Abstract
Superficial injury involving the mucosa of the gastrointestinal tract heals by a process termed restitution that involves epithelial sheet movement into the damaged area. The forces that drive epithelial sheet movement are only partially understood, although it is known to involve changes in the morphology of cells bordering the damage, such as the formation of large, flat, cytoplasmic extensions termed lamellae. We investigated the mechanism of epithelial sheet movement by following the response of the actin cytoskeleton and specific integrins (α6β4, α6β1, and α3β1) to wounding. To model this event in vitro, monolayers of T84 cells, well-differentiated colon carcinoma cells, were damaged by aspiration and the ensuing response was analyzed by a combination of time-lapse video microscopy, fluorescence confocal microscopy and antibody inhibition assays. We show that wound healing begins with retraction of the monolayer. α6β4 integrin is localized on the basal surface in structures referred to as type II hemidesmosomes that persist throughout this early stage. We hypothesize that these structures adhere to the substrate and function to retard retraction. Once retraction ceases, the wound is contracted initially by actin purse strings and then lamellae. Purse strings and lamellae produce a pulling force on surrounding cells, inducing them to flatten into the wound. In the case of lamellae, we detected actin suspension cables that appear to transduce this pulling force. As marginal cells produce lamellae, their basal type II hemidesmosomes disappear and the α6 integrins appear evenly distributed over lamellae surfaces. Antibodies directed against the α6 subunit inhibit lamellae formation, indicating that redistribution of the α6 integrins may contribute to the protrusion of these structures. Antibodies directed against the α3β1 integrin also reduce the size and number of lamellae. This integrin’s contribution to lamellae extension is most likely related to its localization at the leading edge of emerging protrusions. In summary, wounds in epithelial sheets initially retract, and then are contracted by first an actin purse string and then lamellae, both of which serve to pull the surrounding cells into the denuded area. The α6 integrins, particularly α6β4, help contain retraction and both the α6 integrins and α3β1 integrin contribute to lamellae formation.
Disruptions in the mucosal lining of the gastrointestinal tract reseal by a process termed restitution. 1,2 Restitution is an important component of the barrier function of the gut lining because it prevents luminal contents from seeping into the underlying intestinal tissue during normal wear and tear of the epithelium. Initial light microscopic studies of fixed tissue discerned that gut epithelial cells respond to injury by altering their morphology. Further work showed that mucosal injuries are resealed by the concerted movement of the surrounding cells, not by cell division or by contributions from blood clot formation. 3,4 These points illustrate that restitution is a very specific example of wound healing in epithelial monolayers.
Epithelial sheets respond to injury by mobilizing their actin cytoskeleton. Two different types of responses have been noted. 5,6 One involves lamellae formation, a key feature of restitution both in vivo and in vitro. 4,7 Lamellae are large, flat, cytoplasmic protrusions that are extended by the marginal cells into the denuded area. Much of what is known about lamellae is derived from the study of solitary migrating cells such as fibroblasts. 8 During fibroblast locomotion the actin cytoskeleton within the lamella associates with integrins on the surface. The traction to pull the cell forward is provided by the adhesion between the integrins and their specific extracellular matrix ligands. Eventually the lamella contracts, detaching the rearward part of the cell and allowing it to translocate. Although this process seems relevant to epithelial wound healing, many normal epithelia reseal defects without loss of cell contact. 9,10 In the case of restitution, marginal cells surrounding gut injuries are thought to use lamellae to migrate into the wound, but cells are not observed to detach from their neighbors and translocate into the injury. 1,2,4,7,11 This observation raises the question of how lamellae participate in the healing process. Other epithelial wounds heal by a process called purse string contraction in which cells marginal to the damage arrange their actin in a belt that tightens to close the injury. 5,6 It is thought that small wounds (<0.008 mm2) heal by purse string contraction, whereas larger wounds use lamellae. 6 However, recent studies of corneal abrasions suggest that the two processes may not be mutually exclusive. 12 It is unclear from previous findings whether actin purse strings form during gut epithelial wound healing or whether lamellae that form during restitution preclude these structures.
We were interested in understanding the underlying molecular processes that drive restitution. To model the processes in vitro we used T84 cells, well-differentiated human intestinal carcinoma cells that are polarized along their apical to basal axis and express well-developed intercellular junctions. 13 Previously we found that the laminin family of extracellular matrix proteins and the integrins that bind these proteins are instrumental in restitution. 14 Our findings targeted the laminin-binding integrins, α6β1, α6β4, and α3β1, as part of the molecular machinery responsible for epithelial wound healing, but the point at which these integrins participate in the healing process is unknown. It would be informative to correlate the changes in cell morphology that occur on wounding with changes in the function and distribution of laminin-binding integrins.
Here, we examine the mechanism of epithelial cell movement during wound closure by following the mobilization of the actin cytoskeleton and laminin-binding integrins in response to injury of T84 monolayers. We show that lamellae formation is a later step in a progression of cytoskeletal rearrangements that begins with actin purse strings. The α6 integrins and α3β1 integrin, which we found to be instrumental in lamellae formation, redistribute to distinctive locales on the lamellae during wound resealing. We conclude that the purpose of both the actin purse string and lamellae is to allow the usually columnar T84 cell to flatten greatly, providing as much cytoplasmic coverage of the denuded area as possible.
Materials and Methods
Culture of T84 Cells
T84 colon carcinoma cells were cultured as described previously. 13,14 Briefly, cells were grown in DME-low glucose/Ham’s F12 (GIBCO, Grand Island, NY) supplemented with 15 mmol/L Hepes, 6% normal calf serum, 2 mmol/L L-glutamine, 50 μg/ml streptomycin, and 50 U/ml penicillin. Cells were grown for 2 to 3 days after reaching confluency before being used in assays.
Antibodies
The following monoclonal antibodies specific for integrin subunits were used in the present study: mouse antibody UM-A9 (integrin β4 subunit), provided by Dr. Thomas Carey (University of Michigan, Ann Arbor, MI); rat antibody GoH3 (integrin α6 subunit) and mouse antibody against CD29 (integrin β1 subunit), purchased from Immunotech (Marseille, France); mouse antibody PIB5 (integrin α3 subunit), purchased from Becton Dickinson (San Jose, CA). Mouse and rat IgG was purchased from Sigma (St. Louis, MO). Fluorescein-conjugated goat anti-rat and goat anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Immunofluorescence Microscopy
T84 cells were dissociated with trypsin-EDTA and, except where mentioned in the figure legends, plated onto glass coverslips. The cells were grown in a 37°C, 5% CO2 atmosphere for 10 to 14 days. Subsequently, the confluent monolayers were wounded by aspiration through a micropipet tip. These were made by pulling a heated glass Pasteur pipet so that a very narrow bore resulted.
At various times after wounding, cells were fixed for 10 minutes with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) with 7% sucrose followed by 0.4% Triton X-100 in Tris-buffered saline (TBS, pH 7.4) for 2 minutes. In some cases, noted in the figure legend, cells were permeabilized before fixation for 30 seconds on ice in F1 buffer (10 mmol/L Pipes, pH 6.8, 0.5% Triton-X 100, 300 mmol/L sucrose, 100 mmol/L potassium chloride, 3 mmol/L magnesium chloride, 10 mmol/L ethyleneglycoltetraacetic acid, and 2 mmol/L phenylmethylsulfonyl fluoride). After fixation, the coverslips were incubated in a blocking solution (3% bovine serum albumin and 1% normal donkey serum in TBS) for 1 hour at room temperature. After incubation with either primary antibody or nonspecific IgG, the coverslips were washed in TBS (3 times, 10 minutes each) and incubated for 1 hour at room temperature in the appropriate fluorescein-conjugated secondary antibody.1:100 In most experiments the secondary antibody was accompanied by rhodamine-phalloidin (1 μg/ml, Sigma) or rhodamine-phalloidin and Hoechst stain (0.5 μg/ml, Sigma). All antibodies and fluorescent compounds were diluted in blocking solution. After staining, the coverslips were washed in TBS and mounted in a mixture (8:2) of glycerol and PBS, pH 8.5, containing 1% propylgallate. Slides were examined by confocal imaging using a Zeiss LSM410 Laserscan Microscope equipped with a peripheral Argon-UV laser for exciting Hoechst fluorescence (Carl Zeiss, Thornwood, NY).
Video Microscopy and Function Blocking Experiments
T84 cells were grown for 10 to 14 days on 35-mm tissue culture dishes. Circular wounds were made using micropipet tips. The healing process was examined by time-lapse video-microscopy using a Nikon Diaphot 300 inverted microscope with phase contrast optics, equipped with a stage warmer. Images were captured using a CCD camera (Dage-MTI, Michigan City, IN), a frame-grabber (Scion) and a 7600 Power Macintosh computer. Films of wounds healing at 37°C, 5% CO2 were made by capturing images every 2 minutes for up to 12 hours. Some wounds were filmed for various amounts of time and then fixed and stained for immunofluorescence. For function-blocking experiments, T84 cell monolayers were wounded, fresh medium containing function-blocking integrin antibodies was added, and then images of the wounds were captured so that their initial diameters could be measured. After incubation for 2 hours in a 37°C, 5% CO2 incubator, images were captured again for a final wound diameter measurement. Alternatively, images of wounds were captured for measurements of initial diameters and then wound healing was allowed to progress until the first appearance of lamellae. At this point, function-blocking antibodies were added. After two hours, images were captured again for measurement of final wound diameters. Wound diameters were measured using IP lab spectrum software (Scanalytics, Fairfax, VA). The initial and final diameters for each wound were used to determine effects of the monoclonal antibodies on wound closure.
Results
Wound Closure in T84 Monolayers Progresses via Retraction, Actin Purse String Formation, and Lamellae Extension
To identify the sequence of events involved in the closure of T84 wounds (in this case, 0.018 mm2, approximately 400 cells), we used time-lapse video microscopy (Figure 1) ▶ . Initially after wounding, the wound edge actually retracted for 10 minutes and the surface area of the wound increased by ∼14%. At this time, a refractile ring that surrounded the wound edge was evident. This ring was similar in appearance to actin purse strings that have been shown to contract smaller wounds. 5,6 Subsequent to the appearance of the refractile ring, the cells surrounding the wound edge elongated and increased their surface area approximately twofold, a process that resulted in the initiation of wound closure. Although the surface area of the wound was reduced by 30% at 40 minutes relative to its largest size at 10 minutes, surface protrusions such as lamellae were not evident in cells at the wound edge. In fact, distinct lamellae extending from a few cells abutting the damage were not seen until 60 minutes after wounding. Lamellae protrusion was not synchronous, but gradually most of the marginal cells produced these extensions, while the refractile ring became less evident. At 120 minutes after wounding, the denuded area was covered entirely by lamellae. Based on this video analysis, we conclude that the closure of T84 wounds involves a sequence of morphologically-defined events that involve an initial retraction of the wound edge, subsequent refractile ring formation accompanied by cell elongation, and, finally, lamellae extension.
Figure 1.
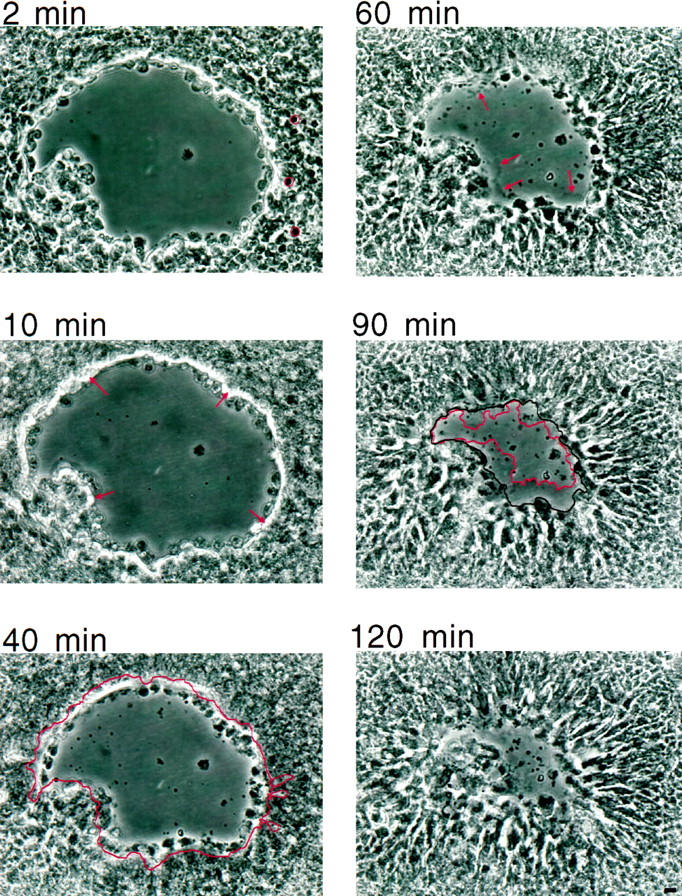
Time-lapse video microscopy reveals the sequence of events during wound healing of a monolayer of T84 cells. Times above the panels refer to the number of minutes elapsed after wounding. The first image of the wound, approximately 0.018 mm2, was taken at 2 minutes. Cell bodies were randomly chosen on the right side of the denuded area and outlined in red. At 10 minutes postwound, retraction reached its fullest extent and the denuded area was now approximately 0.021 mm2. The refractile ring, greatly resembling an actin purse string, is indicated by arrows. By 40 minutes, the wound closed to 0.015 mm2, a 30% reduction from its largest size at 10 minutes. The outline of the injury at 10 minutes is transposed onto the image taken at 40 minutes. Cell bodies, also outlined, were randomly chosen on the right side of the denuded area for comparison with those at 2 minutes. Similar comparisons of 10 randomly chosen cells, followed from 16 minutes to 40 minutes, showed that during this period of wound contraction, cell surface area increased on the average twofold (from 54 ± 3 μm 2 to 116 ± 7 μm2, P < 0.01). By 60 minutes, lamellae, indicated by arrows, were obvious. At 90 minutes, many of the cells adjacent to the wound protruded lamellae. Lamellae are highlighted in red, and the margin of the cell bodies is outlined in black. The area between these two outlines is considered to be the amount of lamella surface area contributing to wound sealing up to this point in time, a measurement used in Figure 5 ▶ . By 120 minutes, the denuded area was nearly completely covered by lamellae. Scale bar, 10 μm.
The possibility that the refractile ring we observed during the closure of a 0.018 mm 2 wound is an actin purse string is intriguing because actin purse strings have been associated with smaller wounds (<0.008 mm2). 6 This possibility was examined by staining monolayers 30 minutes after wounding with rhodamine-phalloidin to visualize F-actin (Figure 2) ▶ . Indeed, an intense band of F-actin, characteristic of an actin purse string, was seen around the circumference of such wounds (Figure 2A) ▶ . In fact, even larger wounds exhibited actin purse string formation within 30 minutes after wounding (Figure 2B) ▶ . Interestingly, cells that were damaged as a result of wounding were excluded and appeared in the denuded area outside the actin purse string. Cross-sectional images revealed that these damaged cells were detached from the substrate (Figure 2C) ▶ .
Figure 2.

Rhodamine-phalloidin fluorescent staining of recently wounded T84 monolayers reveals actin purse string formation. T84 monolayers were wounded by pipet aspiration and fixed 30 minutes later. A: Monolayers grown on porous supports were wounded. This en face view of the injury shows a continuous belt of actin or purse string, indicated by arrows, encircling the denuded area. The purse string is not visible in the upper half of the injury because the porous support at this point declines away from the plane of focus. B: An en face view of a larger injury reveals that it is not yet fully surrounded by an actin belt. The actin purse string (arrows) is in the process of forming. The asterisk marks cells, not included in the purse string, that were damaged and have lifted off the substrate. C: An optical cross-section taken at the point of the upper arrow in B reveals the actin purse string (arrow) forming in the basolateral compartment of the attached cells marginal to the wound. Cells not included in the purse string are detached from the substrate. Scale bars, 10 μm.
Coincident with actin purse string formation, cells surrounding the denuded area elongated with their long axis at right angles to the wound edge. Image analysis indicated that cells as far as 10 cell diameters away from the injury elongated in response to wounding and increased their basal surface area by approximately twofold (Figure 1) ▶ . Moreover, rhodamine-phalloidin staining revealed that cells surrounding the wound rearranged their stress fibers orthogonally to the wound edge such that these stress fibers stretched toward the actin purse string (Figure 3A) ▶ . Cross-sectional images highlighted the impression of an intact monolayer tapered down to the actin purse string (Figure 3B) ▶ . Collectively, these images demonstrate that cells distant from the wound contribute to closure by elongating, and that actin purse strings, which generate a contractive force, are associated with this elongation.
Figure 3.

Rhodamine-phalloidin staining demonstrates that cell elongation is accompanied by actin stress fiber rearrangement. A: T84 monolayers were fixed 3 hours after wounding. In this en face view taken at the basal surface, the arrow points to the actin purse string. Cells rearward of those producing the purse string exhibit stress fibers arrayed at right angles to the injury. B: An optical cross-section of an area similar to that shown in A shows how the monolayer flattens as it reaches the actin purse string, highlighted by an arrow. Scale bars, 10 μm.
The actin rearrangements involved in lamellae formation were also examined by rhodamine-phalloidin staining. En face views of wounds surrounded by lamellae demonstrated the presence of F-actin within the lamellae protruding into the denuded area (Figure 4) ▶ . However, we observed regions in the monolayer adjacent to the lamellae that contained no detectable F-actin (Figure 4A) ▶ . Cross-sectional analysis revealed that these F-actin-deficient regions corresponded to the bodies of the marginal cells that produced the lamellae and actually contained fine F-actin cables that were suspended above the basal plane (Figure 4B) ▶ . For this reason, images taken at this basal plane gave the appearance of F-actin holes in the monolayer. These F-actin suspension cables extended from the marginal cell’s rearward apicolateral cell surface to the dense actin network at the base of the lamella. This unique arrangement of actin suggested that the cables were under tension (Figure 4B) ▶ . A cross-section taken in a neighboring region revealed shorter actin suspension cables (Figure 4C) ▶ . These cross-sectional views imply that the cable’s suspension from the rearward apicolateral cell surface down to the lamella base is related to the extent to which the cell containing it elongates. Together, the images presented in Figure 4 ▶ demonstrate that lamellae serve to stretch the marginal cells that produce them and result in the formation of actin suspension cables that bridge the rearward, lateral cell surface with the lamella base.
Figure 4.

Rhodamine-phalloidin staining of lamellae formation. Monolayers were wounded and then fixed 7 hours later. A: An en face view shows lamellae protruding into the denuded area. The arrows, pointing to lamellae, indicate areas through which optical cross-sections were taken (B and C). Rearward of many of these lamellae lies a relatively actin-deficient area. One such area is denoted by an asterisk. B: A cross-section taken through the monolayer at the point indicated by the lower arrow in A shows fine actin suspension cables (asterisk) reaching down from the rearward, apical-lateral cell surface to the dense actin array at the base of the lamella. The dashed line, level with the substratum, is shown to help visualize the angle at which these cables descend. C: An optical cross-section taken through the monolayer at the point indicated by the upper arrow in A. In this area, where filamentous actin is visible rearward of the lamellae, actin cables are present but not as elongated as those in B. D: A wound surrounded by lamellae. These lamellae exhibit a dense actin array at their base and actin-rich lamellopodia at their leading edges, to which the arrows point. Areas behind the lamellae that appear actin-deficient are denoted by asterisks. Based on cross-sections in B and C, these areas correspond to cell bodies that contain fine actin suspension cables extending down to the bases of the lamellae. Scale bar, 10 μm.
Correlating Function and Localization of the α6 Integrins and α3β1 Integrin during Wound Healing with Changes in Cell Morphology Induced by Wounding
Previously we demonstrated that α3β1 and the α6 integrins were involved in wound resealing of T84 cell monolayers. In the current study, we determined at which stage (retraction, actin purse string formation or lamellae extension) these integrins contributed to the healing process. Wounds were allowed to reseal for 2 hours in the presence of nonspecific antibody or antibodies against the α3 or α6 integrin subunit (Figure 5a) ▶ . All of the wounds, regardless of treatment, exhibited refractile actin purse strings, suggesting that neither the α3β1 integrin nor the α6 integrins were involved in the formation of this structure. Wounds treated with nonspecific antibody closed 35%, whereas those treated with α6 subunit antibody were 11% larger compared with their initial sizes. These data demonstrate that cells treated with the α6 subunit antibody were unable to halt retraction within 2 hours of wounding, suggesting that the α6 integrins promote the cessation of retraction. In contrast, wounds treated with α3 subunit antibody were 12% smaller. Thus, injured monolayers treated with α3 subunit antibody were able to halt retraction within the 2-hour time span, but resealed more slowly than those treated with nonspecific antibody.
Figure 5.

Antibody inhibition of T84 monolayer wound healing. a: Wounds were made in the presence of 20 μg/ml of nonspecific IgG or monoclonal antibodies against the α3 or α6 integrin subunit. The area of the denuded region measured immediately after wounding was compared to that value measured 2 hours later to determine the percentage of total closure. The mean percent closure of wounds treated with α3 and α6 integrin-specific antibodies was statistically significantly different from those treated with nonspecific IgG (P < 0.01). b: Wounds were allowed to close until lamellae appeared and then 20 μg/ml nonspecific IgG, antibody against the α3 subunit or antibody against the α6 integrin subunit was added. After 2 hours, the percent closure due to lamella surface area was determined by subtracting the area of the denuded region from the area bounded by the cell bodies of the marginal cells. (See the 90 minute panel in Figure 1 ▶ , where the red line encircles the denuded region and the black line traces the area bounded by the cell bodies of the marginal cells). The mean percent closure due to lamellar surface area of wounds treated with α3 and α6 integrin-specific antibodies was statistically significantly different from those treated with nonspecific IgG (P < 0.01).
We next assessed the effects of the integrin-specific antibodies on lamellae protrusion. To determine the contribution of α3β1 integrin and the α6 integrins to this process, inhibitory antibodies were added just as lamellae were beginning to emerge, rather than immediately after wounding (Figure 5b) ▶ . This strategy allowed wound resealing to proceed past the retraction and purse string stages before the inhibitory antibodies were added. Wounds were analyzed 2 hours later for the contribution of lamellae to wound closure. In the presence of an α3-specific antibody, lamellae contributed only 10% of the surface area to wound healing as compared to 25% in the presence of nonspecific antibody. When antibodies against the α6 integrin subunit were added at this later time point, lamellae formation was also reduced by 50% relative to that seen with nonspecific antibody. These findings demonstrate that both the α6 integrins and α3β1 integrin participate in lamellae formation.
We examined the localization of the α3, α6, and β4 subunits in cells at distinct stages of wound closure to obtain additional insights into their contribution to wound healing. We focused on the α6β4 integrin because a significant fraction of the α6 signal colocalizes with the β4 subunit on the basal surface of intact T84 monolayers in plaque-like structures. 14 The basal surface was examined by immunofluorescence microscopy soon after wounding, as retraction ceased and purse strings formed (Figure 6A) ▶ . Superimposing a relatively apical section containing the forming actin purse string (in blue) on a basal section containing actin stress fibers (in red) and α6β4 integrin (in green) demonstrated that intact plaques remained on the basal surface of marginal cells during retraction. Higher magnification revealed that basal plaques occupied almost the entire basal surface of T84 cells and contained striations through which actin stress fibers ran (Figure 6B) ▶ . These actin-striated plaques are most likely Type II hemidesmosomes, described in several mammary and colon carcinoma cell lines. 15,16 Besides resembling type II hemidesmosomes in appearance, they contained the protein HD-1, also known as plectin, but lacked the proteins BP-1 and BP-2 (data not shown). These findings indicate that α6β4 integrin is found in type II hemidesmosomes, basal structures that survive injury and thus are present during retraction.
Figure 6.

The α6β4 integrin resides in basal plaques that are disassembled during lamellae formation. T84 monolayers, either intact or injured, were fixed and then stained with rhodamine-phalloidin and by immunofluorescence for the β4 integrin subunit (A−C and E) or for the β4 integrin subunit alone (D) or by immunofluorescence for the β1 and α6 integrin subunit (F). A: An injured T84 monolayer was fixed during wound retraction and stained with rhodamine-phalloidin (red and blue) and for the β4 integrin subunit (green). An en face view of an actin purse string (blue, denoted by the white arrow) forming in a relatively apical plane is superimposed over the corresponding view of the basal compartment showing actin stress fibers (red, denoted by black arrow) running through α6β4 integrin (green). This image demonstrates that α6β4-containing plaques remain intact after wounding, including marginal cells that form the purse string during retraction. Scale bar, 5 μm. B: An intact T84 monolayer was fixed and permeabilized in F1 buffer. Treatment with F1 buffer allowed antibody access to the basal surface but left the actin cytoskeleton intact. This en face view of the basal plane was stained with rhodamine-phalloidin (red) and for β4 integrin subunit (green). The α6β4 integrin is found in ovoid plaques that are interrupted by actin stress fibers, three of which are indicated by arrows. The outline of a single cell demonstrates that almost the entire basal surface expresses α6β4 integrin. Scale bar, 5 μm. C: An en face view of the basal surface of an injured T84 monolayer fixed 6 hours after wounding and stained with Hoechst stain for nuclei (blue) as well as rhodamine-phalloidin (red) and for the β4 integrin subunit (green). (Not every cell in this image exhibits a nucleus because many nuclei lie above the basal plane.) Many of the cells adjacent to the wound extend lamellae (long arrow). The relatively actin-deficient areas that contain thin actin cables as described in Figure 4 ▶ are here seen to contain nuclei, one of which is denoted by the short arrow. This image demonstrates that the actin-deficient areas, which contain nuclei and therefore are the bodies of marginal cells, do not exhibit plaques. Rearward of the marginal cells lies a second tier of cells that surround the wound but are not directly adjacent to it. One of the nuclei of these cells is indicated by the dashed arrow. Cells in the second tier do exhibit plaques, some of which are circled. Scale bar, 10 μm. D: An enlargement of the basal surface of cells in the second tier stained for the β4 integrin subunit. The wound is out of view to the left of the image and the asterisks are placed in F-actin-deficient areas. This image shows plaques stretched toward the wound edge. Scale bar, 10 μm. E: Higher magnification reveals that the β4 subunit (green) is scattered over the surface of a lamella (contained within the box) but does not colocalize with actin filaments (red, arrows) radiating out from the base. Scale bar, 10 μm. F: Cells were double-stained for the β1 (green) and α6 (red) integrin subunits to detect α6β1 integrin (yellow). Yellow points of colocalization are dispersed across the lamella, contained within the lines. Scale bar, 10 μm.
We next analyzed the distribution of the α6 integrins on lamellae to determine how they participate in the formation of these processes. Examination of marginal cells revealed that α6β4 integrin does not reside in plaques on those producing lamellae. Rather, α6β4 integrin was dispersed on the marginal cell surface and was not found in the characteristic plaque pattern, in which it alternates with actin. This was seen in a wound fully encircled by lamellae and stained for nuclei (Figure 6C ▶ , blue), actin (red), and α6β4 integrin (green). Marginal cells exhibited a uniform distribution of the β4 subunit, although cells located rearward to the marginal cells displayed this integrin in basal plaques stretched toward the injury (Figure 6D) ▶ . Close inspection of a lamella revealed the β4 subunit distributed between the fine actin filaments radiating out from the lamella base (Figure 6E) ▶ . To detect α6β1 integrin on lamellae, double-staining experiments were performed with antibodies to the α6 and β1 integrin subunit. Points of colocalization, indicating the presence of α6β1 integrin, were dispersed over these extensions (Figure 6F) ▶ . These images demonstrate that the α6 integrins respond to injury by distributing themselves over lamellae.
The localization of α3β1 integrin during lamellae formation was also analyzed. Before the appearance of large, adherent lamellae, small cytoplasmic protrusions were often found emerging into the denuded region in areas where the actin purse string was no longer intact (Figure 7) ▶ . A distinctive feature of these small protrusions was that they were observed when wounds were stained for the α3 integrin subunit, but not when they were stained for the α6 or β4 integrin subunit. They also stained weakly for actin. Approximately 1 out of 5 of these emerging lamellae exhibited more intense α3 integrin subunit staining at their leading edge than on their bodies. Optical cross-sectioning revealed that this intense band corresponded to a concentration of α3 integrin subunit at the outermost edge into which thin actin filaments ran (Figure 7D) ▶ . The α3 subunit on more mature lamellae was evenly distributed across these larger extensions and was not found as concentrated at the edge of larger lamellae as it was on small emerging ones (Figure 7E) ▶ . The localization of α3β1 integrin on the leading edge of small protrusions and the antibody inhibition data suggest that this integrin plays a distinct role in lamellae formation.
Figure 7.

Emerging lamellae are characterized by intense α3β1 integrin staining at their leading edge. Wounded T84 monolayers were fixed and stained with rhodamine-phalloidin and by immunofluorescence for the β1 integrin subunit (A) or the α3 integrin subunit (B−E). A: In this en face image, small protrusions, indicated by arrows, are intensely labeled for the β1 integrin subunit (green) and emerge past the actin purse string (red) denoted by the asterisk. To the right of the purse string are broader extensions in which the actin purse string is no longer evident. B: An en face view of emerging lamellae showing that α3β1 integrin (green) is prevalent on the outermost edge of some of these protrusions, as indicated by the arrows. The asterisk denotes actin filaments (red) either disbanding from the purse string or assembling into the base of a forming lamella. C: An en face image of an emerging lamella shows that it is intensely labeled for the α3 integrin subunit (green) at its outer edge, as indicated by the arrow. D: An optical cross-section taken at the point of the arrow in C reveals that α3β1 integrin is intensely concentrated at the outermost edge of the lamella to which the arrow points. An actin filament (red) runs into this concentration of integrin. E: An en face image shows the α3 subunit (green) evenly scattered over a lamella, part of which is contained within the box. Scale bars, 10 μm.
Discussion
In this paper, we document the progression of events leading to wound closure of an epithelial sheet. These findings yield new insight into gastrointestinal restitution, a specific example of epithelial sheet wound healing. Previous studies on restitution focused primarily on the morphology of cells involved in wound closure. Our study extends this work by describing not only the morphology but also changes in cytoskeleton organization and integrin localization in the context of a progression that leads to wound closure. More specifically, we examined filamentous actin and the laminin-binding integrins α6β1, α6β4, and α3β1 because they have been implicated in epithelial sheet healing. 14 We found that after an initial retraction, wound closure progressed by a series of actin rearrangements that generated a contractive or pulling force on the cells surrounding the defect, inducing them to flatten into the denuded area, thereby sealing it. The laminin-binding integrins participated at different points during the healing process. The α6 integrins, particularly α6β4, helped to contain retraction, and both the α6 integrins and α3β1 integrin contributed to lamellae formation.
Actin purse strings were first described in studies that demonstrated the ability of small epithelial wounds to heal without any contribution from lamellae. 5,6 These studies show that actin purse strings mechanically pull on marginal cells, drawing them together. When purse string formation is inhibited by the inactivation of endogenous Rho, small, embryonic skin wounds fail to heal. 17 In this study we show that purse strings also participate in the resealing of larger wounds that later produce lamellae. Our results demonstrate that closure begins after purse strings form and before lamellae appear. In the case of larger wounds, the force generated by the purse string appears to be transduced to more remote cells, probably via cell-to-cell contacts. The result is that remote cells stretch toward the injury, increasing their surface area on the average twofold as their basal stress fibers become aligned at right angles to the purse string. These findings suggest that actin purse strings contribute to the resealing of larger wounds by providing a pulling force that induces surrounding cells to flatten, thus increasing coverage of the denuded area.
Lamellae, a key feature of restitution, appear after the purse string completely encircles the wound. We suggest that the wounds made in this study (with surface area of >50 cells), cannot be closed solely by actin purse contraction but require further efforts provided by lamellae. The signal to extend lamellae may be related to the down-regulation of adherens junctions that link the filamentous actin components together to form the purse string. 12 It has been inferred from studies of migrating, isolated cells that the primary function of lamellae during epithelial wound healing is that of a motile apparatus. In contrast, the images presented here indicate that the primary function of lamellae is to generate a pulling force on distal cells, much like purse strings. In fibroblasts, the pulling force generated by lamellae results in the detachment of the cell tail and translocation of the cell. 18,19 However, in intestinal epithelium, the marginal cells do not detach from their rearward neighbors. 2,4,7,11 Rather, the force generated by lamellae is transferred rearward, inducing cells that are distant from the wound to flatten as they stretch toward the injury. This force can be detected by the fact that α6β4-containing basal plaques become aligned orthogonally to the wound edge in cells that are rearward to those abutting the damage. Cell flattening serves to cover the denuded area in addition to surface area provided by the lamellae themselves.
A novel finding in our study is that marginal cells rearrange their actin cytoskeleton to produce an actin suspension cable. There is no equivalent of this structure in solitary migrating cells, and to our knowledge it has not been described previously. We propose that this actin suspension cable is used to pull the rearward cells toward the lamellae at the wound margin. Actin cables course down from the rearward, apicolateral surface of the marginal cell into the dense actin array at the base of the lamellae. This array in the lamellae of solitary migrating fish keratocytes consists of actin and myosin arranged to produce the contractive force required for detachment of the trailing edge. 20 Our data suggest that this contractive force in T84 cell monolayers and other epithelial sheets is transduced along actin suspension cables to cell-cell contacts at the surface distal from the lamellae. In this way the contractive force is communicated to surrounding cells, inducing them to flatten.
Past work by our group pertaining to extracellular matrix interactions during wound healing demonstrated that laminins secreted by T84 cells, particularly laminin-5, and the laminin-binding integrins α6β1 and α6β4 contribute to closure. 14 However, these studies did not pinpoint the stages during resealing at which these integrins act. Here we show that the α6 integrins act during retraction and lamellae formation. We hypothesize that α6β4 integrin within type II hemidesmosome-basal plaques is responsible for generating substratum adhesion that helps contain retraction. This is suggested by the observation that Type II hemidesmosomes share important features with classical hemidesmosomes, complex structures that mediate very tight adhesion of the epidermis to the underlying basal lamina of the skin. 21-26 Both types of hemidesmosome link the keratin cytoskeleton to the cell substratum via α6β4 integrin. 16,27 Our hypothesis that the α6β4 integrin is responsible for containing retraction is supported by the sharp distinction seen during retraction between nonadherent cells lying in the damaged area and the adherent cells that express basal plaques. It is likely that α6β4 integrin within the type II hemidesmosome attaches to laminin-5, one of its ligands that is secreted as a basal sheet by T84 cells. 14,28 Our previous finding that antibodies to laminin-5 added at the time of injury, like those against α6 integrin subunit, allow the monolayer to retract unimpeded lend credence to the idea that α6β4 integrin within the basal plaques mediates attachment to laminin-5, helping to halt retraction. 14
Antibody inhibition data also demonstrated that the α6 subunit-containing integrins participate in lamellae protrusion during T84 wound resealing. Localization studies revealed that on extending lamellae, marginal cells no longer exhibit alternating actin and α6β4 integrin characteristic of type II hemidesmosomes, nor the lateral cell surface expression of α6β1 integrin seen in intact monolayers. 14 Instead, α6β4 and α6β1 integrin are found dispersed over the lamellae, indicating that both integrins redistribute as lamellae extend. A similar observation has been made during corneal wound healing. In this case, α6β4 integrin relocates over the cell surface and classical hemidesmosomes disassemble on corneal epithelial cells bordering the injury. 10,24,29 We suggest that redistribution of α6β4 and α6β1 integrin is important for lamellae formation and that the basis for α6-subunit antibody inhibition is interference with the rearrangement of either one or both of these integrins. In agreement with this conclusion, α6β4 integrin has been shown to promote the formation and stabilization of lamellae in carcinoma cells by stimulating cyclic AMP-related and phosphoinositide 3-OH kinase pathways. 30-32 It is intriguing to speculate that the surface relocation and release of α6β4 integrin from hemidesmosome-like structures coincides with its signaling to these pathways during epithelial wound healing.
Antibody inhibition assays also demonstrate that α3β1 integrin participates in lamellae formation. Images presented here suggest that α3β1 integrin plays a prominent role in lamellae formation because they show this integrin concentrated at the leading edge of small protrusions where actin filaments terminate. Similarly, α3β1 integrin associates with actin in keratinocytes and in doing so participates in cell spreading as opposed to cell anchoring. 23 Its role in lamellae formation may also help explain the finding that α3β1 integrin is important for pancreatic carcinoma cell migration. 33 The observed concentration of α3β1 integrin at the leading edge of small lamellae suggests that it moves over the cell surface during T84 wound resealing similarly to β1 integrins during fibroblast migration. 34 In fibroblast migration, integrins are preferentially transported to the cell’s leading edge where they bind to their extracellular matrix ligand and then associate with the actin cytoskeleton. The actin cytoskeleton exerts a rearward force on the adherent integrin resulting in the traction required to move the cell forward. Our data indicate that α3β1 integrin on T84 cells preferentially moves toward the leading edge of emerging lamellae. We hypothesize that it then binds to laminin-5, one of its ligands, helping to generate the traction or a pulling force required for lamella growth. 35 Eventually, this force is transmitted rearward and serves to pull on surrounding cells.
Our findings on T84 epithelial sheet wound healing can be summarized in the following model. On injury, the monolayer first retracts. Retraction ceases, at least in part, due to the adhesion mediated by α6β4 integrin in type II hemidesmosomes on the basal surface of the cells. Once retraction ceases, the actin purse string exerts a pulling force on the monolayer. The result is that cells flatten into the denuded area and the wound begins to contract. Type II hemidesmosomes remain intact during this process even though the cells stretch to cover 2× more surface area. Some wounds do not close entirely by the efforts of actin purse strings and, in these cases, lamellae are extended by marginal cells. During lamellae extension, type II hemidesmosomes disassemble and actin suspension cables are formed. Disassembly of type II hemidesmosomes is part of lamellae formation and is accompanied by redistribution of the α6 integrins. α3β1 integrin contributes to the initial phase of lamellae protrusion by helping to create the adhesive traction required to form actin suspension cables. The actin suspension cables pull on the rearward cells and flatten them, similarly to the actin purse string, creating more cytoplasmic coverage of the denuded area.
Footnotes
Address reprint requests to Margaret M. Lotz, Dana Bldg., Research West, Room 813, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215. E-mail: mlotz@caregroup.harvard.edu.
M. M. L. was supported by a Crohn’s and Colitis Foundation of America Career Development Award. I. R. and A. M. were supported by National Institutes of Health grant CA44704.
References
- 1.Svanes K, Ito S, Takeucchi D, Silen W: Restitution of the surface epithelium of in vitro frog gastric mucosa after damage with hyperosmolar NaCl: morphological and physiological characteristics. Gastroenterology 1982, 82:1409-1426 [PubMed] [Google Scholar]
- 2.Rutten MJ, Ito S: Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol 1983, 244:G171-G182 [DOI] [PubMed] [Google Scholar]
- 3.Lacy ER: Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 1988, 10:S72-S77 [DOI] [PubMed] [Google Scholar]
- 4.Nusrat A, Delp C, Madara JL: Intestinal epithelial restitution: characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 1992, 89:1501-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin P, Lewis J: Actin cables and epidermal movement in embryonic wound healing. Nature 1992, 360:179-183 [DOI] [PubMed] [Google Scholar]
- 6.Bement WM, Forscher P, Mooseker MS: A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol 1993, 121:565-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacy ER, Ito S: Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest 1984, 51:573-583 [PubMed] [Google Scholar]
- 8.Sheetz MP, Felsenfeld DP, Galbraith CG: Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol 1998, 8:51-54 [DOI] [PubMed] [Google Scholar]
- 9.Dipasquale A: Locomotory activity of epithelial cells in culture. Exp Cell Res 1975, 94:191-215 [DOI] [PubMed] [Google Scholar]
- 10.Buck RC: Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Visual Sci 1979, 18:767-784 [PubMed] [Google Scholar]
- 11.Moore R, Carlson S, Madara JL: Rapid barrier restitution in an “in vitro” model of intestinal epithelial injury. Lab Invest 1989, 60:237-244 [PubMed] [Google Scholar]
- 12.Danjo Y, Gipson IK: Actin purse string filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci 1998, 111:3323-3331 [DOI] [PubMed] [Google Scholar]
- 13.Madara JL, Stafford J, Dharmsathaphorn K, Carlson S: Structural analysis of a human intestinal epithelial cell line. Gastroenterol 1987, 92:1133-1145 [DOI] [PubMed] [Google Scholar]
- 14.Lotz MM, Nusrat A, Madara JL, Ezzell R, Wewer UM, Mercurio AM: Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol 1997, 150:747-760 [PMC free article] [PubMed] [Google Scholar]
- 15.Uematsu J, Nishizawa Y, Sonnenberg A, Owaribe K: Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J Biochem 1994, 115:469-476 [DOI] [PubMed] [Google Scholar]
- 16.Fontao L, Dirrig S, Owaribe K, Kedinger M, Launay JF: Polarized expression of HD-1: Relationship with the cytoskeleton in cultured human colonic carcinoma cells. Exp Cell Res 1997, 231:319-327 [DOI] [PubMed] [Google Scholar]
- 17.Brock J, Midwinter K, Lewis K, Martin P: Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996, 135:1097-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W-T: Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol 1981, 90:187-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauffenberger DA, Horwitz AF: Cell migration: a physically integrated molecular process. Cell 1996, 84:359-369 [DOI] [PubMed] [Google Scholar]
- 20.Svitkina TM, Verhovsky AB, McQuade KM, Borisy GG: Analysis of the actin-mysosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol 1997, 139:397-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK: α6β4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci USA 1990, 87:8970-8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LMH, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, Garrod D: Integrin α6β4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol 1991, 113:907-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA: Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol 1990, 111:3141-3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurpakus MA, Quaranta V, Jones JCR: Surface relocation of α6β4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol 1991, 115:1737-1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowling J, Yu Q-C, Fuchs E: β4 integrin is required for hemidesmosome formation, cell adhesion, and cell survival. J Cell Biol 1996, 134:559-572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A: Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet 1993, 13:366-369 [DOI] [PubMed] [Google Scholar]
- 27.Green JK, Jones JCR: Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB J 1996, 10:871-881 [DOI] [PubMed] [Google Scholar]
- 28.Neissen CM, Hogervost F, Jaspars LH, de Melker AA, Delwell GO, Hulsman EHM, Kuikman I, Sonnenberg A: The α6β4 integrin is a receptor for both laminin and kalinin. Exp Cell Res 1994, 211:360-367 [DOI] [PubMed] [Google Scholar]
- 29.Gipson IK, Spurr-Michaud S, Tisdale A, Elwell J, Stepp MA: Redistribution of the hemidesmosome components α6β4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp Cell Res 1993, 207:86-98 [DOI] [PubMed] [Google Scholar]
- 30.Shaw LM, Rabinovitz I, Wang HH-F, Toker A, Mercurio AM: Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 1997, 91:949-960 [DOI] [PubMed] [Google Scholar]
- 31.Rabinovitz I, Mercurio AM: The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol 1997, 139:1873-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor KL, Shaw L, Mercurio AM: Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and chemotactic migration of invasive carcinoma cells. J Cell Biol 1998, 143:1749-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I: Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5 and migrate on the newly deposited basement membrane. Am J Pathol 1997, 151:1289-1302 [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt CE, Horwitz AF, Lauffenberger DA, Sheetz MP: Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric and regulated. J Cell Biol 1993, 123:977-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter WG, Ryan MC, Gahr PJ: Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell 1991, 65:599-610 [DOI] [PubMed] [Google Scholar]


