Abstract
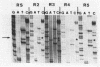
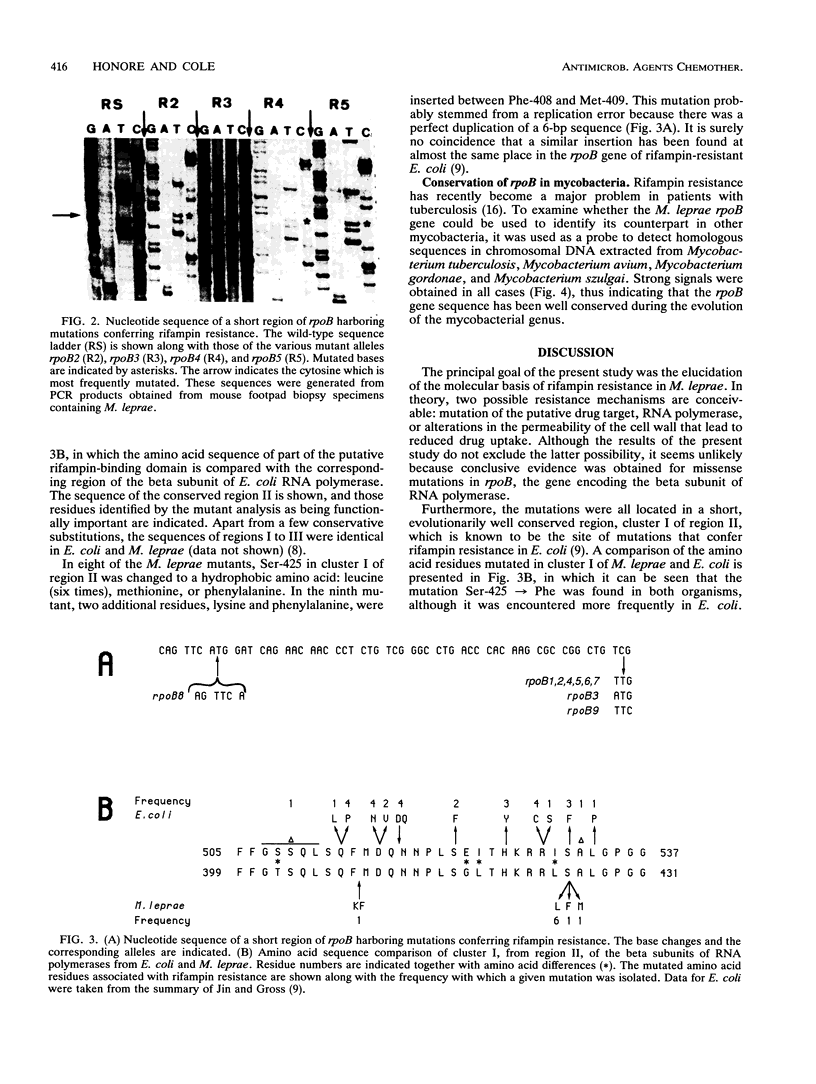
Rifampin is currently the most potent drug used in leprosy control programs. We show that the rifampin resistance which emerged in nine patients with lepromatous leprosy, who had received rifampin monotherapy, stemmed from mutations in the rpoB gene, which encodes the beta subunit of RNA polymerase of Mycobacterium leprae. In eight cases missense mutations were found to affect a serine residue, Ser-425, while in the remaining mutant a small insertion was found close to this site. These findings will be of use for the development of a rapid screening procedure, involving the polymerase chain reaction, for monitoring the emergence of rifampin-resistant M. leprae strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Walsh G. P. Conservation of genomic sequences among isolates of Mycobacterium leprae. J Bacteriol. 1989 Sep;171(9):4844–4851. doi: 10.1128/jb.171.9.4844-4851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Cole S. T. Towards the integration of foreign DNA into the chromosome of Mycobacterium leprae. Res Microbiol. 1991 Jul-Aug;142(6):617–622. doi: 10.1016/0923-2508(91)90074-k. [DOI] [PubMed] [Google Scholar]
- Falkenburg D., Dworniczak B., Faust D. M., Bautz E. K. RNA polymerase II of Drosophila. Relation of its 140,000 Mr subunit to the beta subunit of Escherichia coli RNA polymerase. J Mol Biol. 1987 Jun 20;195(4):929–937. doi: 10.1016/0022-2836(87)90496-7. [DOI] [PubMed] [Google Scholar]
- Grosset J. H., Guelpa-Lauras C. C., Bobin P., Brucker G., Cartel J. L., Constant-Desportes M., Flageul B., Frédéric M., Guillaume J. C., Millan J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int J Lepr Other Mycobact Dis. 1989 Sep;57(3):607–614. [PubMed] [Google Scholar]
- Honoré N., Bergh S., Chanteau S., Doucet-Populaire F., Eiglmeier K., Garnier T., Georges C., Launois P., Limpaiboon T., Newton S. Nucleotide sequence of the first cosmid from the Mycobacterium leprae genome project: structure and function of the Rif-Str regions. Mol Microbiol. 1993 Jan;7(2):207–214. doi: 10.1111/j.1365-2958.1993.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Ji B., Grosset J. H. Recent advances in the chemotherapy of leprosy. Lepr Rev. 1990 Dec;61(4):313–329. doi: 10.5935/0305-7518.19900029. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J Bacteriol. 1989 Sep;171(9):5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Levy L. Studies of the mouse foot pad technique for cultivation of Mycobacterium leprae. 3. Doubling time during logarithmic multiplication. Lepr Rev. 1976 Jun;47(2):103–106. doi: 10.5935/0305-7518.19760019. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A., Sverdlov E. D., Moiseyeva E. P., Danilevskaya O. N., Nikiforov V. G. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196(1):173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Lipkin V. M., Sverdlov E. D., Kiver I. F., Bass I. A., Mindlin S. Z., Danilevskaya O. N., Khesin R. B. Primary structure of Escherichia coli RNA polymerase nucleotide substitution in the beta subunit gene of the rifampicin resistant rpoB255 mutant. Mol Gen Genet. 1981;184(3):536–538. doi: 10.1007/BF00352535. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Guriev S. O., Kalinina N. F., Sverdlov E. D., Gragerov A. I., Bass I. A., Kiver I. F., Moiseyeva E. P., Igumnov V. N. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol Gen Genet. 1983;190(2):344–348. doi: 10.1007/BF00330662. [DOI] [PubMed] [Google Scholar]
- Snider D. E., Jr, Roper W. L. The new tuberculosis. N Engl J Med. 1992 Mar 5;326(10):703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Gillis T. P., Portaels F. Geographically distinct isolates of Mycobacterium leprae exhibit no genotypic diversity by restriction fragment-length polymorphism analysis. Mol Microbiol. 1990 Oct;4(10):1653–1659. doi: 10.1111/j.1365-2958.1990.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Woods S. A., Cole S. T. A family of dispersed repeats in Mycobacterium leprae. Mol Microbiol. 1990 Oct;4(10):1745–1751. doi: 10.1111/j.1365-2958.1990.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Woods S. A., Cole S. T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol Lett. 1989 Dec;53(3):305–309. doi: 10.1016/0378-1097(89)90235-8. [DOI] [PubMed] [Google Scholar]
- Woychik N. A., Young R. A. RNA polymerase II: subunit structure and function. Trends Biochem Sci. 1990 Sep;15(9):347–351. doi: 10.1016/0968-0004(90)90074-l. [DOI] [PubMed] [Google Scholar]
- Yepiz-Plascencia G. M., Radebaugh C. A., Hallick R. B. The Euglena gracilis chloroplast rpoB gene. Novel gene organization and transcription of the RNA polymerase subunit operon. Nucleic Acids Res. 1990 Apr 11;18(7):1869–1878. doi: 10.1093/nar/18.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]