Abstract
In alcoholic hepatitis, a severe form of alcohol-induced toxic liver injury, as well as in experimental intoxication of mice with the porphyrinogenic drugs griseofulvin and 3,5-diethoxycarbonyl-1,4-dihydrocollidine, hepatocytes form cytoplasmic protein aggregates (Mallory bodies; MBs) containing cytokeratins (CKs) and non-CK components. Here we report that mice lacking the CK8 gene and hence CK intermediate filaments in hepatocytes, but still expressing the type I partner, ie, the CK18 gene, do not form MBs but suffer from extensive porphyria and progressive toxic liver damage, leading to the death of a considerable number of animals (7 of 12 during 12 weeks of intoxication). Our observations show that 1) in the absence of CK8 as well as in the situation of a relative excess of CK18 over CK8 no MBs are formed; 2) the loss of CK8 is not compensated by other type II CKs; and 3) porphyria and toxic liver damage are drastically enhanced in the absence of CK8. Our results point to a protective role of CKs in certain types of toxic liver injury and suggest that MBs by themselves are not harmful to hepatocytes but may be considered as a product of a novel defense mechanism in hepatocytes.
Cytokeratins (CKs) represent a multigene family of cytoskeletal proteins, typically and abundantly present in epithelial cells, in which they form bundles of intermediate-sized filaments (IFs). 1-5 In human tissues 21 different CK polypeptides expressed in development- and cell-type-specific patterns have to date been identified. 6-8 Based on amino acid sequence homology, relative charge, size, and association affinities, two CK subfamilies can be distinguished, the type I and type II CKs. For IF assembly, at least one member of each subfamily has to be present. 8,9-16
The first clues to the biological CK functions have been obtained by discoveries that a variety of blistering skin diseases, such as epidermolysis bullosa simplex, are caused by mutations in the epidermal CK genes. 8,17,18 Such mutations caused a disturbance of IF assembly and a reduced mechanical stability of keratinocytes, resulting in blister formation. The essential contribution of CK IFs to the specific tissue architecture and mechanical stability has also been seen in mice lacking one of the CKs normally present in epidermis and some other stratified squamous epithelia. For instance, ablation of the gene encoding CK14 (−/−) induces severe epidermolytic blistering and a thinned corneal epithelium, 19 in several aspects also resembling the blistering skin disease observed in patients with defects resulting from premature termination of CK14. 20-22 Moreover, severe skin blistering with erythema and local epidermal erosions, often associated with postnatal death, has been noted in CK10−/− mice. 23
Among the various CKs, the pair of CK8 (type II) and CK18 (type I) is of special interest. These two polypeptides are not only the most widespread in internal organs, in tumors, and in cell culture lines, 10,11,24-27 and the only CKs present in certain simple epithelial cells such as hepatocytes, 3,4,10,11,25-29 but they are also the first to appear in vertebrate embryogenesis. 30-36 The biological function of the simple epithelial CKs is less clearly elucidated than that of epidermal CKs. However, an important role of CK8 and CK18 in liver disease has recently been shown in transgenic and gene knockout mice and highlighted by the report of a mutation in the CK18 gene in a patient with cryptogenic liver cirrhosis. 37-44
Experiments in mice in which the CK8 gene had been inactivated gave interesting, although perplexing results. On a C57Bl/6 genetic background, homozygous CK8 gene knockout (CK8−/−) embryos were retarded in growth, and most of them (94%) died between days 11 and 13 of gestational life. 45 A small proportion of these mice, however, seemed to develop normally and the adult mice did not display any obvious abnormalities. By contrast, on a FVB/N genetic background, ∼50% of CK8−/− mice survived but showed reduced female fertility, a tendency to hyperplasia of colorectal mucosa often followed by anorectal prolapse, and slight elevations of certain serodiagnostic enzymatic markers of disturbed liver functions. 46 These findings, together with the observation that hepatocytes of CK18−/− mice, which as in the CK8−/− mice are devoid of CK filaments, look regular and functionally normal, indicate that CK IFs are not essential for proper liver development. 42
The importance of the hepatocytic CKs, however, becomes obvious when the liver is exposed to a variety of stress conditions. Thus in CK8−/− FVB/N mice, increased susceptibility to liver damage has been noted after treatment with certain anesthesia protocols, partial hepatectomy, or treatment with the phosphatase inhibitor microcystin. 43,44 Furthermore, livers of transgenic mice carrying dominant-negative point mutations of CK18, have shown inflammatory liver disease and a higher susceptibility to certain hepatotoxins. 37,38 Such findings of an increased hepatocyte susceptibility to various kinds of injury were regarded as a consequence of reduced mechanical stability in the absence of CK IFs. The loss or disruption of CK IFs, however, does not only impair cell stability but may also affect other cell functions. Some of these functions are related to the activation of certain protein kinases which phosphorylate CK8 and CK18 at multiple sites. 41,47
In the present study we have investigated the role of CKs in a model for a widespread type of toxic liver injury, alcoholic hepatitis, characterized by the occurrence of variously-sized cytoplasmic aggregates (Mallory bodies; MBs) consisting of CKs and non-CK components such as the MB-specific MM120–1 antigen, a 62- to 65-kd protein recognized by the antibody SMI 31 and ubiquitin. 48-59 Accumulation of MBs is further accompanied by profound alterations of hepatocytic CKs including overexpression and hyperphosphorylation of CK polypeptides as well as a derangement and marked diminution of the CK IF network (Stumptner C, Omary BM, Fickert P, Denk H, Zatloukal K, manuscript submitted). 54,60-63 Highly similar cytoskeletal alterations as seen in human alcoholic hepatitis can be induced in mice by chronic intoxication with griseofulvin or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC). 48,49,52-54,57,58,61,64-66 To elucidate the role of CKs in MB pathogenesis and to obtain new insights into the biological significance of MBs and the associated CK alterations in alcoholic liver injury, we have studied the response of CK8−/− mice to DDC intoxication.
Materials and Methods
Breeding and Intoxication of Mice
Heterozygous CK8(+/−) FVB/N mice 46 were mated and genotypes of offsprings were determined by polymerase chain reaction (PCR) analysis of tail DNA extracts. One μl of the DNA extract was directly used for PCR in a 25-μl reaction mixture, with 1× PCR buffer, 5 μl dimethyl sulfoxide, 0.25 mmol/L of each deoxynucleoside triphosphate (dNTP; Pharmacia Biotech. Inc., Uppsala, Sweden), 1 U of AmpliTaq DNA polymerase (Perkin Elmer Cetus, Norwalk, CT) and 1 μM of each primer: mK8–868 (5′-GGC CCT GCC CTC TAG TGT-3′) and mK8–1377 (5′-AGG GGT CTC ACC TTG TCA AT-3′) for amplification of the wild-type (wt) allele (CK8+), and mK8–868 and neo-249 (5′-CCT TCC CGC TTC AGT TAC-3′) for amplification of the mutated allele (CK8−). The reaction mixture was overlaid with mineral oil, heated to 94°C (where the Taq polymerase was added), and subsequently cycled for 45 cycles of 30 seconds at 94°C, 90 seconds at 55°C, and 2 minutes at 72°C, followed by a final extenuation step of 4 minutes at 72°C. The PCR products were separated on a 1% agarose gel in 1× Tris-borate-EDTA (TBE) buffer. The expected product from mK8–868 and mK8–1377 is 529 bp, from mK8–868 and neo-249 it is 700 bp.
Wild-type, heterozygous, and homozygous CK8 knockout mice as well as mice harboring a neomycin resistance gene under the control of the thymidine kinase promoter (M-Tkneo) 67 were fed a standard diet (Altromin; Marek, Vienna, Austria) and water ad libitum. For experimental induction of MBs the mice received a standard diet containing 0.1% DDC (Aldrich, Steinheim, Germany) for 6 and 12 weeks, respectively. 66
Immunohistochemistry
Mice were sacrificed by cervical dislocation. Tissues were immediately removed and either snap-frozen in methylbutane precooled in liquid nitrogen for immunofluorescence staining and mRNA preparation, or fixed in 4% formaldehyde solution in phosphate-buffered saline (PBS), embedded in paraffin, and 7-μm-thick sections were stained with hematoxylin and eosin. Immunohistochemistry for proliferative cell detection was performed after BrdU incorporation (5-bromo-2′-deoxy-uridine Labeling and Detection kit; Boehringer Mannheim, Mannheim, Germany) and with antibodies to proliferating cell nuclear antigen (PCNA; Dako, Glostrup, Denmark), using standard ABC detection protocols (Dako). Double-label immunofluorescence microscopy was performed as described previously. 58 The following antibodies were applied to cryostat sections (4-μm thick, fixed in acetone at −20°C for 10 minutes): 1) primary mouse monoclonal antibody (mAb) to lamin B2 (X223 ascites, 1:400), 68 mAb MM120–1 reacting with MBs, 58 mAb SMI 31 detecting a phosphorylated epitope of a 62- to 65-kd MB component (1:1000; Sternberger Monoclonals Inc., Baltimore, MA), 57 anti-CK 7 (1:50; Monosan, Am Uden, The Netherlands), anti-CK19 (Amersham, Buckinghamshire, UK), and anti-desmoplakin I and II (Boehringer Mannheim). 2) Secondary fluorescein isothiocyanate-conjugated goat antibodies to mouse immunoglobulin (1:100; Dianova, Hamburg, Germany). 3) Rabbit antibodies 50K160 directed to CK8 and CK18 (1:50) 58 . 4) tetramethylrhodamine isothiocyanate (TRITC)-conjugated porcine antibodies to rabbit immunoglobulin (1:50; Dako). For control, primary antibodies were omitted or replaced by isotype-matched immunoglobulins. Immunofluorescent specimens were analyzed in an MRC 600 (Bio-Rad, Richmond, CA) laser-scanning confocal device attached to a Zeiss Axiophot microscope. The fluorescent images were collected using the confocal photomultiplier tube as full frame (768 × 512 pixels). For dual labeling separate excitation wavelengths (488 nm for fluorescein isothiocyanate, 568 nm for TRITC) from a krypton/argon ion laser were used. Separate filter cubes allowed acquisition and storage of the images of the identical optical focal plane within the cell.
Porphyrin Determination
Total porphyrins were determined fluorometrically after extraction from 10% liver homogenates (in buffer containing 250 mmol/L sucrose; 50 mmol/L Tris; 25 mmol/L KCl; 5 mmol/L MgCl2, pH 7.4) into 0.9 mol/L HClO4:ethanol (1:1, v/v) with protoporphyrin as standard. 69
Clinical Chemistry
Approximately 1 ml of blood was collected after decapitation of anesthetized mice. Serum was processed in an automatic chemistry analyzer (Hitachi, Roche, Vienna, Austria). The respective values were determined by standard enzymatic or colorimetric assays.
Analysis of RNA by Reverse Transcriptase-PCR (RT-PCR)
Total RNA was isolated as described. 70 Primers for PCR were synthesized on a 392 DNA/RNA synthesizer (Applied Biosystems, Foster City, CA). The following primers were used for quantitative RT-PCR, cDNA synthesis and for the construction of RNA standards: mK8–1299, 5′-TGC AGA ACA TGA GCA TTC-3′; mK8–1439, 5′-GGT GCG GCT GAA AGT GTT-3′; mK8–1641, 5′-CAG AGG ATT AGG GCT GAT-3′; mK18–904, 5′-GAC GCT GAG ACC ACA CT-3′; mK18–1023, 5′-TCC ATC TGT GCC TTG TAT-3′; GAPDH-428, 5′-ATG TTT GTG ATG GGT GTG-3′; GAPDH-787, 5′-TAC TTG GCA GGT TTC TCC-3′. Exogenous RNA standards generated for quantitative RT-PCR contained a 29-bp (CK18), 30-bp (GAPDH), or 34-bp (CK8) deletion, respectively. First, strand cDNA synthesis was performed in a 20-μl reaction mixture containing 0.1 μg of total RNA, 0.5 U Inhibit-ACE (5′→3′ Inc., Boulder, CO), 1× RT buffer (100 mmol/L Tris-HCl, pH 8.3, at 42°C; 40 mmol/L KCl; 10 mmol/L MgCl2; 0.5 mmol/L spermidine), 1.25 mmol/L of each dNTP (Pharmacia), 4 mmol/L sodium pyrophosphate, 5 U AMV reverse transcriptase (Boehringer Mannheim), 0.5-μmol/L specific downstream primer and a certain number of RNA standard molecules. Total RNA, RNA standard, and primers were mixed and heated to 65°C for 10 minutes before adding the other components. The reaction was incubated at 42°C for 1 hour, heated to 95°C for 2 minutes, and then immediately chilled on ice. Tubes were centrifuged for 5 seconds in a microcentrifuge. We used 1 μl of cDNA for PCR amplification performed in a volume of 25 μl containing 1× PCR buffer, 2.5 μl DMSO, 1 μmol of each primer, 0.25 mmol/L of each dNTP (Pharmacia) and 1 U AmpliTaq DNA polymerase (Perkin-Elmer). The PCR comprised an initial denaturation step at 94°C for 2 minutes and 45 cycles of 1 minute at 94°C, 1 minute at 55°C, 1 minute at 72°C, and a final extension step of 4 minutes at 72°C using a Perkin-Elmer PCR thermocycler. The PCR products were separated on a 3% ethidium bromide-stained agarose gel in 1× TBE buffer. Band intensities were determined with a Docu Gel V video densitometer (MWG Biotech, Ebersberg, Germany) and Rflp-scan software (Scanalytics, Billerica, MA). After densitometry corrections were made for relative band sizes and differences in amplification efficiencies of standard and mRNA-derived cDNA. 71
Immunoblotting
CK8 and CK18 proteins were analyzed by immunoblotting using rabbit antibodies 50K160 directed to both CKs. In brief, a 5% (w/v) liver homogenate was prepared by sonication of frozen liver in sample buffer (10 mmol/L phosphate buffer containing 5% sodium dodecyl sulfate and 10% 2-mercaptoethanol). Proteins were precipitated by acetone, concentrations determined by the method of Bradford, 72 separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, 73 and electroblotted onto nitrocellulose membranes. 74 After blocking with 5% nonfat milk in PBS, the membranes were incubated with the CK antibodies (1:500) for 2 hours at room temperature. The membranes were then washed for 1 hour with 0.1% Tween in PBS, followed by incubation with horseradish peroxidase-conjugated swine-anti-rabbit secondary antibody (1:1000, Dako) for 1 hour at room temperature. After a further washing step immunoreactive bands were detected by enhanced chemiluminescence (ECL; Amersham).
Statistical Analysis
Statistical evaluation was done by t-test analysis. Differences with an error probability P < 0.05 were considered as significant.
Results
The general phenotype of the CK8−/− FVB/N mice has been described. 46 In the course of the present study, we have confirmed a marked hyperplasia of the colonic mucosa and anorectal prolapse, with a penetrance of >70% in 5-month-old CK8−/− mice. In some mice we also noted hyperplasia of the small intestinal mucosa.
CK7 Does Not Compensate for CK8 in Hepatocytes and Bile Duct Epithelia
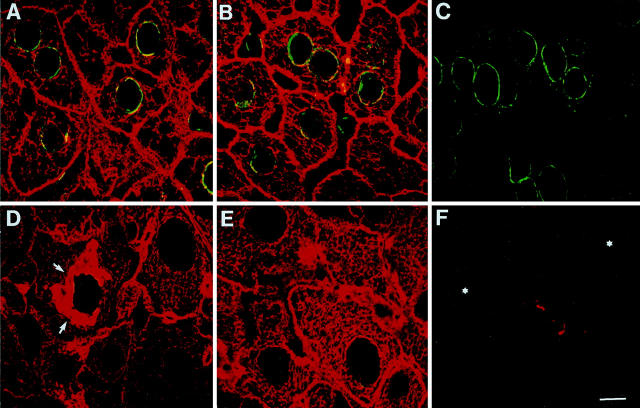
In heterozygous CK8 knockout (CK8+/−) mice fed a standard diet, hepatocytes displayed a regular CK IF network containing CK8 and CK18, indistinguishable from that of wt mice (Figure 1) ▶ . In CK8−/− mice, hepatocytes were devoid of a CK IF network because CK18 does not accumulate in the absence of a type II CK partner. In contrast to hepatocytes, bile duct epithelia, which normally express CK7 and CK19 in addition to CK8 and CK18, showed a residual CK network in the apical region of the cells. In bile duct epithelia of wt and CK8+/− mice, CK7 and CK19 were almost exclusively located in the apical cell portions whereas IFs containing CK8 and CK18 were distributed in the whole cytoplasm (Figure 1, A, B, D, E) ▶ . By contrast, in CK8−/− mice, CK18 was only present in the apical region but not in the residual cytoplasm of bile duct epithelial cells (Figure 1C) ▶ and the same intracellular location was noted for CK7. This also indicates that CK7 did not substitute for CK8 as partner of CK18. Surprisingly, CK19 which is apically enriched (Figure 1, D and E) ▶ in both wt and CK8+/− mice, was not detected in bile duct epithelial cells of CK8−/− mice. This suggests that in bile duct epithelial cells of CK8−/− mice CK18 is the partner of CK7.
Figure 1.
Double-label immunofluorescence microscopy of livers of wt (A and D), CK8+/− (B and E), and CK8−/− (C and F) mice with rabbit antibodies (50K160) to CK8 and CK18 (red, A–F), murine mAb to CK7 (green, A–C), or murine mAb to CK19 (green, D–F), showing that CK7 and CK19 is exclusively expressed in bile duct epithelia (A–E), whereas CK19 seems to be absent in the bile duct epithelium of CK8−/− mice (F); the red immunostaining seen here is due to residual CK18. Arrowheads in C indicate the basal cell border of bile duct epithelia. Scale bar, 10 μm.
Specific Toxic Effects and Cell Biological Changes
Compared to wt animals, we noticed in chronically DDC-intoxicated CK8−/− mice a much earlier and extensive appearance of skin necroses, especially on light-exposed ears, as typical of photosensitization induced by porphyria. 66,75 Moreover, we observed a much higher death rate so that 12 weeks after beginning of intoxication seven out of 12 CK8−/− mice had died (Figure 2) ▶ . By contrast, all of the DDC-treated wt mice and nine out of 11 CK8+/− mice survived. On inspection of the internal organs, we consistently found the livers of the treated animals to be enlarged and dark-brown in color, as typical for DDC-intoxicated mice. 66 The liver to body weight ratio was markedly increased three- to fourfold after 12 weeks of DDC intoxication, but this was similar in all three kinds of mice (wt, CK8+/−, and CK8−/−).
Figure 2.
Graphic presentation showing the increased lethality of CK8 knockout mice with increasing time of DDC intoxication. Wild-type (wt) (CK8+/+), heterozygous (CK8+/−), and homozygous CK8 knockout mice (CK8−/−) were fed a diet containing 0.1% DDC. No wt mice (0 of 13) died within an intoxication period of 12 weeks, whereas 2 of 11 of the CK8+/− mice and seven of 12 of the CK8−/− mice died.
Histological examination revealed enlarged hepatocytes, an increased number of bile ductules, and numerous pigment-containing macrophages in lobules and portal tracts (Figure 3) ▶ . Accumulation of pigment material, predominantly representing N-methyl-protoporphyrin IX, 75,76 was much more pronounced in the CK8−/− mice than in the CK8+/− or in wt mice (Figure 3, A ▶ -C; Figure 4 ▶ ). Moreover, the proliferation pattern of bile ductules differed between wt and CK8−/− mice: In wt mice, increased numbers of bile ductules, mostly with a clearly discernible lumen (typical bile ductular reaction), 77 were found in the periphery of portal tracts (Figure 3D) ▶ , whereas in CK8−/−mice, immature ductules without detectable lumina (atypical bile ductular reaction) 77 prevailed (Figure 3E) ▶ . Another difference between wt and CK8−/− mice was that in wt mice DDC intoxication for 12 weeks led to the appearance of many apoptotic cells (frequency, 3.8 ± 1.6 apoptoses per 10 high-power fields) which was not seen in CK8−/− mice (0.1 ± 0.2 apoptoses per 10 high-power fields). On necropsy and histology of CK8−/− mice we noted, besides the extensive porphyria, marked vacuolization and microvesicular steatosis as well as focal hepatocytic necroses reflecting toxic liver damage. In addition, we found that in CK8−/− mice fed a standard diet the proportion of proliferative hepatocytes was significantly increased, as demonstrable by BrdU-incorporation and PCNA immunostaining (results not shown).
Figure 3.

Light microscopy showing the porphyria and the bile ductular reaction in livers of DDC-intoxicated CK8 knockout mice. H&E-stained sections of formaldehyde-fixed, paraffin-embedded livers of wt (CK8+/+) mice (A and D), of CK8+/− mice (B), and CK8−/− mice (C and E) at 12 weeks of DDC intoxication. Note the differences between the wt and the CK8 knockout mice with respect to pigment-associated porphyrin accumulations (brown depositions; compare A and C). Arrowheads in D indicate typical bile ductular reaction (increased number and elongation of bile ducts with lumina), which is confined to the periphery of the portal tract; the inset in the lower left of D shows a hepatocyte containing a MB (small arrow). Arrowheads in E indicate atypical bile ductular reaction (cords of bile duct epithelia mostly without discernible lumina which extend into the periportal liver parenchyma, thus blurring the border between portal tract and lobule). H&E staining, A–E. Magnifications: A–C, ×80 ; D and E, ×360; inset in D, ×540.
Figure 4.
Histogram presenting porphyrin concentrations in liver. Liver tissue was obtained from wt mice fed a standard diet and from CK8 knockout mice as well as from transgenic mice expressing the neomycin resistance gene after 3 months (3 months) of DDC intoxication. Total porphyrin content in the liver was measured fluorometrically after acid extraction. Porphyrin concentration was calculated per gram of liver tissue. Bars represent mean values (with standard deviations) from five different mice per group (CK8+/+, CK8+/−, CK8−/−) and, for control, three transgenic mice containing the neomycin resistance gene under the control of the thymidine kinase promoter (M-TKneo). Note the significant increase of liver porphyrin in CK8+/− and CK8−/− mice, with the highest liver porphyrin accumulation in the latter. The neomycin resistance gene itself, which is present in the CK8 knockout as well as M-TKneo mice, had no influence on the DDC-induced porphyria. Protoporphyrin is not detected (n.d.) in normal mouse liver (CO).
All serodiagnostic parameters (alanine aminotrans-ferase, aspartate aminotransferase, bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase, cholesterol, triglycerides, and albumin) measured at 12 weeks after onset of intoxication showed no major differences between wt and CK8−/− mice (not shown). However, the hepatic porphyrin content of CK8−/− and CK8+/− mice was drastically increased, compared to wt mice (Figure 4) ▶ . To exclude that the increased porphyria was due to presence of the neomycin resistance gene in the CK8 knockout mice we further analyzed transgenic mice expressing only the neomycin resistance gene under the control of the thymidine kinase promoter. 67 These studies revealed that the neomycin resistance gene did not influence the effect of DDC on the hepatic cytoskeleton (see below), or affect the porphyria seen in the CK8+/− or CK8−/− mice (Figure 4) ▶ .
Alterations of the Hepatocytic Cytoskeleton
At 6 weeks of DDC intoxication, the hepatocytes of wt mice showed severe alterations of the cytoskeleton (Figure 5) ▶ which were similar to those described in human alcoholic hepatitis and in griseofulvin-treated mice. 48,49,52-54, 57, 58, 61, 64-66 The changes observed comprised 1) disturbance and diminution of the IFs containing CKs 8 and 18; 2) reduction of B-type nuclear lamins; 78 3) appearance of MBs containing CKs, the MM120–1 antigen, an abnormally phosphorylated 62- to 65-kd protein, and ubiquitin; and 4) enlargement (ballooning) of hepatocytes.
Figure 5.
Double-label immunofluorescence microscopy showing changes in the cytoskeletal organization of hepatocytes after 6 weeks of DDC intoxication in wt and CK8 knockout mice. Frozen sections were immunostained with mAb X223 against lamin B2 (green) and rabbit CK antibodies reacting with CK8 and CK18 (red). A–C: Animals fed control diet; D–F: intoxicated mice. A and D: wt mice; B and E: CK8+/− mice; C and F: CK8−/− mice. Arrows in D indicate CK-containing MBs, asterisks in F denote nuclei which have lost nuclear lamin B2 reactivity. Note CK network derangement and MB formation as well as loss of nuclear lamin immunoreactivity in CK8+/+ mice. By contrast, the CK network remains intact in intoxicated CK8+/− mice, no MBs are seen but lamin immunoreactivity is lost. Note also some enlargement of hepatocytes in the course of DDC intoxication. In the CK8−/− mice (C and F) neither a CK network nor MBs are demonstrable. Scale bar, 10 μm (A–F).
In the intoxicated CK8−/− mice, these pathological changes were modified. Although the alterations of the nuclear lamins and the enlargement of the hepatocytes were similar to those in wt mice, the accumulation of porphyrin and the bile ductular reaction were more pronounced in comparison to DDC-treated wt mice indicating that the drug had a particularly toxic effect in the CK8−/−mice (Figure 3, C and E ▶ ; Figure 5F ▶ ). The most striking difference, however, was that MBs were absent in the hepatocytes of CK8−/− mice (Figure 5F) ▶ .
Surprisingly, CK8+/− mice, in which hepatocytes do have a CK network, also lacked MB-like aggregations of CKs on intoxication, although other signs of intoxication, such as enlargement of hepatocytes, loss of nuclear lamins, and porphyria were seen (Figure 5E) ▶ . To confirm this phenotype dissociation in DDC-treated wt and CK8+/− mice, ie, an increased porphyrinogenic reaction to DDC (Figure 4) ▶ and the apparent resistance to CK alterations (Figure 5E) ▶ , we analyzed mice at 12 weeks of intoxication. Under these conditions, livers of wt mice revealed pronounced alterations of the CK system and many hepatocytes containing numerous MBs (Figure 6 ▶ A, D). Even after this prolonged period of intoxication, the CK skeleton was largely intact in CK8+/− mice (Figure 6, B and E) ▶ , with only very few hepatocytes (<0.1%) containing small MB-like inclusions (not shown). As already described for mice after 6 weeks of DDC treatment, prolonged intoxication of CK8−/− mice did not induce the accumulation of the MB-specific antigen MM120–1 (Figure 6C) ▶ , or of the 62- to 65-kd SMI 31-reactive protein (Figure 6F) ▶ , or of ubiquitin (not shown). To assess potential effects of the introduced neomycin resistance gene on the DDC-induced cytoskeletal alterations, M-TKneo mice were examined in parallel. The presence of the neomycin resistance gene had no detectable influence on the CK system either in mice fed a standard diet or in DDC-intoxicated mice (Figure 6, G and H) ▶ .
Figure 6.

Double-label immunofluorescence microscopy of livers from 12-week DDC-intoxicated mice showing that MBs do not form in the absence of CK8. A–H: CK8 and CK18 were immunostained with rabbit antibodies (50K160, red). A–C, G, H: The mAb MM120–1 (green) also reacts with MBs (yellow, due to co-localization with CK) found only in wt as well as M-TKneo mice; D–F: mAb SMI 31 (green) recognizes a 62- to 65-kd MB component which co-localizes with CK in MBs. A and D, wt mice; B and E, CK8+/− mice; C and F, CK8−/− mice; G, liver of a M-TKneo mouse fed a standard diet; H, liver of a M-TKneo mouse fed DDC for 12 weeks. Note that the presence of the neomycin resistance gene in M-TKneo mice has no effect on DDC-induced derangement of the CK IF cytoskeleton and MB formation. Scale bar, 20 μm.
To characterize the differences between the effects of DDC on the cytoskeleton in wt, CK8+/−, and CK8−/− mice, we analyzed the mRNAs of CK8 and CK18 by quantitative RT-PCR. In nonintoxicated CK8+/− mice we found, as expected, a reduction of the mRNA concentration to ∼60% of wt mice, whereas CK18 mRNA was unaltered (Figure 7) ▶ . In nonintoxicated CK8−/− mice, CK8 mRNA was not detectable whereas the CK18 mRNA was slightly but not significantly increased. In wt mice, DDC intoxication for 12 weeks led to a more than fourfold increase of the mRNAs for both CK8 and CK18. In the monoallelic CK8+/−, the increase of CK8 mRNA was significantly lower than that of the CK18 mRNA. In CK8−/− mice there was still a threefold increase of the CK18 mRNA. Because these mice did not contain CK IFs, the mRNA increase is not caused by regulatory mechanisms at the CK level but rather reflects a hepatocytic response to the intoxication.
Figure 7.
Concentrations of CK8 and CK18 mRNAs in mice intoxicated with DDC for 12 weeks as determined by quantitative RT-RCR using RNAs with small deletions as standards. Upper panel: PCR results obtained with three mouse livers per group: wt (+/+) mice, in comparison with heterozygous (+/−) and homozygous (−/−) CK8 knockout mice. Control mice (CO) received a standard diet, DDC-treated mice (DDC) were fed a DDC-containing diet for 12 weeks. CK8 PCR products derived from CK8 mRNA present in 100 ng of total RNA. Standard 4 × 105or 4 × 10 6 indicate PCR products derived from 4 × 10 5 or 4 × 10 6 internal standard RNA copies added to the sample before cDNA synthesis. Lower panel: Bars and the corresponding numbers represent corrected mean values of mRNA copy numbers (×105) in 100 ng total RNA from three mice each, with standard deviations indicated. CK8 RNA has not been detected (n.d.) in CK8−/− mice.
This observation raised the question whether the relatively high amounts of CK18 mRNA correlated with an increased accumulation of CK18 protein. Immunoblot analysis of total liver homogenates with polyclonal antibodies recognizing CK8 and CK18 revealed the absence of both CK8 and CK18 in untreated as well as in intoxicated CK8−/− mice (Figure 8) ▶ . The immunoreactive bands at 90 to 180 kd most likely represent cross-linked CKs known to be enriched in griseofulvin- or DDC-intoxicated livers. 79,80 This slight increase of CK content in DDC-exposed CK8+/− mouse livers also corresponded to the histological finding of a more dense CK IF network (Figure 6, B and E) ▶ : after 12 weeks of intoxication, wt mouse livers showed areas with hepatocytes mostly devoid of CK fibrils (Figure 6, A and D) ▶ , whereas in CK8+/− mice the CK network had remained largely unaltered (Figure 6, B and E) ▶ .
Figure 8.
Immunoblot analysis of CK8 and CK18 in total liver homogenates of wt (+/+), heterozygous (+/−), and homozygous (−/−) CK8 knockout mice after 12 weeks of DDC-treatment (12wDDC) and without treatment (control). Rabbit antibodies (50K160) reacting with both CK8 and CK18 (although somewhat more intensely with the latter) were used. Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (left half); corresponding immunoblot (right half).
Discussion
MBs are characteristic features of hepatocyte injury in alcoholic hepatitis but are also seen in nonalcoholic steatohepatitis, 81 Wilson’s disease, primary biliary cirrhosis, and certain forms of drug-induced liver injury such as amiodarone toxicity. 52,53,81 In mice, MBs can be induced by chronic intoxication with griseofulvin or DDC thus providing an experimental system for studies on MB pathogenesis. 64,66 Previous studies have shown that CKs are major constituents of MBs which in addition contain various non-CK components, including the stress-inducible MM120–1 antigen, ubiquitin, and a 62- to 65-kd protein recognized by antibody SMI 31. 48-50,52-54,57,58,61,65
In the present study we have shown with the help of CK8−/− mice that CK is a key protein in MB formation. The observed absence of MBs in hepatocytes of CK8−/− mice is not merely a consequence of the lack of the CK network because CK18−/− mice, in which hepatocytes are also devoid of a CK system, are able to form MBs consisting of CK8 and the various non-CK components. 42 This different behavior of CK8−/− and CK18−/− mice furthermore demonstrates that the formation of MBs is not dependent on the presence of CK IF fibrils but involves, at least in CK18−/− mice, nonassembled CK molecules. To our surprise, the hepatocytes of CK8+/− mice, which do have a CK system, did not respond with MB formation on prolonged DDC intoxication. One likely explanation for this observation is that because of the lack of one CK8 allele, these mice produce a relative excess of CK18 (see also Figure 7 ▶ ), and that the relative ratio of CK8 to CK18 determines whether MBs can be formed.
Our findings also allow some general conclusions on type I:type II CK dimerization and oligomer formation. In the CK8−/− hepatocytes we have not seen a special structure formed by CK18 in the absence of its partner CK8. Clearly, in the absence of a type II CK, CK18 does not accumulate at desmosomes or other distinct places. Correspondingly, CK8 present in CK18−/− mice also does not form IFs but tends to aggregate in structures reminiscent of MBs. 42 Moreover, the sustained production of CK18 in CK8−/− mice does not induce expression of genes coding for type II CKs, such as CK7, in contrast to a report for other CK pairs. 82 This is the more remarkable as it has been shown that hepatocytes are basically able to synthesize CK7 and CK19 during liver development and under certain disease conditions. 83-85
The present study also provides new insights into the involvement of CKs in the cellular response to toxic injury. We found that hepatocytes respond to the toxic challenge with DDC with an up-regulation of CK synthesis. This overexpression of CK genes is apparently independent of the status of the IF system present and thus reflects a direct or indirect response to the toxin (Figure 7) ▶ . Interestingly, CK8+/− mice, which have a reduced ability of CK8 formation, showed an increased sensitivity to the noxious agent although the CK fibril network was maintained. Therefore, one must assume that the up-regulation of CK synthesis, which is an active response of the hepatocyte, is associated with a better tolerance for toxic stress. Based on the observations in CK8+/− and CK8−/− mice, we suggest that CK protects hepatocytes from toxic injury. As in the hepatocytes of the CK8−/− mice the residual CK18 alone cannot form IFs, we presently cannot decide whether the toxicity protection is due to CK8 as a protein or to IF structures. At any rate, the demonstration of a major contribution of CK8 or CK8-containing IFs to protection against toxic liver-cell damage extends our view of IF protein functions to nonarchitectural roles (for architectural CK functions see also the studies on epidermolysis bullosa cited in the Introduction). 43,44
Our observations in CK8−/− mice are also in line with the previous report of Bauman et al 86 on the induction of multidrug resistance in cultured mouse fibroblasts by transfection with CK8 and CK18 cDNAs, suggesting that the phenomenon of protection from toxicity is not restricted to DDC. The resulting basic question how CK8 or CK8:CK18 IFs counteract the toxic effects of DDC and other substances cannot be answered at present. Whether CK8 or CK IFs act by absorption of the toxic principle, by stabilization of detoxifying components involved, or through indirect effects remains to be elucidated. Because the detoxification of DDC leads to the generation of methyl radicals that are known to be responsible for the porphyria 75,87 but could also adversely affect other cell components, the absence of CK8 or CK8-containing IFs might render cells more susceptible to radical injury. Another mechanism by which CK8 could interfere with toxic cell damage is that CKs become highly phosphorylated under a variety of stress conditions 38,40,47,88-90 including DDC feeding (Stumptner et al, manuscript in preparation) so that CK8, as a major substrate of protein kinases, can prevent other proteins from deleterious phosphorylation effects (see “phosphate sink” hypothesis of Lai et al 91 ).
In summary, the results presented provide evidence that in the absence of CK8, MBs do not appear and the relative ratio of CK8 to CK18 decides whether MBs are formed under certain conditions. On the other hand, the progressive liver injury in the intoxicated CK8−/−mice shows that MBs as such are not instrumental in, or needed for, the development of the liver damage. This leads to the concept that the MB is the product of yet unknown defensive response to toxic injury that involves CK, particularly CK8.
Note added in proof:
The 62- to 65-kd MB component was identified as p62, which is a stress-inducible, ubiquitin-binding protein (Zatloukal, manuscript in preparation).
Acknowledgments
The scientific advice of Dr. R. Eferl for quantitative RT-PCR, the help of Dr. M. Moser with statistical analysis, as well as the technical assistance of Ms. A. Fuchsbichler are gratefully acknowledged. Furthermore we thank Dr. E. F. Wagner (Institute for Molecular Pathology, Vienna, Austria) for providing the M-TKneo mice.
Footnotes
Address reprint requests to Dr. Kurt Zatloukal, Division of Experimental Cell Research and Oncology, Department of Pathology, University of Graz, Auenbruggerplatz 25, A-8036 Graz, Austria. E-mail: kurt.zatloukal@kfunigraz.ac.at.
Supported by Austrian Science Foundation grant S7401-MOB (K. Z.).
References
- 1.Franke WW, Weber K, Osborn M, Schmid E, Freudenstein C: Antibody to prekeratin. Decoration of tonofilament-like arrays in various cells of epithelial character. Exp Cell Res 1978, 116:429-445 [DOI] [PubMed] [Google Scholar]
- 2.Franke WW, Schmid E, Osborn M, Weber K: Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci USA 1978, 75:5034-5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K: Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation 1979, 15:7-25 [DOI] [PubMed] [Google Scholar]
- 4.Franke WW, Schmid E, Kartenbeck J, Mayer D, Hacker HJ, Bannasch P, Osborn M, Weber K, Denk H, Wanson JC, Drochmans P: Characterization of the intermediate-sized filaments in liver cells by immunofluorescence and electron microscopy. Biol Cell 1979, 34:99-110 [Google Scholar]
- 5.Sun TT, Shih C, Green H: Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci USA 1979, 76:2813-2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin C, Moll R, Kubicka S, Ouhayoun JP, Franke WW: Characterization of human cytokeratin 2, an epidermal cytoskeletal protein synthesized late during differentiation. Exp Cell Res 1992, 202:132-141 [DOI] [PubMed] [Google Scholar]
- 7.Collin C, Ouhayoun JP, Grund C, Franke WW: Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 1992, 51:137-148 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs E, Weber K: Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 1994, 63:345-382 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs E, Coppock S, Green H, Cleveland D: Two distinct classes of epidermal keratin genes and their evolutionary significance. Cell 1981, 27:75-84 [DOI] [PubMed] [Google Scholar]
- 10.Moll R, Franke WW, Schiller D, Geiger B, Krepler R: The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31:11-24 [DOI] [PubMed] [Google Scholar]
- 11.Quinlan RA, Cohlberg LA, Schiller DL, Hatzfeld M, Franke WW: Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol 1984, 178:265-288 [DOI] [PubMed] [Google Scholar]
- 12.Hatzfeld M, Franke WW: Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol 1985, 101:1826-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulombe PA, Fuchs E: Elucidating the early stages of keratin filament assembly. J Cell Biol 1990, 111:153-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatzfeld M, Weber K: The coiled-coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratin: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol 1990, 110:1199-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinert PM: The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem 1990, 265:8766-8774 [PubMed] [Google Scholar]
- 16.Hofmann I, Franke WW: Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: viscometry and determinations of relative affinities. Eur J Cell Biol 1997, 72:122-132 [PubMed] [Google Scholar]
- 17.McLean WHI, Lane EB: Intermediate filaments in disease. Curr Opin Cell Biol 1995, 7:118-125 [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E: The cytoskeleton and disease: genetic disorders of intermediate filaments. Annu Rev Genet 1996, 30:197-231 [DOI] [PubMed] [Google Scholar]
- 19.Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E: The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol 1995, 129:1329-1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YM, Anton-Lamprecht I, Yu QC, Jackel A, Zabel B, Ernst JP, Fuchs E: A human keratin 14 “knockout”: the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev 1994, 8:2574-2587 [DOI] [PubMed] [Google Scholar]
- 21.Rugg EL, McLean WHI, Lane EB, Pitera R, McMillan JR, Dopping-Hepenstal PJC, Navsaria HA, Leigh IM, Eady RAJ: A functional “knockout” of human keratin 14. Genes Dev 1994, 8:2563-2573 [DOI] [PubMed] [Google Scholar]
- 22.Fuchs E, Cleveland DW: A structural scaffolding of intermediate filaments in health and disease. Science 1998, 279:514-519 [DOI] [PubMed] [Google Scholar]
- 23.Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RAJ, Magin TM: Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol 1996, 132:925-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franke WW, Schmid E, Weber K, Osborn M: HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res 1979, 118:95-109 [DOI] [PubMed] [Google Scholar]
- 25.Franke WW, Denk H, Kalt R, Schmid E: Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res 1981, 131:299-318 [DOI] [PubMed] [Google Scholar]
- 26.Franke WW, Mayer D, Schmid E, Denk H, Borenfreund E: Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res 1981, 134:345-365 [DOI] [PubMed] [Google Scholar]
- 27.Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, Denk H, Krepler R, Platzer B: Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol 1981, 153:933-959 [DOI] [PubMed] [Google Scholar]
- 28.Borenfreund E, Schmid E, Bendich A, Franke WW: Constitutive aggregates of intermediate-sized filaments of the vimentin and cytokeratin type in cultured hepatoma cells and their dispersal by butyrate. Exp Cell Res 1980, 127:215-235 [DOI] [PubMed] [Google Scholar]
- 29.Denk H, Krepler R, Lackinger E, Artlieb U, Franke WW: Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic neoplastic nodules of mice. Lab Invest 1982, 46:584-596 [PubMed] [Google Scholar]
- 30.Brûlet P, Babinet C, Kemler R, Jacob F: Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci USA 1980, 77:4113-4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson BW, Grund C, Schmid E, Bürki K, Franke WW, Illmensee K: Formation of cytoskeletal elements during mouse embryogenesis. Differentiation 1980, 17:161-179 [DOI] [PubMed] [Google Scholar]
- 32.Jackson BW, Grund C, Winter S, Franke WW, Illmensee K: Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation 1981, 20:203-216 [DOI] [PubMed] [Google Scholar]
- 33.Kemler R, Brûlet P, Schnebelen MZ, Gaillard J, Jacob F: Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morph 1981, 64:45-60 [PubMed] [Google Scholar]
- 34.Franz JK, Gall L, Williams MA, Picheral B, Franke WW: Intermediate-size filaments in germ cells: expression of cytokeratins in oocytes and eggs of the frog Xenopus. Proc Natl Acad Sci USA 1983, 80:6254-6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshima RG, Howe WE, Klier FG, Adamson ED, Shevinsky LH: Intermediate filament protein synthesis in preimplantation murine embryos. Dev Biol 1983, 99:447-455 [DOI] [PubMed] [Google Scholar]
- 36.Chisholm JC, Houliston E: Cytokeratin filament assembly in the preimplantation mouse embryo. Development 1987, 101:565-582 [DOI] [PubMed] [Google Scholar]
- 37.Ku NO, Michie SA, Oshima RG, Omary MB: Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a cytokeratin 18 conserved arginine mutant. J Cell Biol 1995, 131:1303-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Oshima RG, Omary MB: Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest 1996, 98:1034-1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku NO, Wright TL, Terrault NA, Gish R, Omary MB: Mutation of human keratin 18 in association with cryptogenic cirrhosis. J Clin Invest 1997, 99:19-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Omary MB: Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J Cell Biol 1998, 143:2023-2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omary MB, Ku NO: Intermediate filament proteins of the liver: emerging disease association and functions. Hepatology 1997, 25:1043-1048 [DOI] [PubMed] [Google Scholar]
- 42.Magin TM, Schröder R, Leitgeb S, Wanninger F, Zatloukal K, Grund C, Melton DW: Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J Cell Biol 1998, 140:1441-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loranger A, Duclos S, Grenier A, Price J, Wilson-Heiner M, Baribault H, Marceau N: Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pathol 1997, 151:1673-1683 [PMC free article] [PubMed] [Google Scholar]
- 44.Toivola DM, Omary MB, Ku NO, Peltola O, Baribault H, Eriksson JE: Protein phosphatase inhibition in normal and keratin 8/18 assembly-incompetent mouse strains supports a functional role of keratin intermediate filaments in preserving hepatocyte integrity. Hepatology 1998, 28:116-128 [DOI] [PubMed] [Google Scholar]
- 45.Baribault H, Price H, Miyai K, Oshima RG: Mid-gestational lethality in mice lacking keratin 8. Genes Dev 1993, 7:1191-1202 [DOI] [PubMed] [Google Scholar]
- 46.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M: Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 1994, 8:2964-2974 [DOI] [PubMed] [Google Scholar]
- 47.Omary MB, Ku NO, Liao J, Price D: Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem 1998, 31:105-140 [PubMed] [Google Scholar]
- 48.Denk H, Franke WW, Kerjaschki D, Eckersdorfer R: Mallory bodies in experimental animals and man. Int Rev Exp Pathol 1979, 20:77-121 [PubMed] [Google Scholar]
- 49.Denk H, Franke WW, Dragosics B, Zeiler I: Pathology of cytoskeleton of liver cells: demonstration of Mallory bodies (alcoholic hyalin) in murine and human hepatocytes by immunofluorescence microscopy using antibodies to cytokeratin polypeptides from hepatocytes. Hepatology 1981, 1:9-20 [DOI] [PubMed] [Google Scholar]
- 50.Denk H, Krepler R, Lackinger E, Artlieb U, Franke WW: Immunological and biochemical characterization of the keratin-related component of Mallory bodies: a pathological pattern of hepatocytic cytokeratins. Liver 1982, 2:165-175 [DOI] [PubMed] [Google Scholar]
- 51.French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, Mendenhall CL, : VA Cooperative Study Group 119: Pathology of alcoholic liver disease. Semin Liver Dis 1993, 13:154-169 [DOI] [PubMed] [Google Scholar]
- 52.Jensen K, Gluud C: The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey). Hepatology 1994, 20:1061-1077 [DOI] [PubMed] [Google Scholar]
- 53.Jensen K, Gluud C: The Mallory body: theories on development and pathological significance (Part 2 of a literature survey). Hepatology 1994, 20:1330-1342 [PubMed] [Google Scholar]
- 54.Katsuma Y, Swierenga SHH, Khettry U, Marceau N, French SW: Changes in the cytokeratin intermediate filament cytoskeleton associated with Mallory body formation in mouse and human liver. Hepatology 1987, 7:1215-1223 [DOI] [PubMed] [Google Scholar]
- 55.Lowe J, Blanchard A, Morell K, Lennox G, Reynolds L, Billet M, Landon M, Mayer RJ: Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibers in cerebellar astrocytomas, cytoplasmic bodies in muscle, and Mallory bodies in alcoholic liver disease. J Pathol 1988, 155:9-15 [DOI] [PubMed] [Google Scholar]
- 56.Ohta M, Marceau N, Perry G, Manetto V, Gambetti P, Autilio-Gambetti L, Metuzals J, Kawahara H, Cadrin M, French SW: Ubiquitin is present on the cytokeratin filaments and Mallory bodies of hepatocytes. Lab Invest 1988, 59:848-856 [PubMed] [Google Scholar]
- 57.Preisegger KH, Zatloukal K, Spurej G, Riegelnegg D, Denk H: Common epitopes of human and murine Mallory bodies and Lewy bodies as revealed by a neurofilament antibody. Lab Invest 1992, 66:193-199 [PubMed] [Google Scholar]
- 58.Zatloukal K, Denk H, Spurej G, Lackinger E, Preisegger KH, Franke WW: High molecular weight component of Mallory bodies detected by a monoclonal antibody. Lab Invest 1990, 62:472-434 [PubMed] [Google Scholar]
- 59.Zatloukal K, Böck G, Rainer I, Denk H, Weber K: High molecular weight components are main constituents of Mallory bodies isolated with a fluorescence activated cell sorter. Lab Invest 1991, 64:200-206 [PubMed] [Google Scholar]
- 60.Denk H, Franke WW: Rearrangement of the hepatocyte cytoskeleton after toxic damage: involution, dispersal and peripheral accumulation of Mallory body material after drug withdrawal. Eur J Cell Biol 1981, 23:241-249 [PubMed] [Google Scholar]
- 61.Denk H, Lackinger E, Cowin P, Franke WW: Maintenance of desmosomes in mouse hepatocytes after drug-induced rearrangement of cytokeratin filament material. Exp Cell Res 1985, 161:161-171 [DOI] [PubMed] [Google Scholar]
- 62.Hazan R, Denk H, Franke WW, Lackinger E, Schiller DL: Change of cytokeratin organization during development of Mallory bodies as revealed by a monoclonal antibody. Lab Invest 1986, 54:543-553 [PubMed] [Google Scholar]
- 63.Zatloukal K, Spurej G, Rainer I, Lackinger E, Denk H: Fate of Mallory body-containing hepatocytes: disappearance of Mallory bodies and restoration of the hepatocytic intermediate filament cytoskeleton after drug withdrawal in the griseofulvin-treated mouse. Hepatology 1990, 11:652-661 [DOI] [PubMed] [Google Scholar]
- 64.Denk H, Gschnait F, Wolff K: Hepatocellular hyalin (Mallory bodies) in long term griseofulvin-treated mice: a new experimental model for the study of hyalin formation. Lab Invest 1975, 32:773-776 [PubMed] [Google Scholar]
- 65.Franke WW, Denk H, Schmid E, Osborn M, Weber K: Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbrated rods of filaments containing precytokeratin-like polypeptides. Lab Invest 1979, 40:207-220 [PubMed] [Google Scholar]
- 66.Tsunoo C, Harwood TR, Arak S, Yokoo H: Cytoskeletal alterations leading to Mallory body formation in livers of mice fed 3,5-diethoxycarbonyl-1,4-dihydrocollidine. J Hepatol 1987, 5:85-97 [DOI] [PubMed] [Google Scholar]
- 67.Stewart CL, Schuetze S, Vanek M, Wagner EF: Expression of retroviral vectors in transgenic mice obtained by embryo infection. EMBO J 1987, 6:383-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoeger TH, Zatloukal K, Waizenegger I, Krohne G: Characterization of a second highly conserved B-type lamin present in cells previously thought to contain only a single B-type lamin. Chromosoma 1990, 99:379-390 [DOI] [PubMed] [Google Scholar]
- 69.Abbritti G, De Matteis F: Decreased levels of cytochrome P-450 and catalase in hepatic porphyria caused by substituted acetamides and barbiturates. Importance of the allyl group in the molecule of the active drugs. Chem Biol Interact 1971/72, 4:281–286 [DOI] [PubMed]
- 70.Krieg PA, Amtmann E, Sauer G: The simultaneous extraction of high molecular weight DNA and of RNA from solid tumors. Anal Biochem 1983, 134:288-294 [DOI] [PubMed] [Google Scholar]
- 71.Eferl R, Lehner M, Kenner L, Kapfer I, Guertl B, Zatloukal K: Evaluation of different standard types for quantitative reverse transcription polymerase chain reaction: possible pitfalls and strategies for avoidance. Technical Tips Online 1997, http://tto.trends.com:T01214
- 72.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 73.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1979, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 74.Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci 1979, 76:4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tephly TR, Coffman BL, Ingall G, Abou Zeit-Har MS, Goff HM, Tabba HD, Smith KM: Identification of N-methylprotophorphyrin IX in livers of untreated mice and mice treated with 3,5-diethoxycarbonyl-1,4-dihydrocollidine: source of the methyl group. Arch Biochem Biophys 1981, 212:120–126 [DOI] [PubMed]
- 76.Ortiz de Montellano PR, Beilan HS, Kunze KL: N-Methylprotoporphyrin IX: Chemical synthesis and identification as the green pigment produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine treatment. Proc Natl Acad Sci USA 1981, 78:1490–1494 [DOI] [PMC free article] [PubMed]
- 77.Desmet V, Roskams T, Van Eyken P: Ductular reaction in the liver. Pathol Res Pract 1995, 191:513-524 [DOI] [PubMed] [Google Scholar]
- 78.Zatloukal K, Denk H, Spurej G, Hutter H: Modulation of protein composition of nuclear lamina. Lab Invest 1992, 66:589-597 [PubMed] [Google Scholar]
- 79.Zatloukal K, Denk H, Lackinger E, Rainer I: Hepatocellular cytokeratins as substrates of transglutaminase. Lab Invest 1989, 61:603-608 [PubMed] [Google Scholar]
- 80.Cadrin M, Marceau N, French SW: Cytokeratin of apparent high molecular weight in livers from griseofulvin-fed mice. J Hepatol 1992, 14:226-231 [DOI] [PubMed] [Google Scholar]
- 81.James OFW, Day CP: Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol 1998, 29:495-501 [DOI] [PubMed] [Google Scholar]
- 82.Giudice GJ, Fuchs E: The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell 1987, 48:453-463 [DOI] [PubMed] [Google Scholar]
- 83.Van Eyken P, Sciot R, Desmet VL: A cytokeratin immunohistochemical study of alcoholic liver disease: evidence that hepatocytes can express “bile duct-type” cytokeratins. Histopathology 1988, 13:605-617 [DOI] [PubMed] [Google Scholar]
- 84.Van Eyken P, Sciot R, Desmet VJ: A cytokeratin immunohistochemical study of cholestatic liver disease: evidence that hepatocytes can express “bile duct-type” cytokeratins. Histopathology 1989, 15:125-135 [DOI] [PubMed] [Google Scholar]
- 85.Dinges HP, Zatloukal K, Denk H, Smolle J, Mair S: Alcoholic liver disease. Parenchyma to stroma relationship in fibrosis and cirrhosis as revealed by three-dimensional reconstruction and immunohistochemistry. Am J Pathol 1992, 141:69-83 [PMC free article] [PubMed] [Google Scholar]
- 86.Baumann PA, Dalton WS, Anderson JM, Cress AE: Expression of cytokeratin confers multiple drug resistance. Proc Natl Acad Sci USA 1994, 91:5311-5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marks GS, Powles J, Lyon M, McCluskey S, Sutherland E, Zelt D: Patterns of porphyrin accumulation in response to xenobiotics. Parallels between results in chick embryo and rodents. Ann N Y Acad Sci 1987, 514:113-127 [DOI] [PubMed] [Google Scholar]
- 88.Liao J, Ku NO, Omary MB: Stress, apoptosis, and mitosis induce phosphorylation of human keratin 8 at ser-73 in tissues and cultured cells. J Biol Chem 1997, 272:17565-17573 [DOI] [PubMed] [Google Scholar]
- 89.Liao J, Lowthert LA, Omary MB: Heat stress or rotavirus infection of human epithelial cells generates a distinct hyperphosphorylated form of keratin 8. Exp Cell Res 1995, 219:348-357 [DOI] [PubMed] [Google Scholar]
- 90.Feng L, Zhou X, Liao J, Omary MB: Pervanadate-mediated tyrosine phosphorylation of keratin 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci 1999, 112:2081-2090 [DOI] [PubMed] [Google Scholar]
- 91.Lai YK, Lee WC, Chen KD: Vimentin serves as a phosphate sink during the apparent activation of protein kinases by okadaic acid in mammalian cells. J Cell Biochem 1993, 53:161-168 [DOI] [PubMed] [Google Scholar]