Abstract
Although the roles of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in angiogenesis are well described, the putative roles of these factors in retinopathy of prematurity (ROP) remain unknown. We evaluated VEGF and HGF protein levels in subretinal fluid of eyes with ROP, and expression of their corresponding receptors in retrolental membranes associated with stage 5 ROP. We examined subretinal fluid samples from eyes using rhegmatogenous retinal detachment as a control. VEGF and HGF were differentially elevated in eyes with ROP. In Stage 5 ROP (n = 22), the mean VEGF and HGF levels were 14.77 ± 14.01 ng/ml and 16.56 ± 9.62 ng/ml, respectively. Interestingly, in patients with active stage 4 ROP, mean VEGF levels were highly elevated (44.16 ± 18.72 ng/ml), whereas mean HGF levels remained very low (4.77 ± 2.50 ng/ml). Next, we investigated in vivo expression of VEGF receptor-2 and HGF receptor in retrolental membranes from 16 patients with stage 5 ROP. Both VEGF receptor-2 and HGF receptor proteins were detected mainly in posterior portions of the membrane as well as in vessel walls and along the retinal interface where angiogenesis was active. These findings together suggest that VEGF and HGF play important roles in the pathogenesis of ROP.
Angiogenesis plays an essential role not only in normal physiology, but also in many pathological conditions such as proliferative diabetic retinopathy, age-related macular degeneration, and tumor growth. 1-4 In recent years much progress has been made in identifying factors that regulate neovascularization and angiogenesis. Two such factors are vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF). VEGF is a dimeric glycoprotein expressed by normal epithelial and tumor cells. 5 Recent studies have revealed the importance of VEGF in many angiogenic processes under normal and abnormal conditions, including experimental retinal neovascularization in animal models of retinopathy of prematurity (ROP). 6-12
Expression of VEGF is regulated by hypoxia. 13 Both retinal pigment epithelium and retinal glial cells, including Muller cells, release VEGF in response to hypoxic conditions. 14-16 In vitro, VEGF is a potent mitogenic factor for endothelial cells 17,18 and induces permeability of capillaries and blood vessels. 19 In vivo, VEGF is required for vasculogenesis 20,21 and tumor-induced angiogenesis. 22 These biological activities are mediated by binding of VEGF to two high-affinity receptors, VEGF receptors 1 (VEGFR-1 or FLT-1) and 2 (VEGFR-2 or FLK-1/KDR-1), which are expressed mainly by endothelial cells. 23,24
Unlike VEGF, HGF is expressed predominantly in cells of stromal origin, 25 including fibroblasts, vascular smooth muscle cells, and glial cells. 26 HGF exhibits pleiotropic biological functions as mitogenic, motogenic, morphogenic factor in epithelial cells and angiogenic factor in epithelial and as angiogenic factor in endothelial cells. 25-27 Recent studies demonstrate that HGF stimulates both growth and migration of endothelial cells in vitro, 28 and is a potent inducer of angiogenesis both in vitro and in vivo. 29,30 HGF exerts its actions through activation of a high-affinity tyrosine kinase receptor, hepatocyte growth factor receptor (HGFR). 31 HGFR is the 190-kd product of the met proto-oncogene composed of a 45-kd α chain disulfide linked to a 145-kd β chain. 32 Many cell types express HGFR, especially cells of epithelial and endothelial origin. 33 To date, the putative roles of angiogenic factors such as HGF and VEGF have not been firmly established in clinical cases of advanced ROP.
ROP is a major cause of newborn blindness. 34 It is seen almost exclusively in premature infants and is associated with low birth weight and oxygen supplementation during the postnatal period. 35 These conditions foster an intense proliferation of the vascular endothelium and glial cells at the junction of avascular and vascularized portions of the retina, 36 a process thought to result from liberation of angiogenic factors such as VEGF. 37 Advanced ROP is characterized by retinal neovascularization leading to traction retinal detachment (stage 4) or further proliferation of fibrovascular tissue on the retinal surface, resulting in formation of a retrolental fibrovascular membrane (RLF) and total retinal detachment with a white pupillary reflex (stage 5). 38,39
To investigate the putative roles of VEGF and HGF in ROP, we quantified samples of subretinal fluid (SRF) for presence of VEGF and HGF and examined RLF membranes from eyes with stage 5 ROP for expression of their corresponding receptors, VEGFR-2 and HGFR. Our study demonstrated that VEGF and HGF were both significantly elevated in eyes with stage 5 ROP. However, in eyes with stage 4 ROP, only VEGF levels in SRF were highly elevated, whereas HGF levels remained low, similar to those of rhegmatogenous retinal detachment (RRD). These observations suggest that VEGF and HGF may play an important role in pathogenesis of ROP. However, their contribution to the progression of this disease may be temporally related to a particular stage of this disease.
Materials and Methods
Cells and Culture Media
All cells were grown on 10-cm dishes in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, Grand Island, NY) containing 10% fetal bovine serum (FBS), supplemented with a mix of L-glutamate (100 mg/L), penicillin (100 U/ml), and streptomycin (100 mg/L) (GPS). Starvation medium consisted of DMEM containing 0.1% calf serum and GPS.
Reagents and Antibodies
Active, heterodimeric recombinant human HGF was purchased from R&D Systems (Minneapolis, MN). For immunoprecipitation of HGFR, a polyclonal anti-human HGFR antibody was kindly provided by Bruce Elliott, Queen’s University (Kingston, ON). This antibody was raised against the cytoplasmic domain of HGFR. For Western blotting and immunohistochemistry of the HGFR, a polyclonal antibody was purchased (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-phosphotyrosine antibody, pY20, was purchased from Transduction Laboratories (Lexington, KY) and used according to the suggestions provided. Anti-VEGFR-2 was made in-house. It is a polyclonal antibody raised against the kinase insert domain of VEGFR-2. This antibody does not cross-react with other members of VEGFRs (Rahimi N, Lashkari K, unpublished data). Nonimmune polyclonal rabbit IgG was purchased from Vector Laboratories (Burlingame, CA).
Patient Selection
ROP was diagnosed and staged according to patient history and an established international staging guideline. 38,39 Eyes with ROP were examined under anesthesia, including slit-lamp and indirect ophthalmoscopy. B-scan sonography was performed to image the extent and location of retinal detachment in stage 5 eyes in which the RLF membrane precluded a view of the fundus. In stage 4 ROP, a large fundus drawing was also made to document the extent and location of retinal detachment. Twenty-two eyes of 21 consecutive patients with stage 5 ROP undergoing open-sky vitrectomy or scleral buckling with drainage, and 5 eyes of 5 patients with stage 4 (A or B) ROP who were undergoing surgery scleral buckling with drainage were selected. Both eyes of one patient with bilateral stage 5 ROP were also included and analyzed. The number of stage 4 ROP samples was limited because of the use of scleral buckling without external drainage in treatment of most of these cases.
Twenty-one consecutive patients with uncomplicated RRD, in the absence of any evidence of proliferative vitreoretinopathy, were also analyzed. Subretinal fluid (SRF) of eyes with RRD was chosen as control for the following reasons: (i) in normal eyes, the subretinal space is a potential space that does not contain an appreciable amount of fluid, and is therefore not amenable to sampling; (ii) it is generally agreed that uncomplicated retinal detachment is not associated with any neovascular process, in the absence of an underlying systemic or local disease (such as diabetes mellitus, uncontrolled hypertension, hypoxia, blood dyscrasias, and proliferative vitreoretinopathy), whereas in RRD, VEGF, or HGF levels likely represent a basal level of secretion; and (iii) SRF from uncomplicated RRD eyes is readily available for comparative analysis.
Careful medical histories were taken of these patients to exclude any underlying systemic disease including diabetes mellitus, congestive heart failure, renal or hepatic insufficiency, and advanced or untreated hypertension. The duration and extent of retinal detachment were also recorded.
Collection of Subretinal Fluid Specimens
The use of human tissues was approved by the Investigational Review Board of the Schepens Eye Research Institute and adheres to the Declaration of Helsinki. SRF from patients with either ROP or RRD was collected during scleral buckling or open-sky vitrectomy procedures by making a full-thickness, linear incision through the sclera (posterior sclerotomy) under direct observation, and a small knuckle of choroid was exposed. SRF was expressed by choroidal puncture and collected directly into a tuberculin syringe connected to a 19-gauge irrigating cannula, which was held directly at the sclerotomy site. This technique does not generally result in choroidal hemorrhage even though the choroidal layer is vascular. Effort was made not to bring the tip of the tuberculin syringe in contact with the surgical bed to avoid contamination of the specimen with blood products or irrigation fluids. After surgery, SRF was transported to the laboratory and stored at −20°C until the time of assay.
Enzyme-Linked Immunosorbent Assay (ELISA) for HGF and VEGF
ELISA assay for VEGF and HGF were purchased from R&D Systems and used according to instructions. SRF samples were empirically diluted in phosphate buffered saline (PBS) to place the calculated concentration of growth factor within the standard curve established by the manufacturer. Levels of VEGF and HGF were read off an automated ELISA reader and corrected for their original concentrations. Readings were repeated and similar results were obtained.
Immunoprecipitation and Western Blot Analysis of HGFR
A549 cells were used because they express readily detectable levels of HGFR. In A549 cells, the HGFR is tyrosine-phosphorylated only in response to exogenous HGF stimulation. Cells were grown to 80% confluence in 10-cm plates and incubated overnight in starving medium. Serum-starved cells were stimulated for 10 minutes with 50 ng/ml HGF (positive control), 0.1% bovine serum albumin (negative control), or subretinal fluid from three independent samples of ROP and three samples of RRD. Cells were washed twice with iced 20 mmol/L HEPES buffer supplemented with 150 mmol/L NaCl (pH 7.4), and then lysed in extraction buffer (EB) (10 mmol/L Tris-HCl, pH 7.4, 5 mmol/L EDTA, 50 mmol/L NaCl, 50 mmol/L NaF, 0.1% bovine serum albumin, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L Na3VO4, 20 μg/ml aprotinin, 2 μg/ml leupeptin). 40 The lysates were centrifuged at 13,000 rpm in a microcentrifuge and the supernatants were incubated with 5 μg/ml of antiphosphotyrosine antibody for 1.5 hours at 4°C. Immune complexes were collected on protein A sepharose and washed three times in extraction buffer. 40 Immunoprecipitates were resolved on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel under reducing conditions and transferred to Immobilon membrane (Millipore, Bedford, MA). The membranes were incubated for 1 hour at room temperature in Block solution (137 mmol/L NaCl, 20 mmol/L Trizma base, pH 7.6, 10 mg/ml BSA, 10 mg/ml ovalbumin, 0.05% Tween-20, 0.5% NaN3) and probed with a human anti-HGFR antibody that recognizes the tail region of the 145-kd subunit of the HGFR for 2 hours at 4°C (Santa Cruz; 1:100). The membranes were then washed 3 times with Western rinse (137 mmol/L NaCl, 20 mmol/L Trizma base, pH 7.6), incubated with horseradish peroxidase-labeled secondary donkey anti-rabbit IgG (Amersham; 1:4000) mixed in Block, and washed three times in TBST buffer (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.1% Tween 20), and once in Western buffer containing 0.1% Tween 20. The membranes were then developed with enhanced chemiluminescent reagent (ECL, Amersham).
Tissue Preparation and Immunohistochemical Staining
Sixteen RLF membranes were collected from eyes with stage 5 ROP after en bloc dissection during open-sky vitrectomy and immediately fixed in phosphate-buffered formaldehyde (10% v/v, pH 7.2). Specimens were embedded in paraffin, sectioned, and mounted on 3-amino-propyl-trioxysilane-coated glass slides (Sigma, St. Louis, MO). Serial paraffin sections were prepared and representative sections were stained with hematoxylin and eosin.
For immunohistochemical staining, the Vector ABC kit was used. Unstained sections were deparaffinized, rehydrated, washed in PBS, pH 7.4, and incubated in 3% hydrogen peroxide solution to inhibit intrinsic peroxidase activity. They were placed in a moisture chamber, blocked for 30 minutes, and probed with the primary antibody (against VEGFR-2 or HGFR) for 1 hour. Sections were washed in PBS and incubated with biotinylated secondary antibody for 1 hour, washed again, and stained with diaminobendizine (DAB reagent, brown), and counterstained with hematoxylin (blue). Appropriate positive and negative controls were also run. For positive controls, sections of paraffin-embedded diabetic neovascular membranes and human eye were stained for VEGFR-2 and HGFR (data not shown). For negative controls, a polyclonal nonimmune antibody was substituted for the primary antibody at the same dilution.
Results
VEGF and HGF Levels in Subretinal Fluid of Eyes with RRD
To begin assessing the role of VEGF and HGF, we measured their respective levels in SRF of eyes with stages 4 and 5 ROP and in eyes with RRD. In 21 eyes of 21 patients with primary, uncomplicated RRD, VEGF and HGF titers were 0.45 ± 0.87 ng/ml and 6.45 ± 4.95 ng/ml, respectively (Table 1) ▶ . VEGF and HGF levels did not significantly correlate with age. Pearson’s correlation coefficient for VEGF and HGF versus age were r = 0.3505, P = 0.13 and r = 0.287, P = 0.207, respectively. Regression analysis was performed to test the influence of duration and extent of retinal detachment on VEGF and HGF levels. The duration and extent of retinal detachment did not significantly affect these levels or influence the relationship between age and these levels. We also calculated the mean ratio of VEGF to HGF (V/H ratio) in these eyes. The V/H ratio was 0.07 ± 0.12, suggesting that mean HGF concentration was nearly 14-fold higher than VEGF concentration in eyes with RRD.
Table 1.
VEGF and HGF Levels in Subretinal Fluid of Patients with Rhegmatogenous Retinal Detachment (RRD)
| Patient no. | Age (years) | Duration of RRD (days) | Extent of RRD (C hours) | VEGF (ng/ml) | HGF (ng/ml) | VEGF/HGF ratio |
|---|---|---|---|---|---|---|
| 1 | 5 | 180 | 12 | 0.22 | 0.68 | 0.32 |
| 2 | 5 | 30 | 12 | 0.34 | 11.4 | 0.03 |
| 3 | 6 | − | 12 | 0 | 0.8 | 0 |
| 4 | 27 | 180 | 3.5 | 0.13 | 15.2 | 0.01 |
| 5 | 28 | 7 | 6 | 0.3 | 3.1 | 0.10 |
| 6 | 32 | 7 | 6 | 0.05 | 3.02 | 0.02 |
| 7 | 41 | 30 | 6 | n/a | 5.15 | 0 |
| 8 | 43 | 30 | 6 | 2.21 | 4.8 | 0.46 |
| 9 | 44 | 5 | 6 | 0.41 | 3.1 | 0.13 |
| 10 | 46 | 180 | 6 | 0.04 | 6.1 | 0.01 |
| 11 | 52 | 75 | 12 | 0 | 4.35 | 0 |
| 12 | 52 | 3 | 12 | 0.36 | 6.01 | 0.06 |
| 13 | 55 | 7 | 2.5 | 0.05 | 5.3 | 0.01 |
| 14 | 59 | 14 | 8 | 0 | 2.1 | 0 |
| 15 | 59 | 21 | 6 | 0 | 5 | 0 |
| 16 | 62 | 30 | 6 | 0.25 | 19.4 | 0.01 |
| 17 | 63 | 30 | 6 | 0 | 7.5 | 0 |
| 18 | 70 | 14 | 6 | 0 | 4.3 | 0 |
| 19 | 75 | 3 | 6 | 0.8 | 9.2 | 0.09 |
| 20 | 78 | 180 | 4 | 0.25 | 4.1 | 0.06 |
| 21 | 91 | − | 12 | 3.50 | 14.9 | 0.23 |
−, not available due to insufficient sample volume; 0, not detectable by ELISA.
VEGF And HGF Titers Are Elevated in Subretinal Fluid of ROP Eyes
Table 2 ▶ shows titers of VEGF and HGF and the mean calculated V/H ratios in patients with stages 5 and 4 ROP. In 22 eyes with stage 5 ROP, the mean VEGF titer was 14.77 ± 14.01 ng/ml, and the mean HGF titer was 16.56 ± 9.62 ng/ml. The mean V/H ratio was 1.04 ± 1.11. In 5 eyes with stage 4 ROP, the VEGF titers were markedly elevated at 44.16 ± 18.72 ng/ml, whereas HGF levels remained low at 4.77 ± 2.50 ng/ml. Although sample size for stage 4 ROP was limited for reasons discussed previously, the mean V/H ratio was 8.16 ± 4.63, indicating that VEGF concentration was approximately eightfold higher than HGF concentration (Table 3) ▶ . The Pearson’s correlation coefficient showed no statistically significant relationship between age and VEGF or HGF levels. Their respective r values were 0.1556, P = 0.501 and 0.2763, P = 0.213. A two-sample independent t-test was used to compare the mean VEGF in stage 5 ROP with the mean VEGF in RRD. The differences between mean value of HGF for stage 5 ROP (14.76 ng/ml) and RRD (0.45 ng/ml) were highly significant, with P < 0.001. Similarly, the mean difference between the value of HGF for stage 5 ROP (16.56 ng/ml) and RRD (6.45 ng/ml) was statistically significant, with P < 0.001.
Table 2.
HGF and VEGF Levels in Subretinal Fluid of Patients with Stage 5 and 4 Retinopathy of Prematurity
| Patient no. | Age (months) | Eye | VEGF ng/ml | HGF ng/ml | VEGF/HGF ratio |
|---|---|---|---|---|---|
| Stage 5 ROP | |||||
| 1 | 2 | OS | 8.62 | 47.33 | 0.18 |
| 2 | 3 | OD | n/a | 15.10 | − |
| 3 | 3 | OD | 22.25 | 23.45 | 0.95 |
| 4 | 4 | OS | 6.41 | 5.10 | 1.26 |
| 5 | 4 | OD | 0.12 | 10.20 | 0.01 |
| 6 | 4 | OS | 8.82 | 12.00 | 0.74 |
| 7 | 6 | OS | 25.22 | 12.50 | 2.02 |
| 8 | 6 | OD | 12.37 | 15.70 | 0.79 |
| 9 | 6 | OS | 0.76 | 7.15 | 0.11 |
| 10 | 6 | OD | 0.68 | 3.40 | 0.20 |
| 11 | 6 | OS | 15.38 | 19.00 | 0.81 |
| 12 | 7 | OS | 16.28 | 19.50 | 0.83 |
| 13 | 9 | OS | 32.23 | 12.70 | 2.54 |
| 14 | 9 | OD | 2.66 | 10.26 | 0.26 |
| 15 | 9 | OS | 39.60 | 11.00 | 3.60 |
| 16 | 12 | OD | 11.91 | 16.00 | 0.74 |
| 17 | 12 | OS | 3.65 | 15.10 | 0.24 |
| 18 | 12 | OS | 49.9 | 13.70 | 3.64 |
| 19A | 12 | OD | 3.48 | 25.60 | 0.14 |
| 19B | 12 | OS | 3.38 | 22.60 | 0.15 |
| 20 | 24 | OD | 32.25 | 14.80 | 2.18 |
| 21 | 48 | OS | 14.16 | 32.16 | 0.44 |
| Stage 4 ROP | |||||
| 1 | 2 | OS | 16.92 | 6.56 | 2.58 |
| 2 | 3 | OS | n/a | 0.60 | − |
| 3 | 3 | OD | 59.70 | 4.30 | 13.88 |
| 4 | 4 | OD | 50.01 | 5.90 | 8.48 |
| 5 | 4 | OD | 50.00 | 6.50 | 7.69 |
−, not available due to insufficient sample volume.
Table 3.
Comparison of Mean VEGF and HGF Levels in Rhegmatogenous Retinal Detachment and Advanced Retinopathy of Prematurity
| Condition | Sample size (n) | Mean VEGF (ng/ml) | Mean HGF (ng/ml) | VEGF/HGF ratio |
|---|---|---|---|---|
| Rhegmatogenous retinal detachment | 21 | 0.45 ± 0.87 | 6.45 ± 4.95 | 0.07 ± 0.12 |
| Stage 5 ROP | 22 | 14.77 ± 14.01 | 16.56 ± 9.62 | 1.04 ± 1.11 |
| Stage 4 ROP | 5 | 44.16 ± 18.72 | 4.77 ± 2.50 | 8.16 ± 4.63 |
HGF in Stage 5 ROP Is Secreted in its Active Form
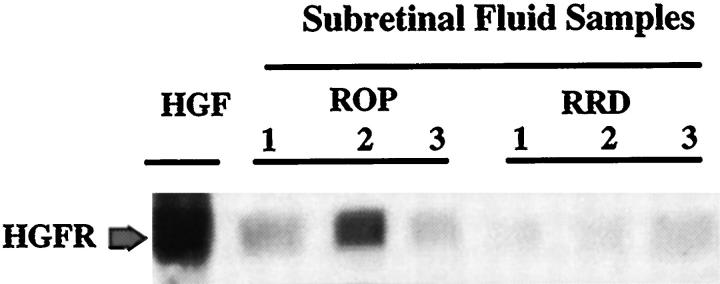
Unlike VEGF, HGF is secreted as a promolecule that requires activation by serum proteases. 41 We examined whether the HGF detected in SRF from stage 5 ROP eyes was biologically active. A549 cells are lung carcinoma cells with high levels of HGFR. 42 Serum-starved A549 cells expressing HGFR were stimulated with 50 ng/ml HGF (for positive control) or with SRF samples chosen from three patients with stage 5 ROP and three patients with RRD. Cells were stimulated for 10 minutes, immunoprecipitated with anti-phosphotyrosine antibody, and immunoblotted with anti-HGFR antibody (Figure 1) ▶ . We found that SRF from stage 5 ROP enhanced the tyrosine phosphorylation of HGFR in A549 cells. The HGFR could be recovered with an anti-phosphotyrosine antibody from SRF- or HGF-stimulated cells, but not from unstimulated cells (data not shown).
Figure 1.
HGF-dependent tyrosine phosphorylation of the HGF receptor (HGFR) in response to stimulation with subretinal fluid from stage 5 retinopathy of prematurity (ROP) and rhegmatogenous retinal detachment (RRD). A549 cells were grown to 80% confluence and serum-starved overnight in DMEM containing 0.1% calf serum. Cells were stimulated for 10 minutes with either 50 ng/ml HGF (as control), or with subretinal fluid collected from 3 eyes with stage 5 ROP and 3 eyes with RRD. Cells were lysed and immunoprecipitated with anti-phosphotyrosine antibody and then subjected to Western blot analysis using anti-HGFR antibody. The blot shows that subretinal fluid from both stage 5 ROP and RRD were able to trigger tyrosine phosphorylation of HGFR, suggesting that HGF is secreted in an active form within the subretinal space. The arrow shows the HGFR (145 kd) protein.
Immunolocalization of VEGFR-2 and HGFR in RLF Membranes
To investigate in vivo expression of VEGFR-2 and HGFR, 16 RLF membranes were collected from 16 patients with stage 5 ROP and analyzed by immunohistochemistry (Figures 2 and 3) ▶ ▶ . RLF membrane was comprised of a fibrous component with interspersed intrastromal cells with spindle morphology. Overall, membranes were more vascular in their posterior portions adjacent to the retina. Numerous feeder vessels were observed, probably originating from the underlying retina. We examined the expression of these receptors with respect to their localization within the stromal or vascular areas of the RLF membranes. Both VEGFR-2 and HGFR were highly expressed within posterior portions of the RLF membranes. They were heavily concentrated in the vascular portions, within vessel walls, and along the posterior lining of the membranes at the retinal interface (Figure 2, A and B) ▶ . To identify the cell types, we stained the membranes with an anti-Factor VIII antibody, an endothelial marker, and demonstrated that majority of these cells expressed Factor VIII (data not shown). Data suggest that the cells expressing VEGFR-2 and HGFR are mostly of endothelial origin. Interestingly, VEGFR-2 and HGFR were also identified in intrastromal spindle cells within the central fibrous component of the membranes (Figure 3, A and B) ▶ .
Figure 2.
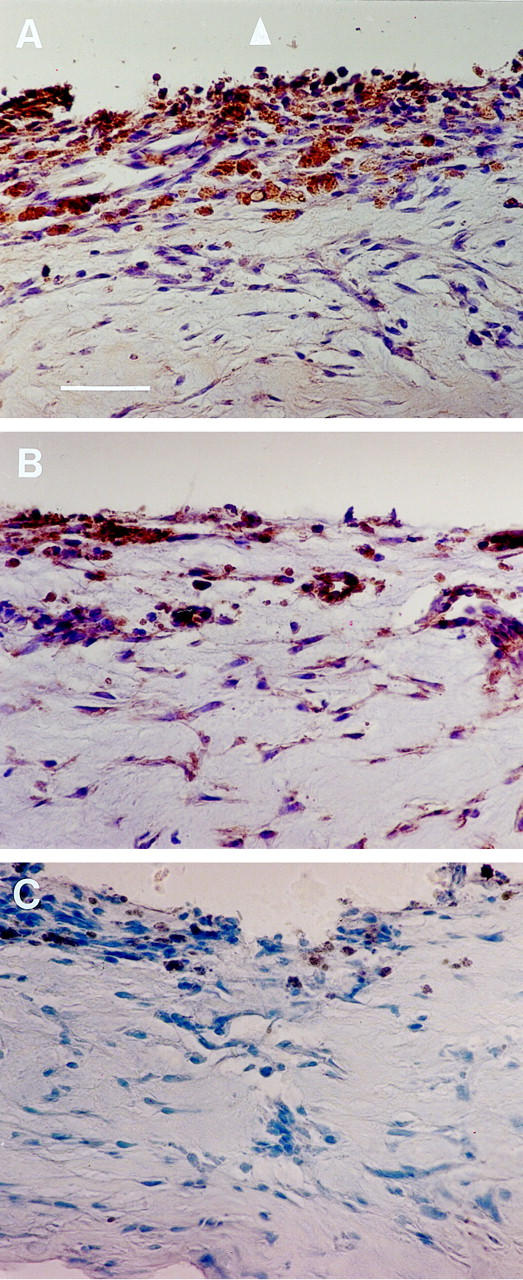
Immunohistochemical localization of vascular endothelial growth factor receptor-2 (VEGFR-2) and hepatocyte growth factor receptor (HGFR) in retrolental membranes (RLF) obtained from patients with stage 5 retinopathy of prematurity (ROP). Protein expression was detected using the DAB reagent (peroxidase; brown color) and tissue was counterstained with hematoxylin. Bar, 100 μm. A: Using an antibody that recognizes VEGFR-2, its expression is seen in population of cells and in the vascular compartment within the posterior segment of the RLF adjacent to the retinal interface (arrowhead). B: HGFR expression is seen in vessel walls and within stromal cells, and more concentrated along the retinal interface. C: Negative control using nonimmune polyclonal rabbit antibody. Trace pigment deposition is along the posterior segment of the RLF membrane.
Figure 3.
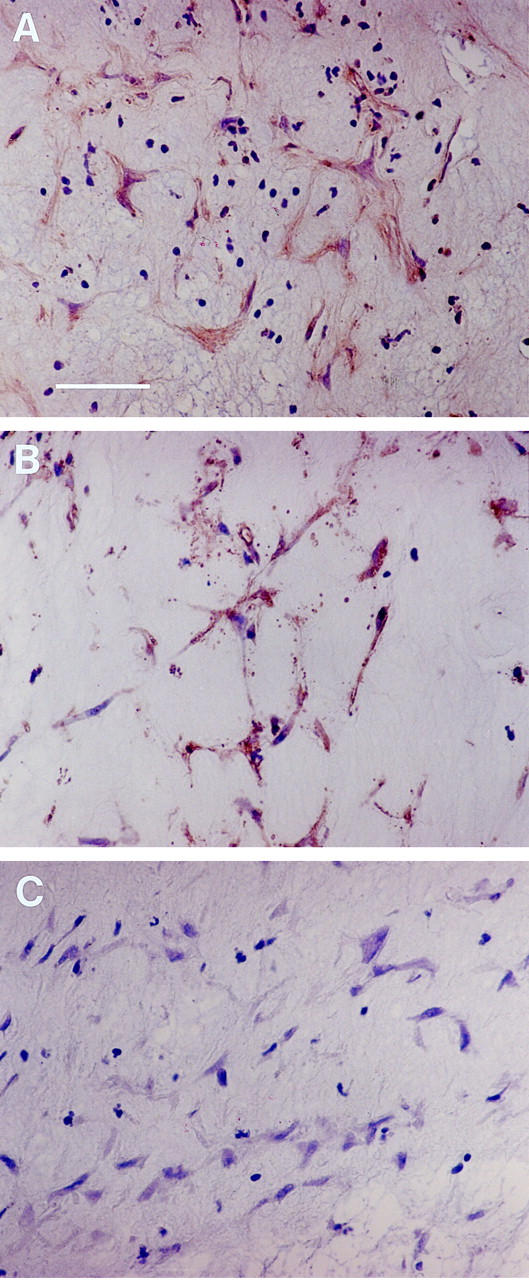
Expression of vascular endothelial growth factor receptor-2 (A) and hepatocyte growth factor receptor (B) by intrastromal spindle cells within the retrolental membrane from stage 5 retinopathy of prematurity. Proteins were detected using DAB reagent (peroxidase; brown color), and tissue was counterstained with hematoxylin. Bar, 50 μm. C: Negative control using nonimmune polyclonal rabbit antibody.
Discussion
Factors that contribute to normal vascularization during development are the molecules likely involved in pathological neovascularization. Both HGF and VEGF have been implicated in vasculogenesis and angiogenesis. 20-22,29,30 However, the role of HGF in this process is less understood. We have demonstrated that VEGF and HGF levels were elevated in SRF of patients with ROP. VEGF levels were significantly elevated (90-fold) in stage 4 ROP as compared with RRD eyes, whereas HGF levels remained unchanged. In contrast, high levels of HGF were detected only in stage 5 of ROP. Stage 4 ROP is characterized by partial retinal detachment in which the neovascularization and fibrovascular proliferation processes are still active. 39 Our findings that VEGF levels were elevated in stage 4 of ROP but greatly reduced in stage 5 suggests that VEGF is highly active in stage 4 and is more likely to play a key role in the maintenance of neovascularization. It is likely that VEGF contributes continually to the progression of disease in the form of vascular growth and vasodilation of retinal vessels. This idea is further reinforced by our detection of VEGFR-2 in ROP membranes, which are the target tissue.
The finding that HGF levels are elevated only in stage 5 ROP, but reduced in stage 4, implicates different roles for these molecules in the pathophysiology of this disease. HGF can induce a variety of cellular responses in endothelial and epithelial cells including scattering, proliferation, and migration. 25,27-29 Because HGF functions are not limited to endothelial cells, HGF is likely to be involved in various aspects of ROP, including growth of pericytes and glial and spindle cells. Spindle cells are believed to be of mesenchymal origin and contribute to retinal vascularization. 43-45
For example, HGF may directly influence vascularization by mediating or modulating interactions between endothelial cells and pericytes. HGF may also modulate extracellular matrix production and thereby contribute to retinal detachment. The clinical importance of HGF in clinical cases of retinal detachment is highlighted by our recent study that implicates HGF in retinal detachment associated with proliferative vitreoretinopathy. 40
Further studies are required to characterize fully the nature of HGF activity in ROP development. Our present work represents the first evidence that HGF and VEGF levels are both elevated in patients with ROP. These findings are important to understanding the role of these factors in initiation and progression of this disease.
Acknowledgments
We thank Ms. Patricia Pearson and Ms. Holly Goolsby for technical assistance.
Footnotes
Address reprint requests to Kameran Lashkari, M.D., Schepens Eye Research Institute, 20 Staniford Street, Boston, MA 02114. E-mail: lashkari@vision.eri.harvard.edu.
Supported in part by Clinical Investigator Award, Schepens Eye Research Institute (to K. L.), a grant from the Marsh Charitable Fund (to J. W. M.), and a departmental grant from the Massachusetts Lions Eye Research Fund, Inc. (to N.R.).
References
- 1.Wiedemann P: Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol 1992, 36:373-384 [DOI] [PubMed] [Google Scholar]
- 2.Forrester JV, Shafiee A, Schroder S, Knott R, McIntosh L: The role of growth factors in proliferative diabetic retinopathy. Eye 1993, 7:276-287 [DOI] [PubMed] [Google Scholar]
- 3.Zarbin MA: Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol 1998, 8:199-206 [DOI] [PubMed] [Google Scholar]
- 4.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 5.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 6.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE: Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995, 92:10457-10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorey CK, Aouididi S, Reynaud X, Dvorak HF, Brown LF: Correlation of vascular permeability factor/vascular endothelial growth factor with extraretinal neovascularization in the rat. Arch Ophthalmol 1996, 114:1210-1217 [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata, Sueishi K: The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest 1996, 74:68-77 [PubMed] [Google Scholar]
- 9.Stone J, Chan-Ling T, Pe’er J, Itin A, Gnessin H, Keshet E: Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1996, 37:290-299 [PubMed] [Google Scholar]
- 10.Donahue ML, Phelps DL, Watkins RH, LoMonaco MB, Horowitz S: Retinal vascular endothelial growth factor (VEGF) mRNA expression is altered in relation to neovascularization in oxygen induced retinopathy. Curr Eye Res 1996, 15:175-184 [DOI] [PubMed] [Google Scholar]
- 11.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA: Trangenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997, 151:281-291 [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins SG, Rajaratnam VS, Penn JS: Evidence for upregulation and redistribution of vascular endothelial growth factor (VEGF) receptors flt-1 and flk-1 in the oxygen-injured rat retina. Growth Factors 1998, 16:1-9 [DOI] [PubMed] [Google Scholar]
- 13.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E: Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol 1998, 18:3112-3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA: Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 1995, 113:1538-1544 [DOI] [PubMed] [Google Scholar]
- 15.Kociok N, Heppekausen H, Schraermeyer U, Esser P, Thumann G, Grisanti S, Heimann K: The mRNA expression of cytokines and their receptors in cultured iris pigment epithelial cells: a comparison with retinal pigment epithelial cells. Exp Eye Res 1998, 67:237-250 [DOI] [PubMed] [Google Scholar]
- 16.Behzadian MA, Wang XL, Shabrawey M, Caldwell RB: Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia 1998, 24:216-225 [PubMed] [Google Scholar]
- 17.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246:1306-1309 [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW: The vascular endothelial growth factor family of polypeptides. J Cell Biochem 1991, 47:211-218 [DOI] [PubMed] [Google Scholar]
- 19.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT: Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989, 246:1309-1312 [DOI] [PubMed] [Google Scholar]
- 20.Breier G, Albrecht U, Sterrer S, Risau W: Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114:521-532 [DOI] [PubMed] [Google Scholar]
- 21.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A: High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 22.Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS: Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res 1999, 59:1592-1598 [PubMed] [Google Scholar]
- 23.Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR: A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci USA 1991, 88:9026-9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fong GH, Rossant J, Gertsenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 25.Birchmeier C, Meyer D, Riethmacher D: Factors controlling growth, motility, and morphogenesis of normal and malignant epithelial cells. Int Rev Cytol 1995, 160:221-226 [DOI] [PubMed] [Google Scholar]
- 26.Koochekpour S, Jeffers M, Rulong S, Taylor G, Klineberg E, Hudson EA, Resau JH, VandeWoude GF: Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 1997, 57:5391-5398 [PubMed] [Google Scholar]
- 27.Bhargava M, Joseph A, Knesel J, Halaban R, Li Y, Pang S, Goldberg I, Setter E, Donovan MA, Zarnegar R, et al: Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ 1992, 3:11-20 [PubMed] [Google Scholar]
- 28.Rosen EM, Grant D, Kleinman H, Jaken S, Donovan MA, Setter E, Luckett PM, Carley W, Bhargava M, Goldberg ID: Scatter factor stimulates migration of vascular endothelium and capillary-like tube formation. EXS 1991, 59:76-88 [DOI] [PubMed] [Google Scholar]
- 29.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM: Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA 1993, 90:1937-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen EM, Zitnik RJ, Elias JA, Bhargava MM, Wines J, Goldberg ID: The interaction of HGF-SF with other cytokines in tumor invasion and angiogenesis. EXS 1993, 65:301-310 [PubMed] [Google Scholar]
- 31.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, VandeWoude G: Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA 1987, 84:6379-6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AM, King HW, Tempest PR, Deakin EA, Cooper CS, Brookes P: Primary structure of the met protein tyrosine kinase domain. Oncogene 1987, 1:229-233 [PubMed] [Google Scholar]
- 33.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993, 123:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen RA, Hunter DG, Mukai S: Retinopathy of prematurity. Principles and Practice of Ophthalmology, vol 4, ch 224. Edited by Albert DM and Jakobiec FA. Philadelphia, Saunders, 1994, pp 2799–2812
- 35.Lucey JL, Dangman B: A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics 1984, 73:82-96 [PubMed] [Google Scholar]
- 36.Flynn JT, O’Grady GE, Herrera J, Kushner BJ, Cantolino S, Milam W: Retrolental fibroplasia. I. Clinical observations. Arch Ophthalmol 1977, 95:217-223 [DOI] [PubMed] [Google Scholar]
- 37.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995, 1:1024-1028 [DOI] [PubMed] [Google Scholar]
- 38.: The Committee for the Classification of Retinopathy of Prematurity: An international classification of retinopathy of prematurity. Arch Ophthalmol 1984, 102:1130-1134 [DOI] [PubMed] [Google Scholar]
- 39.: The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity: An international classification of retinopathy of prematurity. II. The classification of retinal detachment. Arch Ophthalmol 1987, 105:906-912 [PubMed] [Google Scholar]
- 40.Lashkari K, Rahimi N, Kazlauskas A: Hepatocyte growth factor receptor in human RPE cells: Implications in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1999, 40:149-156 [PubMed] [Google Scholar]
- 41.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S: Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342:440-443 [DOI] [PubMed] [Google Scholar]
- 42.Wang NS, Liu C, Emond J, Tsao MS: Annulate lamellae in a large cell lung carcinoma cell line with high expression of tyrosine kinsase receptor and proto-oncogenes. Ultrastruct Pathol 1992, 16:439-449 [DOI] [PubMed] [Google Scholar]
- 43.Cogan DG, Kuwabara T: Accessory cells in vessels of the prenatal human retina. Arch Ophthalmol 1986, 104:747-752 [DOI] [PubMed] [Google Scholar]
- 44.Kretzer FL, Hittner HM: Retinopathy of prematurity: Clinical implications of retinal development. Arch Dis Child 1988, 63:1151-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fielder AR, Moseley MJ, Ng YK: The immature visual system and premature birth. Br Med Bull 1988, 44:1093-1118 [DOI] [PubMed] [Google Scholar]