Abstract
Human neuroblastoma cell lines typically consist of heterogenous subpopulations of cells that are morphologically and biochemically distinct. The cell types are characterized as neuroblastic (N-type), substrate-adherent Schwann-like (S-type), or intermediate (I). These cell types can undergo spontaneous or induced transdifferentiation in vitro. We investigated the complement sensitivity of different neuroblastoma cell lines and of matched sets of cloned N- and S-type neuroblastoma cell lines. Human neuroblastoma cell lines that consisted predominantly of a neuroblastic phenotype were shown to be significantly more susceptible to human complement-mediated lysis than cell lines of other cancer types. Complement sensitivity of neuroblastoma cell lines was correlated with low levels of CD59, decay-accelerating factor, and membrane cofactor protein expression. We found that cloned S-type neuroblastoma cells were much more resistant to complement-mediated lysis than cloned N-type cells. The increased complement resistance of S-type cells was shown to be due to increased expression of membrane-bound complement inhibitors. CD59 was the single most important protein in providing S-type cells with protection from complement lysis. S-type cells were also found to express lower levels of GD2, a target antigen for a complement activating monoclonal antibody currently in clinical trials for neuroblastoma immunotherapy. The ability of S-type cells to evade complement, and the ability of S-type cells to differentiate into the more tumorigenic N-type cells, may represent a mechanism of tumor survival and regrowth, a phenomenon often observed with this cancer.
Neuroblastoma is one of the most common extracranial solid tumors of children and is often lethal in patients who present with metastatic disease. Although these tumors may respond well to chemotherapy and spontaneous tumor regression is sometimes observed, neuroblastoma has a propensity to recur, sometimes after long periods of quiescence, eventually killing the patient. The molecular mechanisms underlying disease progression and tumor regrowth are not well understood. 1
Neuroblastomas exhibit diverse morphologies with tumors composed generally of a mixture of neuroblasts, ganglion cells, Schwann-like cells, and stromal cells. Since the initial description of distinct N- and S-type cells by Biedler et al, subclones have been derived from established neuroblastoma cell lines. 2 Cells with intermediate morphology (I-type) have also been cloned, and the three subtypes can interconvert or transdifferentiate either spontaneously or after chemical induction. In vivo correlates of these various clonal subtypes have not been definitively determined, but it is generally believed that S-type cells exist and may be masquerading as stromal or Schwann-like cells. Although some stromal cells in human neuroblastoma may derive from normal tissues, the presence of S-type cells in human neuroblastoma is a real possibility.
It is not clear how transdifferentiation between the different morphological phenotypes might modify tumor behavior and response to treatment. It has been hypothesized that S-type cells represent a more differentiated benign cell type and that tumor regression, either spontaneous or as a result of therapy, may parallel transdifferentiation from N to S cells. 2 It is also possible that S-type cells, and their ability to differentiate to more tumorigenic N cells, represent an important link between tumor regression and frequently observed tumor recurrence. There have been many studies on N and S cell differentiation and on the molecular basis for N cell tumorigenicity. However, these studies have not addressed the relative resistance of the cell types to anti-tumor reagents and to host defense mechanisms.
Complement resistance is likely to play an important role in tumor cell survival, and may contribute to tumor cell escape from immune surveillance and present obstacles to effective antibody-mediated immunotherapy. The Complement effector systems involved in the immune response to tumor cells include amplification of inflammatory response, recruitment and activation of immune effector cells and direct complement-mediated cytolysis. Complement activation is controlled on the surface of host cells by the membrane-bound proteins decay-accelerating factor (DAF), membrane cofactor protein (MCP), and complement receptor 1 (CR1). These proteins inhibit formation of the C3 convertase, an enzymatic complex that amplifies the complement cascade. The terminal complement pathway is inhibited by membrane-bound CD59, which binds to the assembling membrane attack complex (MAC or C5b-9) and prevents cytolysis. CD59, together with DAF and/or MCP, is expressed by almost all primary tumors and tumor cell lines that have been examined; they are often up-regulated on tumor cells. In this study we investigate the expression of complement inhibitors by various neuroblastoma cell types and the susceptibility of these cells to complement-mediated lysis.
Materials and Methods
Cell Lines
SK-N-ER is a neuroblastoma cell line established at Memorial Sloan-Kettering Cancer Center. LAN-1 neuroblastoma cell line was obtained from Dr. Robert Seeger of the University of California-Los Angeles. Seven clones of the neuroblastoma cell line LAN-1 were derived as previously described, 3 and the derived N-type and S-type cloned cell populations (55N, 5S, 66N, 6S) were kindly provided by Dr. Robert Ross, Fordham University (New York, NY). NMB-7 (neuroblastoma) was provided by Dr. Liao of McMaster University (Hamilton, ON). The melanoma cell line HTB-63 was provided by Dr. A. N. Houghton (Memorial Sloan-Kettering Cancer Center). The ovarian cell line SKOV3 was provided by Dr. M. L. Disis (University of Washington, Seattle, WA). The breast cancer cell line BT474 was purchased from the American Type Culture Collection. HTB-63 and SKVO3 were maintained in McCoy’s S5A medium (GIBCO BRL, Grand Island, NY) containing 10% fetal calf serum. All other cell lines were passaged in RPMI 1640 media supplemented with 10% heat-inactivated defined bovine calf serum (Hyclone, Logan, UT), 2 mmol/L glutamine. All media contained 100 U/ml of penicillin and 100 μg/ml of streptomycin and incubation was at 37°C in 5% CO2.
Antibodies and Complement
Rabbit antisera to tumor cell membranes used to sensitize the various tumor cell lines to complement were prepared by standard techniques. 4 Cell membranes of each cell line were prepared by Dounce homogenization of cells in hypotonic media (10 mmol/L sodium phosphate, pH 8) and subcellular fractionation to remove nuclei and mitochondria. Anti-GD2 3F8 monoclonal antibody 5 and the tumor-selective 8H9 monoclonal antibody 6 were described previously. Anti-human CD59 monoclonal antibody YTH53.1 7 was a gift from Dr. B. P. Morgan (University of Wales, Cardiff, UK), anti-DAF polyclonal antibody and monoclonal antibody 1H4 8 were gifts from Dr. T. Kinoshita (Osaka University, Osaka, Japan) and anti-MCP monoclonal antibody M75 9 was a gift of Dr. D. M. Lublin (Washington University, St. Louis, MO). Anti-DAF monoclonal antibody 1A10 was described previously. 8 F(ab)2 antibody fragments of anti-CD59 YTH53.1 and anti-DAF 1H4 were prepared by pepsin digestion using an F(ab)2 preparation kit from Pierce (Rockford, IL) according to supplied instructions. FITC-conjugated antibodies used for flow cytometry were purchased from Sigma (St. Louis, MO). Normal human serum was obtained from the blood of healthy volunteers in the laboratory and stored in aliquots at −70°C until use.
Flow Cytometry and Western Blot Analyses
Analysis of cell surface protein expression was performed by flow cytometry using appropriate antibodies as previously described. 10 Isotype-matched control antibodies were used in experiments. Anti-DAF Western blotting was performed on cell membrane preparations that were prepared as described above. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed as described in a previous study that analyzed DAF expression on neuroblastoma cell lines. 11 The anti-DAF monoclonal antibody 1H4 was used for Western blot analysis. Membrane preparations from the equivalent of approximately 3 × 10 5 cells were loaded per lane for the neuroblastoma cell lines and from the equivalent of approximately 1 × 10 5 cells for SKOV3; the Western blot data shown are qualitative and were obtained to confirm data on DAF expression obtained from flow cytometry.
Complement Lysis Assays
Complement-mediated cell lysis was determined by a standard 51Cr release assay. 12 Briefly, cells at 50 to 80% confluency were detached with versene/EDTA (Gibco), washed once, and resuspended in Eagle’s minimal essential medium (EMEM)/10% heat-inactivated fetal calf serum. Cells were preloaded with51Cr at a concentration of 1 × 107/ml (2 hours/37°C), washed in complete media and resuspended to 1 × 106/ml. Rabbit anti-cell membrane antisera at a final concentration of 10% diluted in EMEM/10% fetal calf serum, or monoclonal antibody 3F8 at 15 μg/ml was added and the cells incubated on ice for 30 minutes. Cells were centrifuged and resuspended to 1 × 106/ml in EMEM/10% fetal calf serum. Equal volumes of cells and serum dilutions were incubated for 60 minutes at 37°C, and cell lysis determined by measuring released radioactivity. In some experiments, lysis was determined by trypan blue exclusion 13 with similar results. Complement lysis assays of neuroblastoma cell lines were also performed using monoclonal antibody 8H9 together with anti-IgG1 polyclonal antibody to sensitize cells to complement. Monoclonal antibody 8H9 is IgG1 and recognizes a tumor-selective surface antigen on neuroblastoma cells. 6 Cells were incubated first with 8H9 at 10 μg/ml for 30 minutes at 4°C, and purified rabbit anti-mouse IgG1 polyclonal antibody at 15 μg/ml (Sigma) was then added. A secondary anti-IgG1 complement activating antibody was necessary because mouse IgG1 does not activate complement. At similar antibody concentrations, similar levels of anti-IgG1 bound to both 5S and 55N cells as determined by flow cytometry (see Results).
The effect of anti-complement inhibitor blocking antibodies and F(ab)2 fragments on complement-mediated lysis was performed essentially as described. 14-16 The function blocking activity of anti-CD59 YTH53.1, 14,17 anti-DAF 1H4 15 and anti-MCP M75 16 has been previously characterized. Cells were preincubated with 50 μg/ml blocking antibody or F(ab)2 fragment for 30 minutes before the addition of sensitizing antibody, and lysis was then determined. Complement inhibitor blocking experiments were performed with whole antibodies, and for YTH53.1 and 1H4, with F(ab)2 fragments. The results were essentially similar whether whole antibody or F(ab)2 fragments were used.
Results
Sensitivity of Neuroblastoma and Other Cancer Cell Lines to Lysis by Complement
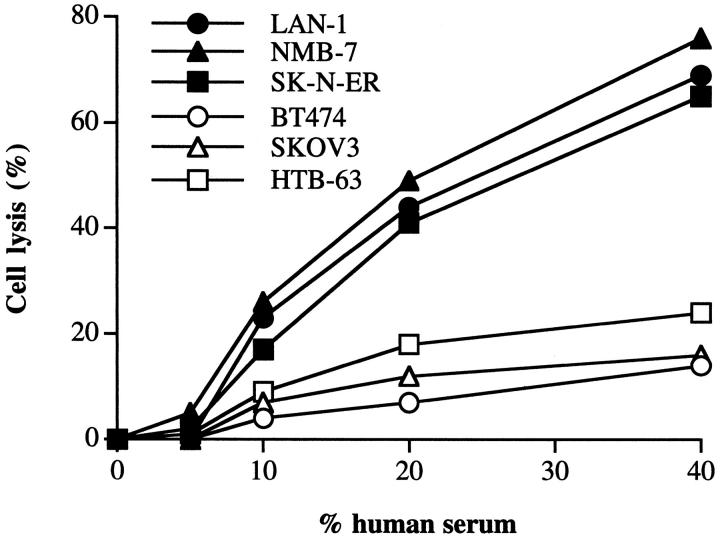
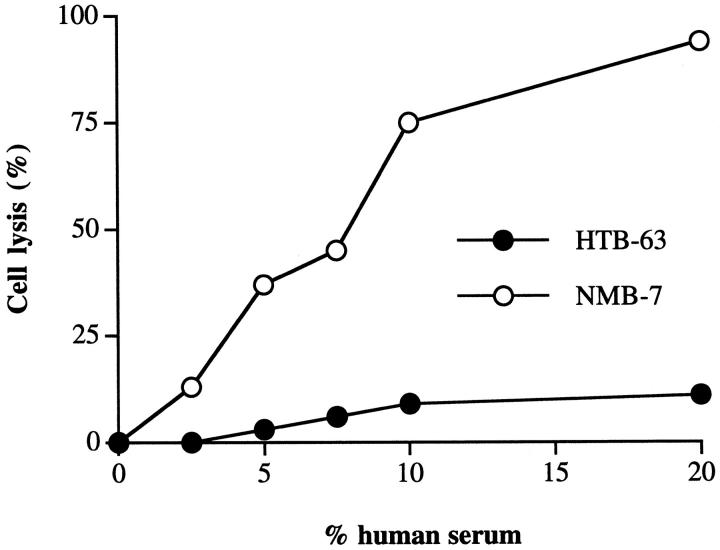
Three neuroblastoma cell lines and a representative cell line from three other types of cancer were assayed for their sensitivity to human serum. All of the neuroblastoma cell lines tested were effectively lysed by human complement (Figure 1) ▶ . In contrast, the other cancer cell lines were relatively complement-resistant, even at high concentrations of human serum. This finding is generally consistent with studies using various other cancer cell lines. Rabbit antisera raised against membrane preparations from each cell line were used to sensitize the tumor cells to complement, and at the antiserum concentration used in complement lysis assays, all cell lines stained with a similar saturating mean fluorescence when analyzed by flow cytometry. However, it is possible that differences in the sensitizing antibodies may account for the difference in the observed lysis. The antisera may also contain antibodies against membrane complement inhibitors that may bias the results, although we could not detect purified CD59 on a Western blot using the antisera (not shown). For this reason, we compared the complement susceptibility of HTB-63 (melanoma) and NMB-7 (neuroblastoma) using the anti-GD2 monoclonal antibody, 3F8, as sensitizing antibody. These two cell lines were found to exhibit a similar mean fluorescence when stained by means of 3F8 (HTB-63 = 486, NMB-7 = 437), but the melanoma cell line was considerably more resistant to lysis by human complement when sensitized with 3F8 (Figure 2) ▶ , consistent with data obtained using polyclonal antisera. Also, the relative sensitivities of the cell lines to complement was the same when various other complement activating anti-tumor antigen monoclonal antibodies were used to sensitize tumor cells (anti-HER2 for BT474 and SKOV3 cell lines that both express high levels of the HER2/erbB2 antigen, anti-GD3 for HTB-63, and anti-GD2 for neuroblastoma cell lines), although antigen density and relative binding of the different antibodies was not quantitated (data not shown).
Figure 1.
Lysis of tumor cell lines by human complement. Cells were sensitized to complement by preincubation in 10% anti-membrane rabbit antiserum. Sensitized cells were washed, exposed to the indicated concentration of human serum (37°C/60 minutes), and cell lysis determined. Either the omission of sensitizing antibody or the use of heat-inactivated human serum in cell lysis assays resulted in a background lysis of <10% of test value. Figure ▶ shows representative data from three separate experiments.
Figure 2.
Lysis of anti-GD2-sensitized HTB-63 and NMB-7 by human complement. Cells were sensitized to complement by preincubation in anti-GD2 3F8 monoclonal antibody at 15 μg/ml. Sensitized cells were washed, exposed to the indicated concentration of human serum (37°C/60 minutes), and cell lysis determined. ▶ shows representative data from three separate experiments.
Expression of Complement Inhibitors
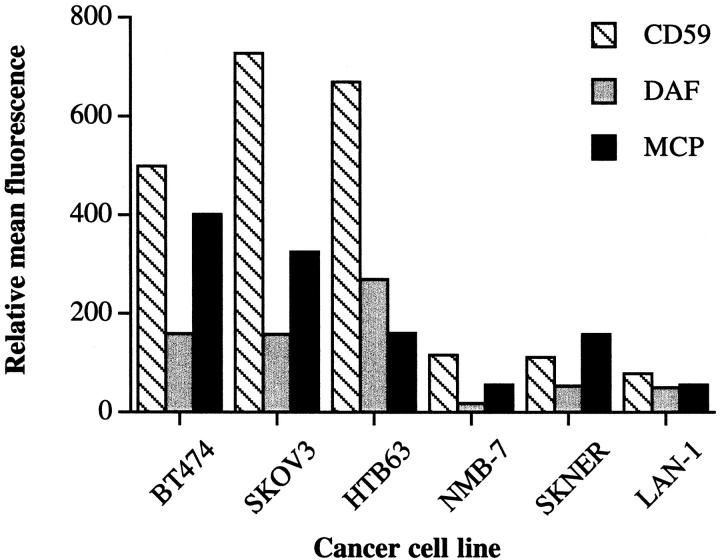
Each cell line was assayed for relative expression of membrane-bound complement inhibitors by flow cytometry. Data in Figure 3 ▶ show that the relative expression of CD59, MCP, and DAF on neuroblastoma cell lines were all low compared to expression of these complement inhibitory proteins on the other cancer cell types. Thus, the complement resistance of neuroblastoma and the other cancer cell lines correlated with the relative overall expression levels of complement inhibitors. Of note, we detected DAF expression on the surface of the neuroblastoma cell lines, whereas previous studies have failed to detect expression of DAF on various neuroblastoma cell lines. 11,18
Figure 3.
Surface expression of complement inhibitory proteins on tumor cell lines. Cells were stained by immunofluorescence using monoclonal antibodies to human CD59 (YTH53.1), MCP (M75), and DAF (1H4) as primary antibodies. ▶ shows relative mean fluorescence by flow cytometric analysis. Isotype-matched antibodies of irrelevant specificity were used for controls.
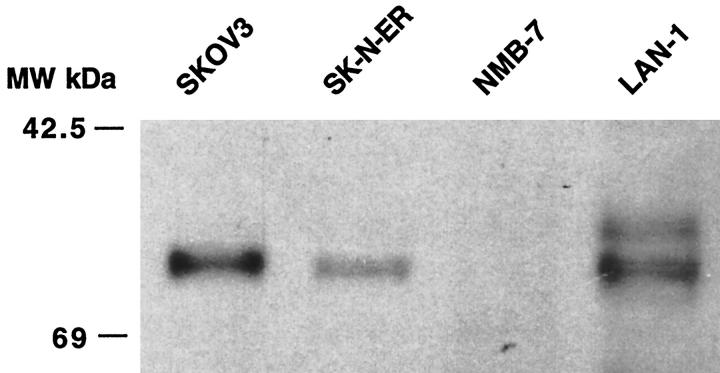
To ensure that the 1H4 anti-DAF monoclonal antibody that we used for flow cytometry was not cross-reacting with a neuroblastoma cell surface antigen, we performed flow cytometric analysis using different anti-DAF antibodies (1A10 monoclonal antibody and anti-DAF polyclonal); the same relative levels of DAF expression were found (not shown). To further confirm our data, we also performed anti-DAF Western blot analysis of neuroblastoma cell membranes. Figure 4 ▶ shows the presence of DAF of the expected molecular weight (60 kd) in LAN-1 and SK-N-ER neuroblastoma cell membranes. We did not detect DAF in NMB-7 membrane preparations by this method, but the level of DAF expression by this cell line as indicated from flow cytometry was extremely low. The second band of lower molecular weight reacting with anti-DAF antibody observed in the LAN-1 membrane may be a degradation product.
Figure 4.
Western blot analysis of DAF expression by tumor cell lines. Membrane preparations from the indicated neuroblastoma cell lines and the SKOV3 ovarian cancer cell line were analyzed by Western blot using anti-DAF monoclonal antibody 1H4.
Analysis of N- and S-Type Cells
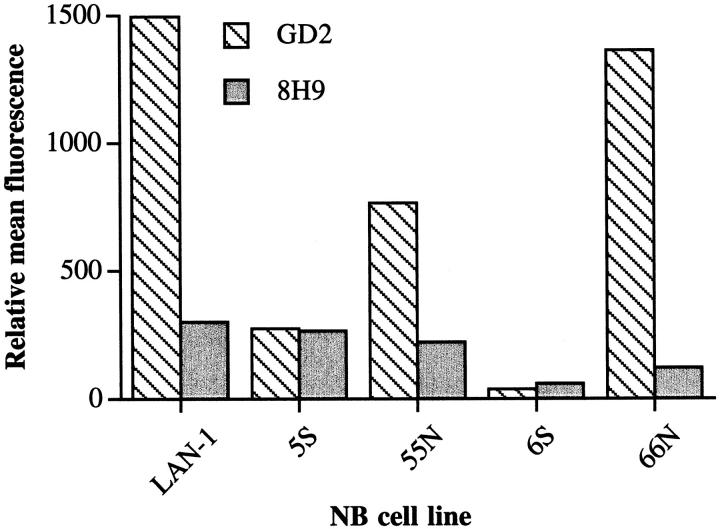
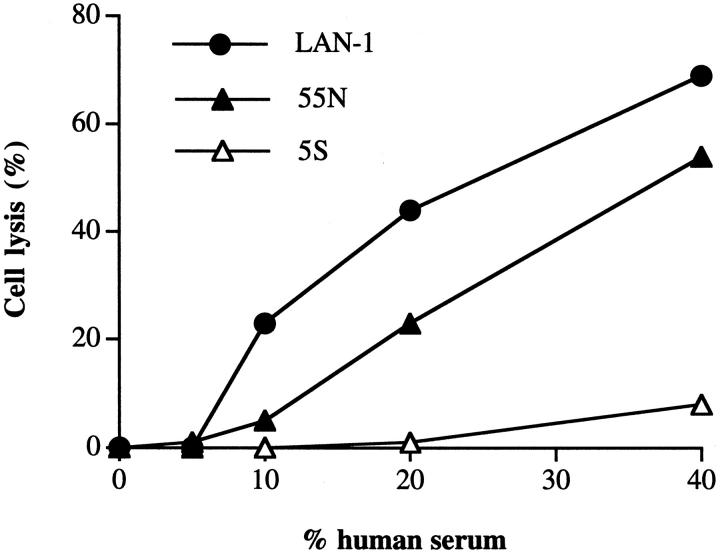
As noted, neuroblastoma consists of diverse morphologies. We next analyzed cloned matched sets of N- and S-type cells, derived from the LAN-1 cell line, for their susceptibility to complement-mediated lysis. We wished to use the clinically relevant antibody 3F8 to sensitize the N and S type cells to complement. 3F8 is a complement activating antibody currently in clinical trials that recognizes GD2, an antigen overexpressed on neuroblastoma. However, S cells express significantly lower levels of GD2 relative to N type cells and the parental LAN-1 cell line (Figure 5) ▶ . 19 This finding means that 3F8 is not a suitable complement-activating antibody for comparing the complement sensitivity of N and S cells, but also has possible implications regarding the survival of S type cells in vivo after 3F8 immunotherapy. A different tumor-selective antibody, 8H9, 6 was found to stain LAN-1 and the matched set of 5S and 55N cells with a similar mean fluorescence when analyzed by flow cytometry (Figure 5) ▶ . 8H9 recognizes an undefined tumor-selective antigen and is a candidate for antibody-targeted therapies. 6 Therefore, 8H9 was used to target complement to the cell surface in complement lysis assays of 5S, 55N, and LAN-1. Because 8H9 is a non-complement-activating mouse IgG1 isotype, we used 8H9 together with polyclonal anti-IgG1 antibody to sensitize the cells to complement (see Materials and Methods). Figure 6 ▶ shows that 55N and the parental cell line LAN-1 were sensitive to lysis by human complement, with 55N being slightly more resistant. The 5S cells, however, were almost completely resistant to lysis by complement. The fact that the parental LAN-1 cell line is the most sensitive to complement-mediated lysis likely just represents the average of a heterogeneous population containing highly sensitive clones. Cell lines in passage become heterogeneous over time and the LAN-1 cell line has been in passage over several years, whereas the N and S cell variants were cloned out with a limited number of passages.
Figure 5.
Surface expression of tumor-associated antigens on neuroblastoma tumor cell lines. Cells were stained by immunofluorescence using monoclonal antibodies to GD2 (monoclonal antibody 3F8) and an undefined tumor-selective antigen (monoclonal antibody 8H9) as primary antibodies. ▶ shows relative mean fluorescence by flow cytometric analysis. Isotype-matched antibodies of irrelevant specificity were used for controls.
Figure 6.
Lysis of neuroblastoma tumor cell lines by human complement. Cells were sensitized to complement by preincubation with 8H9 and anti-IgG1 antibodies. Sensitized cells were then exposed to the indicated concentration of human serum (37°C/60 minutes), and cell lysis determined. Either the omission of sensitizing antibodies or the use of heat-inactivated human serum in cell lysis assays resulted in a background lysis <10% of test value. ▶ shows representative data from three separate experiments.
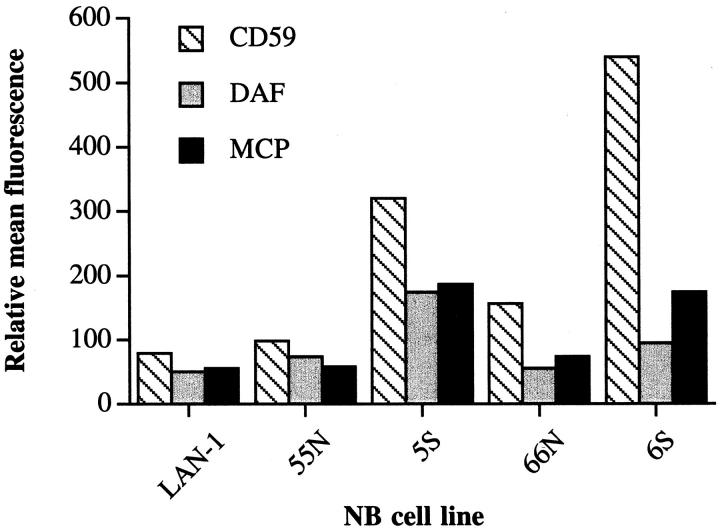
We next analyzed 5S and 55N for expression of complement inhibitors. Figure 7 ▶ shows that the intensity of staining for CD59, DAF, and MCP is considerably higher (between two- and fourfold) for complement-resistant 5S compared to complement-sensitive 55N. Similar relative staining intensities were found for another cloned set of N- and S-type cells, 6S and 66N (Figure 7) ▶ . Thus, the complement resistance of 5S compared to 55N correlated with a higher relative level of complement inhibitor expression on 5S. To establish firmly that the resistance of 5S cells to complement was due to the increased expression of complement inhibitors, the complement susceptibility of the 5S cells was determined in the presence of antibody or F(ab)2 antibody fragments that block the function of the different complement inhibitors. The data presented in Figure 8a ▶ show that blocking CD59 function on 5S enhanced complement-mediated lysis to a level comparable to that seen with 55N. Blocking DAF function had a more modest effect on enhancing complement lysis, whereas blocking MCP function had no effect. The function-blocking activity of each of the monoclonal antibodies and F(ab)2 fragments has been previously characterized (see Materials and Methods). Blocking the function of complement inhibitors on 55N also enhanced complement-mediated lysis (Figure 8b) ▶ . Blocking CD59 function on 55N cells had a particularly significant effect. As with 5S, blocking DAF function on 55N resulted in a more modest enhancement of complement lysis, and blocking MCP function had no effect. F(ab)2 fragments of the anti-CD59 and anti-DAF antibodies were used to prevent any contribution to cell lysis from complement activation by the whole antibodies. F(ab)2 fragments of the anti-MCP antibody were not tested, because the whole antibody did not enhance lysis of antibody-sensitized neuroblastoma cells.
Figure 7.
Surface expression of complement inhibitory proteins on neuroblastoma tumor cell lines. Cells were stained by immunofluorescence using monoclonal antibodies to human CD59 (YTH53.1), MCP (M75), and DAF (1H4) as primary antibodies. ▶ shows relative mean fluorescence by flow cytometric analysis.
Figure 8.
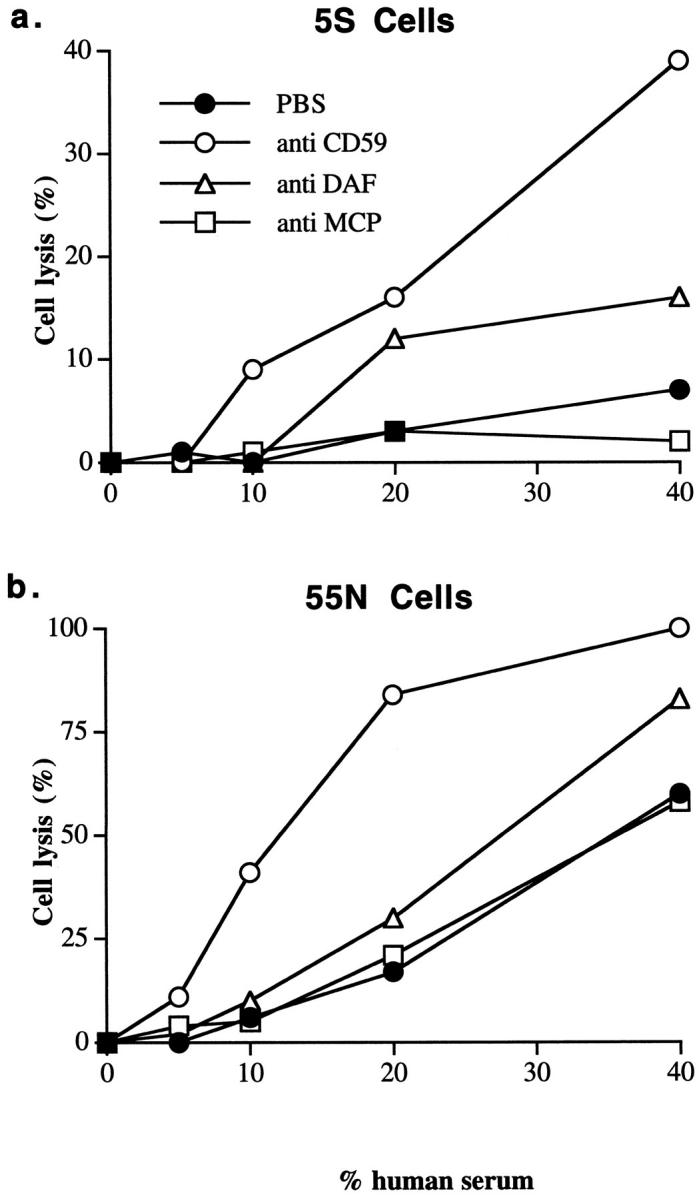
Effect of blocking the function of complement inhibitory proteins on complement-mediated lysis of 5S and 55N cells. 5S cells (a) or 55N cells (b) were preincubated with F(ab)2 fragments of anti-CD59, anti-DAF, or whole IgG anti-MCP monoclonal antibody at 50 μg/ml. Cells were then sensitized to complement, exposed to the indicated concentration of human serum (37°C/60 minutes), and cell lysis determined. Increasing the concentration of function blocking anti-complement inhibitor F(ab)2 fragments or antibody did not further enhance complement-mediated lysis. ▶ shows representative data from three experiments.
Discussion
Neuroblastoma tumors are morphologically diverse. Neuroblastic cells predominate, but nonneuronal Schwann-like cells have been observed. 20,21 When human neuroblastoma cell lines are established in culture they spontaneously give rise to heterogeneous populations of neuroblastic (N-type) and Schwann-like (S-type) cells that have distinct biochemical and morphological characteristics. 2,22,23 N-type cells predominate in established neuroblastoma cell lines. The N- and S-type cells observed in vitro may have in vivo correlates.
S cells have limited growth potential in vivo and in vitro. 23,24 It has been suggested that S cells represent a more differentiated state, and that N-to-S differentiation parallels in vivo differentiation and tumor regression. 2 However, the survival of S-type cells in vivo and their ability to differentiate back into tumorigenic N-type cells may represent a mechanism of tumor cell survival and regrowth. In this context, complement evasion may be an important mechanism of survival from immune surveillance effector mechanisms or from antibody-mediated immunotherapy. We compared a matched set of N-type and S-type cell clones (5S and 55N) for their resistance to human complement, and found that S cells were much more resistant to complement-mediated lysis. Compared to the N cell clone, the S cells were found to express significantly increased levels of all three major membrane-bound inhibitors of complement. Similar relative levels of complement inhibitor expression were found on a second cloned set of S and N cells (6S and 66N). Additional data established that the increased expression level of complement inhibitors on the 5S cells was responsible for their increased resistance to complement lysis.
Results from experiments in which the function of each complement inhibitor was individually blocked indicated that CD59 was the most effective single molecule at providing protection from complement-mediated lysis. Of the two inhibitors of complement activation (DAF and MCP), only DAF neutralization enhanced complement-mediated lysis of S cells, albeit less than CD59 neutralization. Nevertheless, CD59, unlike DAF and MCP, does not directly effect complement activation and the generation of C3 and C5 activation products. It should be noted that these complement activation products, either deposited on the cell surface (C3 fragments) or released as soluble inflammatory mediators (C3a and C5a), may be important for promoting or enhancing cell-mediated cytotoxic mechanisms in vivo.
The complement susceptibility of neuroblastoma may be a significant factor in the outcome of neuroblastoma immunotherapy using unmodified monoclonal antibodies, and in this respect, the anti-GD2 monoclonal antibody 3F8 has proven relatively successful in clinical trials. 25,26 The role of S-type cells in neuroblastoma is not clear, but the low level of GD2 expression and the high levels of complement inhibitor expression on S-type cells may provide a mechanism for their survival from anti-GD2 and complement-mediated immunotherapy. Of further interest, data show that S cells are also more resistant to the cytostatic and cytotoxic effects of radiation and anthracyclines (N. K. V. Cheung, unpublished data). GD2 and complement inhibitor expression levels on S cells may also have implications for diagnostic procedures and bone marrow purging.
The complete elimination of S cell types may be important for long-term patient survival, and tumor regrowth may be related to the ability of S-type cells to survive and subsequently transdifferentiate into the more tumorigenic N-type. Differential antigen expression by S-type cells and their increased complement resistance may provide the basis for the ability of neuroblastoma to survive as microscopic residual disease.
Footnotes
Address reprint requests to Stephen Tomlinson, Ph.D., New York University School of Medicine, Department of Pathology, MSB 126, 550 First Avenue, New York, NY 10016. E-mail: tomlis01@popmail.med.nyu.edu.
Supported by National Institutes of Health grants AI 34451 and CA16087 and Department of the Army grant DAMD179717273.
References
- 1.Brodeur GM, Castleberry RP: Principles and practice in pediatric oncology. 1997:pp 761-797 JB Lippincott Company, Philadelphia
- 2.Biedler JL, Spengler BA, Ross RA: Human neuroblastoma cell differentiation. Principles and Practice of Genitourinary Oncology. 1999, :pp 1053-1061 Lippincott-Raven, Philadelphia [Google Scholar]
- 3.Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA: Phenotype diversification in human neuroblastoma cells; expression of distinct neural crest lineages. Cancer Res 1989, 49:219-225 [PubMed] [Google Scholar]
- 4.Harlow E, Lane D: Antibodies: A Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory, 1988
- 5.Cheung N-KV, Saavinen UM, Neely JE, Landmeier B, Donovan D, Coocia PF: Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res 1985, 45:2642-2650 [PubMed] [Google Scholar]
- 6.Modak SI, Gultekin SH, Kramer K, Guo HF, Rosenfeld MR, Ladanyi M, Larson SM, Cheung N-KV: Novel tumor-associated surface antigen: broad distribution among neurectodermal, mesenchymal and epithelial tumors, with restricted expression among normal tissues. Proc Am Soc Clin Oncol 1998, 17:445a(abstr) [Google Scholar]
- 7.Davies A, Simmons DL, Hale G, Harrison RA, Tighe H, Lachmann PJ, Waldmann H: CD59, an Ly-6 protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex of homologous cells. J Exp Med 1989, 170:637-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita T, Medof ME, Silber R, Nussenzweig V: Distribution of decay-accelerating factor in peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med 1985, 162:75-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seya T, Hara T, Matsumoto M, Akedo H: Quantitative analysis of membrane cofactor protein (MCP) of complement. J Immunol 1990, 145:238-245 [PubMed] [Google Scholar]
- 10.Yu J, Abagyan RA, Dong S, Gilbert A, Nussenzweig V, Tomlinson S: Mapping the active site of CD59. J Exp Med 1997, 185:745-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasque P, Thomas A, Fontaine M, Morgan BP: Complement activation on human neuroblastoma cell lines in vitro: route of activation and expression of functional complement regulatory proteins. J Neuroimmunol 1996, 66:29-40 [DOI] [PubMed] [Google Scholar]
- 12.Helfand SC, Hank JA, Gan J, Sondel PM: Lysis of human tumor cell lines by canine complement plus monoclonal antiganglioside antibodies or natural cannine xenoantibodies. Cell Immunol 1996, 167:99-107 [DOI] [PubMed] [Google Scholar]
- 13.Rushmere NK, Tomlinson S, Morgan BP: Expression of rat CD59: functional analysis confirms lack of species specificity and reveals that glycosylation is not required for function. Immunology 1997, 90:640-646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakulinen J, Meri S: Expression and function of the complement membrane attack complex inhibitor protectin (CD59) on human breast cancer cells. Lab Invest 1994, 71:820-827 [PubMed] [Google Scholar]
- 15.Coyne KE, Hall SE, Thompson S, Arce MA, Kinoshita T, Fujita T, Antsee DJ, Rosse W, Lublin DM: Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol 1992, 149:2906-2913 [PubMed] [Google Scholar]
- 16.Seya T, Hara T, Matsumoto M, Sugita Y, Akedo H: Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein. J Exp Med 1990, 172:1673-1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldemann H, Lachmann PJ: Human protectin (CD59), an 18–20 kD complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 1990, 72:1-9 [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung N-KV, Walter EI, Smith-Mensah WH, Ratnoff WD, Tykocinski ML, Medof ME: Decay-accelerating factor protects human tumor cells from complement mediated cytotoxicity in vitro. J Clin Invest 1988, 81:1122-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung NK, Usmani N, Cordon-Cardo C: Monoclonal antibody detection of ganglioside expression in human neuroblastoma. Gangliosides and Cancer. 1989, :pp 103-108 VCH Verlagsgesellshaft, Weinheim [Google Scholar]
- 20.Pochedly M, ed: Histogenesis and histopathology of neuroblastoma. Neuroblastoma, Clinical and Biological Manifestations. New York, Elsevier, 1982
- 21.Russell DS, Rubenstein LJ: Peripheral tumors of the neurone series. Pathology of the Nervous System. 1971, :pp 305-323 Williams and Wilkins, Baltimore [Google Scholar]
- 22.Ross RA, Spengler BA, Chang TD: Transdifferentiation of neuroblastoma cells. J Natl Cancer Inst 1983, 71:741-747 [PubMed] [Google Scholar]
- 23.Spengler BA, Lazarova DL, Ross RA, Biedler JL: Cell lineage and differentiation state are primary determinants of MYCN gene expression and malignant potential in human neuroblastoma cells. Oncol Res 1997, 9:467-476 [PubMed] [Google Scholar]
- 24.Biedler JL, Spengler BA, Tien-ding C, Ross RA: Transdifferentiation of human neuroblastoma cells results in coordinate loss of neuronal and malignant properties. Prog Clin Biol Res 1988, 271:265-276 [PubMed] [Google Scholar]
- 25.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, Bonilla MA, Finn R, Yeh SJ, Larson SM: Anti-GD2 antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol 1998, 16:3053-3060 [DOI] [PubMed] [Google Scholar]
- 26.Cheung NK, Kushner BH, Yeh SJ, Larson SM: 3F8 monoclonal antibody treatment of patients with stage IV neuroblastoma: a phase II study. Int J Oncol 1998, 12:1299-1306 [DOI] [PubMed] [Google Scholar]