Abstract
Pulmonary hypoplasia associated with congenital diaphragmatic hernia (CDH) remains a major therapeutic problem. Moreover, the pathogenesis of pulmonary hypoplasia in case of CDH is controversial. In particular, little is known about early lung development in this anomaly. To investigate lung development separate from diaphragm development we used an in vitro modification of the 2,4-dichlorophenyl-p-nitrophenylether (Nitrofen) animal model for CDH. This enabled us to investigate the direct effects of Nitrofen on early lung development and branching morphogenesis in an organotypic explant system without the influence of impaired diaphragm development. Epithelial cell differentiation of the lung explants was assessed using surfactant protein-C and Clara cell secretory protein-10 mRNA expression as markers. Furthermore, cell proliferation and apoptosis were investigated. Our results indicate that Nitrofen negatively influences branching morphogenesis of the lung. Initial lung anlage formation is not affected. In addition, epithelial cell differentiation and cell proliferation are attenuated in lungs exposed to Nitrofen. These data indicate that Nitrofen interferes with early lung development before and separate from (aberrant) diaphragm development. Therefore, we postulate the dual-hit hypothesis, which explains pulmonary hypoplasia in CDH by two insults, one affecting both lungs before diaphragm development and one affecting the ipsilateral lung after defective diaphragm development.
Congenital diaphragmatic hernia (CDH) has a mean prevalence of approximately 1 in 3000 newborns. 1 Even sophisticated management techniques, such as extracorporeal membrane oxygenation (ECMO) and fetal surgery, have not significantly influenced the mortality rate of CDH in high-risk patients and consequently, CDH remains a major problem in pediatric surgery and neonatology. A recent review shows that the mortality remains high at 50 to 60% in high-risk patients. 2 However, other reports suggest a modest reduction in mortality to approximately 30% in comparable series of patients. 3,4 A combination of morphological features characterizes this anomaly: pulmonary hypoplasia and persistent pulmonary hypertension are held responsible for the high mortality. In particular, the degree of pulmonary hypoplasia is almost impossible to evaluate pre- and immediately postnatal, and in most cases the degree of pulmonary hypoplasia is an important determinant of the outcome. 5
The pathogenetic events resulting in the diaphragmatic defect and pulmonary hypoplasia are unknown. Classically, the primary defect is believed to be located in the diaphragm. Abdominal organs that herniate through this defect will interfere with normal pulmonary development. This results in a secondary defect: pulmonary hypoplasia and abnormal pulmonary vascular development. 6,7 However, since the introduction of the 2,4-dichlorophenyl-p-nitrophenylether (Nitrofen) animal model for CDH, an alternative hypothesis has been suggested. 8,9 This animal model is based on the teratogenic effects of the herbicide Nitrofen. When administered in the right dosage and at the right time to pregnant rats and mice, Nitrofen interferes with development of the lungs and the diaphragm of the offspring. 9-11 Studies with this animal model have suggested that CDH might be due to primary disturbance of pulmonary growth into the pleuroperitoneal canal, thereby disturbing the growth of the posthepatic mesenchymal plate, the main origin of the diaphragm. 8 After exposure to Nitrofen, 100% of the litter has a variable amount of lung hypoplasia, whereas depending on animal strain and timing of Nitrofen administration, a smaller percentage, varying from 60 to 90% in rats and 40 to 60% in mice, has a diaphragmatic hernia as well. 9-11 Observations that both the contralateral and ipsilateral lung are hypoplastic suggest that proper pulmonary development in CDH is already impaired before the failed closure of the diaphragm. On the ipsilateral side, growth of the lung is hampered at a later phase by the presence of abdominal organs in the thoracic cavity and eventually interference with fetal breathing movements.
We hypothesized that the lungs are the primary target organ in the pathogenesis of pulmonary hypoplasia in case of Nitrofen-induced CDH. This implies that the lungs have to be hypoplastic before defective closure of the diaphragm has occurred. To test this hypothesis, we investigated early pulmonary development in an in vitro modification of the Nitrofen model. We have set up a foregut/lung explant system in which it is possible to study lung formation and branching morphogenesis without the influence of normal or defective closure of the diaphragm. The direct effects of Nitrofen on early pulmonary development in vitro were investigated by culturing explants in the presence of Nitrofen. In addition, the effects of removal of physical constraint on branching morphogenesis after Nitrofen treatment in vivo were tested. We show herein that Nitrofen interferes with branching morphogenesis both in vitro and in vivo. In contrast, initial lung bud outgrowth is not disturbed. Epithelial cell differentiation is attenuated even before effects of a defective closure of the diaphragm could have occurred. Furthermore, the interplay between proliferation and apoptosis is disturbed in lungs exposed to Nitrofen and therefore contributes to pulmonary hypoplasia.
Materials and Methods
Animals
Female (200–250 g) and male (250–300 g) Wistar rats were obtained from Charles River (St. Constant, PQ). The animals were kept in a controlled light-dark cycle and food and water were supplied ad libitum. Rats were mated overnight and the finding of a sperm-positive vaginal smear was designated day 0 of gestation. At 11 and 13 days of gestation (term = 22 days of gestation), timed-pregnant rats were killed by cervical dislocation after a short exposure to diethyl ether to anesthetize them. The fetuses were delivered by Caesarian section using aseptic surgical techniques and kept in Hanks’ balanced salt solution (Gibco, Burlington, ON). All protocols were evaluated and approved by the Animal Care Committee of the Hospital for Sick Children.
Explant Cultures
Foreguts and lungs were cultured according to previously reported studies. 12 In short, foreguts (at 11 days of gestation) and lungs (at 13 days of gestation) were harvested from fetal rats under a dissection microscope using microsurgical techniques. The foreguts and lungs were transferred to porous membrane inserts (4 μm pore size) from Millipore (Bedford, MA), and incubated in 4-well cell culture plates from Nunc (Intermed, Denmark). The membrane inserts were pre-soaked in MEM (Gibco) for 1 hour before the explants were placed on them. The explants were incubated as floating cultures in 200 μl Dulbecco’s modified Eagle’s medium, nutrient mixture F-12 (Gibco) supplemented with 100 μg/ml streptomycin, 100 units/ml penicillin, 0.25 mg/ml ascorbic acid and 10% (v/v) heat-inactivated fetal bovine serum (Gibco). Heat inactivation of the serum was required to support long-term culture. The explants were cultured at 37°C in 95% air and 5% CO2.
Nitrofen
The herbicide 2,4-dichloro-phenyl-p-nitrophenylether (Nitrofen) was obtained from Rohm & Haas Co. (Philadelphia, PA). To induce congenital diaphragmatic hernia and pulmonary hypoplasia in vivo, 100 mg Nitrofen dissolved in 1 ml olive oil was administered orally on day 9 of gestation. To investigate the effects of Nitrofen in vitro, the explants were exposed during the first day of culture to a concentration of Nitrofen, which is similar to that used in vivo: 0.25 mg.200 μl−1 medium. To mimic the half-life of Nitrofen in vivo, the explants were exposed to half of this during the second day of culture. 13,14 During the remaining culture period the explants were cultured in medium alone. Nontreated explants and explants cultured in the presence of vehicle dimethyl sulfoxide (DMSO; BDH, Toronto, ON) in the same concentration as was used to dissolve the Nitrofen served as controls (Table 1) ▶ . Because Nitrofen is extremely toxic, all handling was done in a fume hood or laminar flow using protective gear to prevent inhalation and contact with the skin. Disposal of all waste products containing Nitrofen was done according to local regulations of The Hospital for Sick Children and all experimental procedures were approved by the department of Occupational Health and Safety of the Hospital for Sick Children.
Table 1.
Organotypic Explant Cultures
Conditions for the organotypic explant cultures. For details see Materials and Methods.
Quantification of Branching Morphogenesis
During the entire period of culture, lung formation and branching morphogenesis was monitored daily by phase-contrast microscopy. At approximately the same time point each day, branching morphogenesis was assessed by manually counting the number of terminal buds, and the lung explants were photographed.
Tissue Preparation
After 8 days (foregut) or 4 and 6 days (lung) of culture, explants were removed from the inserts and fixed in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 16 to 18 hours, dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraplast (Oxford Labware, St. Louis, MO). Sections 5 μm thick were cut and mounted on Superfrost slides (Fisher Scientific, Unionville, ON) and baked at 42°C for 16 to 18 hours.
In Situ Hybridization
After dewaxing and rehydrating, tissue sections were postfixed in 4% (w/v) paraformaldehyde in PBS and permeabilized with proteinase K (20 μg·ml−1 in 50 mmol/L Tris/HCl, 5 mmol/L EDTA, pH 8.0) for 15 minutes at room temperature. After another postfixation step in 4% (w/v) paraformaldehyde in PBS for 5 minutes, the sections were carbethoxylated by 0.1% (v/v) active diethylpyrocarbonate treatment twice for 15 minutes to reduce background by inactivating RNases in the sections. Subsequently, the sections were equilibrated in 5× SSC (NaCl 0.75 mol/L, sodium citrate 0.075 mol/L) for 15 minutes and prehybridized for 2 hours at 58°C in 50% (v/v) formamide, 5× SSC and 40 μg·ml−1 salmon sperm DNA. The sections were hybridized overnight at 58°C with digoxigenin (DIG)-labeled Surfactant protein-C (SP-C) and Clara cell secretory protein-10 (CC-10) RNA probes in the same hybridization mixture. Rat specific SP-C and CC-10 cDNA fragments (330 and 315 bases, respectively) were DIG-labeled according to a protocol provided by the manufacturer (Boehringer Mannheim, Montreal, PQ). The next day, sections were washed in 2× SSC for 30 minutes at room temperature, 1 hour at 58°C and in 0.1× SSC for 1 hour at 58°C. The DIG Nucleic Acid Detection Kit from Boehringer Mannheim was used for immunological detection of the hybridized probe. Unspecific labeling was removed in 95% ethanol and the sections were counterstained with methyl green for 1.5 minutes. After dehydration in a graded series of ethanol and xylene, the sections were mounted with coverslips using Permount (Fisher Scientific).
Immunohistochemistry
Sections were dewaxed in xylene after heating them at 60°C for 5 minutes and rehydrated in a graded series of ethanol. Subsequently, antigen retrieval was performed by boiling the sections in a 10 mmol/L sodium citrate solution, pH 6.0 for two periods of 5 minutes in a microwave at medium high. Between the boiling periods, the sections cooled down for 20 minutes. After rinsing the slides in PBS endogenous peroxidase activity was blocked by exposing the slides to a 3% (v/v) hydrogen peroxide in methanol solution for a period of 10 minutes. After two rinses in PBS, the sections were blocked with 5% (v/v) normal goat serum and 1% (w/v) bovine serum albumin in PBS for the period of 1 hour. The sections were incubated overnight at 4°C with 1:1000 diluted primary monoclonal antibody to proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology, Santa Cruz, CA). The sections were washed in PBS containing 0.05% (v/v) Tween followed by two washes in PBS alone. The sections were then incubated with a 200-fold dilution of biotinylated anti-mouse IgG for 1 hour at room temperature. After washing, the sections were incubated with avidin-biotin peroxidase complex (Vectastain) kit from Vector Laboratories (Burlingame, CA) for 2 hours at room temperature. Subsequently, after washes in PBS and Tris-buffered saline (TBS), the sections were developed using 3,3′-diaminobenzidine as substrate. Following washes in TBS and PBS, sections were counterstained with Carazzi’s hematoxylin. Subsequently, the sections were dehydrated in a graded series of ethanol and xylene and mounted with coverslips using Permount.
Terminal Deoxyribonucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
After dewaxing and rehydrating, tissue sections were incubated with proteinase K, 20 μg·ml−1 in 10 mmol/L Tris/HCl, pH 7.6 for 15 minutes at 37°C. Following two washes with PBS, the sections were incubated with 25 μl TUNEL reaction mixture (Boehringer Mannheim) for 1 hour at 37°C. Subsequently, the sections were washed in PBS and mounted with coverslips using Vectashield mounting medium with DAPI from Vector Laboratories (Burlingame, CA).
Data Presentation
All results are expressed as mean ± SE. Statistical significance was determined by one way analysis of variance followed by assessment of differences using a Tukey test for pairwise multiple comparison procedures. 15 Linear regression was performed after transformation of the curves in straight lines by changing the numerical y axis scaling into a natural log scale. The rate of branching (slope) was depicted by the coefficient ± SE and differences were assessed using Student’s t-test. Significance was defined as P < 0.05.
Results
Lung Formation and Branching Morphogenesis
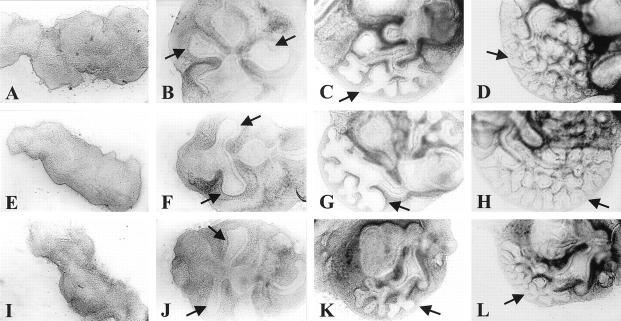
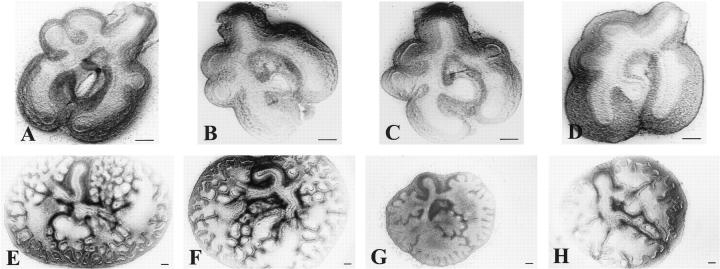
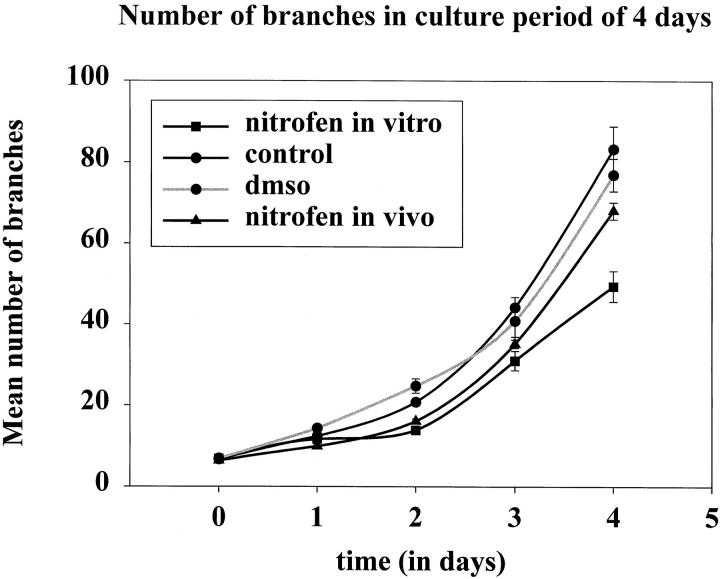
After 72 hours of culture, lung formation was observed in all foregut explants as a diverticulum arising from the foregut to form two primary lung buds (Figure 1, B, F, and J) ▶ . The timing of lung bud appearance was approximately the same in all explants. The same holds true for initial lung bud outgrowth after Nitrofen treatment in vivo, since the endodermal part of the lungs in this group appeared similar at the moment of isolation (Figure 2D) ▶ . However, the mesenchymal parts of the lungs treated with Nitrofen in vivo appeared much thicker than the mesenchymal parts of the control lungs (Figure 2D) ▶ . During the remaining period of culture, dichotomous branching was observed in all lung explants (Figure 2, E ▶ -H). In the Nitrofen-exposed explants, only lobular branching was observed (Figure 2, G and H) ▶ , and the rate of branching was reduced (Figure 3 ▶ and Table 2 ▶ ). The explants exposed to Nitrofen in vitro showed an arrest in branching during the first 2 days of culture, the period of exposure to Nitrofen. However, the rate of branching was also reduced during the subsequent 2-day culture period (Figure 3) ▶ . The number of branches of explants exposed to Nitrofen in vitro was significantly reduced when compared to control explants (P < 0.05; Table 2 ▶ ). Exposure to Nitrofen in vivo did not result in a statistically significantly reduced number of branches. When only the 75% lowest mean numbers of branches were used for comparison, statistical significance was reached (Table 2) ▶ . No significant differences were observed between control and DMSO-exposed explants. The size of the explants was reduced after exposure to Nitrofen, suggesting lung hypoplasia. Nitrofen exposure in vitro resulted in more severe reduction in branching and size than exposure in vivo.
Figure 1.
Lung formation and branching morphogenesis of the lung in an explant system of rat foregut. Foreguts of rats were harvested at 11 days of gestation and cultured in a semidry system. Pictures representative of a series of experiments of untreated (A−D), vehicle DMSO-exposed (E−H) and Nitrofen-exposed (I−L) foreguts developing into branching lungs are shown at culture day 0 (A, E, I), 2 (B, F, J), 6 (C, G, K), and 8 (D, H, L), respectively. The Nitrofen-exposed group was exposed to Nitrofen in a concentration similar to that used in vivo: 0.25 mg.200 μl−1 medium on the first culture day, and to half of this on the second culture day. Lung formation in this group occurred at the same time point as in the control groups (J), which were cultured in medium with vehicle DMSO (F) or in medium alone (B). Branching morphogenesis was clearly reduced in the explants exposed to Nitrofen during the remaining culture period (L). Arrow indicates lung. All images at same magnification.
Figure 2.
Branching morphogenesis in a rat lung explant system. At 13 days of gestation, lungs were removed from the fetuses and cultured in a semidry system. The upper row of pictures are representative for lungs at 13 days of gestation at culture day 0 just after isolation of the lungs from the fetus. The bottom row of pictures represents lungs after a culture period of 4 days. One group was treated with Nitrofen in vivo by administration of the Nitrofen to the mother at 9 days of gestation (D and H) Another group was treated with Nitrofen in vitro as described before (G). Branching morphogenesis was compared to groups cultured in medium alone (E), and groups cultured in medium plus vehicle DMSO (F). In all Nitrofen-exposed explants a statistically significant (P < 0.05) reduced number of branches was observed after 4 days of culture (G and H), whereas no effects of exposure to DMSO were observed (F; see also Figure 3 ▶ and Table 2 ▶ ). In addition, the size of the explants exposed to Nitrofen was clearly reduced (G and H). The effects of Nitrofen exposure in vitro were more severe than the effects after Nitrofen exposure in vivo. All pictures are representative of a series of experiments. Scale bar represents magnification level.
Figure 3.
Branching morphogenesis plotted as a graph. Number of branches, mean ± SE, is plotted against days of culture. Explants exposed to Nitrofen in vitro showed an arrest in branching during the first 2 days of culture, the period of exposure to Nitrofen. In addition, the rate of branching (slope) was also reduced after the first 2 days of culture during the remaining culture-period. After exposure to Nitrofen in vivo explants showed also a reduced number of branches after 3 days of culture and a reduced rate of branching (slope) although to a lesser extent than the ones exposed to Nitrofen in vitro.
Table 2.
Branching Morphogenesis Is Reduced in Nitrofen-Exposed Rat Lung Explants, 13 Days of Gestation after 4 Days of Culture
| Treatment | No. of branches (mean ± SE) | n | Slope |
|---|---|---|---|
| Control | 83.278 5.576 | 16 | 0.63 |
| DMSO | 76.917 4.008 | 12 | 0.58 |
| Nitrofen in vitro | 49.385 3.790* | 13 | 0.49* |
| Nitrofen in vivo | 64.207 2.099* | 40 | 0.60 |
The number of branches after 4 days of culture and the rate of branching (slope) were significantly reduced in the explants exposed to Nitrofen in vitro. When only the 75% of animals with lowest number of branches (corresponding to the frequency of diaphragmatic hernias in the animal model) was taken into account for the in vivo treated group, statistical significance was also reached for this group. However, the slope in this group remained unchanged. No difference was detected between the control explants and the explants exposed to vehicle DMSO. n, number of lungs taken from different fetuses of different litters and in different experiments; *, P < 0.05.
Epithelial Cell Differentiation
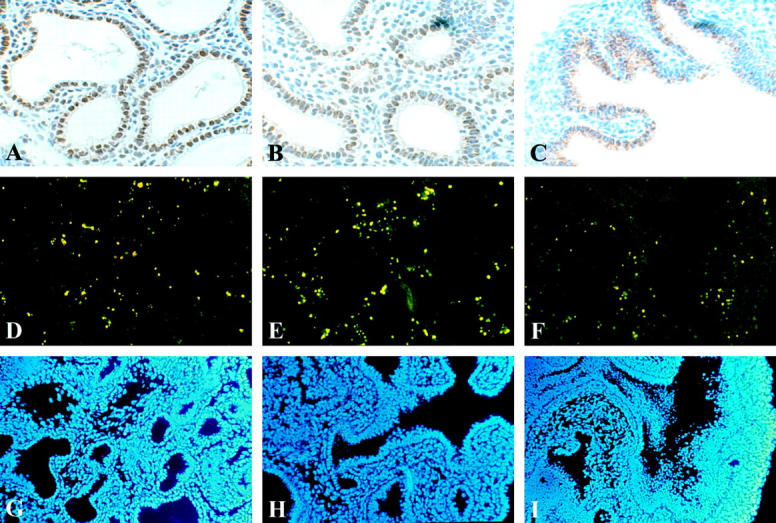
In the sections of the control explants and DMSO explants, major airways and a pseudoglandular aspect were distinguished (Figure 4, A, B, E, and F) ▶ . In the sections of Nitrofen exposed explants, large air spaces, due to lobular branching were observed (Figure 4, C, D, G, and H) ▶ . After 4 days of culture, SP-C mRNA was present in the control and DMSO exposed explants as well as in the explants exposed to Nitrofen in vivo (Figure 4, A, B, and D) ▶ . Expression was observed in the epithelial lining of the distal airways. In contrast, no expression was observed in the explants exposed to Nitrofen in vitro (Figure 4C) ▶ . No differences in the spatial expression pattern of SP-C mRNA were observed between DMSO, control and Nitrofen in vivo treated explants. After 6 days of culture, the same expression pattern was observed in the explants exposed to Nitrofen in vitro (results not shown). After 4 days of culture, CC-10 mRNA expression was only observed in the DMSO and control explants in the epithelial lining of the major airways (Figure 4, E and F) ▶ . This time, expression was not observed in any of the explants exposed to Nitrofen (Figure 4, G and H) ▶ .
Figure 4.
Expression of markers for distal and proximal epithelial cell differentiation, SP-C (upper row) and CC-10 (bottom row) mRNA respectively, in lung explants after 4 days of culture. Expression of SP-C mRNA was observed in control, DMSO, and Nitrofen in vivo treated explants in the epithelial lining of the terminal lung buds after 4 days of culture (A, B, and D). No expression was observed in the explants exposed to Nitrofen in vitro (C). Expression of CC-10 mRNA was observed in control and DMSO explants in the epithelial lining of the major proximal airways. (E and F). No expression was observed in the explants exposed to Nitrofen both in vitro (G) and in vivo (H). All pictures are representative of a series of experiments. All images at same magnification.
Proliferation and Apoptosis
To investigate whether the interplay between proliferation and apoptosis played a role in the early pathogenesis of Nitrofen-induced pulmonary hypoplasia in vitro, we investigated proliferation, using immunohistochemistry with an antibody against PCNA. 16 Apoptotic cells were detected using TUNEL assay. 17 Proliferating cells (PCNA-positive nuclei) were localized mainly in the epithelial lining of the branching terminal lung buds (Figure 5, A and B) ▶ . A smaller number of proliferating cells was observed in the mesenchyme (Figure 5, A and B) ▶ . In mesenchyme of the Nitrofen-exposed explants, attenuated PCNA immunoreactivity was observed (Figure 5C) ▶ . Unexpectedly, in the epithelial lining of the terminal buds, PCNA protein was localized perinuclearly and in the cytoplasm, and not in the nuclei (Figure 5C) ▶ .
Figure 5.
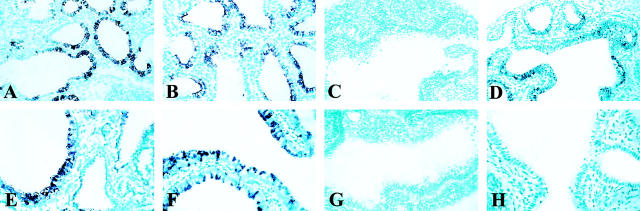
Following a culture period of 4 days, proliferation (A−C) and apoptosis (D−F) were investigated. Immunolocalization of PCNA indicates proliferating cells. Strong PCNA immunoreactivity was observed in the epithelial lining of the terminal buds of the control and DMSO explants, and also, but lower in the mesenchyme (A and B). Surprisingly immunoreactivity was not observed in the mesenchyme and only perinuclear in the explants exposed to Nitrofen (C). Apoptosis in the explants was assessed using TUNEL assay. TUNEL positive cells were observed in all explants in the mesenchyme only, and the number of TUNEL positive cells appeared similar in all explants (D−F). All nuclei were stained using DAPI mounting solution (G−I). All pictures are representative of a series of experiments. All images at same magnification.
In all explants TUNEL-positive cells were observed mainly in the mesenchyme and occasionally in the epi- thelium. Although not quantitated, the number of apoptotic cells appeared similar in all explants, including the ones exposed to Nitrofen (Figure 5, D ▶ -F). All nuclei of the cells were shown by staining with DAPI (Figure 5, G ▶ -I).
Discussion
Despite many years of clinical efforts to find alternative treatment modalities, the mortality rate of CDH has not really changed during the past decades. A poor understanding of the pathogenesis of CDH might be partly responsible for this. It is still not clear whether the diaphragmatic defect is the cause or result of the pulmonary hypoplasia. We hypothesized that the lungs are the primary target organ in Nitrofen-induced CDH, and to test this hypothesis, we separated the pathogenesis of CDH in events happening before and after (defective) closure of the diaphragm. In this study we investigated early pulmonary development in an in vitro modification of the Nitrofen model 4 to 6 days before normal closure of the diaphragm (day 17 of gestation) takes place in the rat fetus. 18 We studied the direct effects of Nitrofen on lung formation, branching morphogenesis and epithelial cell differentiation in our organ culture system.
We report here that Nitrofen exposure both in vitro and in vivo reduces branching morphogenesis before the time that closure of the diaphragm would normally occur in the rat (day 17 of gestation). Only the later phases of branching morphogenesis (ie, dichotomous branching) are affected, since initial lung bud outgrowth (ie, primary monopodial branching) and subsequent formation of the lobar bronchi (ie, secondary branching) were not affected in the Nitrofen-treated explants. The timing and degree of interference with branching morphogenesis by Nitrofen are in accordance with case reports of CDH in which the number of airway generations were assessed by the group of Lynne Reid. 19,20 Both studies report a reduction in airway generations to about half the normal number. Because development of the bronchial tree is normally complete by the 16th week of intrauterine life, timing of the insult in affected human babies should be early in gestation.
Although in vivo exposure to Nitrofen at first did not result in a statistically significant reduction in the number of branches, the statistical significance was observed when the lower 75% of the lungs with reduced branching were considered from among all lungs evaluated. In a previous study, we have shown that in our hands administration of Nitrofen results in a rate of up to 80% diaphragmatic defects. 21 Therefore, the lower 75% of the lungs with reduced branching might correspond to the most severe cases of pulmonary hypoplasia. All these data indicate that Nitrofen has a direct effect on branching morphogenesis and Nitrofen treatment might thus result in hypoplastic lungs before a diaphragmatic hernia occurs during development. This implies that in case of Nitrofen-induced CDH, the existing hypoplastic lungs might even induce the diaphragmatic defect, which has been suggested before by Iritani 8 and by Cilley et al. 9 However, results of the study of Iritani were based on experiments performed in the presence of Nitrofen during a period from day 5 of gestation until the day of sacrifice. In addition, both studies were done in mice, and the effects of Nitrofen were investigated in an in vivo situation, where influences of aberrant diaphragm development cannot be ruled out. To our knowledge, our study is the first to investigate the effects of Nitrofen on lung branching in vitro and after a limited period of exposure to Nitrofen, which is similar to the exposure of Nitrofen in the well established in vivo animal model.
In addition to the effects of Nitrofen on branching morphogenesis, the epithelial cell differentiation was also disturbed. Again, the effects of Nitrofen exposure in vitro were more severe than exposure in vivo, in that SP-C mRNA expression was not observed in the explants exposed to Nitrofen in vitro after 4 days, but only after 6 days of culture. In the Nitrofen-treated explants no CC-10 mRNA expression could be observed. Thus, in vitro treatment specifically resulted in a delay of epithelial cell differentiation. Whereas apoptotic processes did not change when evaluated by the number and localization of TUNEL-positive cells, PCNA immunoreactivity was clearly reduced in the mesenchymal component of the Nitrofen-treated explants. In addition, PCNA reactivity was only observed perinuclear and in the cytoplasm of epithelial cells of the explants exposed to Nitrofen. This unexpected result clearly indicated that proliferation is disturbed in explants exposed to Nitrofen. Consequently, a disturbed interplay between cell proliferation and apoptosis may be contributing to the observed hypoplasia in the lung explants exposed to Nitrofen.
Taken together, these data suggest that the primary defect in Nitrofen-induced CDH is located in the lungs and that the lungs are already hypoplastic before the formation of a diaphragmatic defect in the rat model. Our results corroborate with those of Cilley et al, 9 who demonstrated in their murine model that Nitrofen-induced pulmonary hypoplasia exists with or without diaphragmatic hernia. The asymmetry of both pulmonary hypoplasia and the diaphragmatic defect can be explained by the fact that there is a difference in timing of closure of the diaphragms. The right diaphragm closes a little earlier than the left. 22 It might well be that timing of the insult resulting in hypoplastic lungs and a diaphragmatic defect is crucial and consequently that it occurs more often on the left than on the right side. Strangely, administration of Nitrofen later during gestation results in a higher percentage of diaphragmatic defects occurring on the right side. 10,11 In addition, the right lung is much bigger and consists of more lobes (4 in the rat and 3 in the human) than the left lung (1 in the rat and 2 in the human). This might result in a right lung providing a more sufficient basis for communication between the lungs and the diaphragm when the lungs grow into the pleuroperitoneal canal, which is disturbed in CDH as suggested by Iritani. 8 Another possibility is that CDH should be considered as the result of a shared or a separate mechanism that affects, for instance, the mesenchyme of the lungs and diaphragm in a similar way. Although these problems are not clarified yet, it is clear that the occurrence of a diaphragmatic defect and pulmonary hypoplasia requires a sensitive and crucial interplay between several factors not yet identified.
Besides isolated or familial case reports describing chromosomal aberrations, until now a genetic origin or an etiological-environmental factor for CDH has been lacking. 23 Therefore, the pathogenesis of pulmonary hypoplasia in the Nitrofen model of CDH should be extrapolated to the human situation with a fair amount of reserve. We postulate that the crucial event resulting in human CDH takes place during early branching morphogenesis in a susceptible genetic environment. There have been reports of families with a higher incidence of CDH, suggesting that a genetic defect is in part responsible for the etiology of the CDH cases. 24,25 Therefore, more research efforts to unravel the genetic defects in this anomaly are justified and will lead to a better understanding of the pathogenesis of CDH.
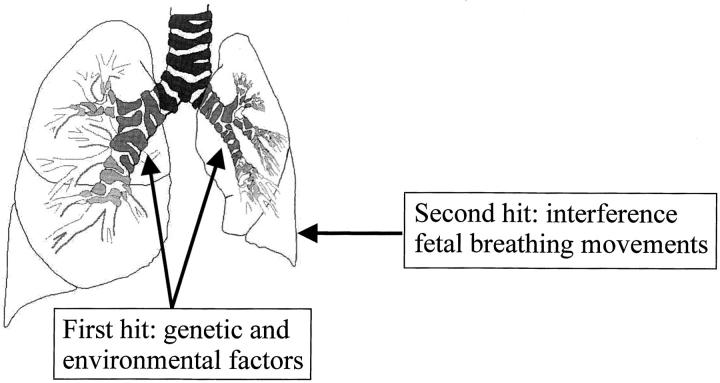
Finally, we would like to postulate a new hypothesis describing the pathogenesis of pulmonary hypoplasia in case of CDH: the dual-hit hypothesis. In this hypothesis the pathogenesis of pulmonary hypoplasia in case of diaphragmatic hernia is explained by two developmental insults. The first insult occurs early on in development, before diaphragm development, given a still unidentified background of genetic and environmental factors. This insult affects both lungs during branching morphogenesis in a similar fashion. After defective development of the diaphragm, the second insult affects the ipsilateral lung only at a later stage of development. In this scenario, herniated abdominal organs will interfere with fetal breathing movements of the ipsilateral lung, resulting in greater impairment of the development of the ipsilateral lung than that of the contralateral lung (Figure 6) ▶ .
Figure 6.
The dual-hit hypothesis: pulmonary hypoplasia in case of CDH is explained by two developmental insults. The first hit affects both lungs before and separate from diaphragm development in a background (unidentified until now) of genetic and environmental factors. The second hit affects only the ipsilateral lung after defective development of the diaphragm because of interference of the herniated abdominal organs with fetal breathing movements of this lung.
In our view, this hypothesis best describes the pathogenesis of pulmonary hypoplasia in case of CDH based on current available data, which were obtained mainly from studies in the Nitrofen model. However, more research will be necessary both in humans and in the Nitrofen model to solve the chicken-or-egg question that is still associated with this anomaly. In conclusion, in the Nitrofen model of CDH, pulmonary hypoplasia precedes the diaphragmatic defect and might even be the cause of the diaphragmatic defect, instead of the result. This insight in the pathogenesis of pulmonary hypoplasia in case of CDH leads to a different perspective on the hypoplastic lungs that are characteristic of this anomaly.
Acknowledgments
We thank Bonnie Welsh for administration of Nitrofen to the rats, Frans P. Lohman for assistance during preparation of the manuscript, and Carel Meijers for carefully reading the manuscript.
Footnotes
Address reprint requests to Dick Tibboel, M.D., Ph.D., Professor of Experimental Pediatric Surgery, Head Pediatric Surgical Intensive Care, Sophia Children’s Hospital, Erasmus University Medical Centre Rotterdam, Dr. Molewaterplein 60, 3015 GJ Rotterdam, The Netherlands. E-mail: illsley@chis.azr.nl.
Supported by a Medical Research Council of Canada Group Grant in Developmental Lung Biology, the David Vervat Foundation, and the Sophia Foundation for Medical Research.
References
- 1.Torfs CP, Curry CJ, Bateson TF, Honore LH: A population-based study of congenital diaphragmatic hernia. Teratology 1992, 46:555-565 [DOI] [PubMed] [Google Scholar]
- 2.Katz AL, Wiswell TE, Baumgart S: Contemporary controversies in the management of congenital diaphragmatic hernia. Clin Perinatol 1998, 25:219-248 [PubMed] [Google Scholar]
- 3.Reickert CA, Hirschl RB, Atkinson JB, Dudell G, Georgeson K, Glick P, Greenspan J, Kays D, Klein M, Lally KP, Mahaffey S, Ryckman F, Sawin R, Short BL, Stolar CJ, Thompson A, Wilson JM: Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery 1998, 123:305-310 [PubMed] [Google Scholar]
- 4.Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ: Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg 1995, 30:406-409 [DOI] [PubMed] [Google Scholar]
- 5.Thebaud B, Mercier JC, Dinh-Xuan AT: Congenital diaphragmatic hernia: a cause of persistent pulmonary hypertension of the newborn which lacks an effective therapy. Biol Neonate 1998, 74:323-336 [DOI] [PubMed] [Google Scholar]
- 6.Harrison MR, Adzick NS, Nakayama DK, deLorimier AA: Fetal diaphragmatic hernia: pathophysiology, natural history, and outcome. Clin Obstet Gynecol 1986, 29:490-501 [PubMed] [Google Scholar]
- 7.Allan DW, Greer JJ: Pathogenesis of nitrofen-induced congenital diaphragmatic hernia in fetal rats. J Appl Physiol 1997, 83:338-347 [DOI] [PubMed] [Google Scholar]
- 8.Iritani I: Experimental study on embryogenesis of congenital diaphragmatic hernia. Anat Embryol (Berl) 1984, 169:133-139 [DOI] [PubMed] [Google Scholar]
- 9.Cilley RE, Zgleszewski SE, Krummel TM, Chinoy MR: Nitrofen dose-dependent gestational day-specific murine lung hypoplasia and left-sided diaphragmatic hernia. Am J Physiol 1997, 272:L362-L371 [DOI] [PubMed] [Google Scholar]
- 10.Kluth D, Kangah R, Reich P, Tenbrinck R, Tibboel D, Lambrecht W: Nitrofen-induced diaphragmatic hernias in rats: an animal model. J Pediatr Surg 1990, 25:850-854 [DOI] [PubMed] [Google Scholar]
- 11.Tenbrinck R, Tibboel D, Gaillard JL, Kluth D, Bos AP, Lachmann B, Molenaar JC: Experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg 1990, 25:426-429 [DOI] [PubMed] [Google Scholar]
- 12.Souza P, Sedlackova L, Kuliszewski M, Wang J, Liu J, Tseu I, Liu M, Tanswell AK, Post M: Antisense oligodeoxynucleotides targeting PDGF-B mRNA inhibit cell proliferation during embryonic rat lung development. Development 1994, 120:2163-2173 [DOI] [PubMed] [Google Scholar]
- 13.Costlow RD, Manson JM: Distribution and metabolism of the teratogen nitrofen (2,4-dichloro-4′-nitro dephenyl ether) in pregnant rats. Toxicology 1983, 26:11-23 [DOI] [PubMed] [Google Scholar]
- 14.Manson JM: Mechanism of nitrofen teratogenesis. Environ Health Perspect 1986, 70:137-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochberg Y, Tamhane AC: Multiple Comparison Procedures. 1987. John Wiley & Sons, New York
- 16.Takasaki Y, Deng JS, Tan EM: A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med 1981, 154:1899-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluth D, Keijzer R, Hertl M, Tibboel D: Embryology of congenital diaphragmatic hernia. Semin Pediatr Surg 1996, 5:224-233 [PubMed] [Google Scholar]
- 19.Kitagawa M, Hislop A, Boyden EA, Reid L: Lung hypoplasia in congenital diaphragmatic hernia: a quantitative study of airway, artery, and alveolar development. Br J Surg 1971, 58:342-346 [DOI] [PubMed] [Google Scholar]
- 20.Areechon W, Reid L: Hypoplasia of lung with congenital diaphragmatic hernia. Br Med J 1963, 1:230-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijsselstijn H, Pacheco BA, Albert A, Sluiter W, Donahoe PK, De Jongste JC, Schnitzer JJ, Tibboel D: Prenatal hormones alter antioxidant enzymes and lung histology in rats with congenital diaphragmatic hernia. Am J Physiol 1997, 272:L1059-L1065 [DOI] [PubMed] [Google Scholar]
- 22.Moore KL: Development of body cavities, primitive mesenteries, and the diaphragm. Wonsiewicz M eds. The Developing Human: Clinically Oriented Embryology. 1988, :pp 159-169 WB Saunders Company, Philadelphia [Google Scholar]
- 23.Bos AP, Pattenier AM, Grobbee RE, Lindhout D, Tibboel D, Molenaar JC: Etiological aspects of congenital diaphragmatic hernia: results of a case comparison study. Hum Genet 1994, 94:445-446 [DOI] [PubMed] [Google Scholar]
- 24.Tibboel D, Gaag AV: Etiologic and genetic factors in congenital diaphragmatic hernia. Clin Perinatol 1996, 23:689-699 [PubMed] [Google Scholar]
- 25.Enns GM, Cox VA, Goldstein RB, Gibbs DL, Harrison MR, Golabi M: Congenital diaphragmatic defects and associated syndromes, malformations, and chromosome anomalies: a retrospective study of 60 patients and literature review. Am J Med Genet 1998, 79:215-225 [PubMed] [Google Scholar]