increased Bax levels has been demonstrated in a model of diabetic embryopathy. 12 The combined in vivo and in vitro data of our and Moley’s 12 studies highlight once again molecular similarities in two diabetic pathologies, vascular disease and embryopathy, that are vastly different phenotypically. We had reported in the past that overexpression of extracellular matrix molecules, a hallmark of the microangiopathy that develops chronically in diabetes, 26,27 also occurs within the rapid time frame of embryonic development in diabetic rats. 28 The response to high glucose of both Bax and extracellular matrix molecules appears to be much slower and of lesser magnitude in adult cells 29 (and this work) than in the developing embryo, 12,28 possibly reflecting the different rates of metabolism and/or transcriptional activity in the two settings.
Increased Bax levels may play a role in the apoptosis of retinal cells by tilting the cellular balance of apoptosis regulators in a direction that increases susceptibility to stressful stimuli, but may also be sufficient to kill cells directly. 7,30 The cultured pericyte model will permit us to address if glucose-induced Bax overexpression is the event responsible for pericyte apoptosis, and if so, what mechanisms are involved. In vivo studies would then be justified to test whether, in the absence of Bax, diabetes can still accelerate the death of vascular and neural cells in the retina and cause retinopathy.
Footnotes
Address reprint requests to Mara Lorenzi, M.D., Schepens Eye Research Institute, Harvard Medical School, 20 Staniford Street, Boston, MA 02114. E-mail: lorenzi@vision.eri.harvard.edu.
Supported by U.S. Public Health Service grant EY09122, a research grant of the American Diabetes Association, and the George and Frances Levin Endowment.
F. P. and G. R. contributed equally to this work.
References
- 1.Shepro D, Morel NML: Pericyte physiology. FASEB J 1993, 7:1031-1038 [DOI] [PubMed] [Google Scholar]
- 2.Cogan DG, Toussaint D, Kuwabara T: Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 1961, 66:366-378 [DOI] [PubMed] [Google Scholar]
- 3.Engerman RL: Pathogenesis of diabetic retinopathy. Diabetes 1989, 38:1203-1206 [DOI] [PubMed] [Google Scholar]
- 4.Bloodworth JMB: Diabetic retinopathy. Diabetes 1962, 11:1-22 [PubMed] [Google Scholar]
- 5.Mizutani M, Kern TS, Lorenzi M: Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996, 97:2883-2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW, : The Penn State Retina Research Group: Neural apoptosis in the retina during experimental and human diabetes. J Clin Invest 1998, 102:783-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 1998, 281:1322-1326 [DOI] [PubMed] [Google Scholar]
- 8.Rodeck U, Jost M, DuHadaway J, Kari C, Jensen PJ, Risse B, Ewert DL: Regulation of Bcl-XL expression in human keratinocytes by cell-substratum adhesion and the epidermal growth factor receptor. Proc Natl Acad Sci USA 1997, 94:5067-5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsiades N, Poulaki V, Kotoula V, Mastorakos G, Tseleni-Balafouta S, Koutras DA, Tsokos M: Fas/Fas ligand up-regulation, and BCL-2 down-regulation may be significant in the pathogenesis of Hashimoto’s thyroiditis. J Clin Endocrinol Metab 1998, 83:2199-2203 [DOI] [PubMed] [Google Scholar]
- 10.Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, Dšrken B: Overexpression of the death-promoting gene bax-α which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest 1996, 97:2651-2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius V-M, Niskanen E, Nordling S, Reed JC: Reduced expression of proapoptotic gene Bax is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res 1995, 55:4471-4478 [PubMed] [Google Scholar]
- 12.Moley KH, Chi MM-Y, Knudson CM, Korsmeyer SJ, Mueckler MM: Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med 1998, 4:1421-1424 [DOI] [PubMed] [Google Scholar]
- 13.Mizutani M, Gerhardinger C, Lorenzi M: Müller cell changes in human diabetic retinopathy. Diabetes 1998, 47:445-449 [DOI] [PubMed] [Google Scholar]
- 14.Gerhardinger C, Brown LF, Roy S, Mizutani M, Zucker CL, Lorenzi M: Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am J Pathol 1998, 152:1453-1462 [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL: The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984, 102:527-532 [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson RA, Mandel TE: Anatomy of the mouse retina. Endothelial cell-pericyte ratio and capillary distribution. Invest Ophthalmol Vis Sci 1986, 27:1659-1664 [PubMed] [Google Scholar]
- 17.Mandarino LJ, Finlayson J, Hassel JR: High glucose downregulates glucose transport activity in retinal capillary pericytes but not endothelial cells. Invest Ophthalmol Vis Sci 1994, 35:964-972 [PubMed] [Google Scholar]
- 18.Bradford MA: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 19.Krajewski S, Krajewska M, Shabaik A, Wang H-G, Irie S, Fong L, Reed JC: Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 1994, 54:5501-5507 [PubMed] [Google Scholar]
- 20.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74:609-619 [DOI] [PubMed] [Google Scholar]
- 21.Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed JC, Miyawaki T: Immunoblot analysis of cellular expression of Bcl-2 family proteins, Bcl-2, Bax, Bcl-X and Mcl-1, in human peripheral blood and lymphoid tissues. Int Immunol 1995, 7:1817-1825 [DOI] [PubMed] [Google Scholar]
- 22.Krajewski S, Mai JK, Krajewska M, Sikorska M, Mossakowski MJ, Reed JC: Upregulation of Bax protein levels in neurons following cerebral ischemia. J Neurosci 1995, 15:6364-6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneda K, Kashii S, Kurosawa T, Kaneko S, Akaike A, Honda Y, Minami M, Satoh M: Apoptotic DNA fragmentation, and upregulation of Bax induced by transient ischemia of the rat retina. Brain Res 1999, 815:11-20 [DOI] [PubMed] [Google Scholar]
- 24.Isenmann S, Wahl C, Krajewski S, Reed JC, Bähr M: Upregulation of Bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur J Neurosci 1997, 9:1763-1772 [DOI] [PubMed] [Google Scholar]
- 25.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC: Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 1994, 145:1323-1336 [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Maiello M, Lorenzi M: Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest 1994, 93:438-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Cagliero E, Lorenzi M: Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci 1996, 37:258-266 [PubMed] [Google Scholar]
- 28.Cagliero E, Forsberg H, Sala R, Lorenzi M, Eriksson UJ: Maternal diabetes induces increased expression of extracellular matrix components in rat embryos. Diabetes 1993, 42:975-980 [DOI] [PubMed] [Google Scholar]
- 29.Cagliero E, Maiello M, Boeri D, Roy S, Lorenzi M: Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest 1988, 82:735-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang J, Chao DT, Korsmeyer SJ: BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci USA 1996, 93:14559-14563 [DOI] [PMC free article] [PubMed] [Google Scholar]