Abstract
Enhanced hepatocyte growth factor (HGF) receptor (Met) signaling has been suggested to play an important role in the development and progression of various epithelial and nonepithelial tumors. N-terminally truncated forms of the HGF receptor have been shown to be constitutively activated and tumorigenic in animal experiments. In the present study, 102 benign and malignant human musculoskeletal tumors were examined for expression of the HGF receptor by Western blotting and/or immunohistochemistry. A clear predominance of HGF receptor expression was seen in malignant as compared to benign tumors (Western blotting, P < 0.001; immunohistochemistry, P < 0.02). For the first time we show HGF receptor expression in the following four tumor types: dermatofibrosarcoma protuberans, clear cell sarcoma of tendons, malignant primitive neuroectodermal tumor, and benign fibrous histiocytoma. In three cases of sarcoma with high HGF receptor expression by Western blotting, we found indications of a short 85-kd N-terminally truncated HGF receptor that was tyrosine phosphorylated and located in the cytoplasm. Although fragments of this length were seen in 18 of 65 tumors, most were not tyrosine-phosphorylated. Northern blotting revealed only the 7.5-kb full-length HGF receptor transcript, suggesting that the 85-kd fragment is generated by an alternative initiation of translation or by proteolytic cleavage. Southern blotting detected no amplification of the Hgfr/Met gene in the 35 tumors examined, in contrast to our recent report of Hgfr/Met gene amplification in 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat sarcomas. The present data suggest that the locally aggressive and malignant properties of human mesenchymal tumors maybe related, in part, to high levels of full-length HGF receptors, and in some cases to the occurrence of N-terminally truncated HGF receptors, activated independently of HGF.
The hepatocyte growth factor (HGF) regulates biological functions such as cell growth, cell migration, and morphogenesis by activation of its tyrosine kinase receptor Met. 1 The HGF receptor/Met is a 190-kd heterodimeric transmembrane receptor composed of a 50-kd α- and a 140-kd β-subunit. The β-subunit is a transmembrane spanning protein with an intracellular tyrosine kinase domain that can be activated by autophosphorylation of two tyrosine residues. 2 Although the Hgfr/Met proto-oncogene was originally identified as a transforming gene isolated from a carcinogen-treated human osteosarcoma cell line, 3 it is primarily expressed in epithelial cells. Many types of human carcinomas overexpress this receptor, which suggests this receptor is involved in the development and progression of epithelial tumors. 4-6
Because HGF is ubiquitously expressed in mesenchymal tissues and released to the extracellular compartment, paracrine and/or autocrine signaling would be a possible mechanism of tumorigenesis in mesenchymal tumors that express the HGF receptor. 7,8 Recent studies have shown that the Hgfr/Met proto-oncogene is frequently overexpressed in spontaneously transformed NIH/3T3 fibroblasts, 7,9 and amplified and overexpressed in chemically induced sarcomas in rat. 10 HGF receptor expression has also been reported in human mesenchymal tumor cell lines. 11,12 Furthermore, several studies have demonstrated overexpression of the HGF receptor in human nonepithelial tumors, including osteosarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, synovial sarcoma, epithelioid sarcoma, and peripheral nerve sheath tumors. 8,11-18 However, most of these studies of human musculoskeletal tumors have been based solely on immunohistochemistry and/or have dealt with a limited number of mesenchymal tumor cell lines or small series of selected tumors. 7,8,11-18 The status of the HGF receptor in several types of human mesenchymal tumors has never been studied and remains controversial for some tumors, eg, giant cell tumor (GCT) of bone. 16,18
In light of our recent report of amplification and overexpression of the HGF receptor in 7,12-dimethylbenz(a)anthracene (DMBA) induced rat sarcomas, 10 we carried out the present study to analyze the possible overexpression and/or amplification of the HGF receptor in a wide spectrum of benign and malignant human mesenchymal and neuroectodermal tumors with a range of biological behaviors using immunohistochemistry as well as Western, Northern, and Southern blotting techniques. The tumorigenicity of short intracellular non-ligand-binding autoactivated HGF receptor fragments has been shown in experimental studies of cultured cells and in transgenic mice. 19,20 This study reports, for the first time, similar autoactivated HGF receptor fragments in high grade human musculoskeletal tumors.
Materials and Methods
Tumor Specimens
Of a total of 102 bone and soft tissue tumors, 81 cases were retrieved from the Department of Pathology, Sahlgrenska University Hospital, and 21 cases from Lund University Hospital. In 65 cases, frozen tumor samples were analyzed by Western blotting. Formalin-fixed, paraffin-embedded tumor tissue from 88 cases was used for the immunohistochemical analysis. Diagnoses were based on clinical information, light microscopy, immunohistochemical analysis, and, in selected cases, electron microscopy.
Western Blotting
All Western blotting experiments were performed under reducing conditions, mainly as previously described. 10 In brief, proteins were prepared from fresh-frozen tumor samples and human liver tissue by sonication in a buffer containing 10 mmol/L K2HPO4, 10 mmol/L KH2PO4, 10 mmol/L EDTA, 6.0 mg/ml CHAPS (3-[(3-Cholamidopropyl) dimethyl ammonio]-l-propanesulfonate), and 1 Complete protease inhibitor cocktail tablet (Roche Molecular Biochemicals, Mannheim, Germany) per 25 ml of buffer. Then 50 mmol/L dithiothreitol (DTT) were added to the samples. Thirty micrograms of protein per sample were run on NuPage 10% Bis-Tris gels using a Novex Xcell II system (Novex, San Diego, CA), and then transferred to polyvinylidene difluoride membranes (Novex) by electroblotting. The membranes were blocked overnight in 10% dry milk in a TBS-Tween buffer (50 mmol/L Tris-HCl, 137 mmol/L NaCl, 0.1% Tween 20).
Antibodies
Blots were probed with two different rabbit polyclonal anti-Met antibodies (C-28 and C-12, Santa Cruz Biotechnology, Santa Cruz, CA) raised against peptides corresponding to the carboxyl terminus (amino acids 1366–1390 and 1379–1390, respectively) of the human HGF receptor. Thus, both C-terminal anti-Met antibodies were directed against domains of the carboxyl terminus of the HGF receptor distal to the tyrosine kinase domain, located approximately at amino acids 1100–1350. 21,22 An anti-rabbit immunoglobulin F(ab′)2 fragment antibody conjugated with horseradish peroxidase (Amersham Life Science, Buckinghamshire, UK) was used for detection of the primary antibodies. The membranes were washed and exposed to the enhanced chemiluminescence detection system (ECL+, Amersham Life Science). The specificity of the primary anti-Met antibody (C-28) was confirmed by incubating tumor samples with different concentrations of human Met blocking peptide (sc-161P; Santa Cruz Biotechnology). The primary antibodies were stripped from the membranes in a stripping buffer containing 62.5 mmol/L Tris, 2% sodium dodecyl sulfate (SDS), and 100 mmol/L β-mercaptoethanol. The membranes were blocked in 3% bovine serum albumin and reprobed with a mouse monoclonal anti-phosphotyrosine IgG (PY 20; Santa Cruz Biotechnology). A different secondary anti-mouse F(ab′)2 fragment antibody was used for the second probing (Amersham Life Science). A mouse monoclonal antibody raised against the extracellular region of human Met 23 (DL-21; Upstate Biotechnology, Lake Placid, NY) was used for Western blotting of the subcellular protein preparations from the tumors (see below).
Subcellular Protein Preparation
One ml of buffer containing 0.154 mol/L KCl and 0.01 mol/L K2HPO4 (pH 7.4) with 1 tablet of Complete per 25 ml of buffer was added to approximately 250 μg of tumor tissue before homogenization. Then the samples were centrifuged at 12,000 × g for 30 minutes at 4°C. The pellets were resuspended and used as membranous fractions. The supernatants containing cytoplasmic proteins were transferred to new tubes and ultracentrifuged at 105,000 × g for 1 hour to remove microsomal proteins from the cytosolic preparations.
Densitometry of Western Blottings
The Western blots were scanned, and relative levels of HGF receptors in individual samples was estimated using the Image Quant software (Molecular Dynamics, Sunnyvale, CA) and compared to the HGF receptor level in normal liver tissue. Each tumor was assigned one of the following values: +, mild; ++, moderate; or +++, strong positivity. Normal liver value was set as +++.
Immunohistochemical Analysis
After deparaffinization, tissue sections were treated in a microwave oven for conventional antigen retrieval and then immunostained for human Met using a NexES automated staining system (Ventana, Tucson, AZ). A standard avidin-biotin complex method was used, and the sections were incubated with an anti-human Met antibody, C-28, at a dilution of 1:100 at 37°C. Appropriate positive and negative controls were included in this analysis. Cases with unequivocal cytoplasmic staining were regarded as positive, while cases with faint, diffuse background staining of cellular as well as extracellular structures were regarded as negative.
Southern Blotting
Southern blotting was performed as previously described 10 with minor modifications. In brief, high molecular weight genomic DNA was extracted from 35 tumor samples and human liver tissue using an extraction kit (Genepure, Perkin-Elmer, Foster City, CA). Ten micrograms of DNA were digested with EcoRI and loaded on agarose gels. After electrophoresis, the DNA was transferred to membranes and probed with a radioactively labeled HGF receptor probe. 21
Northern Blotting
Total RNA was extracted from homogenized frozen tumor samples and human liver tissue using a guanidium thiocyanate-phenol-chloroform method. 24 The RNA was size-fractionated on 1% agarose-formaldehyde gels and then transferred to nylon filter membranes (Hybond N+, Nycomed Amersham plc, Buckinghamshire, UK) in 10× SSC. The membranes were prehybridized in a hybridization buffer (5× SSC, 10 mmol/L NaPO4, 10× Denhard, 30 mg/ml salmon sperm DNA, 2% SDS) at 45°C for 3 hours and then hybridized with a 32P-labeled cDNA probe for the Hgfr/Met gene for 18 hours at 45°C. Finally, the filters were washed in 1× SSC containing 0.1% SDS at room temperature for 2 × 15 minutes and in 0.1× SSC containing 0.1% SDS for 2 × 15 minutes at 45°C. Signals were detected autoradiographically.
Results
Both the Western blotting and the immunohistochemical staining results indicated an increased frequency and amount of HGF receptors in malignant bone and soft tissue tumors compared to benign ones (Table 1) ▶ . In Western blotting under reducing conditions, 8/21 benign tumors (38%) and 35/44 malignant tumors (80%) were positive for the 140-kd band corresponding to the native HGF receptor β-chain. Immunohistochemistry showed similar results: 16/29 benign tumors (55%) and 47/59 malignant tumors (80%) were HGF receptor-positive. The higher frequency of HGF receptor expression in the malignant versus benign tumors was assessed statistically using a χ 2 test and found to be significant for both Western blotting and immunohistochemistry (Table 1) ▶ .
Table 1.
Comparison in HGF Receptor Expression between Benign and Malignant Bone and Soft Tissue Tumors
| Tumor type | Immunohistochemistry | Western blot | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | |
| Benign | 16 | 13 | 29 | 8 | 14 | 21 |
| Malignant | 47 | 12 | 59 | 35 | 9 | 44 |
| χ2 value (P value) | 5.733 (<0.02) | 12.428 (<0.001) |
In the immunohistochemical study (Table 2) ▶ , the 16 HGF receptor-positive benign tumors included 5 benign fibrous histiocytomas, 2 desmoid tumors, 2 schwannomas, 1 fibrous hamartoma of infancy, and 6 giant cell tumors (GCT) of bone. Thus, desmoid and GCT of bone comprised 8/16 of the HGF receptor-positive benign tumors. Four of the eight HGF receptor-positive cases in Western blotting were GCT of bone or desmoid (Table 2) ▶ . Although these two tumor types are classified as benign, they are clinically characterized by locally aggressive behavior with a propensity for local recurrences and, in the case of GCT of bone, rare metastases. Thus, many of the benign tumors that express HGF receptors are comparatively aggressive. The intensity of HGF receptor expression measured by densitometry was relatively higher in malignant as compared to benign tumors, and was especially marked in high grade tumors, such as malignant fibrous histiocytoma, malignant peripheral nerve sheath tumor, and clear cell sarcoma (Table 2) ▶ . With the exception of three benign lesions, the results of Western blotting were in complete agreement with the immunohistochemical findings (Table 2) ▶ .
Table 2.
Full-Length HGF Receptor Expression in Human Soft Tissue and Bone Tumors
| Tumors (n) | Immunohistochemistry | Western blot | Relative expression* | ||
|---|---|---|---|---|---|
| + | ++ | +++ | |||
| Benign soft tissue tumors | |||||
| Benign fibrous histiocytoma (6) | 5 /6 | ND | |||
| Desmoid (6) | 2 /6 | 1 /5 | 1 | 0 | 0 |
| Lipoma (3) | 0 /1 | 0 /2 | 0 | 0 | 0 |
| Schwannoma (3) | 2 /3 | 3 /3 | 2 | 0 | 1 |
| Neurofibroma (2) | 0 /2 | 0 /2 | 0 | 0 | 0 |
| Hibernoma (2) | 0 /1 | 0 /2 | 0 | 0 | 0 |
| Hemangioma (2) | 0 /2 | 0 /2 | 0 | 0 | 0 |
| Fibrous hamartoma of infancy (1) | 1 /1 | 0 /1 | 0 | 0 | 0 |
| Intramuscular myxoma (1) | 0 /1 | 1 /1 | 1 | 0 | 0 |
| Subtotal (26) | 10 /23 (43%) | 5 /18 (28%) | 4 | 0 | 1 |
| Benign bone tumors | |||||
| Giant cell tumor (GCT) of bone (6) | 6 /6 (100%) | 3 /3 (100%) | 3 | 0 | 0 |
| Total benign tumors (32) | 16 /29 (55%) | 8 /21 (38%) | 7 | 0 | 1 |
| Malignant soft tissue tumors | |||||
| Malignant fibrous histiocytoma (MFH) (14) | 7 /8 | 13 /14 | 7 | 3 | 3 |
| Liposarcoma (11) | 8 /9 | 6 /9 | 3 | 3 | 0 |
| Dermatofibrosarcoma protuberans (6) | 2 /6 | 1 /1 | 1 | 0 | 0 |
| Angiosarcoma (5) | 4 /5 | ND | |||
| Leiomyosarcoma (4) | 2 /4 | 1 /1 | 1 | 0 | 0 |
| Extraskeletal myxoid chondrosarcoma (4) | 4 /4 | 1 /1 | 1 | 0 | 0 |
| Clear cell sarcoma (3) | 3 /3 | 1 /1 | 0 | 0 | 1 |
| Epitheloid sarcoma (2) | 2 /2 | ND | |||
| Malignant peripheral nerve sheath tumor (2) | 2 /2 | 2 /2 | 0 | 1 | 1 |
| Fibrosarcoma (1) | 1 /1 | 1 /1 | 1 | 0 | 0 |
| Malignant hemangiopericytoma (1) | 0 /1 | 0 /1 | 0 | 0 | 0 |
| Primitive neuroectodermal tumor (1) | 1 /1 | 1 /1 | 1 | 0 | 0 |
| Extraskeletal osteosarcoma (1) | Not detected | 0 /1 | 0 | 0 | 0 |
| Subtotal (55) | 36 /46 (78%) | 27 /33 (82%) | 15 | 7 | 5 |
| Malignant bone tumors | |||||
| Chondrosarcoma (6) | 5 /5 | 4 /6 | 2 | 1 | 0 |
| Osteosarcoma (5) | 3 /4 | 1 /2 | 1 | 0 | 0 |
| Chordoma (4) | 3 /4 | 3 /3 | 1 | 2 | 0 |
| Subtotal (15) | 11 /13 (85%) | 8 /11 (73%) | 4 | 3 | 0 |
| Total malignant tumors (70) | 47 /59 (80%) | 35 /44 (80%) | 19 | 10 | 5 |
| Total (102) | 63 /88 (72%) | 43 /65 (66%) | 26 | 10 | 6 |
*Relative expression estimated by densitometry in Western blotting: +, mild; ++, moderate; +++, strong. ND, not detected.
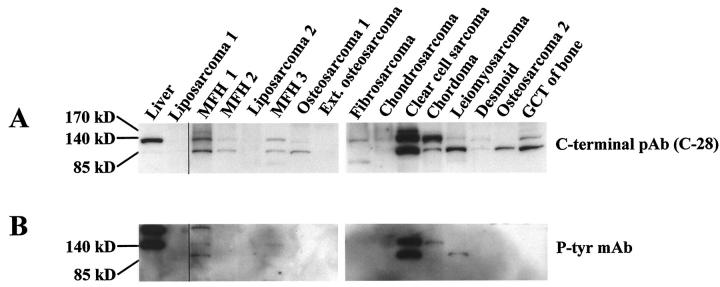
Of the 65 tumors examined by Western blotting using an antibody directed against the carboxyl terminus of the HGF receptor (C-28), 18 (28%) showed distinctive positive bands of an approximate size of 85 kd. Apart from three cases (eg, osteosarcoma 2 in Figure 1A ▶ ), the 85-kd band was detected together with the native 140-kd band that corresponds to the full-length HGF receptor β-chain. Shorter fragments less than 64 kd in size were also present in many cases independent of the presence of the native HGF receptor. After stripping of the anti-HGF receptor antibody, 3 of the 85-kd bands detected with the anti-HGF receptor antibody were also detected with the anti-phosphotyrosine antibody (MFH 1, the clear cell sarcoma, and the leiomyosarcoma in Figure 1B ▶ ). None of the other short fragments of various sizes was tyrosine-phosphorylated. A blocking experiment to test the specificity of the carboxyl terminal HGF receptor antibody (C-28) demonstrated that the blocking peptide competitively blocked the reactivity of the antibody. The blocking of the shorter 85-kd band was nearly identical to that of the full-length β-chain using three different concentrations of blocking peptide (not shown).
Figure 1.
Western blotting for the HGF receptor (A) and tyrosine phosphorylation after stripping of the anti-HGF receptor antibody C-28 (B) in various human musculoskeletal tumors and liver tissue as a positive control. Mainly two bands are visible, the 140-kd native β-chain and a shorter 85-kd fragment. In some samples a third band is seen, corresponding to the single-chain 170-kd HGF receptor precursor (A). In three tumors, the 85-kd fragment was also detected with an anti-phosphotyrosine antibody (B). MFH, malignant fibrous histiocytoma; GCT, giant cell tumor; Ext. osteosarcoma, extraskeletal osteosarcoma; pAb, polyclonal antibody; MAb, monoclonal antibody.
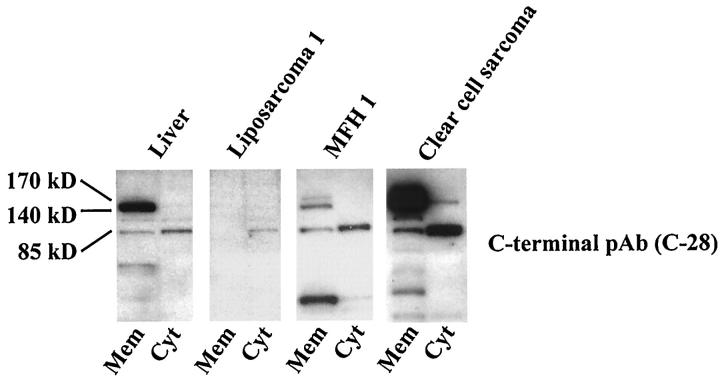
Two of the 3 sarcomas with the phosphorylated short fragments and one HGF receptor-negative tumor (liposarcoma) in Western blotting were further examined by preparation of subcellular protein fractions. By this method, the 85-kd fragments were found to be located in the cytoplasm (Figure 2) ▶ . Although the 85-kd fragment was also detectable in the cytosolic preparations of both liver tissue and the full-length HGF receptor-negative liposarcoma, the signal intensity of their short fragments was weak in contrast to that of the HGF receptor-positive sarcomas simultaneously examined (Figure 2) ▶ .
Figure 2.
Western blotting for HGF receptor expression using subcellular protein preparations of selected tumor samples with a C-terminal anti-HGF receptor antibody C-28. Note the full-length 140-kd β-chain of the HGF receptor located mainly in the membranous fraction (Mem) and the shorter 85-kd fragment located predominantly in the cytoplasmic fraction (Cyt). pAb, polyclonal antibody; MAb, monoclonal antibody.
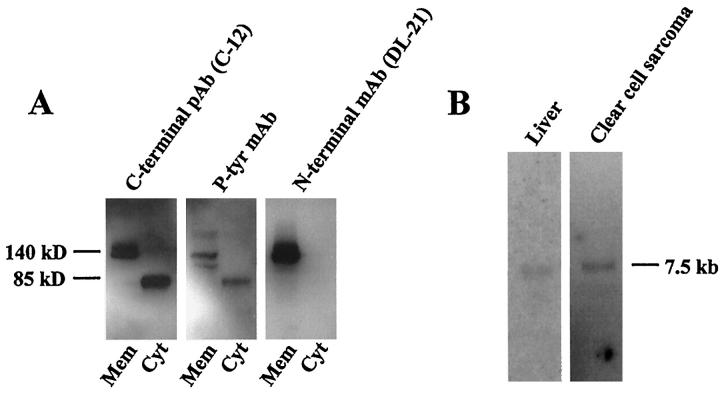
To further characterize the short cytoplasmic 85-kd HGF receptor fragment, we used another C-terminal antibody (C-12) and the phosphotyrosine antibody, for Western blotting of the strongest HGF receptor-positive tumor, a clear cell sarcoma (Figure 3A) ▶ . Both the full-length 140-kd and the short 85-kd HGF receptor fragments were recognized by this second C-terminal HGF receptor antibody (C-12), as well as by the anti-phosphotyrosine antibody (Figure 3A) ▶ . Another antibody recognizing the N-terminal, extracellular region of the HGF receptor protein detected no fragments other than the 140-kd native bands, further indicating that the 85-kd band represents an HGF receptor fragment with a truncated N-terminus rather than C-terminus (Figure 3A) ▶ .
Figure 3.
Two further HGF receptor specific antibodies were used for Western blotting of subcellular proteins from a clear cell sarcoma shown in Figure 1 and 2 ▶ ▶ (A). Both the full-length 140-kd β-chain of the HGF receptor and the 85-kd fragment were recognized by a C-terminal antibody (C-12) and by a phosphotyrosine antibody, whereas only the 140-kd β-chain was recognized by an N-terminal antibody, DL-21 (A). Northern blotting revealed only the full-length 7.5-kb HGF receptor transcript in RNA preparations from human liver and a clear cell sarcoma (B). pAb, polyclonal antibody; MAb, monoclonal antibody.
Northern blotting failed to reveal any alternative splicing variants of the gene transcript in the liver specimen or in the clear cell sarcoma that had the strongest positivity for both native and short (85-kd) forms of the HGF receptor protein (Figure 3B) ▶ . Northern blotting was not successful in other tumors displaying the shorter HGF receptor bands by Western blotting, probably due to insufficient amounts of intact mRNA.
Generally, the immunohistochemical staining for the HGF receptor was cytoplasmic and diffuse, with or without linear membranous staining (Figure 4) ▶ . The staining was most intense in the malignant tumors such as clear cell sarcoma (Figure 4, a and b) ▶ and malignant fibrous histiocytoma (Figure 4, c and d) ▶ and the locally aggressive GCT of bone (Figure 4, e and f) ▶ , and of moderate intensity in chordoma (Figure 4, g and h) ▶ as well as benign lesions. In some malignant fibrous histiocytomas, there was distinct immunostaining of mitotic figures (Figure 4d ▶ , insert).
Figure 4.
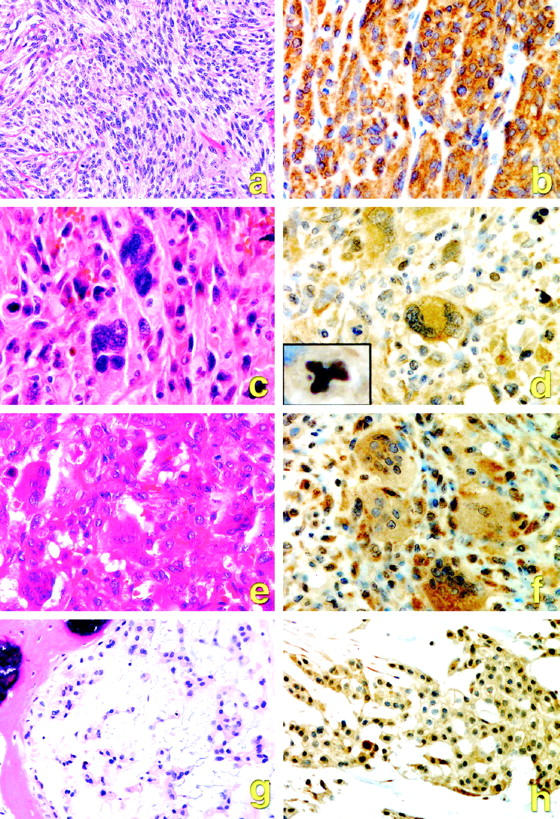
Representative morphology (a, c, e, g) and positive HGF receptor immunostaining (b, d, f, h) in human musculoskeletal tumors. a, b: Clear cell sarcoma. c, d: Malignant fibrous histiocytoma. e, f: Giant cell tumor of bone. g, h: Chordoma. Inset, d: Met positivity seen in an abnormal mitotic figure in malignant fibrous histiocytoma.
The 35 cases analyzed by Southern blotting revealed no change in the copy number of the Hgfr/Met gene, although 33 of these samples expressed high levels of HGF receptor protein by immunohistochemistry and Western blotting.
Discussion
In light of our recent report of amplification and overexpression of the HGF receptor in 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat sarcomas, 10 we carried out the present study to analyze the possible overexpression and/or amplification of the HGF receptor in human mesenchymal and neuroectodermal tumors. We systematically examined a large number of benign and malignant soft tissue and bone tumors (102 cases) and found that a statistically significantly larger proportion of malignant tumors overexpressed the HGF receptor, compared to benign tumors. Some earlier studies have demonstrated HGF receptor expression in musculoskeletal tumors. Most of these were based solely on immunohistochemistry or a limited number of mesenchymal tumor cell lines or small series of selected tumors. 7,8,11-18
In the present study, HGF receptor overexpression was observed in a wide variety of bone and soft tissue sarcomas. No clear correlation with tumor lineage or differentiation was seen. We detected HGF receptor expression in dermatofibrosarcoma protuberans, clear cell sarcoma of tendons, and malignant primitive neuroectodermal tumor as well as benign fibrous histiocytoma. These findings have not previously been described in the literature. Our study also corroborates reports of HGF receptor positivity in malignant fibrous histiocytoma, leiomyosarcoma, liposarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, epithelioid sarcoma, fibrosarcoma, osteosarcoma, chondrosarcoma, and chordoma. 8,11-18
Although HGF receptor positivity was observed in a few entirely benign lesions, most of the tumors classified as benign with HGF receptor expression are known to be locally aggressive, such as desmoid 25 and GCT of bone. 26 Desmoids are known to be locally aggressive, benign fibrous lesions characterized by rapid, infiltrative growth and a tendency toward recurrence but no metastasis. 25 GCT of bone is a locally aggressive benign tumor and shows extensive bone destruction, cortical expansion, and invasion into surrounding soft tissue. 26 Moreover, pulmonary metastases, or so-called benign pulmonary implants, may develop in approximately 1 to 2% of patients with GCT of bone. 26 HGF receptor expression was also found in some non-aggressive, benign tumors. The finding of HGF receptor expression in benign fibrous histiocytomas has not, to our knowledge, been previously described. This observation may be related to a low-level expression of HGF receptor in cultured human diploid fibroblasts. 7,12 We also detected HGF receptor expression in schwannoma, another example of a benign lesion that is, with the exception of paraspinal cellular schwannomas, a non-aggressive tumor. The detection of HGF receptor expression in both benign schwannomas and malignant peripheral nerve sheath tumors in this study is in accordance with a previous study of this receptor in peripheral nerve sheath tumors. 17 HGF has been identified as a potent mitogen for rat Schwann cells and its receptor has also been demonstrated in benign neurofibromas. 13,27 The reason why neurofibromas in this study were negative for HGF receptor expression both by immunohistochemistry and Western blotting may be related to differences in the methods used; eg, higher amounts of protein (100 μg) were loaded in the Western blotting study of Fukuda et al than in ours. 13 Neurofibromas consist of perineurial cells, fibroblasts, and varying numbers of Schwann cells. This could also explain differences in the level of HGF receptor expression between neurofibromas and schwannomas, particularly if HGF receptor expression occurs predominantly in the Schwann cells of benign peripheral nerve sheath tumors.
HGF, the ligand, is ubiquitously expressed in mesenchyme-derived cells. 1 The production of HGF in fibroblasts and other cell types can be up-regulated by inflammatory and tumor-associated proteins like basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), interleukin-1 (IL-1), and insulin-like growth factor-1 (IGF-1). 28,29 Thus, enhanced HGF receptor/MET expression in sarcomas could result in autocrine or paracrine stimulation of tumor cell proliferation. As HGF has both motogenic and mitogenic effects, HGF produced in surrounding mesenchymal tissues may attract the tumor cells and contribute to invasiveness of sarcomas via a paracrine signaling pathway as suggested by Comoglio and coworkers 1 and Vande Woude and coworkers. 12 Therefore, the level of HGF expression in the tumor cells themselves may be of less importance.
In contrast to carcinogen-induced sarcomas in rats, 10 no amplification of the Hgfr/Met gene was detected in the 35 human tumors examined by Southern blotting. The discrepancy between the human and rat tumors is not surprising, considering that DMBA was the only causative agent in the rat model and that genetic heterogeneity in the rat model is much less than that of humans. In the study by Scotlandi et al 18 on a smaller number of human musculoskeletal tumors, neither amplification nor major rearrangement of the Hgfr/Met gene was found, similar to our study. Specific amplification of the Hgfr/Met gene or rearrangement of the chromosome region 7q21–31 encompassing this gene has not been recognized in spontaneous human mesenchymal tumors, although such genetic alterations have recently been demonstrated in some types of human carcinomas and gliomas. 30-34 The molecular mechanisms contributing to HGF receptor overexpression in the absence of Hgfr/Met gene amplification are still largely unknown.
In the present study we observed an 85-kd fragment with HGF receptor immunoreactivity, ie, a markedly shorter protein than the 140-kd HGF receptor β-chain. This fragment was recognized in Western blotting by two antibodies directed against the C-terminus of the HGF receptor, and by a monoclonal anti-phosphotyrosine antibody. Both C-terminal antibodies (C-28 and C-12), that were used in the present study, are directed toward the carboxyl terminus (amino acids 1366–1390 and 1379–1390, respectively) distal to the tyrosine kinase domain of Met (amino acids ∼1100–1350). 21,22 The 85-kd fragment was not detectable with an N-terminal HGF receptor antibody, which also strongly suggests a truncation site in the N-terminal rather than C-terminal portion of the HGF receptor protein. According to the findings of the subcellular protein preparations, the 85-kd fragment did not appear to be membrane bound, but was clearly located in the cytoplasmic protein fraction. Although this shorter HGF receptor fragment was also present in human liver tissue, it was expressed at a very low level compared to the full-length β-chain. On the basis of its molecular size and the putative site of truncation, this short HGF receptor fragment appears to be different from other altered or truncated HGF receptor variants, such as the chimeric tpr-met and the C-terminally truncated receptor of similar size reported by Prat et al 23 and Crepaldi et al 35 The 85-kd size of this fragment could suggest a truncation site outside of the membrane spanning part of the native receptor β-chain. However, the size of the fragment could be affected by postproteolytic modifications, eg, the covalent attachment of ubiquitin. 36 It seems unlikely that noncovalently bound proteins, eg, intracellular signaling molecules, were coprecipitated with the HGF receptor fragment, because all Western blotting experiments were conducted under reducing conditions. Prediction of the exact site of the truncation in Western blotting is difficult, and further analysis such as protein sequencing is needed to address this issue.
One possible explanation for the generation of truncated HGF receptor fragments is that the cellular deactivation mechanism could be overloaded when the production and turnover of the HGF receptor is high, resulting in cytoplasmic accumulation of shorter HGF receptor fragments. However, this size (85 kd) of a putative N-terminally truncated degradation product of the HGF receptor protein has not been previously described, to our knowledge. 36 Another possibility is that this fragment is generated by alternative initiation of translation or splicing of the HGF receptor mRNA. Although alternative variants of the transcript have been reported, they are different in size from the short form of the HGF receptor that we detected. 37,38 Moreover, we did not see any alternative transcripts by Northern blotting. The possibility of a protein other than an HGF receptor fragment recognized by two different HGF receptor antibodies and an anti-phosphotyrosine antibody seems unlikely. Moreover, the specificity of one of the C-terminal primary antibodies used in our study was confirmed by a blocking experiment.
Expression of activated, truncated forms of the HGF receptor could be a novel mechanism for uncontrolled, ligand-independent HGF receptor signaling that could contribute to tumor development and progression in some human musculoskeletal tumors. The 85-kd cytoplasmic fragment of the HGF receptor was detected by an anti-phosphotyrosine antibody, indicating that it was tyrosine-phosphorylated. Therefore, this receptor protein seemed to be autoactivated without binding to its ligand, HGF. This assumption is based on the finding that the site of truncation was in the N-terminal, ligand-binding portion of the receptor. Moreover, phosphorylation inhibitors were used during protein preparation, therefore in vitro tyrosine phosphorylation of intracellular degradation products should not have occurred. 36 It has been shown in several studies that cell lines that are transfected with and express high levels of HGF receptors acquire tumorigenic and metastatic properties. 39 Interestingly, Zhen et al reported that the cytoplasmic domain of the HGF receptor, artificially truncated immediately below the transmembrane region, acquired constitutive tyrosine kinase activity in vivo and was even more tumorigenic than the full-length receptor. 20 Also, mice transgenic for the chimeric non-ligand binding autoactivated tpr-met develop tumors. 19 Thus, the significance of short autoactivated HGF receptor fragments has been shown in experimental studies on cell lines and in mice, but never in human tumors. Further efforts are needed to characterize the 85-kd fragment found in this study and to determine its possible significance in human tumors. However, the present study shows, for the first time, the presence of a short, 85-kd, non-ligand-binding autoactivated protein with HGF receptor immunoreactivity in human tumors. Although we found this phosphorylated 85-kd HGF receptor-like fragment in only about 5% of the tumor samples examined by Western blotting, it was present in some malignant tumors that are particularly aggressive, and could clearly be an important factor in tumor progression.
Acknowledgments
We thank Professor Sven-Olof Olofsson for valuable comments and Karin Karlsson for excellent technical assistance.
Footnotes
Address reprint requests to Ville Wallenius, M.D., RCEM, Gröna stråket 8, S-413 45 Gothenburg, Sweden. E-mail: ville.wallenius@medic.gu.se.
Supported by grants from the Swedish Medical Research Council (9894), Swedish Cancer Society, Bergvall Foundation, Novo Nordisk Pharma, Assar Gabrielsson Foundation, Swedish Medical Society, Gothenburg Medical Society, the Swedish Society for Medical Research, the Lundberg Foundation, and the Johan Jansson Foundation for Cancer Research.
References
- 1.Comoglio PM, Boccaccio C: The HGF receptor family: unconventional signal transducers for invasive cell growth. Genes Cells 1996, 1:347-354 [DOI] [PubMed] [Google Scholar]
- 2.Naldini L, Vigna E, Ferracini R, Longati P, Gandino L, Prat M, Comoglio PM: The tyrosine kinase encoded by the MET proto-oncogene is activated by autophosphorylation. Mol Cell Biol 1991, 11:1793-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF: Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311:29-33 [DOI] [PubMed] [Google Scholar]
- 4.Beviglia L, Matsumoto K, Lin CS, Ziober BL, Kramer RH: Expression of the c-Met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer 1997, 74:301-309 [DOI] [PubMed] [Google Scholar]
- 5.Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA: Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene 1992, 7:2549-2553 [PubMed] [Google Scholar]
- 6.Giordano S, Zhen Z, Medico E, Gaudino G, Galimi F, Comoglio PM: Transfer of motogenic and invasive response to scatter factor/hepatocyte growth factor by transfection of human MET protooncogene. Proc Natl Acad Sci USA 1993, 90:649-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortner J, Vande Woude GF, Rong S: The Met-HGF/SF autocrine signaling mechanism is involved in sarcomagenesis. Goldberg ID Rosen EM eds. In Epithelial-Mesenchymal Interactions in Cancer. 1995, :pp 89-121 Switzerland, Birkauser Verlag, Basel [Google Scholar]
- 8.Ferracini R, Di Renzo MF, Scotlandi K, Baldini N, Olivero M, Lollini P, Cremona O, Campanacci M, Comoglio PM: The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995, 10:739-749 [PubMed] [Google Scholar]
- 9.Cooper CS, Tempest PR, Beckman MP, Heldin CH, Brookes P: Amplification and overexpression of the met gene in spontaneously transformed NIH3T3 mouse fibroblasts. EMBO J 1986, 5:2623-2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helou K, Wallenius V, Qiu Y, Ohman F, Stahl F, Klinga-Levan K, Kindblom LG, Mandahl N, Jansson JO, Levan G: Amplification and overexpression of the hepatocyte growth factor receptor (HGFR/MET) in rat DMBA sarcomas. Oncogene 1999, 18:3226-3234 [DOI] [PubMed] [Google Scholar]
- 11.Ferracini R, Olivero M, Di Renzo MF, Martano M, De Giovanni C, Nanni P, Basso G, Scotlandi K, Lollini PL, Comoglio PM: Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 1996, 12:1697-1705 [PubMed] [Google Scholar]
- 12.Rong S, Jeffers M, Resau JH, Tsarfaty I, Oskarsson M, Vande Woude GF: Met expression and sarcoma tumorigenicity. Cancer Res 1993, 53:5355-5360 [PubMed] [Google Scholar]
- 13.Fukuda T, Ichimura E, Shinozaki T, Sano T, Kashiwabara K, Oyama T, Nakajima T, Nakamura T: Coexpression of HGF and c-Met/HGF receptor in human bone and soft tissue tumors. Pathol Int 1998, 48:757-762 [DOI] [PubMed] [Google Scholar]
- 14.Kuhnen C, Tolnay E, Steinau HU, Voss B, Muller KM: Expression of c-Met receptor and hepatocyte growth factor/scatter factor in synovial sarcoma and epithelioid sarcoma. Virchows Arch 1998, 432:337-342 [DOI] [PubMed] [Google Scholar]
- 15.Motoi T, Ishida T, Kuroda M, Horiuchi H, Oka T, Matsumoto K, Nakamura T, Machinami R: Coexpression of hepatocyte growth factor and c-Met proto-oncogene product in synovial sarcoma. Pathol Int 1998, 48:769-775 [DOI] [PubMed] [Google Scholar]
- 16.Naka T, Iwamoto Y, Shinohara N, Ushijima M, Chuman H, Tsuneyoshi M: Expression of c-met proto-oncogene product (c-MET) in benign and malignant bone tumors. Mod Pathol 1997, 10:832-838 [PubMed] [Google Scholar]
- 17.Rao UN, Sonmez-Alpan E, Michalopoulos GK: Hepatocyte growth factor and c-MET in benign and malignant peripheral nerve sheath tumors. Hum Pathol 1997, 28:1066-1070 [DOI] [PubMed] [Google Scholar]
- 18.Scotlandi K, Baldini N, Oliviero M, Di Renzo MF, Martano M, Serra M, Manara MC, Comoglio PM, Ferracini R: Expression of Met/hepatocyte growth factor receptor gene and malignant behavior of musculoskeletal tumors. Am J Pathol 1996, 149:1209-1219 [PMC free article] [PubMed] [Google Scholar]
- 19.Liang TJ, Reid AE, Xavier R, Cardiff RD, Wang TC: Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest 1996, 97:2872-2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhen Z, Giordano S, Longati P, Medico E, Campiglio M, Comoglio PM: Structural and functional domains critical for constitutive activation of the HGF-receptor (Met). Oncogene 1994, 9:1691-1697 [PubMed] [Google Scholar]
- 21.Wallenius VR, Rawet H, Skrtic S, Helou K, Qiu Y, Levan G, Ekberg S, Carlsson B, Isaksson OG, Nakamura T, Jansson JO: Chromosomal localization of rat hepatocyte growth factor (Hgf) and HGF receptor (Met) and characterization of HGF receptor cDNA. Mamm Genome 1997, 8:661-667 [DOI] [PubMed] [Google Scholar]
- 22.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G: Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA 1987, 84:6379-6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prat M, Crepaldi T, Gandino L, Giordano S, Longati P, Comoglio P: C-terminal truncated forms of Met, the hepatocyte growth factor receptor. Mol Cell Biol 1991, 11:5954-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 25.Enzinger FM, Weiss SW: Fibromatoses. 3rd ed. Enzinger FM Weiss SW eds. Soft Tissue Tumors, 1995, :pp 201-229 Mosby, St. Louis [Google Scholar]
- 26.Dorfman HD, Czerniak B: Giant cell lesions. Dorfman HD Czerniak B eds. Bone Tumors. 1999, :pp 559-606 Mosby, St. Louis [Google Scholar]
- 27.Krasnoselsky A, Massay MJ, DeFrances MC, Michalopoulos G, Zarnegar R, Ratner N: Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J Neurosci 1994, 14:7284-7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T: Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res 1997, 57:3305-3313 [PubMed] [Google Scholar]
- 29.Skrtic S, Wallenius V, Ekberg S, Brenzel A, Gressner AM, Jansson JO: Insulin-like growth factors stimulate expression of hepatocyte growth factor but not transforming growth factor betal in cultured hepatic stellate cells. Endocrinology 1997, 138:4683-4689 [DOI] [PubMed] [Google Scholar]
- 30.Fischer U, Muller HW, Sattler HP, Feiden K, Zang KD, Meese E: Amplification of the MET gene in glioma. Genes Chromosomes Cancer 1995, 12:63-65 [DOI] [PubMed] [Google Scholar]
- 31.Houldsworth J, Cordon-Cardo C, Ladanyi M, Kelsen DP, Chaganti RS: Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res 1990, 50:6417-6422 [PubMed] [Google Scholar]
- 32.Knuutila S, Bjorkqvist AM, Autio K, Tarkkanen M, Wolf M, Monni O, Szymanska J, Larramendy ML, Tapper J, Pere H, El-Rifai W, Hemmer S, Wasenius VM, Vidgren V, Zhu Y: DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol 1998, 152:1107-1123 [PMC free article] [PubMed] [Google Scholar]
- 33.Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E: Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun 1992, 189:227-232 [DOI] [PubMed] [Google Scholar]
- 34.Zhuang Z, Park WS, Pack S, Schmidt L, Vortmeyer AO, Pak E, Pham T, Weil RJ, Candidus S, Lubensky IA, Linehan WM, Zbar B, Weirich G: Trisomy 7-harbouring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Genet 1998, 20:66-69 [DOI] [PubMed] [Google Scholar]
- 35.Crepaldi T, Prat M, Giordano S, Medico E, Comoglio PM: Generation of a truncated hepatocyte growth factor receptor in the endoplasmic reticulum. J Biol Chem 1994, 269:1750-1755 [PubMed] [Google Scholar]
- 36.Jeffers M, Taylor GA, Weidner KM, Omura S, Vande Woude GF: Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol 1997, 17:799-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CC, Yamada KM: Identification of a novel type of alternative splicing of a tyrosine kinase receptor. Juxtamembrane deletion of the c-met protein kinase C serine phosphorylation regulatory site. J Biol Chem 1994, 269:1945-1946 [PubMed] [Google Scholar]
- 38.Rodrigues GA, Naujokas MA, Park M: Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol 1991, 11:2962-2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asai T, Ueda T, Itoh K, Yoshioka K, Aoki Y, Mori S, Yoshikawa H: Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int J Cancer 1998, 76:418-422 [DOI] [PubMed] [Google Scholar]