Abstract
Mesenchymal chondrosarcomas are small-cell malignancies named as chondrosarcomas due to the focal appearance of cartilage islands. In this study, the use of in situ detection techniques on a large series of mesenchymal chondrosarcoma specimens allowed the identification of tumor-cell differentiation pathways in these neoplasms. We were able to trace all steps of chondrogenesis within mesenchymal chondrosarcoma by using characteristic marker genes of chondrocytic development. Starting from undifferentiated cells, which were negative for vimentin and any other mesenchymal marker, a substantial portion of the cellular (undifferentiated) tumor areas showed a chondroprogenitor phenotype with an onset of expression of vimentin and collagen type IIA. Cells in the chondroid areas showed the full expression panel of mature chondrocytes including type X collagen indicating focal hypertrophic differentiation of the neoplastic chondrocytes. Finally, evidence was found for transdifferentiation of the neoplastic chondrocytes to osteoblast-like cells in areas of neoplastic bone formation. These results establish mesenchymal chondrosarcoma as the very neoplasm of differentiating premesenchymal chondroprogenitor cells. The potential of neoplastic bone formation in mesenchymal chondrosarcoma introduces a new concept of neoplastic (chondrocytic) osteogenesis in musculoskeletal malignant neoplasms, which qualifies the old dogma that neoplastic bone/osteoid formation automatically implies the diagnosis of osteosarcoma.
Mesenchymal chondrosarcoma is an uncommon malignant chondrogenic neoplasm with an overall poor prognosis. 1-3 It represents about 1% of all chondrosarcomas 1,4,5 and affects all ages (5 to 74 years) with a peak occurrence in the second decade of life. 3 As a peculiarity of this neoplasm, about one third of the cases develop outside the bone including a significant number arising in the meninges. 1,5-9
Mesenchymal chondrosarcoma was first described as mesenchymoma/polyhistioma by Jacobson 10 and is composed of two characteristic tumor components: one being highly cellular and the other showing cartilage formation with abundant extracellular matrix.
So far, only histological and ultrastructural studies have been performed 5,11-14 and no studies on the biochemical composition of the extracellular tumor matrix and pattern of cell differentiation in mesenchymal chondrosarcomas are available. Hence, cell differentiation is so far poorly understood. 15 To define a phenotypic profile of these heterogeneous tumors, techniques allowing in situ analysis on the cellular level are required. This is not possible with conventional biochemical or molecular techniques. Here, therefore, besides conventional histological and histochemical techniques we used in situ localization methods for both, protein and mRNA, enabling identification of matrix components and their gene expression pattern in correlation to the different tumor compartments. More importantly, these techniques enable the identification of the cellular differentiation pattern in situ. This is particularly practical for studying chondrogenesis because distinct markers of different developmental stages of chondrogenic cell differentiation have been identified (for review see Cancedda and colleagues 16 ). Thus, chondroprogenitor cells in the limb-bud mesenchyme start to express a specific splice variant of collagen type II (COL2A) 17,18 even before any cartilage matrix formation is observable. Differentiated chondrocytes form abundant, histologically visible extracellular cartilage matrix by expressing collagen types II (COL2B), IX (COL9), and XI (COL11) as well as the cartilage-typical large aggregating proteoglycan aggrecan. 19 In the terminal phase of chondrocyte differentiation, the cells become hypertrophic and start to express type X collagen (COL10). 20,21 Most of the terminally differentiated chondrocytes in the fetal growth plate subsequently undergo apoptotic cell death. More recent experimental evidence suggests, however, that at least part of these cells can transdifferentiate to osteoblast-like cells. 22,23 Transdifferentiated chondrocytes can be identified by the onset of the expression of type I collagen (COL1) and the deposition of bone matrix. Thus, chondrogenesis and endochondral bone formation can be traced on the basis of specific marker gene products such as COL2A for chondroprogenitor cells and COL10 for terminally differentiated hypertrophic chondrocytes. In our recent work, we were able to phenotype neoplastic cells of conventional and clear cell chondrosarcomas using these marker genes. 24,25 In this study, we analyzed the expression of these marker genes in a large series of mesenchymal chondrosarcomas to establish matrix biochemistry and cell differentiation pattern in these neoplasms.
Materials and Methods
Tissue Preparation and Histology
Forty-eight specimens of 25 patients with mesenchymal chondrosarcomas from the Mayo bone tumor registry (Rochester, MN) and the Department of Pathology, University of Erlangen-Nürnberg, Germany, were used for the study. Twenty-nine specimens derived from primary, six specimens from recurrences, and 10 specimens from metastatic lesions. Nineteen specimens derived from primary skeletal lesions and seven from primary soft-tissue lesions (one of them meningeal). The material was routinely fixed with 10% formalin, decalcified, and embedded in paraffin. Five-μm-thick paraffin sections were cut and stored at room temperature until use. Conventional hematoxylin and eosin (H&E) staining was performed to establish the diagnosis according to diagnostic criteria described elsewhere 26 and to evaluate histomorphological features of the neoplasms.
Histochemical Methods
Histochemical techniques were used to estimate the total content of cartilage-typical glycosaminoglycans and collagens on a semiquantitative basis (see Table 2 ▶ ).
Table 2.
Distribution of Cytoproteins and Extracellular Matrix Components in Matrix-Poor and Matrix-Rich Small-Cell, Cartilaginous, and Bone-Forming Tumor Compartments in Mesenchymal Chondrosarcomas
| Areas | Vim | S-100 | Col | GAGs Agg | COL1 | COL2 | COL2A | COL3 | COL6 | COL10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Matrix-poor small cell areas | − | − | − | − | (+) | (+) | (++) | (+) | (+) | − |
| Matrix-rich small cell areas | + | −* | ++ | (+) | (+) | ++ | +++ | (+) | (+) | −* |
| Cartilaginous | ++ | ++ | +++ | +++ | (+) | +++ | + | (+) | ++pc | (+++)† |
| Bony | ++ | (+) | +++ | (pc) | +++ | (pc) | (pc) | pc | pc | (pc) |
+++, strongly positive; ++, positive; +, weakly positive; −, negative; 0, focally positive; vim, vimentin; col, collagen content; GAGs, glycosaminoglycans; agg, aggrecan; I (II, IIA, III, VI, X); collagen type I (II, IIA, III, VI, X).
*Only single large rounded cells positive.
†In particular calcified areas.
Glycosaminoglycans
The cartilage-typical glycosaminoglycans were visualized by toluidine blue staining (10 minutes, 0.3% toluidine blue [Merck, Germany]; pH 3.65, room temperature). 27
Collagens
The presence of collagens in the extracellular tumor matrix was demonstrated by Masson-Goldner’s stain.
Immunohistochemistry
Deparaffinized sections were enzymatically pretreated (Table 1) ▶ , incubated with primary antibodies (Table 1) ▶ overnight at 4°C, and visualized using a streptavidin-biotin-complex technique (Super Sensitive Immunodetection System for mouse or rabbit primary antibodies; Biogenex, Mainz, Germany) with alkaline phosphatase as detection enzyme and 3-hydroxy-2-naphthylacid 2,4-dimethylanilid as substrate. Nuclei were counterstained with hematoxylin.
Table 1.
Primary Antibodies and Enzymatic Pretreatments Used for Immunohistochemical Analysis
| Antigen | Type | Dilution | Digestion | Source |
|---|---|---|---|---|
| Vimentin (V9.1) (human) | m | 1:200 | Pt | Dako (Denmark) |
| S-100 protein (bovine) | r | 1:20000 | P | Dako (Denmark) |
| Collagen I (human) | r | 1:200 | H, Pt | Synbio (Germany) |
| Collagen II (chick) | m | 1:50 | H, P | Dr. Holmdahl53 (Uppsala, Sweden) |
| Collagen IIA (human) | p | 1:1000 | H, Pt | Dr. L. Sandell54 |
| Collagen III (human) | r | 1:2000 | H, P | Dr. Günzler (Höchst, Frankfurt, FRG) (prepared and characterized according to Nowack et al55) |
| Collagen VI (human) | r | 1:5000 | H, P | Dr. R. Timpl (MPI for Biochemistry, Munich, FRG)56 |
| Collagen X (X-36, X-54) (human) | m | 1:100 | H, Pt | Dr. von der Mark (Erlangen, Germany)57 |
| Aggrecan (5G5) (human) | m | 1:5000 | H, Pt | Dr. R. Perris (Avioli, Italy; manuscript in preparation) |
m, mouse monoclonal; r, rabbit polyclonal; H, hyaluronidase (ovine testis in 2 mg/ml, phosphate-buffered saline, pH 5, 60 minutes at 37°C; Sigma, Deisenhofer, Germany); P, pronase (2 mg/ml, phosphate-buffered saline, pH 7.3, 60 minutes at 37°C; Boehringer Mannheim); P, pepsin (0.4% in 0.01N HCl; Sigma); Pt, protease XXIV (0.02 mg/ml, phosphate-buffered saline, pH 7.3, 60 minutes at RT; Sigma).
As negative control for immunohistochemical stainings, the primary antibody was replaced by nonimmune mouse or rabbit serum (BioGenex, San Ramon, CA) or Tris-buffered saline (pH 7.2) in selected cases. Specificity of antibodies was tested by using test tissues (eg, fetal growth-plate cartilage) with established staining pattern in parallel experiments.
cDNA Probe Generation
Suitable fragments of human collagen chains α1(I), α1(II), and α1(X), and aggrecan core protein mRNA were selected and transcribed in vitro to generate digoxigenin-labeled antisense and sense riboprobes as described previously. 19,28 The rather long (>1 kb) primary transcripts for aggrecan core protein and type X collagen were reduced to an average length of 300 bp by standard alkaline hydrolysis.
To control probe specificity, all probes were tested on fetal growth-plate specimens in parallel experiments. 19 A probe for 18S rRNA 29 was used as a positive control. Negative samples were discarded from in situ mRNA analysis.
In Situ Hybridization
In situ hybridization was performed as described elsewhere. 28 Briefly, deparaffinized and rehydrated sections were digested with proteinase K (200 μg/ml in 50 mmol/L Tris), postfixed in paraformaldehyde, acetylated, and dehydrated. The sections were hybridized for 12 to 16 hours at 44°C with riboprobes (final concentration, 1 ng/ml) in ECL-gold-hybridization buffer (Amersham, Freiburg, Germany) supplemented with 0.3 mol/L NaCl. After hybridization, the tissue sections were washed at 40°C in 1× standard saline citrate (SSC) and 0.3× SSC, treated with RNases A and T1, and washed again at 50°C in 0.1× SSC. The immunological detection of the digoxigenin-labeled probes was performed using the Digoxigenin-Detection-Kit (Boehringer-Mannheim, Mannheim, Germany). The exposure time was 3 days for all three probes.
Determination of Programmed Cell Death (Apoptosis)—TUNEL-Reaction
For the detection of in situ DNA breaks, the TUNEL-reaction was applied using the Apoptosis-Detection Kit from Oncor (Gaithersburg, MD). According to the suggestions of the manufacturer, the proteinase K pretreatment as well as the terminal deoxytransferase concentration was carefully titrated to ensure specificity and sensitivity of the procedure. Control sections of fetal growth-plate cartilage were processed in parallel revealing specific apoptotic labeling selectively in the lower hypertrophic zone.
Results
The main results of the study are summarized in Table 2 ▶ . These results were consistent among the specimens examined demonstrating a high consistency regarding the various phases of mesenchymal differentiation in between the different specimens.
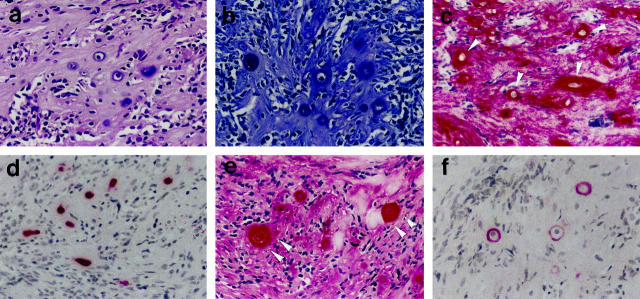
Histomorphologically, the investigated mesenchymal chondrosarcomas showed the typical morphological features of this tumor entity. In principle, in most samples two tumor compartments could be distinguished as described previously: 5,26 First, the noncartilaginous compartment showed either loose sheets of small neoplastic cells (Figure 1a ▶ , lower part) or small cells located around sinusoidal vascular proliferates (hemangiopericytoma-like pattern; Figure 1a ▶ , upper part). The abundance of the extracellular tumor matrix varied in these areas from hardly any to moderate (Figure 1h) ▶ . Notably, rarely single larger round cells surrounded by a rim of hyaline matrix forming a lacunar space were found in the areas showing more abundant extracellular matrix (Figure 2a) ▶ .
Figure 1.
Analysis of small-cell areas. a: H&E staining showing small-cell areas with and without hemangiopericytoma-like growth pattern (upper and lower part). h: H&E staining showing moderately abundant extracellular tumor matrix in small-cell areas. b, c: Immunostaining for vimentin with negativity of tumor cells in the matrix-poor (b: only cells of the vasculature positive) and positivity in the matrix-rich small-cell tumor areas (c). d–g, i, j: Immunodetection showing the absence of COL2 (d), COL10 (e), and aggrecan (f) in matrix-poor small-cell tumor areas, the weak presence of aggrecan (g) and the strong presence of COL2 (i) and COL2A (j) in the matrix-rich small-cell tumor areas. Original magnifications: a, d–g, i, j, ×50; b, c, h, ×100.
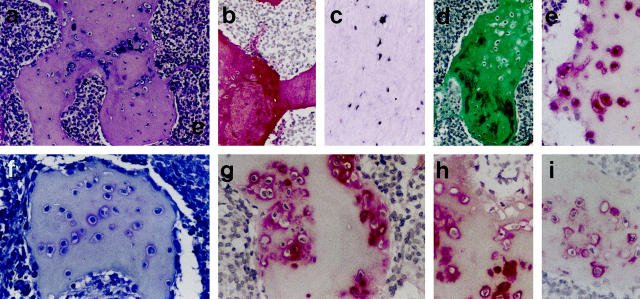
Figure 2.
Analysis of single chondrocytic tumor cells in otherwise matrix-rich small-cell areas. a: Conventional H&E staining. b: Histochemical demonstration of pericellular glycosaminoglycans (toluidine blue). c, e, f: Immunodetection of aggrecan (c), COL2 (e), and COL10 (f) in the extracellular tumor matrix. d: Immunodetection of S-100 protein selectively in the neoplastic chondrocytic cells. Original magnification, ×100.
Secondly, areas of cartilaginous matrix formation with cells sitting in lacunae similar to chondrocytes in physiological fetal cartilage were found (Figure 3e) ▶ . Within the cartilaginous compartment of some samples, focal calcification and areas of bone formation occurred. The presence of mature bone matrix was confirmed by polarized light microscopy. The transition in between small-cell areas and cartilage and bone formation was either rather sharp or smooth.
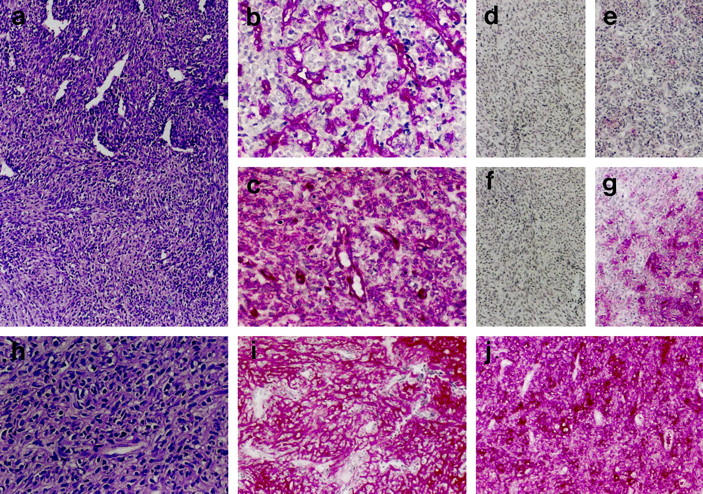
Figure 3.
Analysis of cartilaginous tumor areas. a: Immunodetection of S-100 protein selectively in the cells of cartilaginous areas (in this case islands within cellular areas). b–d, f, g: Immunodetection of COL2A (b: note COL2A largely absent), COL6 (c), COL2 (f), and COL10 (g) as well as aggrecan (d). e: Histochemical demonstration of glycosaminoglycans (toluidine blue). l: TUNEL-positive apoptotic cells in cartilaginous areas (k: same area stained with H&E on a parallel section). Original magnifications: a–f, ×50; g–l, ×100.
Histochemical matrix analysis showed the absence of glycosaminoglycan staining in the cellular areas. Focal staining was found in the matrix-rich small-cell areas, in particular around the large single cells (Figure 2b) ▶ . Abundant staining was seen in the cartilaginous tumor areas (Figure 3e) ▶ . Collagen staining was not significant in the cellular matrix-poor areas and moderate to high in the matrix-rich cellular and cartilaginous areas, respectively. The highest amount of collagens was seen in areas of bone matrix formation (Figure 4d) ▶ . Here some cells showed pericellular staining for glycosaminoglycans (Figure 4f) ▶ whereas others as well as the bone matrix were negative in the toluidine blue reaction.
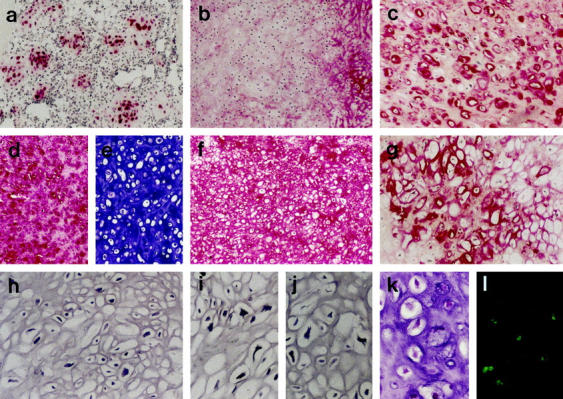
Figure 4.
Analysis of a bone-forming tumor area. a: H&E staining. d: Histochemical demonstration of abundant collagen in the bony tumor matrix (van Gieson’s stain). b, g–i: Immunodetection of COL1 (b), COL2 (h), and COL10 (i) as well as aggrecan (g). COL2, COL10, and aggrecan were seen around some of the osteocytic cells. f: Histochemical demonstration of glycosaminoglycans (toluidine blue). e: immunodetection of S-100 protein in part of the osteocytic cells. Original magnifications: a, b, d, ×50; c, e–i, ×100.
Matrix Protein Analysis—Collagen Subtypes
No principal difference in matrix composition was found in the different small-cell tumor areas irrespective of their morphological appearance, hemangiopericytoma-like or not.
Cellular areas, in which hardly any collagen could be detected histochemically, showed no or only minor amounts of COL1, COL2, COL2A, COL3, and COL6 (Figure 1d, 1e) ▶ and were consistently negative for COL10.
Cellular areas, in which histochemically significant intercellular collagen was detectable, significant staining for COL2 (Figure 1i) ▶ , in particular COL2A (Figure 1j) ▶ , was detectable together with some staining for COL1, COL3, and COL6. COL10 was again not detectable. The large round single cells in these areas were surrounded by a COL6 pericellular matrix. Further away from the cells, but still in their immediate neighborhood, COL2 (Figure 2c) ▶ and less often COL10 (Figure 2f) ▶ were found.
The cartilaginous tumor areas showed strong staining for COL2 throughout the extracellular tumor matrix (Figure 3f) ▶ . Notably, a much less intense or absent staining was found in these areas for the isoform COL2A (Figure 3b) ▶ indicating a switch to predominantly the COL2B variant in these areas, which is characteristic for fully differentiated chondrocytes. The cells were mostly lying in cell lacunae and were surrounded by a COL6 positive pericellular matrix (Figure 3c) ▶ . Multifocally, COL10 deposition was found including the areas of matrix calcification (Figure 3g) ▶ , which expression of COL10 seemed to precede.
In areas of bone formation, the bone matrix was, as expected, positive for COL1, but negative for COL2 or COL10. This was easily seen in one of the cases which showed areas of organoid bone formation (Figure 4a) ▶ . Also here, the bone matrix was positive for COL1 (Figure 4b) ▶ and largely negative for COL2 and COL10. But both collagen types were, however, found in the pericellular matrix of some cells within the bone (Figure 4h, 4i) ▶ suggesting the chondrocytic origin of these cells. COL3 and COL6 were found in the pericellular area of the newly formed bone, but not within the bone matrix itself.
Matrix Proteoglycan Analysis
Immunodetection for aggrecan proteoglycan showed a distribution of aggrecan core protein virtually identical to the histochemical glycosaminoglycan staining. No aggrecan was detectable in the matrix-poor cellular areas (Figure 1f) ▶ . Focal staining was visible in the matrix-rich, histochemically glycosaminoglycan-positive areas (Figure 1g) ▶ , in particular around the single round cells (Figure 2e) ▶ . Overall, clearly less staining for aggrecan (Figure 1g) ▶ was found compared to type II collagen in the small-cell areas (Figure 1i) ▶ . The extracellular matrix in cartilaginous areas was strongly stained for aggrecan (Figure 3d) ▶ . The neoplastic bone was again negative for aggrecan except the pericellular area around some tumor cells (Figure 4g) ▶ .
Gene Expression Analysis
In situ hybridization analysis on the mRNA level confirmed the expression pattern found by immunoanalysis. COL2 mRNA expression was localized to the matrix-rich small-cell and in the cartilaginous areas (Figure 3i) ▶ . COL10 mRNA was restricted to the cartilaginous areas, in particular the foci of beginning or ongoing matrix calcification (Figure 3j) ▶ . Type I collagen mRNA expression was seen in areas of bone formation (Figure 4c) ▶ . Aggrecan mRNA expression was observed mostly in the chondrocytic cells of cartilaginous areas (Figure 3h) ▶ . Most small cells as well as all nonneoplastic cells were negative for aggrecan mRNA.
Cytoprotein Analysis
Immunodetection of vimentin was positive in the matrix-rich noncartilaginous small-cell (Figure 1c) ▶ , cartilaginous, and osteoid areas, but not in the cellular matrix-poor areas (Figure 1b) ▶ . S-100 protein was positive in the single rounded cells in the matrix-rich small-cell areas (Figure 2d) ▶ and in most cells of the cartilaginous areas (Figure 3a) ▶ as well as some cells in the areas of neoplastic bone formation (Figure 4e) ▶ . Small cells and the majority of osteoblast-like cells were negative for S-100 protein.
Analysis of Apoptotic Cell Death
DNA fragmentation detected by the TUNEL-reaction, indicated apoptotic cell death throughout the tumor, but was most prominent in the chondroid tumor areas (Figure 3k, 3l) ▶ .
Discussion
This study identifies mesenchymal chondrosarcoma as the neoplasm of very early prechondrogenic cells, which multifocally undergo full chondrocytic differentiation analogous to limb bud development. The most undifferentiated cells are even vimentin-negative and express none of the chondrocytic marker genes and hardly any matrix components at all. A large proportion of the morphologically undifferentiated cells, however, expresses the marker of chondroprogenitor cells, COL2A 17 and also vimentin. The expression of COL2B together with aggrecan proteoglycan is the hallmark of differentiated chondrocytes in the areas of microscopically visible cartilaginous matrix formation. In these areas, cells are positive for S-100 protein, which is one marker for physiological and neoplastic chondrocytic differentiation. 30,31 The formed cartilage shows histochemically and immunohistochemically all features of fetal hyaline cartilage, thus, confirming previous morphological and ultrastructural studies. 14,32,33 Also, type VI collagen is concentrated pericellularly in these areas, which is characteristic for cartilage and most likely involved into the formation of the cartilage-typical cell lacunae found in the cartilaginous tumor areas. 34,35 Significant portions of the neoplastic chondrocytes in mesenchymal chondrosarcomas become hypertrophic as indicated by the increased cell size and demonstrated by the expression of COL10. In these areas, the cartilage matrix calcifies 1,26,36-38 as does the COL10-positive hypertrophic cartilage in the fetal growth plate. 21 Similar to the terminally differentiated hypertrophic chondrocytes in the fetal growth plate, 39,40 a significant number of the neoplastic chondrocytes of mesenchymal chondrosarcoma undergo apoptotic cell death. In some cases, however, focal bone formation is also observed, 15,26,36 which at least in part is suggested by our data to result from transdifferentiation of neoplastic chondrocytes into osteoblast-like cells. This has been recently shown to be a potential differentiation pathway of hypertrophic chondrocytes in vivo and in vitro. 22,23 Although recruitment of nonneoplastic cells for osteogenesis cannot be excluded by our study, part of the osteogenic cells are positive for S-100 protein and pericellularly for aggrecan, COL2, and COL10. All of these features could best be explained as remnants from a previous chondrocytic differentiation of the now osteocytic cells, although final proof would require a sequential analysis of cell differentiation, which is not feasible with our techniques. Our study suggests a new concept of neoplastic bone formation, ie, chondrocytic neoplastic bone formation as opposed to osteoblastic neoplastic bone formation found in primary osteogenic tumors: chondrosarcomas can form bone matrix (osteoid), qualifying the old dogma, that neoplastic bone formation directly implicates osteosarcoma irrespective of cartilaginous tumor compartments.
The outlined differentiation processes involve mostly larger cell groups, but on occasion isolated cells in noncartilaginous areas also undergo similar cellular processes. They lie in typical cell lacunae, express COL2 as well as aggrecan proteoglycan, and thus, are surrounded by a cartilaginous matrix. 41 They are also positive for S-100 protein and vimentin 41 and show COL6 pericellularly. Part of them even differentiate to hypertrophic chondrocytes, expressing COL10.
Our results explain and specify previous findings on the histochemical and ultrastructural level. Although the extracellular tumor matrix is partly very sparse in some small-cell tumor areas, almost lacking collagen fibrils, 32 it is rather abundant in most parts of the small-cell tumor compartment. 9,12,33 The extracellular tumor matrix is clearly cartilaginous in areas of chondroid differentiation 14,32,33 and contains glycosaminoglycan-rich proteoglycans, 12,41-43 which were identified at least in part as aggrecan in our study. The fine filamentous pericellular material 12,14 observed in these areas is most likely the ultrastructural correlate to COL6, which is known to form fine filamentous networks around chondrocytic cells. 44 The observation that collagen fibrils throughout the tumor are thin and of the same appearance 12,14,32 fits very well to our finding that COL2/2A, which is known to form rather thin fibrils, 45,46 is found throughout most tumor areas.
Our findings support ultrastructural data suggesting that the primary tumor cell represents a very primitive mesenchymal cell type. 38 In a later stage of the development, chondroprogenitor cells 32,47 are found. In cartilaginous areas, the cells are ultrastructurally and functionally similar to fully differentiated chondrocytic cells. 32 The notion of mesenchymal chondrosarcoma as a neoplasm of focally differentiating prechondrogenic cells is also supported by the cytoprotein profile of the cells. We and others found S-100 protein confined to cells of chondrocytic differentiation. 13,42,48 Thus, S-100 is a marker of differentiated chondrocytes and not chondroprogenitor cells, which fits to the negativity of S-100 protein in the epichondral cells in the fetal growth plate (our unpublished results), which are supposed to represent chondroprogenitor cells.
The outlined broad range in cellular differentiation features are the biological explanation of the heterogeneity of the morphology and the nondiagnostic radiographic picture of mesenchymal chondrosarcomas. 15,26 So far, the clinical and biological significance of the different morphological appearances of, eg, the small-cell components—cellular versus hemangiopericytoma-like—are unclear. 15 Notably, in our study, we did not find any significant difference between the different growth patterns as far as extracellular matrix expression and cell differentiation is concerned.
The fact that many mesenchymal chondrosarcomas arise outside the bony skeleton and even in the meningeal areas supports the notion that early mesenchymal precursor cells are not restricted to bone, but found throughout the body. 13,49-51 The factors involved in these differentiation processes are rather unclear at the moment and a matter of future studies. They might involve potent factors such as bone morphogenetic proteins. Many members of this protein family have been demonstrated to be able to initiate chondrogenesis in vivo and in vitro 52 and, recently, we were able to show that COL2A is able to bind bone morphogenetic proteins (BMPs). 46 Mesenchymal chondrosarcoma might well be the paradigmatic in vivo model for investigating involved mechanisms.
Currently, the histological diagnosis of bone tumors is nearly exclusively based on conventional histology. In the future, the use of markers of mesenchymal cell differentiation may also have an impact on the differential diagnosis in critical cases. Our study indicates that in mesenchymal chondrosarcomas without overt cartilage formation, which are at present almost impossible to diagnose, 9,48 the staining of COL2 or COL2A might be a safe differential criterion to exclude other small-cell mesenchymal malignancies such as synovial sarcoma, small-cell osteosarcoma, and Ewing’s sarcoma, which histomorphologically might show a similar growth pattern. This, however, would require more extensive studies.
Overall, our study suggests mesenchymal chondrosarcoma as the prototypic in vivo model to study chondrogenesis starting from the earliest stages of undifferentiated precursor cells. It will be highly interesting to elucidate which factors are involved in committing the differentiation at these early stages selectively toward chondrocytic cell differentiation lineage.
Acknowledgments
We thank Dr. L. A. McKenna for critical reviewing of the manuscript and Ms. G. Herbig, Ms. D. Andrischewski, and S. Stegner for expert photographic and technical help.
Footnotes
Address reprint requests to Dr. T. Aigner, Institute of Pathology, University of Erlangen-Nürnberg, Krankenhausstrasse 8–10, D-91054 Erlangen, Germany. E-mail: thomas.aigner@patho.imed.uni-ERLANGEN.DE.
Supported by the Wilhelm Sander-Stiftung, Munich, Germany.
References
- 1.Salvador AH, Beabout JW, Dahlin DC: Mesenchymal chondrosarcoma—observations on 30 new cases. Cancer 1971, 28:605-615 [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Picci P, Bacchini P, Capanna R, Innao V, Bacci G, Campanacci M: Mesenchymal chondrosarcoma of bone and soft tissues. Cancer 1983, 52:533-541 [DOI] [PubMed] [Google Scholar]
- 3.Dabska M, Huvos AG: Mesenchymal chondrosarcoma in the young. A clinicopathological study of 19 patients with explanation of histogenesis. Virchows Arch A Pathol Anat Histopathol 1983, 399:89-104 [DOI] [PubMed] [Google Scholar]
- 4.Huvos AG, Rosen G, Dabska M, Marcove RC: Mesenchymal chondrosarcoma—a clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer 1983, 51:1230-1237 [DOI] [PubMed] [Google Scholar]
- 5.Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC: Mesenchymal chondrosarcoma of bone and soft tissue—a review of 111 cases. Cancer 1986, 57:2444-2453 [DOI] [PubMed] [Google Scholar]
- 6.Guccion JG, Font RL, Enzinger FM, Zimmerman LE: Extraskeletal mesenchymal chondrosarcoma. Arch Pathol Lab Med 1973, 95:336-340 [PubMed] [Google Scholar]
- 7.Rollo JL, Green WR, Kahn LB: Primary meningeal mesenchymal chondrosarcoma. Arch Pathol Lab Med 1979, 103:239-243 [PubMed] [Google Scholar]
- 8.Seth HN, Singh M: Intracranial mesenchymal chondrosarcoma. Acta Neuropathol 1973, 24:86-89 [DOI] [PubMed] [Google Scholar]
- 9.Scheithauer BW, Rubinstein LJ: Meningeal mesenchymal chondrosarcoma. Cancer 1978, 42:2744-2752 [DOI] [PubMed] [Google Scholar]
- 10.Jacobson SA: Polyhistioma. Cancer 1977, 40:2116-2130 [DOI] [PubMed] [Google Scholar]
- 11.Dobin SM, Donner LR, Speights VO: Mesenchymal chondrosarcoma. A cytogentic, immunohistochemical and ultrastructural study. Cancer Genet Cytogenet 1995, 83:56-60 [DOI] [PubMed] [Google Scholar]
- 12.Fu Y-S, Kay S: A comparative ultrastructural study of mesenchymal chondrosarcoma and myxoid chondrosarcoma. Cancer 1974, 33:1531-1542 [DOI] [PubMed] [Google Scholar]
- 13.Kurotaki H, Takeoka H, Takeuchi M, Yagihashi S, Kamata Y, Nagai K: Primary mesenchymal chondrosarcoma of the lung: a case report with immunohistochemical and ultrastructural studies. Acta Pathol Jpn 1992, 42:353-358 [PubMed] [Google Scholar]
- 14.Steiner GC, Mirra JM, Bullough PG: Mesenchymal chondrosarcoma—a study of the ultrastructure. Cancer 1973, 32:926-939 [DOI] [PubMed] [Google Scholar]
- 15.Huvos AG: Bone Tumors. 1991:pp 1-784 W. B. Saunders, Philadelphia
- 16.Cancedda R, Descalzi-Cancedda F, Castagnola P: Chondrocyte differentiation. Int Rev Cytol 1995, 159:265-358 [DOI] [PubMed] [Google Scholar]
- 17.Sandell LJ, Morris NP, Robbins JR, Goldring MB: Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol 1991, 114:1307-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandell LJ, Nalin AM, Reife RA: The alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dys 1994, 199:129-140 [DOI] [PubMed] [Google Scholar]
- 19.Vornehm SI, Dudhia J, von der Mark K, Aigner T: Expression of collagen types IX and XI as well as other major cartilage matrix components by human fetal chondrocytes in vivo. Matrix Biol 1996, 15:91-98 [DOI] [PubMed] [Google Scholar]
- 20.Linsenmayer TF, Chen Q, Gibney E, Gordon MK, Marchant JK, Mayne R, Schmid TM: Collagen types IX and X in the developing chick tibiotarsus: analyses of mRNAs and proteins. Development 1991, 111:191-196 [DOI] [PubMed] [Google Scholar]
- 21.Reichenberger E, Aigner T, von der Mark K, Stöβ H, Bertling W: In situ hybridization studies on the expression of type X collagen in fetal human cartilage. Dev Biol 1991, 148:562-572 [DOI] [PubMed] [Google Scholar]
- 22.Roach HI, Erenpreisa J, Aigner T: Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol 1995, 131:483-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancedda FD, Gentili C, Manduca P, Cancedda R: Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol 1992, 117:427-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aigner T, Dertinger S, Vornehm SI, Dudhia J, von der Mark K, Kirchner T: Phenotypic diversity of neoplastic chondrocytes and extracellular matrix gene expression in cartilaginous neoplasms. Am J Pathol 1997, 150:2133-2141 [PMC free article] [PubMed] [Google Scholar]
- 25.Aigner T, Dertinger S, Belke J, Kirchner T: Chondrocytic cell differentiation in clear cell chondrosarcoma. Hum Pathol 1996, 27:1301-1305 [DOI] [PubMed] [Google Scholar]
- 26.Unni KK: Dahlin‘s bone tumors. 1996:pp 1-463 Lippincott-Raven, Philadelphia-New York
- 27.Shepard N, Mitchell NS: Simultaneous localization of proteoglycan by light and electron microscopy using toluidine blue O—a study of epiphyseal cartilage. J Histochem Cytochem 1976, 24–5:621-629 [DOI] [PubMed] [Google Scholar]
- 28.Aigner T, Neureiter D, Müller S, Küspert G, Belke J, Kirchner T: Extracellular matrix composition and gene expression in collagenous colitis. Gastroenterology 1997, 113:136-143 [DOI] [PubMed] [Google Scholar]
- 29.Aigner T, Stöβ H, Weseloh G, Zeiler G, von der Mark K: Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol 1992, 62:337-345 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Becker LE, Marks A: S-100 protein in tumors of cartilage and bone. Cancer 1983, 52:1820-1824 [DOI] [PubMed] [Google Scholar]
- 31.Stefansson K, Wollmann RL, Moore BW, Arnason BGW: S-100 protein in human chondrocytes. Nature 1982, 295:63-64 [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Tello FJ, Navas-Palacios JJ: Ultrastructural study of conventional chondrosarcomas and myxoid- and mesenchymal-chondrosarcomas. Virchows Arch A Pathol Anat Histopathol 1982, 396:197-211 [DOI] [PubMed] [Google Scholar]
- 33.Sato NL, Minase T, Yoshida Y, Narimatsu E, Muroya K, Asaishi K, Kikuchi K: An ultrastructural study of extraskeletal mesenchymal chondrosarcoma. Acta Pathol Jpn 1984, 34:1355-1366 [DOI] [PubMed] [Google Scholar]
- 34.Poole CA, Ayad S, Schofield JR: Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci 1988, 90:635-643 [DOI] [PubMed] [Google Scholar]
- 35.Hambach L, Neureiter D, Zeiler G, Kirchner T, Aigner T: Severe disturbance of the distribution and expression of type VI collagen chains in osteoarthritic articular cartilage. Arthritis Rheum 1998, 41:986-997 [DOI] [PubMed] [Google Scholar]
- 36.Schajowicz F: Tumors and Tumorlike Lesions of Bone. 1994. Springer-Verlag, Berlin-Heidelberg
- 37.Aikawa T, Shirasuna K, Iwamoto M, Watatani K, Nakamura T, Okura M, Yoshioka H, Matsuya T: Establishment of bone morphogenetic protein 2 responsive chondrogenic cell line. J Bone Miner Res 1996, 11:544-553 [DOI] [PubMed] [Google Scholar]
- 38.Mirra JM: Bone Tumors. 1989. Lea & Febinger, Philadelphia-London
- 39.Zenmyo M, Komiya S, Kawabata R, Sasagur Y, Inoue A, Morimatsu M: Morphological and biochemical evidence for apoptosis in the terminal hypertrophic chondrocytes of the growth plate. J Pathol 1996, 180:430-433 [DOI] [PubMed] [Google Scholar]
- 40.Hatori M, Klatte KJ, Teixeira CC, Shapiro IM: End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J Bone Miner Res 1995, 10:1960-1968 [DOI] [PubMed] [Google Scholar]
- 41.Ushigome S, Takakuwa T, Shinagawa T, Takagi M, Kishimoto H, Mori N: Ultrastructure of cartilaginous tumors and S-100 protein in the tumors. With reference to the histogenesis of chondroblastoma, chondromyxoid fibroma and mesenchymal chondrosarcoma. Acta Pathol Jpn 1984, 34:1285-1300 [DOI] [PubMed] [Google Scholar]
- 42.Okajima K, Honda I, Kitagawa T: Immunohistochemical distribution of S-100 protein in tumors and tumor-like lesions of bone and cartilage. Cancer 1988, 61:792-799 [DOI] [PubMed] [Google Scholar]
- 43.Kindblom LG, Angervall L: Histochemical characterization of mucosubstances in bone and soft tissue tumors. Cancer 1975, 36:985-994 [DOI] [PubMed] [Google Scholar]
- 44.Keene DR, Engvall E, Glanville R: Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamenteous network. J Cell Biol 1988, 107:1995-2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P: Cartilage contains mixed fibrils of collagen types II, IX and XI. J Cell Biol 1989, 108:191-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Oganesian A, Keene DR, Sandell LJ: Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J Cell Biol 1998, 144:1069-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fechner RE, Mills SE: Tumors of the Bones and Joints. 1993. DC, Armed Forces Institute of Pathology, Washington
- 48.Swanson PE, Lillemoe TJ, Manivel JC, Wick MR: Mesenchymal chondrosarcoma. Arch Pathol Lab Med 1990, 114:943-948 [PubMed] [Google Scholar]
- 49.Rushing EJ, Armonda RA, Ansari Q, Mena H: Mesenchymal chondrosarcoma. A clinicopathologic and flow cytometric study of 13 cases presenting in the central nervous system. Cancer 1996, 77:1884-1891 [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Campora R, Otal-Salaverri C, Gomez-Pascual A, Hevia-Vasquez A, Galera-Davidson H: Mesenchymal chondrosarcoma of the retroperitoneum. Acta Cytol 1995, 39:1237-1243 [PubMed] [Google Scholar]
- 51.Shimo-Oku M, Okamoto N, Ogita Y, Sashikata T: A case of mesenchymal chondrosarcoma of the orbit. Acta Ophthalmol Copenh 1980, 58:831-840 [DOI] [PubMed] [Google Scholar]
- 52.Chen P, Carrington JL, Hammonds RG, Reddi AH: Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor β1 and β2. Exp Cell Res 1991, 195:509-515 [DOI] [PubMed] [Google Scholar]
- 53.Klareskog L, Johnell O, Hulth, Holmdahl R, Rubin K: Reactivity of monoclonal anti type II collagen antibodies with cartilage and synovial tissue in rheumatoid arthritis and osteoarthritis. Arthritis Rheum 1986, 29–6:730-738 [DOI] [PubMed] [Google Scholar]
- 54.Oganesian A, Zhu Y, Sandell LJ: Characterization of type type IIA procollagen amino-peptide antiserum and immunofluorescence localization in human cartilageninous and non-cartilageninous embryonic tissues. J Histochem Cytochem 1997, 45:1469-1480 [DOI] [PubMed] [Google Scholar]
- 55.Nowack H, Gay S, Wick G, Becker U, Timpl R: Preparation and use in immunohistology of antibodies specific for type I and III collagen and procollagen. J Immunol Methods 1976, 12:117-124 [DOI] [PubMed] [Google Scholar]
- 56.Specks U, Mayer U, Nischt R, Spissinger T, Mann K, Timpl R, Engel J, Chu M-L: Structure of recombinant N-terminal globule of type VI collagen α3 chain and its binding to heparin and hyaluronan. EMBO J 1992, 11:4281-4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girkontaité I, Frischholz S, Lammi P, Wagner K, Swoboda B, Aigner T, von der Mark K: Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol 1996, 15:231-238 [DOI] [PubMed] [Google Scholar]