Abstract
The Smyth line (SL) chicken, an animal model for autoimmune human vitiligo, is characterized by a spontaneous posthatch pigment loss, determined to be the result of an autoimmune phenomenon. Because endogenous virus (EV) genes have been reported to be associated with a number of autoimmune diseases of human and animal models, we designed this experiment to investigate the role of EV in the SL vitiligo by using the complete sequence of Rous-associated virus-2 as a probe for EV. An F2 resource population was developed by the matings of SL and parental control (BL) chickens. Linkage disequilibrium between vitiligo and EV was apparent (16.2-kb SacI fragment, P ≤ 0.05 and a 19-kb HindIII fragment, P ≤ 0.03). Methylation analyses revealed that the EV and endogenous avian retroviral (EAV) genes were methylated in both the SL and BL sublines of chickens; therefore, methylation does not appear to be responsible for the differences in the expression of vitiligo between SL and BL sublines. Expression of the EV genes correlated with the disease in vitiliginous SL101 birds and also in 5-Azacytidine-induced vitiliginous BL101 parental control chickens. Only one EV locus was detected in the unrelated Light Brown Leghorn control chickens (1q14) by in situ hybridization, whereas 3 EV loci were identified in SL101 and BL101 chickens (1p25, 2q26, and an unidentifiable microchromosome). Our observations indicate that EV genes may play a role in the induction of autoimmune vitiligo in the SL chicken model.
The Smyth line (SL) chicken, an animal model for autoimmune vitiligo in humans, is characterized by a spontaneous posthatch epidermal pigment loss (vitiligo) determined to be the result of an autoimmune phenomenon. 1,2 An intrinsic melanocyte defect, characterized by melanosomes with abnormal, irregular surfaces, predisposes SL chickens to the pigment disorder. 3 Both B cell 4 and T cell 5 compartments of the immune system are involved in the pathology of the disease. Melanocyte-specific SL autoantibodies have been reported in the serum of SL chickens. 6 These autoantibodies cross-react with mouse and human melanocytes and are able to bind to pigment cells within tissues. 7 They also recognize antigens expressed in the cytoplasm and on the surface of the melanocytes and melanoblasts. 7 It was later found that mammalian tyrosinase-related protein-1 (TRP-1) is recognized by the SL autoantibody, 8 although its contribution to the onset and progression of the disease has not been completely determined.
Three SL sublines each homozygous for a different major histocompatibility complex (MHC) haplotype have been developed along with respective MHC-matched BL parental controls. 2 The SL and BL lines were developed from the same base population by divergent selection for the development of vitiligo. The incidence in the SL sublines is 70 to 90%, whereas there is only a minimal incidence in the BL parental control lines (1 to 2%). Molecular analysis of each SL and corresponding BL control subline showed that they are different not only at the MHC loci but also at a number of different loci, including those associated with endogenous viruses. 9,10 Genomes of all organisms, from yeast to humans, contain endogenous retroviral sequences, although the copy number varies in different species ranging from as few as 1 to 10 to more than 100. 11 EV genes have been implicated as etiological agents of autoimmune diseases because of their structural and sequence similarities to exogenous retroviruses associated with immune dysregulation and their tissue-specific or differentiation-dependent expression. 12 Quantitatively or structurally aberrant expression of normally cryptic EVs, induced by environmental or endogenous factors, could initiate autoimmunity by direct or indirect mechanisms. EV genes have been reported to be associated with a number of autoimmune diseases of human and animal models. 12,13 We have previously found, using restriction fragment length polymorphism (RFLP) analyses, that the EV genes were different in vitiliginous SL chickens from those in their MHC-matched normal parental control (BL). 9,10 Because the expression of vitiligo in SL chickens is influenced by a variety of environmental factors including stress, housing conditions, and vaccination against viral diseases, 2,14 it is possible that the EV genes could have an important effect on the initiation of autoimmunity in SL chickens. The present study was undertaken with the aim of detecting the role of endogenous viruses in the expression of autoimmune vitiligo in the SL chicken model.
Materials and Methods
Animals
Birds from the SL101 and BL101 sublines were used for this study. All of the animals used in these experiments were serotyped using alloreactive polyclonal antisera to verify the serological homogeneity of the MHC haplotypes. Light Brown Leghorn (LBL) chickens were used as non-MHC-matched, unrelated controls. Four SL101 males were mated to 25 BL101 females to produce an F1 generation. All of the matings were by artificial insemination and pedigreed by sire and dam. At sexual maturity, 3 F1 males and 30 females were randomly selected and housed in individual cages. These birds were used to produce the F2 generation. Because of the large number of available F2 animals, only a select group of 46 animals was used for the mapping panel. This included 3 grandparents, 4 F1 parents (1 sire and 3 dams selected for large family size) used to generate the F2 population, and 39 F2 animals selected at random within the three dam families.
RFLP Analysis
DNA Extraction
Whole blood samples were collected by brachial venipuncture with EDTA as an anticoagulant. Blood samples were diluted 1:20 in SET buffer (0.16 mol/L NaCl, 0.05 mol/L Tris and 1 mmol/L EDTA, pH 8.0) and stored at −70°C. DNA was extracted according to the standard protocols. 15
Restriction Digestion
The restriction enzymes BamHI, HindIII, SacI, and TaqI (Promega, Madison, WI) were used for the RFLP analyses. Ten micrograms of DNA from individual birds were digested with 40 units of enzyme in the appropriate buffer and incubated overnight. Small aliquots of each reaction mixture were examined in a minigel to monitor the extent of digestion.
Electrophoresis and Transfer of DNA Fragments
After complete digestion with the restriction enzyme, genomic DNA fragments were separated in 0.8% agarose gel. HindIII-digested λ phage DNA was used as the molecular size marker. After appropriate gel treatment, DNA was transferred to nylon membrane (Magnagraph, MSI, San Diego, CA) by standard protocols. 15 Completeness of the DNA transfer from the gel was verified by staining the gel with ethidium bromide after the transfer. The DNA on the membrane was immobilized by UV cross-linking.
Probe Preparation and Labeling
The pRAV-2 probe cloned into pBR322 plasmid, specific for the endogenous viral genes was obtained from Dr. Eugene Smith 16 (ADOL, East Lansing, MI). A partial restriction map of the probe is given in Figure 1b ▶ . This probe encompasses the gag, pol, env, and long terminal repeat (LTR) sequences of the Rous-associated virus-2. (Complete sequences of the RAV-2 genome can be obtained from the following gene bank sequences: K02374, M14902, V14066, V14067, M11755). The DNA was labeled with [α]32P dCTP by a random primer method using the Prime-a-Gene labeling kit (Promega). The unincorporated nucleotides were removed by passage through a Sephadex G-50 column. The labeled probes were then denatured at 100°C for 5 minutes before hybridization.
Figure 1.
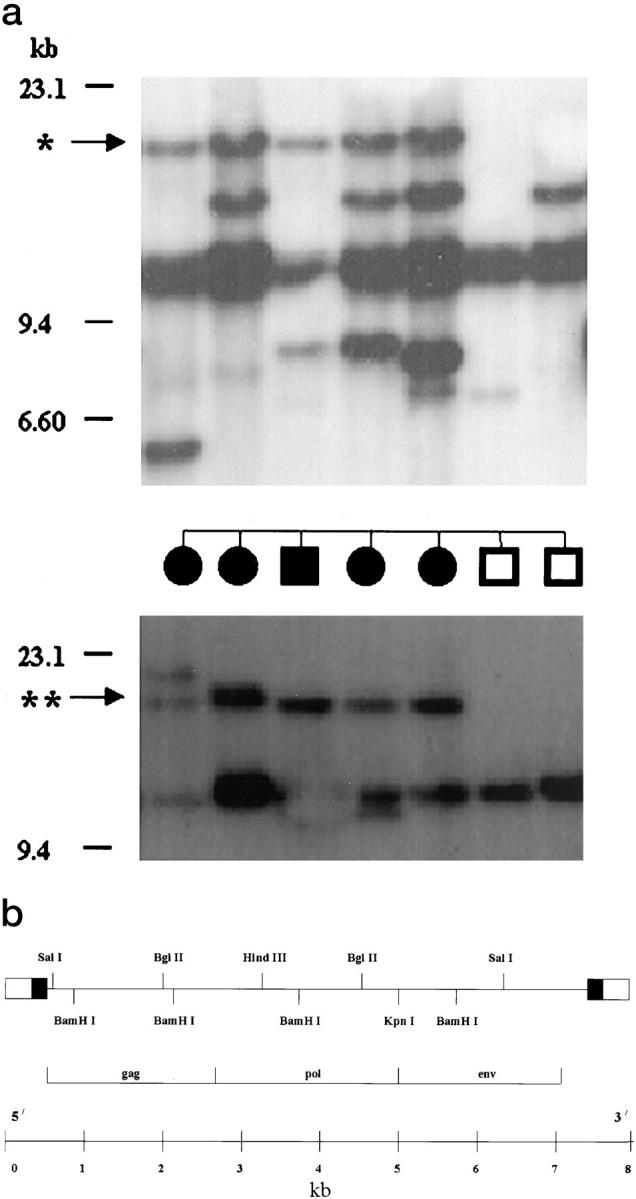
The results of the RFLP analyses of the EV genes in the F2 mapping panel. a, top panel: RFLP analysis using SacI; middle panel: Phenotype of the animals used for the analysis. Circles represent females; squares represent males; solid symbols represent animals affected with vitiligo. Bottom panel: RFLP analysis using HindIII. *, 16.2-kb SacI fragment associated with vitiligo. **, 19-kb HindIII fragment associated with vitiligo. b: Partial restriction map of the pRAV-2 probe used for the RFLP, in situ hybridization, and Northern blot analysis. Flanking boxes represent long terminal repeat sequence generated during viral replication. Open and solid boxes represent sequences unique to the 3′ and 5′ termini of the viral RNA, respectively. (Adapted from reference 16).
DNA Hybridization and Autoradiography
DNA hybridization and washings were carried out as previously described. 17 Briefly, membranes were first prehybridized at 42°C overnight in 20 to 25 ml of a mixture of 50% formamide, 5× Denhart’s solution, 5× SSPE and 0.1% sodium dodecyl sulfate (SDS). Sheared and heat-denatured salmon sperm DNA was added to a final concentration of 200 μg/ml of the prehybridization solution. The membranes were hybridized overnight with a mixture containing 50% formamide, 5× Denhart’s solution, 5× SSPE, and 0.1% SDS and salmon sperm DNA (200 μg/ml) and the [α]32P-labeled DNA probe. The washing conditions were adjusted according to the amount of hybridization of the probe to the membrane and were optimized for high signal-to-noise ratio. The membranes were then exposed to X-ray film with intensifying screen at −70°C.
Analysis of the DNA Methylation of Avian Retroviral Elements
Methylation-sensitive restriction enzymes were used to detect the degree of methylation of chicken EV genes. Two enzyme combinations were used for this analysis: MspI and HpaII, which have same the recognition site (C▾ CGG, GGC▴C) with HpaII being sensitive to methylation; and Sau3AI and MboI (▾GATC, CTAG▴), where MboI is sensitive to methylation. RFLP analyses using these enzymes were done according to the protocol discussed above. To study the methylation pattern of other endogenous retroviral elements, we have used an avian endogenous retroviral (EAV) probe, using the same set of restriction enzymes. The EAV probe was prepared by polymerase chain reaction (PCR) amplification of chicken DNA with the oligomers EAV-1 and EAV-2. 18 (restriction map of the EAV probe has been reported by Benkel and Gavora 18 ). EAVs are a family of endogenous retrovirus-like elements, characterized by short LTRs that have been reported in chicken lines that lack the Rous-associated virus sequences. 19
In Vivo Treatment with 5-Azacytidine (5-AzaC)
Sixteen SL101 and 15 BL101 birds were injected i.p. every 3 days with 3 mg of 5-AzaC/kg body weight from day of hatch to 18 weeks of age. Seventeen SL101 and 16 BL101 control birds received buffered saline injections. Because 5-AzaC is highly unstable in aqueous solution, 20 fresh preparations were made at the time of each injection. All birds were weighed every 3 days and examined for feather pigmentation changes.
Fluorescence in Situ Hybridization (FISH)
Chicken metaphases were prepared from chicken fibroblast cultures following standard procedures, fixed briefly for 5 minutes in 3:1 methanol:acetic acid three times, and dropped on clean coverslips. The 7.5-kb pRAV-2 probe 16 was fluorescently labeled by the nick translation method using biotin 16-dUTP and passed through a Sephadex G-50 column to remove unincorporated nucleotides. The protocol previously described by Ponce de Leon et al 21 was followed. Briefly, 100 ng of labeled pRAV-2 probe was mixed with 11.9 μg of salmon sperm DNA (average size 200–400 bp), precipitated, and resuspended in 12 μl of hybridization buffer consisting of 50% deionized formamide, 1× SSC, and 10% dextran sulfate to achieve a final DNA concentration of 1 μg/μl. The hybridization mix was denatured at 75°C for 5 minutes and hybridized to denatured (70% formamide, 2× SSC at 70°C for 2 minutes) chicken metaphases, mounted, sealed with rubber cement, and incubated in a humidified chamber at 37°C for 18 to 20 hours. The slides were washed in 50% formamide/2× SSC at 42°C for 15 minutes and 0.1× SSC at 60°C for 15 minutes. Blocking was done using 2% blocking reagent (Boehringer Mannheim, Indianapolis, IN) and the signals were detected using avidin-fluorescein isothiocyanate (5 μg/ml, Vector Labs, Burlingame, CA) in 1% blocking solution. Slides were washed in 4× SSC/0.1% Tween-20 for 15 minutes at 42°C, stained for 10 minutes in propidium iodide (200 ng/ml in 2× SSC) and rinsed for 5 minutes in 2× SSC/0.05% Tween 20. The slides were mounted in p-phenylenediamine-11 (PPD-11) antifade and observed under a Zeiss Axioscope fluorescent microscope.
Northern Blot Analysis
Feather tissue from the 5-AzaC-treated and control animals was collected and frozen in liquid nitrogen. Total RNA was isolated by using RNAZOL (Tel-Test, Inc., Friendswood, TX). The RNA was separated in 1.2% agarose formaldehyde gels. Calf liver rRNA (Sigma, St. Louis, MO) was used as the RNA size standard. After separation RNA was transferred to nylon membranes (Magna Graph, MSI) and stored at −70°C. The EV (pRAV-2) 16 and β-actin (Oncor, Gaithersburg, MD) probes were labeled by random priming with [α]32P dCTP by the Prime-a-Gene labeling system (Promega). The membranes were prehybridized for 5 to 10 hours in a hybridization buffer consisting of 50% formamide, 5× SSPE, 2× Denhart’s solution, 0.1% SDS, and 15 μg/ml of salmon sperm DNA. The radio-labeled probe was added directly to the hybridization solution and was incubated for 12–15 hrs at 42°C. The membranes were washed twice with 2× SSC buffer at 42°C followed by several washes with 0.5× SSC at 55°C. The membranes were then washed with 0.1× SSC and exposed to X-ray films at −70°C.
Linkage Analysis
χ 2 test was applied for the linkage disequilibrium analysis. 22
Results
RFLP Analysis of Endogenous Viral Genes in SL and BL Lines
Earlier results from our lab suggested that EV genes are different in SL and BL sublines of chickens. 9,10 In the present study, we have conducted RFLP analyses of the EV genes in an F2 mapping panel to detect the possible association of EV genes with the incidence of spontaneous autoimmune vitiligo. The RFLP analysis was studied in an F2 mapping panel of 46 animals, and differences were found between vitiliginous SL and normally pigmented BL parental control sublines of chickens. All of the restriction enzymes used in this study showed polymorphic bands in the mapping panel. The results of the linkage disequilibrium analysis in the F2 mapping panel are presented in Table 1 ▶ . Linkage disequilibrium between vitiligo and a 16.2-kb SacI fragment (P ≤ 0.05) and a 19 Kb HindIII fragment (P ≤ 0.03) was apparent. The scorable bands were present in 4/12 normal and 17/23 vitiliginous F2 animals for the 16.2-kb SacI locus and 4/10 normal and 21/25 vitiliginous F2 animals for the 19-kb HindIII locus. The polymorphic bands from the SacI and HindIII restriction digestion are shown in Figure 1a ▶ . None of the TaqI and BamHI fragments appeared to be associated with vitiligo. The results from this experiment suggest that EV genes play a major role in the development of vitiligo in SL chickens.
Table 1.
Linkage Disequilibrium Analysis of the Endogenous Viral Genes and Vitiligo in a Smyth line F2 Mapping Population
| Restriction enzyme | Band size | χ2 | P value |
|---|---|---|---|
| Sac1 | 16.2 | 3.901 | 0.050* |
| 14.6 | 0.590 | 0.552 | |
| 5.8 | 0.066 | 0.797 | |
| BamHI | 23 | 0.830 | 0.362 |
| 12.2 | 0.409 | 0.522 | |
| 9.2 | 0.175 | 0.676 | |
| 8.0 | 0.573 | 0.449 | |
| HindIII | 21.6 | 0.873 | 0.350 |
| 19.0 | 4.660 | 0.030* | |
| TaqI | 3.3 | 0.029 | 0.864 |
| 2.1 | 1.640 | 0.200 |
Only those bands that were polymorphic between SL and BL were used for the analysis.
Methylation Analysis of Avian Retroviral Elements
DNA methylation is one of the major mechanisms regulating gene expression and in many systems hypomethylation of regulatory sequences has been shown to correlate with increased gene transcription. 23 Methylation analyses of avian retroviral elements were conducted to detect any association of DNA methylation and the expression of retroviral elements on the incidence of vitiligo in SL chickens. Methylation analyses of EV and EAV elements were carried out by using a combination of methylation-insensitive and -sensitive restriction enzymes. The two enzyme combinations used here, MspI, HpaII and Sau3AI, MboI, revealed that EV genes are methylated in both SL and BL sublines of chickens. However, there was no apparent relationship between the methylation of EV genes and the incidence of vitiligo in the F2 mapping panel. The EAV genes also showed methylation in the SL and BL sublines, and here again, the methylation was not associated with the incidence of vitiligo. Therefore, although both EV and EAV are methylated, this factor alone does not appear to be associated with the SL vitiligo.
Effect of 5-AzaC Treatment on the Expression of EV Genes
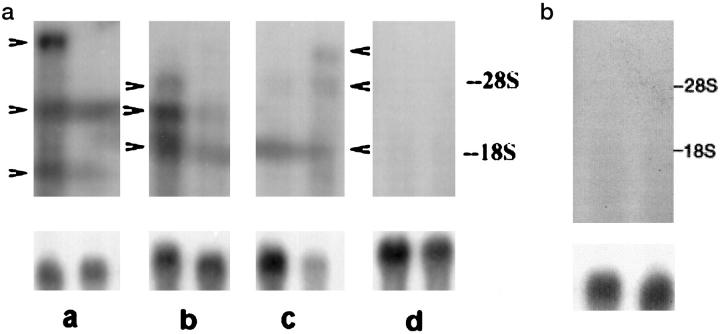
Treatment of chicken embryo fibroblasts with 5-AzaC, an inhibitor of DNA methylation, has been shown to induce the expression of EV genes, 24 but the effect of in vivo 5-AzaC treatment on the expression of EV genes in chickens has not been documented. Because 5-AzaC treatment induces the expression of autoimmune vitiligo in the typically normal parental control lines of chickens, 25 we looked at the expression of EV genes in 5-AzaC-treated and control chickens. The results of the phenotypic manifestation of 5-AzaC treatment on SL101 and BL101 sublines has been described. 25 Vitiligo was observed in 71% of the 5-AzaC-treated BL101 birds, whereas none of the saline-injected controls became vitiliginous. In the SL101 subline, 5-AzaC treatment did not affect the incidence of vitiligo (75%) compared to saline-injected controls. Results of the Northern blot analysis of the 5-AzaC-treated and untreated chickens are shown in Figure 2a ▶ . In this case 5-AzaC treatment induced the expression of the endogenous viral genes in the genetically susceptible but normally nonexpressing BL control lines. EV genes were also expressed in the vitiliginous untreated SL101 chicken and in the 5-AzaC-treated SL101 chickens. The number of transcripts varied between individuals; 1 to 3 transcripts were detected per bird. A transcript of about 4 kb appeared to be expressed in untreated SL chickens and 5-AzaC-treated SL chickens. Two transcripts (∼2 and ∼5 kb) were common in 5-AzaC-treated SL and BL chickens. The expression of the EV genes was associated with the development of vitiligo in vitiliginous SL101 birds and also in BL101 control chickens with 5-AzaC-induced vitiligo. The expression of MHC Class I and Class II genes was not affected by the treatment (data not shown), nor was there any differences in expression of the β-actin gene in either the SL101 or BL101 sublines. The EV transcripts were not detected in spleen tissue (Figure 2b) ▶ . These results indicate that the expression of EV genes has an etiological role in the development of vitiligo in SL chickens.
Figure 2.
a: Northern blot analysis of the total RNA from the feather tissue of SL101 and BL101 chickens treated with 5-Azacytidine. Upper panel: EV genes. Lower panel: β-actin gene. Lane a, SL untreated; lane b, SL 5-AzaC-treated; lane c, parental control (BL)-5-AzaC-treated; lane d, BL untreated. Arrowheads indicate the position of EV transcripts. Calf liver rRNA (28S and 18S) was used as the RNA size standard. b: Northern blot analysis of the total RNA from the spleen tissue of SL101 birds treated with 5-AzaC and showing vitiligo. Upper panel: EV genes. Lower panel: β-actin gene.
FISH Analysis
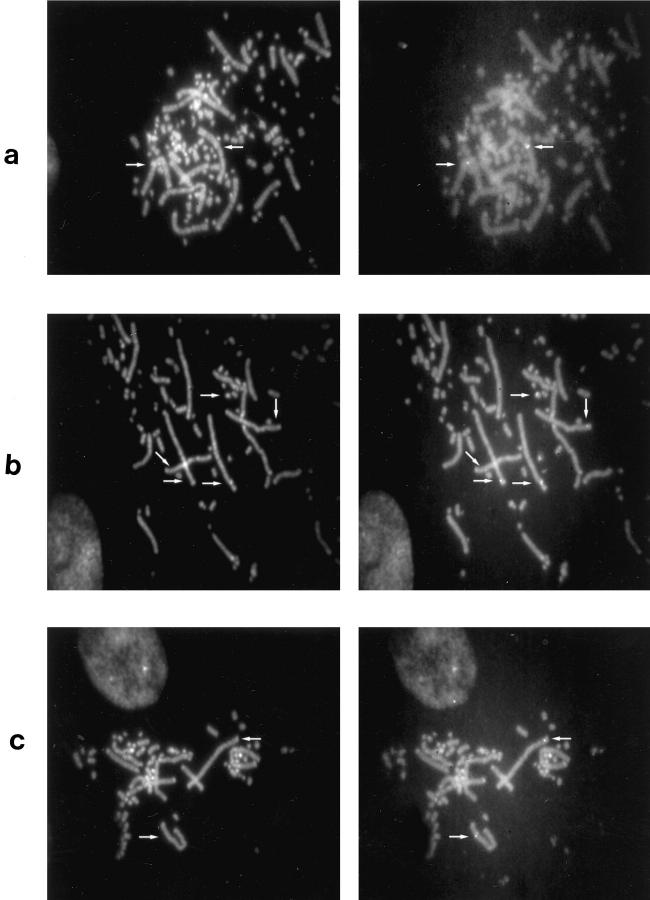
The results of the RFLP analysis in this study confirm our earlier reports 9,10 that EV genes are different in the SL and BL sublines of chickens. In situ hybridization experiments were conducted to detect the number and chromosomal location of the EV genes in SL101 and BL101 sublines of chickens. Results of the FISH analysis using the EV probe in SL101, BL101, and LBL chickens are shown in Figure 3 ▶ . In the unrelated LBL chickens the EV probe hybridized to only one chromosomal location (1q14). In SL101 and BL101 birds, 3 EV loci were identified by in situ hybridization analysis. These were located on chromosome 1 (1p25), chromosome 2 (2q26) and on an unidentifiable microchromosome. The results from the FISH experiments showed that the RFLP differences between the SL101 and BL101 sublines of chicken are not due to the different chromosomal localization of the EV genes, but are due to DNA sequence differences in the EV genes or in their flanking regions, as detected by the presence or absence of restriction sites. It is not clear that the EV gene mapped on the microchromosome is the same in SL and BL chickens. Because the SL and BL sublines were derived from the same base population, it is probable that the SL and BL EV loci are located on the same microchromosome.
Figure 3.
FISH of partial chicken metaphases with the EV probe. Two pictures of each metaphase were given to indicate the banding pattern (on the left) and the FISH signals (on the right). a: Light Brown Leghorn (LBL), unrelated control chicken metaphases hybridized with the EV probe. Signals on chromosome 1 (1q14). b: SL101 chicken metaphases hybridized with the EV probe. Signals appear on chromosome 1 (1p25), chromosome 2 (2q26), and on a michrochromosome. c: BL101 chicken metaphases hybridized with the EV probe. Signals on chromosome 1 (1p25) and chromosome 2 (2q26). The signal on the microchromosome is not shown in this metaphase.
Discussion
Results from the present study show that EV genes are associated with the expression of vitiligo in the SL chicken model. Two SL-specific EV fragments (19-kb HindIII fragment and 16.2-kb SacI fragment) were found to be associated with the incidence of vitiligo. We have previously reported polymorphisms at the EV loci between SL and BL sublines of chickens. 9,10 Because these data were obtained from nonpedigreed stock, it was not possible to associate a particular EV locus to the incidence of vitiligo. The very high level of polymorphisms at the EV locus between SL101 subline selected for high incidence of vitiligo and the BL101 subline selected for the resistance to vitiligo was surprising. This is particularly so considering that EV genes have apparently been present in the chicken genome since the origin of the red jungle fowl, from which the domestic chicken is thought to have evolved. 16,26 This is similar to the situation in mice, where hundreds, even thousands, of EV genes can be present in a single individual. 27 The lack of fixation of the EV genes in the highly inbred SL101 and BL101 populations suggest that EV genes are not neutral, but rather subject to selective forces tending to prevent fixation and to maintain intermediate frequencies. Because the SL and BL sublines were derived from a single base population about 15 generations ago, it is quite unlikely that the polymorphisms observed at the EV loci are due to the presence of different EV genes. We have hypothesized that the differences in the restriction fragment lengths observed at the EV locus in the present study are due to mutational events, generating different RFLP patterns (GP Sreekumar, JR Smyth Jr, FA Pouce de Leon, unpublished data). We have conducted in situ hybridization experiments to examine this hypothesis. Three EV genes have been mapped in the SL101 and BL101 sublines. The EV loci in SL and BL sublines were distinctly different from those of the LBL controls, where only one EV locus was identified. The results of the FISH analysis showed that SL101 and BL101 lines have the same EV loci insofar as their chromosomal locations are concerned. The differences in the RFLP pattern between the SL101 and BL101 lines are thus associated with differences in the DNA sequence in the coding and/or flanking regions. These sequence differences could be one of the important factors contributing to the differences in susceptibility to the autoimmune phenomenon.
One interesting observation from this experiment was that the EV genes were found to be hemizygous in the SL101 and BL101 sublines, ie, in most of the embryos examined, only one chromosome showed the signal per metaphase, the signal being absent in the homologous autosomal chromosome. The mechanism underlying this phenomenon is not clear at the present time. This may be one of the reasons for the higher level of polymorphism at the EV loci, ie, an example of position effect polymorphism.
Virtually all eukaryotic cells harbor DNA sequences that encode structural genes related to species-specific RNA tumor viruses. The endogenous proviral genes homologous to avian leukosis virus (ALV) genomic RNA are inherited as single-copy segments of DNA located at specific chromosomal locations in chickens. 16 During the evolution of chickens, EV genes have been inserted at many, perhaps random, chromosomal locations. Chickens have been observed with zero to five EV genes segregating at different loci. 16,28 EV genes and related retrotransposons could affect normal gene expression by acting as insertional mutagens. Other possible explanations for the association of EV genes and immune function are: (i) viral integration near immunologically important genes and their phenotypic modification through the dominant insertion of promoter or enhancer elements; (ii) close linkage with immunologically important genes; and (iii) direct effect of viral gene expression and superantigen-induced molecular mimicry. 29 The first direct connection between endogenous retrovirus, immune dysregulation, and autoimmunity was observed in MRL/lpr mice, where integration of a retrotransposon sequence into the Fas gene led to abnormal apoptosis that contributed to autoimmunity and lymphocyte accumulation in this model. 30 To date the potential association and significance of EV genes in the development of human autoimmune diseases such as Sjögren’s syndrome, rheumatoid arthritis, and multiple sclerosis has been the subject of much speculation. 31-36 A new EV locus, EV22 was found in the Obese strain chicken model for autoimmune Hashimoto’s thyroiditis, but that locus was not found to be directly associated with the disease. 37 It was hypothesized that EV22 could influence the development of autoimmune thyroiditis by its influence on the glucocorticoid-mediated immunoregulatory process. 37,38 Similarly, EV23 has been reported to be present in the UCD-200 and UCD-206 chicken models for autoimmune systemic scleroderma, but its association with the disease is also unknown. 39 The results from the present study suggest that induction of transcription of EV genes in typically nonvitiliginous BL101 parental controls by 5-AzaC treatment could be one of the reasons for the high incidence of vitiligo observed in this treatment group. We have mapped three EV loci in SL and BL chickens, but at the present time we do not know which of these EV genes are expressed, nor do we know their role in the incidence and severity of vitiligo.
The vitiliginous phenotype in the SL chicken model appears to result from the interaction of aberrant melanocytes 3 and an autoimmune reaction mediated by both B and T cell compartments. 4,5 The results of the earlier studies on morphological and immunological alterations accompanying the vitiligo process in the SL model have led to our working hypothesis. 2 This hypothesis states that an inherent pigment cell defect is basic to the development of vitiligo. Through melanocyte malfunction or death, antigens are presented that lead to an immune response directed specifically to the pigment cells. Thereafter, differentiating and/or melanogenically active melanocytes are destroyed and eliminated by the immune system. The induction of autoimmune vitiligo in the genetically susceptible BL chickens by 5-AzaC treatment and the absence of melanization changes in the unrelated LBL controls further substantiate the above hypothesis. 25 Melanocyte-specific autoantibodies were detectedin the serum of the SL chickens. 6 SL autoantibodies that cross-react with mouse and human melanocytes are able to bind to pigment cells within the tissues and recognize antigens expressed in the cytoplasm and on the surface of the melanocytes and melanoblasts. 7 The SL autoantibodies do not recognize tyrosinase and tyrosinase-related protein-2 (TRP-2), but do cross-react with TRP-1. 7,8 A genetic model for the autoimmune thyroiditis in the Obese strain chicken model has been proposed, whereby an immune system component (thyroglobulin autoantibodies) and a cellular component resulting in thyroid dysfunction are, together, responsible for this autoimmune condition. It has been shown that the autoantibodies have a role in the cellular destruction only when the target tissue is aberrant. 40 If this model is applied to the autoimmune vitiligo in the SL chicken, once the melanocyte autoantibodies are produced, they may have the potential to be involved in further melanocyte destruction if only the aberrant melanocytes are immunological targets. Various melanocyte subpopulations could be expressing various degrees of aberrance, 41 thus explaining observed phenotypic expression differences. 2
The results from the present experiment suggests that EV genes have a major influence in the induction of autoimmune vitiligo in the SL chicken model, and we propose that the basic defect in the melanocytes or a subpopulation of melanocytes is due to the expression of EV genes. However, the mechanism behind this phenomenon is unclear. It is possible that expression of EV genes is tissue-specific and expressed only in the melanocytes. Silencing of the EV genes by DNA methylation, and age-specific demethylation and expression, could possibly result in the development of the disease by 6 to 12 weeks of age. Our results from the 5-AzaC study further support this hypothesis. 25 Such an on/off switch could be reversible, resulting in the sporadic remelanization that occurs spontaneously in this line. 2 Because these viral proteins were not presented to the immune system during T cell development, the host immune system could subsequently mount an immune reaction directed specifically against the melanocytes. Such an event could initiate a cascade of reactions leading to the production of melanocyte-specific antibodies and massive destruction of melanocytes by antibody-mediated cellular cytotoxicity. Another possibility is that one of the melanocyte-specific or immunoregulatory proteins are encoded in part by a retroviral element, leading to molecular mimicry and autoimmunity.
Methylation analyses of the EV genes and EAV genes were conducted. The EAV probe was used in this case to get a full understanding of the methylation of other avian retroviral elements. EAV elements have been shown to have about a 55% nucleotide sequence similarity to the RAV-type endogenous provirus. 42 They are found at an estimated 40 to 100 inserts per bird in modern chicken breeds. 19 Analysis of DNA methylation using methylation-sensitive restriction enzymes has shown that endogenous retroviral elements are methylated. Yet in the present study, the methylation pattern was not found to be correlated with the incidence of vitiligo in the F2 mapping population. However, methylation analysis using restriction enzymes will not give the complete methylation profile of the gene under study. So, even though the methylation of the retroviral elements was not found to be associated with vitiligo, we cannot rule out the effect of methylation of the retroviral elements in the etiology of vitiligo. According to our hypothesis, EV genes are silenced by methylation in a tissue-specific and stage-specific manner. Because the methylation analysis was conducted with DNA from erythrocytes, these results do not eliminate a role for methylation of retroviral sequences in inducing autoimmune disease. More refined experiments with more sensitive methods (ligation-mediated PCR or bisulfite sequencing) could provide critical data for the evaluation of this hypothesis.
Vitiligo in the SL chicken model is influenced by many environmental factors, including vaccination against common viral diseases. Recent unpublished experiments from our lab indicate that day-old vaccination of SL chicks with HVT-Marek’s disease vaccine (live herpes virus of the turkey) has a significant influence on the incidence and severity of vitiligo. Incidence of vitiligo was significantly higher in vaccinated birds reared in a semi-isolated environment compared to those that were not vaccinated. 14 The mechanism by which the vaccine virus induces vitiligo is not known. It is possible that the vaccine, a turkey herpes virus, might be able to trigger the autoimmune response by molecular mimicry. Molecular mimicry occurs frequently with various DNA and RNA viruses. 43
The results from the present study show that the EV genes are associated in the induction of autoimmune vitiligo in the SL chicken model. The RFLP differences between the SL and BL lines were not found to be due to the differences in the chromosomal location of the EV genes. The mechanism involved in the EV gene association with autoimmune vitiligo is not clear at the present time. Stage-specific demethylation and expression of EV genes and superantigen-induced molecular mimicry are possible explanations. However, more experiments are necessary to develop an understanding of the mechanism of autoimmunity that occurs spontaneously in this animal model.
Footnotes
Address reprint requests to J. Robert Smyth Jr., 307A Stockbridge Hall, Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003.
G. P. Sreekumar’s current address: Department of Bio-informatics, Glaxo-Wellcome, 5 Moore Drive, Research Triangle Park, NC 27709.
S. Ambady’s and F. Abel Ponce de Leon’s current address: Department of Animal Science, University of Minnesota, St. Paul, MN 55108.
References
- 1.Smyth JR, Jr, Boissy RE, Fite KV: The DAM chicken: a model for spontaneous postnatal cutaneous and ocular vitiligo. J Hered 1981, 72:150-156 [PubMed] [Google Scholar]
- 2.Smyth JR, Jr: The Smyth line chicken: a model for autoimmune vitiligo. Crit Rev Poult Biol 1989, 2:1-19 [Google Scholar]
- 3.Boissy RE, Smyth JR, Jr, Fite KV: Progressive cytologic changes during the development of the delayed feather vitiligo and associated choroidal defects in DAM chicken line: a vitiligo model. Am J Pathol 1983, 111:197-212 [PMC free article] [PubMed] [Google Scholar]
- 4.Lamont SJ, Boissy RE, Smyth JR, Jr: Humoral immune response and expression of spontaneous postnatal vitiligo in DAM chicken. Immunol Commun 1982, 11:121-127 [DOI] [PubMed] [Google Scholar]
- 5.Erf GF, Trejo-Skalli AV, Smyth JR, Jr: T cells in regenerating feathers of Smyth line chickens with vitiligo. Clin Immunol Immunopathol 1995, 76:120-126 [DOI] [PubMed] [Google Scholar]
- 6.Austin LM, Boissy RE, Jacobson BS, Smyth JR, Jr: The detection of the melanocyte autoantibodies in the Smyth chicken model for vitiligo. Clin Immunol Immunopathol 1992, 64:112-120 [DOI] [PubMed] [Google Scholar]
- 7.Searle EA, Austin LM, Boissy YL, Zhao H, Nordlund JJ, Boissy RE: Smyth chicken melanocyte autoantibodies: cross species recognition, in vivo binding and plasma membrane reactivity of antiserum. Pigment Cell Res 1993, 6:145-157 [DOI] [PubMed] [Google Scholar]
- 8.Austin LM, Boissy RE: Mammalian tyrosinase-related protein-1 is recognized by autoantibodies from vitiliginous Smyth chickens. Am J Pathol 1995, 146:1-13 [PMC free article] [PubMed] [Google Scholar]
- 9.Lakshmanan NK, Smyth JR Jr, Ponce de Leon FA: Incidence of endogenous viral loci (EV) in Smyth line chickens: an avian model for autoimmune vitiligo. Poult Sci 1992, 71(Suppl 1):90
- 10.Sreekumar GP: Mapping of vitiligo genes in the Smyth line chicken model for autoimmune human vitiligo. Ph. 1998, Amherst, D. Thesis. University of Massachusetts
- 11.Abraham GE, Khan AS: Human endogenous retroviruses and immune disease. Clin Immunol Immunopathol 1990, 56:1-8 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Harrison LC: The potential roles of endogenous retroviruses in autoimmunity. Immunol Rev 1996, 152:193-236 [DOI] [PubMed] [Google Scholar]
- 13.Pateince C, Wilkinson DA, Weiss RA: Our retroviral heritage. Trends Genet 1997, 93:3450-3454 [DOI] [PubMed] [Google Scholar]
- 14.Smyth JR, Erf GF, Sreekumar GP: Do viruses and/or growing environment affect the expression of vitiligo in Smyth line chickens? Pigment Cell Res 1997, 10:108. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989. NY, Cold Spring Harbor Laboratory Press, Cold Spring Harbor
- 16.Smith EJ: Endogenous avian leukemia viruses. De Boer GF eds. Avian Leukosis. 1987, :pp 101-129 Martinus Nijhoff, Boston [Google Scholar]
- 17.Smith EJ, Crittenden LB: Endogenous viral genes in a slow-feathering line of white leghorn chickens. Avian Pathol 1986, 15:395-406 [DOI] [PubMed] [Google Scholar]
- 18.Benkel BF, Gavora JS: A novel molecular fingerprint probe based on the endogenous avian retroviral element (EAV) of chickens. Anim Genet 1993, 24:409-413 [DOI] [PubMed] [Google Scholar]
- 19.Resnick R, Bocye-Jacino MT, Fu Q, Faras AJ: Phylogenetic distribution of the novel avian endogenous provirus family EAV-0. J Virol 1990, 84:4640-4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notari RE, DeYoung JL: Kinetics and mechanisms of degradation of the antileukemic agent 5-azacytidine in aqueous solutions. J Pharm Sci 1975, 64:1148-1157 [DOI] [PubMed] [Google Scholar]
- 21.Ponce de Leon FA, Ambady S, Hawkins GA, Kappes SM, Bishop MD, Robl JM, Beattie CW: Development of a bovine X chromosome linkage group and painting probes to assess cattle, sheep, and goat X chromosome segment homologies. Proc Natl Acad Sci USA 1996, 93:3450–3454 [DOI] [PMC free article] [PubMed]
- 22.Terwilliger JD, Ott J: Handbook of Human Genetic Linkage. 1994:pp 199-210 The Johns Hopkins University Press, Baltimore
- 23.Cedar H: DNA methylation and gene expression. DNA methylation. Biochemistry and Biological Significance. Edited by A Razin, H Cedar, AD Riggs. New York, Springer Verlag, 1984, pp 147–164
- 24.Groudine M, Eisenman R, Weintraub H: Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature 1981, 292:311-317 [DOI] [PubMed] [Google Scholar]
- 25.Sreekumar GP, Erf GF, Smyth JR, Jr: In vivo treatment of 5-azacytidine induces autoimmune vitiligo in the susceptible parental control strain of Smyth line model for human vitiligo. Clin Immunol Immunopathol 1996, 81:136-144 [DOI] [PubMed] [Google Scholar]
- 26.Crawford RD: Origin and history of poultry species. Crawford RD eds. Poultry Breeding and Genetics. 1990, :pp 1-42 Elsevier, New York [Google Scholar]
- 27.Stoye JP, Fenner S, Greenoak GE, Moran C, Coffin JM: Role of endogenous retroviruses as mutagens: hairless mutation in mice. Cell 1988, 54:383-391 [DOI] [PubMed] [Google Scholar]
- 28.Crittenden LB: Retroviral elements in the genome of the chicken: implications for poultry genetics and breeding. Crit Rev Poultry Biol 1991, 3:73-109 [Google Scholar]
- 29.Krieg AM, Gourley MF, Perl A: Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J 1992, 6:2537-2544 [DOI] [PubMed] [Google Scholar]
- 30.Adachi M, Watanabe-Fukunaga R, Nagata S: Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA 1993, 90:1756-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg AM, Gause WC, Gourley MF, Steinberg AD: A role of endogenous retroviral sequences in the regulation of lymphocyte activation. J Immunol 1989, 143:2448-2451 [PubMed] [Google Scholar]
- 32.Garry R: Extensive antigenic mimicry by retrovirus capsid proteins. AIDS Res Hum Retroviruses 1990, 6:1361-1362 [DOI] [PubMed] [Google Scholar]
- 33.Krieg AM, Steinberg AD: Analysis of thymic retroviral expression in murine lupus. J Clin Invest 1990, 86:809-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talal N, Garry RF, Alexander SS, Dauphinee MJ, Ballester A, Takei M, Dang H: A conserved interspecies idiotype and autoantibodies to retroviral proteins in systemic lupus erythamatosus. J Clin Invest 1990, 85:1866-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banki K, Maceda J, Hurley E, Abonczy E, Mattson DH, Szegedy L, Hung C, Perl A: Human T-cell lymphotropic virus related to endogenous sequence: HRES-1, encodes a 24 kD protein: a possible autoantigen for HTLV-1 GAG-reactive antibodies. Proc Natl Acad Sci USA 1992, 89:1939-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banki K, Colombo E, Sia F, Halladay D, Mattson DH, Tatum AH, Massa PT, Philips PE, Perl A: Oligodendrocyte-specific expression of autoantigenicity of transaldolase in multiple sclerosis. J Exp Med 1994, 180:1649-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziemiecki A, Kromer G, Mueller RG, Hala H, Wick G: ev22, a new endogenous avian leukosis virus locus found in chickens with spontaneous autoimmune thyroiditis. Arch Virol 1988, 100:267-271 [DOI] [PubMed] [Google Scholar]
- 38.Wick G, Kromer G, Neu N, Fassler R, Ziemiecki A, Muller RG, Ginzel M, Kuhr T, Hala K: The multi-factorial pathogenesis of autoimmune disease. Immunol Lett 1987, 16:249-257 [DOI] [PubMed] [Google Scholar]
- 39.Sgonc R, Dietrich H, Gershwin ME, Colombatti A, Wick G: Genomic analysis of collagen and endogenous viral loci in the UCD-200 and 206 lines of chickens, animal models for scleroderma. J Autoimmun 1995, 8:763-770 [DOI] [PubMed] [Google Scholar]
- 40.Hala K: Immunogenetic analysis of spontaneous autoimmune thyroiditis in obese strain (OS) chickens: an animal model for spontaneous autoimmune thyroiditis. Immunobiology 1988, 177:354-373 [DOI] [PubMed] [Google Scholar]
- 41.Boissy RE, Gecks S, Smyth JR, Jr, Nordlund JJ: Occular pathology in the minimally depigmented subline of the vitiliginous Smyth chicken. Pigment Cell Res 1988, 1:303-314 [DOI] [PubMed] [Google Scholar]
- 42.Dunwiddie CT, Resnick R, Bocye-Jacino MT, Alegre JN, Faras AJ: Molecular cloning and characterization of gag-, pol- and env-related gene sequences in the ev chicken. J Virol 1986, 59:669-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujinami RS, Oldstone MBA: Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 1985, 230:1043-1045 [DOI] [PubMed] [Google Scholar]