Abstract
Giant cell tumor of bone (GCT) is a rare primary osteolytic tumor of bone that is characterized by massive tissue destruction at the epiphysis of long bones. There is no evidence that tumor cells themselves are capable of bone destruction; instead, it appears that the tumor cells of GCT act by promoting osteoclastogenesis and, as a consequence, osteoclastic bone resorption. However, the mechanism by which this is achieved is not understood. Here we attempted to determine whether osteoprotegerin ligand (OPGL), the factor that is necessary and essential for osteoclastogenesis, is involved in tumor cell-recruited osteoclast-like giant cell formation in GCT. Using fluorescence in situ hybridization, we sought to determine mRNA expression of OPGL, its receptor RANK, and its decoy receptor OPG in three major cell types of GCT. We demonstrated that OPG mRNA was expressed in all three cell types of GCT, OPGL transcripts were mainly detected in spindle-shaped stromal-like tumor cells, whereas RANK was expressed only in macrophage-like mononuclear cells and multinuclear osteoclast-like giant cells. By semiquantitative RT-PCR, we also showed that the level of OPGL mRNA in GCT is much higher than that in normal bone and osteogenic osteosarcoma. In contrast, a similar level of OPG transcripts was detected in these three kinds of tissues, and RANK mRNA was detectable only in GCT tissues. We have further examined the regulation of gene expression of OPGL and OPG in tumor cells in response to osteotropic hormones. Administration of 1,25(OH)2D3 and dexamethasone resulted in maximum up-regulation of OPGL level and down-regulation of OPG level in cultured GCT stromal-like tumor cells and the mouse bone marrow-derived ST-2 stromal cell line. Furthermore, we have shown that tumor cells of GCT induce differentiation of RANK-expressing myeloid RAW264.7 cells into osteoclast-like cells in the presence of 1,25(OH)2D3 and dexamethasone. Our findings suggest that OPGL is involved in the tumor cell-induced osteoclast-like cell formation in GCT. The ratio of OPGL/OPG by tumor cells may contribute to the degree of osteoclastogenesis and bone resorption.
Two novel tumor necrosis factor (TNF) superfamilies, osteoprotegerin (OPG) and osteoprotegerin ligand (OPGL), have recently been identified as members of a ligand-receptor system that directly regulates osteoclast differentiation and bone resorption. 1-4 OPGL, also known as osteoclast differentiation factor (ODF), 3 TNF-related activation-induced cytokine (TRANCE), 3,5,6 and receptor activator of NF-κB ligand (RANKL) 5 belong to the membrane-associated TNF-ligand family. It has been shown, using an in vitro culture system, that OPGL can both induce osteoclastogenesis and activate mature osteoclasts. 3,4 The expression of OPGL in osteoblast/stromal cells parallels the formation of osteoclasts in cocultures with bone marrow or spleen cell populations. The recombinant OPGL can replace the requirements for stroma cells in the in vitro model of osteoclastogenesis. 4 Mice with a disrupted opgl gene show severe osteopetrosis and a defect in tooth eruption and completely lack osteoclasts as a result of an inability of osteoblasts to support osteoclastogenesis. 7 It has been assumed that OPGL acts as an osteoclastogenesis-inducing factor linked to interaction between stromal cells and osteoclast progenitors. The cell surface receptor that interacts with OPGL has recently been shown to be the ligand for the TNFR-related protein receptor activator of NF-κB (RANK). 8 Transgenic mice expressing a soluble RANK-Fc fusion protein display osteopetrosis, a defect of osteoclast activity, whereas polyclonal antibody against the RANK extracellular domain promotes osteoclastogenesis in bone marrow cultures. 8 On the other hand, the decoy receptor of OPGL, OPG has been shown to neutralize and interrupt stromal cell-derived OPGL signals, resulting in the reduction of osteoclastogenesis. 1,2 OPG, also known as osteoclastogenesis inhibitory factor (OCIF), 2 is a soluble member of the TNF receptor family. OPG inhibits not only formation of osteoclast-like cells in murine cultures in vitro 2 but also bone resorption in vitro and in vivo. 1,2 OPG knock-out mice exhibited severe osteopenia due to accelerated bone resorption. 1 In short, it is conceivable that OPGL and OPG are key extracellular regulators of osteoclastogenesis and bone resorption. RANK is receptor necessary for the activation of OPGL.
Giant cell tumor of bone (GCT), a rare primary osteolytic tumor of bone, is characterized by massive bone destruction at the epiphysis of long bones. 9 Previous studies have shown that the spindle-shaped stromal-like mononuclear cells of GCT are the most likely candidate cells for the tumor’s neoplastic component. 9-12 However, there is no evidence that tumor cells themselves are capable of bone resorption. 13 Instead, tumor cells of GCT act by recruiting multinucleated osteoclast-like giant cells and hence promoting tumor-induced osteolysis. 9,14,15 We previously showed that tumor cells were capable of recruiting circulating monocytes/osteoclast precursor cells and even osteoclasts through the production of transforming growth factor-β1 (TGF-β1) 9 and monocyte chemoattractant protein 1 (MCP-1). 10 Others have shown that the tumor cells of GCT also produce interleukins-1 and -6 and macrophage colony-stimulating factor, which are related to the induction of osteoclastogenesis and bone resorption. 16-18 It is noteworthy that although individual cytokines play a role in the recruitment of osteoclasts and in osteoclastic bone resorption, none of them are capable of directly inducing the macrophage-like cells to form osteoclasts. In fact, cells from osteogenic tumors such as osteosarcomas can express a similar cytokine profile, 19-21 as do cells from GCT, but induction of osteoclast formation is observed to a lesser degree. Thus the mechanism by which tumor cells of GCT generate osteoclast-like giant cells from recruited macrophage-like cells is unclear.
To determine whether OPGL is involved in the interaction of stromal-like tumor cells and macrophage-like mononuclear cells, which results in the generation of osteoclast-like giant cells in GCT, we have investigated the expression of OPGL, OPG, and RANK in all three cell types of GCT at the mRNA level and examined the regulation of gene expression in response to osteotropic hormones. A coculture system of stromal-like tumor cells and murine osteoclast precursor cell line RAW264.7 cells 8 has been used to evaluate the ability of tumor cells to further induce osteoclastogenesis.
Materials and Methods
Materials
Human GCT from five different cases and normal cancellous bone containing no bone marrow cells were collected fresh from patients after operations at Sir Charles Gairdner Hospital (Nedlands, WA, Australia). The human osteoblast-like osteosarcoma cell line U2OS was purchased from the American Type Culture Collection (Rockville, MD). The mouse stromal cell line ST-2 was provided by Prof. G. C. Nicholson at the Geelong Hospital (Geelong, VIC, Australia). The murine myeloid cell line RAW264.7 was provided by Dr. Ian Cassady at the University of Queensland (Queensland, Australia). Dulbecco’s minimum essential medium (MEM), α-MEM, and fetal bovine serum (FBS) were purchased from TRACE (Sydney, Australia). 1α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) was obtained from Calbiochem-Novabiochem (Alexandria, Australia); dexamethasone was from Sigma (St. Louis, MO); recombinant human RANKL (sRANKL) was from Pepro Tech (NJ, USA); RETROscript was from Ambion (Austin, TX); the DIG RNA Labeling kit and the Fluorescent Antibody Enhancer Set were from Boehringer Mannheim (Sydney, Australia); the diagnostics tartrate resistant acid phosphatase (TRAP) kit was from Sigma; rabbit anti-rat/mouse calcitonin receptor (CTR) polyclonal antibody was provided by Dr. P. M. Sexton at the Department of Pharmacology (University of Melbourne, Victoria, Australia); Texas Red-X-conjugated swine anti-rabbit Ig secondary antibody was purchased from Molecular Probes (Eugene, OR). All other chemicals used were of the highest grade available.
Culture of Giant Cell Tumor of Bone
As described previously, 4 tumor tissues were freshly chopped in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The resultant cell suspension together with small pieces of tissue was transferred to 25-cm 2 flasks for culture at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The pieces of tissue contained many multinuclear giant cells and mononuclear cells that had migrated out of tissue fragments across the culture flask surface and contributed to the cell population in culture. Half of the culture medium was changed every 3 days with fresh DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. On reaching confluence, primary cultures were subcultured, and each passaged culture was stored in liquid nitrogen. To examine the gene regulation of OPGL and OPG in tumor cells of GCT, GCT stromal-like tumor cells obtained after the ninth passage were cultured by the addition of 10−8 mol/L 1,25(OH)2D3 in the absence or presence of 10−7 mol/L dexamethasone. After 7 days of incubation, cells from each group were prepared for RNA extraction.
Culture of ST-2 Cells and Osteoblastic U2OS Cells
Both ST-2 and U2OS cells were cultured in α-MEM containing 10% FBS, 2 mmol/L l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, in a humidified atmosphere of 5% CO2 and 95% air at 37°C. When the effect of 1,25(OH)2D3 and dexamethasone was tested, dose dependence was studied by the addition of 10−8 mol/L 1,25(OH)2D3 in the presence or absence of dexamethasone at doses from 10−9 to 10−6 mol/L. All cultures were harvested and used for total RNA extraction.
Coculture System with Stromal-Like Tumor Cells and RAW264.7 Cells
GCT stromal-like tumor cells at the ninth passage obtained from the liquid nitrogen store were seeded into culture chambers (flask style; Nunc) and treated with 10−8 mol/L 1,25(OH)2D3 in combination with 10−7 mol/L dexamethasone. To the stromal-like tumor cells reaching 50% confluence, 500 RAW264.7 cells were added to each chamber. The cocultures were continuously treated with both 1,25(OH)2D3 and dexamethasone. In separate experiments, RAW264.7 cells were cultured in medium alone or in medium with either 30 ng/ml sRANKL or a combination of 10−8 mol/L 1,25(OH)2D3 and 10−7 mol/L dexamethasone. All cultures were fed every 3 days with fresh medium. After 10 days, all of the cultures were fixed and proceeded to either TRAP histochemistry, CTR immunohistochemistry, or bone resorption pit assay (Zheng et al) 9 to confirm the identity of osteoclasts. TRAP-positive multinuclear cells with more than three nuclei were scored and data were statistically analyzed by Student’s t-tests.
Calcitonin Receptor Immunofluorescence Confocal Microscopy
Calcitonin receptor in cocultures of stromal-like cells and RAW264.7 cells was detected by the method described by Quinn et al. 22 In brief, cocultures prepared as described above were first incubated with 5% sheep serum in 0.5% BSA/PBS for 10 minutes. The cells were then incubated in PBS/BSA containing rabbit anti-rat/mouse CTR antibody (diluted 1:50) preincubated with MBP-CTR antigen. After a 1-hour incubation at room temperature, the cells were rinsed in PBS, and then PBS/BSA containing Texas Red-X-conjugated swine anti-rabbit Ig antibody (diluted 1:100) was added. After another 1-hour incubation in the dark, the antibodies were rinsed away by washing in PBS. Cells were examined under a confocal laser scanning microscope (Bio-Red 10000) for the immunofluorescence of calcitonin receptor.
RNA Extraction and RT-PCR
By the use of RNAzol B (Tel-Test) protocols, total cellular RNA was isolated from solid tumors, cultured cells derived from human GCT, normal bone, U2OS cells, ST-2 cells, RAW264.7 cells, and the coculture system and then reverse-transcribed into cDNA, using 100 units of Moloney-Murine Leukemia Virus (M-MLV) reverse transcriptase (Ambion) according to the manufacturer’s instructions. Primers used for the detection of OPGL, OPG, and RANK are listed in Table 1 ▶ . Housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and acidic ribosomal phosphoprotein (36B4) were used as internal controls in the examinations of human and mouse gene expressions, respectively.
Table 1.
Primers for OPG, OPGL, and RANK
| Region | Sense primer | Antisense primer | Product size (bp) |
|---|---|---|---|
| Human OPG | 5′ GCTAACCTCACCTTCGAG 3′ | 5′ TGATTGGACCTGGTTACC 3′ | 324 |
| Mouse OPG | 5′ AAAGCACCCTGTAGAAAACA 3′ | 5′ CCGTTTTATCCTCTCTACACTC 3′ | 257 |
| Human OPGL | 5′ GCCAGTGGGAGATGTTAG 3′ | 5′ TTAGCTGCAAGTTTTCCC 3′ | 486 |
| Mouse OPGL | 5′ AAGCTTTGGATCCTAACAGAATATCAG 3′ | 5′ AAGCTTCAGTCTATGTCCTGAACTT 3′ | 726 |
| Human RANK | 5′ TTAAGCCAGTGCTTCACGGG 3′ | 5′ ACGTAGACCACGATGATGTCGC 3′ | 497 |
| Mouse RANK | 5′ AAGATGGTTCCAGAAGACGGT 3′ | 5′ CATAGAGTCAGTTCTGCTCGGA 3′ | 350 |
| Human GAPDH | 5′ GGAGTCAACGGATTTGGT 3′ | 5′ GTGATGGGATTTCCATTGAT 3′ | 206 |
| Mouse 36B4 | 5′ TCATTGTGGGAGCAGACA 3′ | 5′ TCCTCCGACTCTTCCTTT 3′ | 832 |
Polymerase chain reaction (PCR) was performed using 1.0 U of Taq polymerase (Boehringer Mannheim, Mannheim, Germany) with 0.4 mmol/L of both human and mouse OPGL, OPG, and RANK primers and 0.2 mmol/L of GAPDH and 36B4 primers, 125 μmol/L of dNTP in 1× PCR buffer (Boehringer Mannheim), and water in a total volume of 25 μl. The amplification was performed in a DNA thermal cycler (model 2400; Perkin-Elmer) under the following conditions: denaturation at 94°C for 5 minutes for the first cycle and for 30 seconds starting from the second cycle, annealing at 55°C (except for human RANK at 65°C, mouse RANK at 58°C) for 30 seconds, and extension at 72°C for 30 seconds. Final extension was at 72°C for 10 minutes. The PCR products were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, and absorbance measured by densitometry.
Fluorescence in Situ Hybridization
The cDNA fragments of human OPGL, OPG, and RANK, which were 486, 324, and 497 bp, respectively, were generated by RT-PCR of total RNA from GCT solid tumor and then separately inserted into pCR2.1 with the Original TA Cloning Kit (Invitrogen). Recombinant plasmids with correct orientation were then purified and transcribed into digoxigenin-labeled antisense riboprobes with T7 RNA polymerase, using a DIG RNA labeling kit (Boehringer Mannheim). All of the clones were sequenced for the confirmation of authentic genes. In situ hybridization was performed as previously described. 23,24 The final concentration of each probe in hybridization solution was 0.3 ng/μl, and RNase treatment (100 μg/ml) before hybridization was used as the negative control. Detection of hybridization products was performed with a fluorescent antibody enhancer set (Boehringer Mannheim). We counterstained slides with 2 μg/ml of propidium iodide for 30 minutes at room temperature to view nuclei or with TRAP histochemistry to confirm the characteristics of both osteoclast-like giant cells and macrophage-like cells. Signals were detected by confocal microscopy (BioRed, 1000).
Results
Expressions of OPG, OPGL, and RANK Genes in GCT
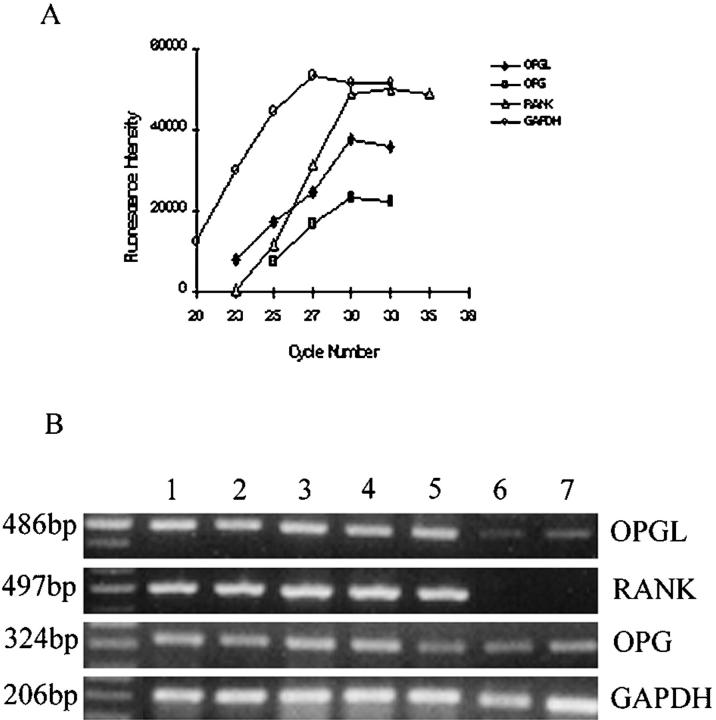
To allow estimation of the level of OPG, OPGL, and RANK gene transcripts in solid tumor and cultures of GCT, cycle-dependent PCR reactions were performed to generate amplification curves for each gene, using specific primers (Figure 1A) ▶ . A total of 28 cycles was selected for semiquantitation of OPGL and OPG gene transcripts, and 33 cycles for RANK and 26 cycles for GAPDH gene expression. As shown in Figure 1B ▶ , all GCT solid tumors expressed OPGL, OPG, and RANK mRNAs, whereas both normal bone and U2OS cells contained much less OPGL gene transcript and no RANK gene transcript. It appears that the ratio of OPGL/OPG in GCT was higher than that in normal bone and osteosarcoma. No correlation of OPGL, OPG, and RANK expression with the Enneking Clinical Stage 25 of GCT was found at presentation. Two cases of GCT at stage III (Figure 1B ▶ , lanes 2 and 3) expressed almost the same level of these gene transcripts as others in GCT at stage I or II (Figure 1B ▶ , lanes 1, 4, and 5).
Figure 1.
Gene expression of OPGL, OPG, and RANK in GCT. A: Cycle-dependent PCR reactions were removed from 20 to 35 cycles at two- to three-cycle intervals and electrophoresed on 1.5% agarose gels stained with EtBr. The intensity of EtBr fluorescence was measured by densitometry and plotted as a function of cycle number to generate amplification curves for OPGL, OPG, and RANK PCR fragments. B: Expression of OPGL, OPG, and RANK mRNA in samples from five cases of GCT (lanes 1–5, cases 1–5, respectively), normal cancellous bone (lane 6), and osteoblast-like osteosarcoma cells U2OS (lane 7). The sizes of OPGL, RANK, OPG, and GAPDH PCR products were 486, 497, 324, and 206 bp, respectively. The GAPDH housekeeping gene determines the variation of loading in the gel.
Localization of OPGL, OPG, and RANK Gene Transcripts in GCT
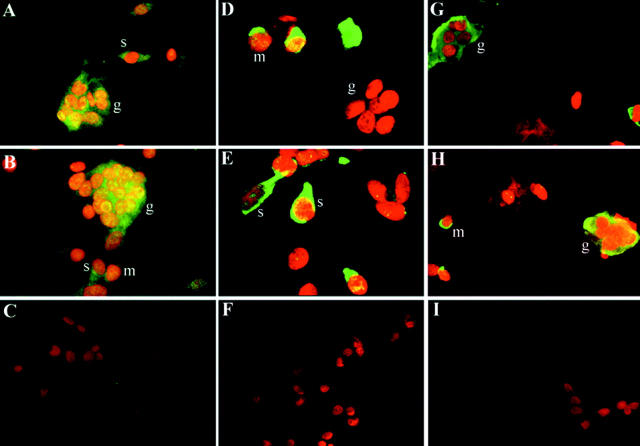
To further examine the localization of OPGL, OPG, and RANK gene transcripts in GCT at the cellular level, fluorescence in situ hybridization (FISH) was performed by using digoxigenin-labeled specific riboprobes (Figure 2) ▶ . Signals for each gene transcript were distributed differently in the cytoplasm of various cell types. OPG signals were detected in spindle-shaped stromal-like tumor cells, macrophage-like mononuclear cells, and multinuclear osteoclast-like giant cells. However, OPGL signals were mainly in stromal-like tumor cells, whereas RANK signals were mainly in macrophage-like mononuclear cells and multinuclear osteoclast-like giant cells. It is noteworthy that the RANK-positive macrophage-like mononuclear cells were also positive for TRAP when double staining of RANK and TRAP was used (data not shown). Treatment with RNase resulted in the absence of signals in all cells, indicating the specificity of the probes for target mRNA sequence (Figure 2) ▶ .
Figure 2.
Localization of OPG, OPGL, and RANK gene transcripts in GCT by FISH. Hybridization signals are in green (FITC), and nuclei staining is in red (PI). A and B: The OPG gene transcript was detected in multinuclear osteoclast-like giant cells (g) and spindle-shaped stromal-like tumor cells (t). It seems that the round mononuclear cells display weak positivity of the OPG mRNA signal. Original magnification, ×400. C: The signal disappeared when the cells were incubated with 100 μg/ml of RNase before hybridization of OPG. Original magnification, ×350. D and E: The OPGL gene transcript was found in stromal-like tumor cells (t) but not in multinuclear osteoclast-like giant cells (g). Original magnification, ×500. F: The signal disappeared when the cells were incubated with 100 μg/ml of RNase before hybridization of OPGL. Original magnification, ×350. G and H: The RANK gene transcript was detected in round macrophage-like cells (m) and multinuclear osteoclast-like giant cells (g), but not in stromal-like tumor cells (t). Original magnification, ×400. I: The signal disappeared when the cells were incubated with 100 μg/ml of RNase before hybridization of RANK. Original magnification, ×350.
Regulation of OPGL and OPG mRNAs by 1,25(OH)2D3 and Dexamethasone in Mesenchymal Stromal Cells
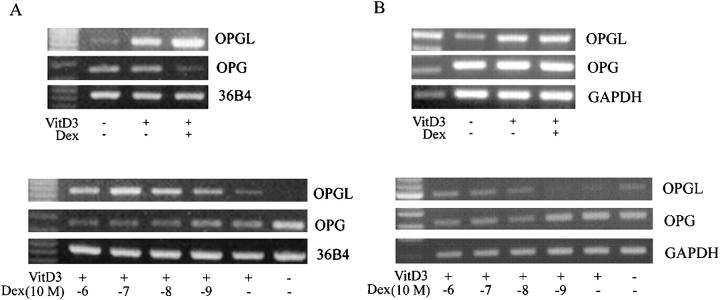
Various osteotropic hormones have been shown to be the regulators of osteoclastogenesis. 3,25,26 Because GCTs are considered to arise from mesenchymal stromal cells that have the capacity to recruit and harbor osteoclasts, 9-16 we attempted to determine whether 1,25(OH)2D3 and dexamethasone were capable of regulating OPGL and OPG expression in the stromal-like tumor cells of GCT and the mouse bone marrow-derived mesenchymal stromal cell line ST-2 cells. Cells were treated with 1,25(OH)2D3 at 10−8 mol/L and dexamethasone at 10−7 mol/L for a period of 7 days. As shown in Figure 3A ▶ , 1,25(OH)2D3 significantly increased OPGL mRNA levels while reducing OPG levels in ST-2 cells. Dexamethasone alone had no effect on OPGL and OPG gene expression (data not shown) but enhanced those effects of 1,25(OH)2D3 on both gene expression in a dose-dependent manner (Figure 3A ▶ , bottom). On the other hand, the effect of 1,25(OH)2D3 and dexamethasone on OPGL and OPG gene expression in primary cultures of GCT tumor cells varied between cases (Figure 3B) ▶ . In case 2 GCT primary culture (Figure 3B ▶ , top), both agents had no obvious effect on the reduction of OPG gene transcription but increased OPGL mRNA levels. On the other hand, dexamethasone increased OPGL levels and decreased OPG levels in a dose-dependent manner when combined with 1,25(OH)2D3 in case 4 GCT primary culture (Figure 3B ▶ , bottom). It appears that the balance between the levels of OPGL and OPG gene transcripts in these cells was significantly altered by the addition of both osteotropic agents.
Figure 3.
Regulation of gene expression of OPGL and OPG by 1,25(OH)2D3 and dexamethasone. Housekeeping genes GAPDH and 36B4 were used as internal controls for tumor cells of GCT and mouse ST-2 cells, respectively. A: Gene expression of OPGL and OPG in ST-2 stromal like cells. Top: 1,25(OH)2D3 at 10−8 mol/L and dexamethasone at 10−7 mol/L increased the level of OPGL mRNA but decreased the level of OPG gene transcript; bottom: dose effects of dexamethasone on 1,25(OH)2D3-regulated OPG and OPGL gene expression. Note that dexamethasone increased the level of OPGL but decreased the level of OPG in a dose-dependent manner. B: Gene expression of OPGL and OPG in cultured stromal-like tumor cells of GCT. Top: 1,25(OH)2D3 at 10−8 mol/L and dexamethasone at 10−7 mol/L increased the level of OPGL mRNA but had no obvious effect on OPG gene expression in case 2 GCT primary culture (ninth passage); bottom: dexamethasone increased OPGL levels and decreased OPG levels in a dose-dependent manner when combined with 1,25(OH)2D3 in case 4 GCT primary culture (ninth passage).
Differentiation of RAW264.7 Cells into Osteoclast-Like Cells in Coculture with Stromal-Like Tumor Cells of GCT
Given that there is no human macrophage cell line that is capable of differentiating into osteoclasts, whereas mouse RAW264.7 cells have been demonstrated to be able to generate osteoclast-like cells in the presence of OPGL but not other cytokines, 8 we have attempted to determine whether tumor cells of GCT are capable of inducing the differentiation of RAW264.7 cells into osteoclast-like cells. As shown in Figure 4 ▶ , RAW264.7 cells alone expressed high levels of RANK (Figure 4 ▶ , top) but did not differentiate into osteoclasts that express TRAP (Figure 4 ▶ , middle, a). RAW264.7 cells cocultured with tumor cells in the presence of 1,25(OH)2D3 and dexamethasone differentiated into TRAP-positive osteoclast-like cells after 10 days (Figure 4 ▶ , middle, d). Treatment of RAW264.7 cells with human sRANKL also induced the formation of TRAP-positive osteoclast-like cells (Figure 4 ▶ , middle, c). These multinuclear cells formed by the fusion of RAW264.7 cells were shown to express calcitonin receptor (Figure 4 ▶ , middle, e) and are capable of bone resorption (Figure 4 ▶ , middle, f). On the other hand, there is no evidence of osteoclast formation when RAW264.7 cells are treated with 1,25(OH)2D3 and dexamethasone in the absence of tumor cells (Figure 4 ▶ , middle, b). These results demonstrated that tumor cells of GCT are capable of inducing osteoclast formation by RAW264.7 cells in the presence of 1,25(OH)2D3 and dexamethasone.
Figure 4.
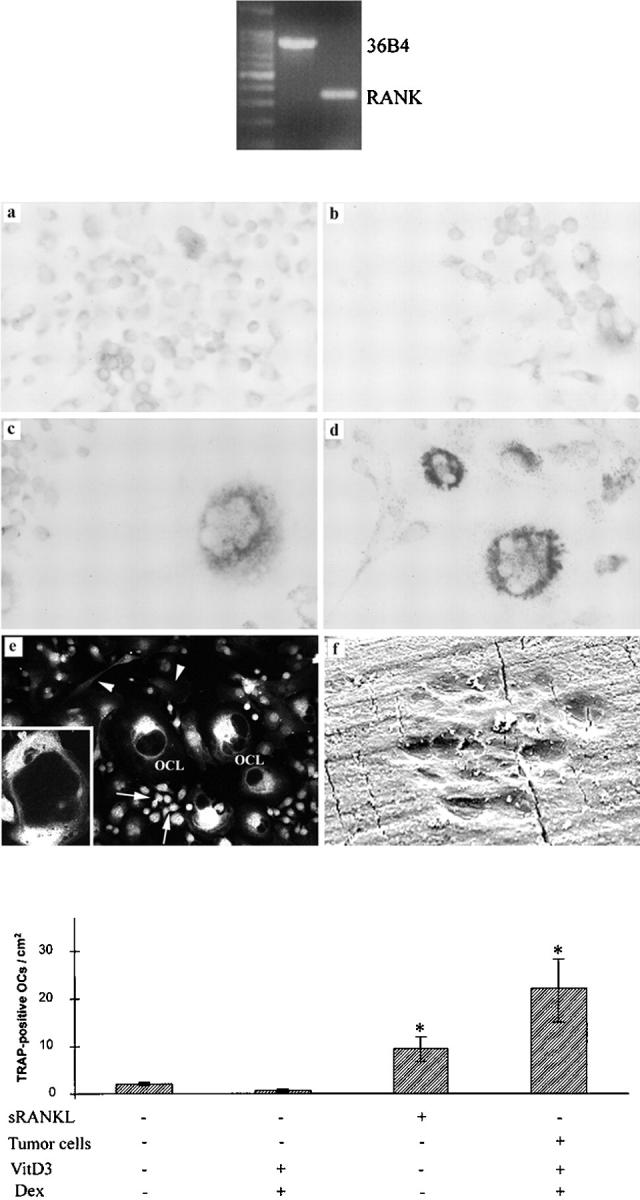
Differentiation of RAW264.7 cells into osteoclast-like cells (OCLs) in coculture with stromal-like tumor cells of GCT. Top: The RAW264.7 cell line was found to express high levels of RANK mRNA by RT-PCR. Middle: Characterization of RAW264.7 cells formed osteoclast-like cells. a: RAW264.7 cells were TRAP-negative. b: Treatment of RAW264.7 cells with 1,25(OH)2D3 and dexamethasone did not induce the formation of TRAP-positive OCLs. c: RAW264.7 cells differentiated into TRAP-positive OCLs when treated with human sRANKL. d: RAW264.7 cells differentiated into TRAP-positive OCLs when co-cultured with stromal-like tumor cells in the presence of 1,25(OH)2D3 and dexamethasone. e: The multinuclear OCLs formed by RAW264.7 cells expressed calcitonin receptor; the signals were mainly located on the surface of OCLs (inset). It is noteworthy that some RAW264.7 cells, apparently mononuclear osteoclast precursor cells, were positive for calcitonin receptor (arrows), whereas the signals in spindle-shaped stromal-like tumor cells were at the background level (arrowheads). f: Bone-resorbing pits by RAW264.7 cells formed osteoclast-like cells. Bottom: The numbers of TRAP-positive osteoclast-like cells formed per treatment group. Values are expressed as mean ± SD, * P < 0.05.
Discussion
Osteoclasts, the bone-resorbing cells, are derived from hematopoietic cells of monocyte/macrophage lineage. 27 The cell-to-cell interaction, the so-called juxtacrine 28 between stromal cells/osteoblasts and osteoclast progenitor cells, is essential for the formation of osteoclasts. OPGL was identified as a membrane-bound TNF ligand family protein necessary for the interaction between stromal cells and osteoclast progenitor cells during osteoclastogenesis. 3,5,6 Unlike any other cytokines, the recombinant soluble OPGL is able to replace the stromal cells/osteoblasts in the induction of osteoclast formation. 1-7 Thus OPGL is considered to be the “master” cytokine that is necessary and sufficient for the induction of osteoclastogenesis. 6
GCT is characterized by abundant multinuclear osteoclast-like giant cells scattered among mononuclear cells. 15 Although the histogenesis of GCT is not fully elucidated, it is generally believed that it is the stromal-like tumor cells that have the ability to recruit circulating monocytes to become multinuclear osteoclast-like giant cells in GCT. 9-16 We reported here the novel findings that OPGL, the osteoclastogenesis-inducing factor, was abundantly expressed in stromal-like tumor cells of GCT, and that RANK, the receptor for OPGL, was expressed in macrophage-like cells and osteoclast-like multinuclear giant cells. On the other hand, OPG, the decoy receptor for OPGL, was also ubiquitously expressed in the stromal-like tumor cells, indicating that a negative feedback loop may exist in which the tumor cells of GCT themselves may modulate the presentation of OPGL molecules on their surface, which in turn can be inhibited by OPG. Thus the ratio of OPGL and OPG gene expression in tumor cells may determine local osteoclastogenesis and osteoclastic bone resorption in the lesion. If the level of OPGL gene expression exceeds that of OPG in the microenvironment, osteoclast formation may be effectively induced. Conversely, if OPG gene expression is at higher levels than OPGL, osteoclast formation may be suppressed. In this study, we have shown a higher ratio of OPGL to OPG mRNA in all cases of GCT than that in normal bone and osteoblast-like osteosarcoma cell lines. This suggests that the production of OPGL may be of great importance for the tumor cell-induced formation of osteoclast-like giant cells in GCT. Bearing in mind that there is still a limitation of using mRNA to assess the expression of OPGL and OPG, further study should be conducted to elucidate their ratio at the protein level.
Previous studies have shown that the combination of 1,25(OH)2D3 and dexamethasone significantly stimulates osteoclast-like cell formation in cocultures of mouse spleen cells or in mouse bone marrow cultures. 29-31 In general accord with these findings, our results demonstrated that the combination of these two agents resulted in maximum up-regulation of OPGL gene expression and down-regulation of OPG expression in the mouse mesenchymal stromal cells tested. It is noteworthy that OPGL mRNA was also remarkably increased in primary cultured tumor cells of GCT when treated with 1,25(OH)2D3 and dexamethasone. Although tumor cells of GCT did not show a consistent decrease in OPG mRNA levels in the presence of both agents, it is reasonable to presume that these tumor cells, which express increased OPGL molecules on their membrane in the presence of 1,25(OH)2D3 and dexamethasone, would induce the differentiation of osteoclast progenitors into osteoclasts. In our present study, we have showed that the murine myeloid RAW264.7 cells differentiated into multinucleated osteoclast-like cells when cocultured with stromal-like tumor cells of GCT in the presence of 1,25(OH)2D3 and dexamethasone. The osteoclast-like cells formed in the coculture satisfied the major criteria of osteoclasts, including multinucleation and the presence of TRAP activity and calcitonin receptor. Given that the induction of osteoclast formation is required by the addition of 1,25(OH)2D3 and dexamethasone, which are the agents for up-regulation of OPGL expression, it is possible that tumor cell-induced osteoclast-like giant cell formation is mediated through the OPGL molecule expressed in stromal-like tumor cells.
In summary, we have shown for the first time that stromal-like tumor cells of GCT express OPGL, whereas macrophage-like cells and multinuclear osteoclast-like giant cells express the receptor for OPGL, the RANK. OPG, the decoy receptor for OPGL, was also found in GCT. However, the ratio of OPGL to OPG mRNA was much higher in GCT than that in normal bone and osteosarcoma cells. Osteotropic agents 1,25(OH)2D3 and dexamethasone up-regulate gene expression of OPGL in tumor cells of GCT. RAW264.7 cells, which express high levels of RANK mRNA, can differentiate into osteoclast-like cells when cocultured with stromal-like tumor cells in the presence of1,25(OH)2D3 and dexamethasone. Our findings suggest that OPGL may be essential for the tumor cell to induce the formation of osteoclast-like giant cells in GCT. It appears that the ratio of OPGL/OPG produced by stromal-like tumor cells may contribute to the degree of osteoclast-like giant cell formation and bone destruction in GCT.
Footnotes
Address reprint requests to Professor Ming H. Zheng, Department of Orthopaedic Surgery, University of Western Australia, Nedlands 6009, WA, Australia. E-mail: zheng@cyllene.uwa.edu.au.
Supported by grants from the National Health and Medical Research Council, the Orthopaedic Research and Education Fund and the Medical Research Fund of Western Australia (to M. H. Z.).
Drs. Huang and Xu contributed equally to this work.
References
- 1.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309-319 [DOI] [PubMed] [Google Scholar]
- 2.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K: Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 1997, 234:137-142 [DOI] [PubMed] [Google Scholar]
- 3.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T: Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998, 95:3597-3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 [DOI] [PubMed] [Google Scholar]
- 5.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L: A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390:175-179 [DOI] [PubMed] [Google Scholar]
- 6.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ: TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 1998, 188:997-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM: OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397:315-323 [DOI] [PubMed] [Google Scholar]
- 8.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ: Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 1999, 96:3540-3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng MH, Fan Y, Wysocki S, Lau ATT, Robertson T, Beilharz M, Wood DJ, Papadimitriou JM: Gene expression of transforming growth factor-β and its type II receptor in giant cell tumor of bone. Am J Pathol 1994, 145:1095-1104 [PMC free article] [PubMed] [Google Scholar]
- 10.Golding SR, Roelke MS, Petrison KK, Bhan AK: Human giant cell tumor of bone: identification and characterisation of cell types. J Clin Invest 1987, 79:483-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Athanasou NA, Bliss E, Gatter KC, Heryet A, Wood CG, McGee JOD: An immunohistological study of giant cell tumor of bone: evidence for an osteoclast origin of the giant cells. J Pathol 1985, 147:153-158 [DOI] [PubMed] [Google Scholar]
- 12.Zheng MH, Siu P, Papadimitriou JM, Wood DJ, Murch A: Telomeric fusion is a major cytogenetic aberration of spindle-shaped mononuclear cells derived from giant cell tumor of bone. Pathology 1999, 31:373-378 [DOI] [PubMed] [Google Scholar]
- 13.Zheng MH, Fan Y, Wysocki S, Wood DJ, Papadimitriou JM: Detection of mRNA carbonic anhydrase II in human osteoclast-like cells by in situ hybridisation. J Bone Miner Res 1993, 8:113-118 [DOI] [PubMed] [Google Scholar]
- 14.Zheng MH, Fan Y, Smith A, Wysocki S, Papadimitriou JM, Wood DJ: Gene expression of monocyte chemoattractant protein-1 in giant cell tumors of bone osteoclastoma: possible involvement in CD68+ macrophage-like cell migration. J Cell Biochem 1998, 70:121-129 [PubMed] [Google Scholar]
- 15.Rosai J: Ackerman’s Surgical Pathology, 8th ed. Edited by J. Rosai. St. Louis, Mosby, 1996, pp 1957–1961
- 16.Chambers TJ, Fuller K, McSheehy PM, Pringle JA: The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol 1985, 145:297-305 [DOI] [PubMed] [Google Scholar]
- 17.Mills BG, Frausto A: Cytokines expressed in multinucleated cells: Paget’s disease and giant cell tumors versus normal bone. Calcif Tissue Int 1997, 61:16-21 [DOI] [PubMed] [Google Scholar]
- 18.Sasaguri Y, Komiya S, Sugama K, Suzuki K, Inoue A, Morimatsu M, Nagase H: Production of matrix metalloproteinases 2 and 3 (stromelysin) by stromal cells of giant cell tumor of bone. Am J Pathol 1992, 141:611-621 [PMC free article] [PubMed] [Google Scholar]
- 19.Mariani E, Tarozzi A, Meneghetti A, Cattini L, Facchini A: TNF-α but not IL-1 and IL-6 modifies the susceptibility of human osteosarcoma cells to NK lysis. Int J Oncol 1998, 13:349-353 [DOI] [PubMed] [Google Scholar]
- 20.Andersen K, Maelandsmo GM, Hovig E, Fodstad O, Loennechen T, Winberg JO: Interleukin-1 α and basic fibroblast growth factor induction of matrix metalloproteinases and their inhibitors in osteosarcoma cells is modulated by the metastasis associated protein CAPL. Anticancer Res 1998, 18:3299-3303 [PubMed] [Google Scholar]
- 21.Franchi A, Arganini L, Baroni G, Calzolari A, Capanna R, Campanacci D, Caldora P, Masi L, Brandi ML, Zampi G: Expression of transforming growth factor β isoforms in osteosarcoma variants: association of TGF β1 with high-grade osteosarcomas. J Pathol 1998, 85:284-289 [DOI] [PubMed] [Google Scholar]
- 22.Quinn JMW, Morfis M, Lam MHC, Elliott J, Kartsogiannis V, Williams ED, Gillespie MT, Martin TJ, Sexton PM: Calcitonin receptor antibodies in the identification of osteoclasts. Bone 1999, 25:1-8 [DOI] [PubMed] [Google Scholar]
- 23.Collier FM, Huang WH, Holloway WR, Hodge JM, Gillespie MT, Daniels LL, Zheng MH, Nicholson GC: Osteoclasts from human giant cell tumours of bone lack estrogen receptors. Endocrinology 1998, 139:1258-1267 [DOI] [PubMed] [Google Scholar]
- 24.Huang WH, Lau ATT, Daniels LL, Fujii H, Seydel U, Wood DJ, Papadimitriou JM, Zheng MH: Detection of estrogen receptor a, carbonic anhydrase II and tartrate-resistant acid phosphatase mRNA in putative mononuclear osteoclast precursor cells of neonatal rats by fluorescence in situ hybridisation. J Mol Endocrinol 1998, 20:211-219 [DOI] [PubMed] [Google Scholar]
- 25.Enneking WF: Staging benign lesions. Musculoskeletal Tumour Surgery, vol 1. Edited by WF Enneking. New York, Churchill Livingstone, 1983
- 26.Horwood NJ, Elliott J, Martin TJ, Gillespie MT: Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblast stromal cells. Endocrinology 1998, 139:4743-4746 [DOI] [PubMed] [Google Scholar]
- 27.Zheng MH, Nicholson GC, Warton A, Papadimitriou JM: What’s new in osteoclast ontogeny? Pathol Res Pract 1991, 187:117-125 [DOI] [PubMed] [Google Scholar]
- 28.Zheng MH, Wood DJ, Papadimitriou JM: What’s new in the role of cytokines on osteoblast proliferation and differentiation? Pathol Res Pract 1992, 188:1104-1121 [DOI] [PubMed] [Google Scholar]
- 29.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T: The bone marrow-derived stromal cell lines MC3T3–G2/PA6 and ST2 support osteoclast-like cell differentiation in co-cultures with mouse spleen cells. Endocrinology 1989, 125:1805-1813 [DOI] [PubMed] [Google Scholar]
- 30.Suda T, Takahashi N, Martin TJ: Modulation of osteoclast differentiation. Endocr Rev 1992, 13:66-80 [DOI] [PubMed] [Google Scholar]
- 31.Shuto T, Kukita T, Hirata M, Jimi E, Koga T: Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte-macrophage colony-stimulating factor production in mouse bone marrow cultures. Endocrinology 1994, 134:1121-1126 [DOI] [PubMed] [Google Scholar]