Abstract
Cyclooxygenase (Cox), the key enzyme of prostanoid synthesis, consists of the two isoforms Cox-1 and Cox-2, both recently noted to be constitutively expressed in rat lungs with a distinct profile of cellular distribution. The responsiveness of pulmonary Cox-1 and Cox-2 expression to intravascular endotoxin lipopolysaccharide (LPS) administration was investigated in isolated, ventilated rat lungs, buffer-perfused with or without admixture of rat plasma. Immunohistochemical staining intensity was measured by a previously described method of silver enhancement and epipolarization image analysis. Both the Cox-1 mRNA, quantified in the whole lung homogenate, and the cellular localization of Cox-1 were unchanged in response to LPS. In contrast, time- and dose-dependent up-regulation of Cox-2 mRNA (lung homogenate) occurred, and differential LPS reactivity at the cellular level was observed. Up-regulation of Cox-2 in cell types expressing this enzyme already under baseline conditions was noted in bronchial epithelial cells, bronchial and vascular smooth muscle cells, cells within the BALT and myocytes of the large hilar veins. De novo induction of Cox-2 occurred in endothelial cells and the majority of alveolar macrophages. Down-regulation of Cox-2 was observed in perivascular and peribronchial macrophage-like cells. Moreover, differential impact of plasma components was noted: for the large majority of cells, CD14 surface expression correlated with Cox-2 responsiveness to LPS independent of plasma, whereas the presence of plasma components was a prerequisite for the LPS response in CD14-negative cells. LPS did not provoke physiological changes in the perfused lungs, but markedly enhanced baseline prostanoid generation. We conclude that LPS-induced Cox-2 regulation occurs in a complex, cell-specific manner, which may be relevant for pathogenetic sequelae in septic lung injury and acute respiratory failure.
Sepsis with multiorgan failure is the leading cause of death in modern intensive care medicine, with Gram-negative bacteria being involved in the majority of cases. Endotoxin, lipopolysaccharide (LPS) of Gram-negative bacteria, is assumed to be mainly responsible for the microcirculatory abnormalities resulting in septic organ failure, among which adult respiratory distress syndrome represents the prototype. 1,2 Some effects of LPS seem to be mediated by the plasma protein LBP (LPS-binding protein) and the membrane receptor CD14. 3-6 Moreover, a soluble form of CD14 has been described, which forms complexes with LPS and LBP and may then bind to cells negative for membrane CD14, such as endothelial cells. 4,5,7 Intracellular signaling via CD14 demands additional coreceptor(s), which may in particular include the family of the recently described toll-like receptors. 8-10 In addition to the LBP/CD14-axis, CD14- and plasma component-independent signal transduction pathways for endotoxin have been suggested, although these are not yet fully characterized. 11-16
During endotoxemia, eicosanoid generation is markedly up-regulated, and these lipid mediators are known to contribute to the vascular abnormalities and changes in bronchomotor tone occurring in LPS-induced lung injury. 17-19 The initial step in the generation of prostaglandins and thromboxane is the conversion of arachidonic acid to prostaglandin H2 via cyclooxygenase. 17,18,20 Two isoforms of this key enzyme have been described, cyclooxygenase-1 (Cox-1), which was known to occur constitutively in various cell types, 21 and the inducible isoform cyclooxygenase-2 (Cox-2), which was initially assumed to be expressed only under inflammatory conditions. 22-24 Recent investigations, however, demonstrated that Cox-2 is also constitutively expressed in various organ systems. 25-32 Performing immunostaining in normal rat lungs, Ermert et al recently described Cox-2 localization to be prominent in vascular smooth muscle cells (VSMC) of partially muscular vessels, macrophage-like cells in the perivascular and peribronchial tissue, and myocytes of the large hilar veins, and lower expression in the epithelial cells of large bronchi; Cox-1 was mainly localized to bronchial epithelial cells, alveolar macrophages, and endothelial cells. 33 Studies with Cox-2-selective inhibitors suggested a prominent role of Cox-2 in basal regulation of pulmonary vascular tone. 34
Studies in distinct cell types in vitro, including macrophages and endothelial and epithelial cell cultures, demonstrated a marked up-regulation of Cox-2 in response to LPS. 22-24,35-37 Priming of intact lungs with low doses of endotoxin, which per se did not result in physiological abnormalities, did result in a markedly enhanced responsiveness to a secondary inflammatory stimulus such as bacterial exotoxins, arachidonic acid, or platelet-activating factor. Up-regulation of Cox-2 was suggested to underlie this phenomenon. 18,38-42 The cellular source of LPS-induced changes in lung prostanoid synthesis has not, however, been addressed in detail. In the current investigation, we used image analysis of the staining intensity of immunoreactive product to characterize endotoxin-elicited changes in rat lung Cox-1 and Cox-2 expression at the cellular level. Studies were performed in the absence and presence of rat plasma to address putative plasma component dependencies of such changes. In essence, Cox-1 expression was found to be unchanged in LPS-challenged rat lungs, whereas Cox-2 immunostaining displayed pronounced changes within 1 to 2 hours of endotoxin exposure, with marked differences in the responsiveness of the various types of cells in the lung. The changes included up-regulation of Cox-2 independent or dependent of plasma constituents, appearance of Cox-2 in cells negative for this enzyme under control conditions, as well as down-regulation of Cox-2 in distinct cell types. This complex response pattern to LPS in the lung, an organ with many different cell types, may well contribute to pulmonary abnormalities occurring in septic lung injury and acute respiratory failure.
Materials and Methods
Reagents
The Cox-2 antibody and preimmune serum (PG26c, polyclonal) were obtained from Oxford Biomedical Research (Oxford, MI). The Cox-2 antibody is directed against a peptide corresponding to the amino acid sequence 581–598 of the carboxy terminal region of Cox-2. 23 The antibodies against Cox-1 (polyclonal IgG, M-20) and CD14 (polyclonal IgG, M-20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The Cox-1 antibody was prepared against a peptide corresponding to the amino acid sequence 583–602 mapping at the carboxy terminus of Cox-1. Both anti-Cox antibodies bind specifically to one of the isoenzymes and do not cross-react. Secondary antibodies were provided by Nanoprobes (Stony Brook, NY) and Biotrend (Cologne, Germany). The silver enhancement solution was acquired from Aurion (Wageningen, The Netherlands). The alkaline phosphatase development substrate Vector Red was obtained from Vector Laboratories (Burlingame, CA). ELISA-kits for the determination of 6-keto prostaglandin F1α (6-keto PGF1α) and thromboxane B2 (TxB2) were obtained from Cayman Chemical Company (Ann Arbor, MI).
Animals
CD rats (Sprague-Dawley) were obtained from Charles River, Germany. All experimental procedures were performed in conformity with the guidelines of the National Institutes of Health set forth in “Guide for the care and use of laboratory animals” (NIH publication no. 86–23, revised 1985, U.S. Government Printing Office, Washington, DC).
Lung Isolation and Perfusion
The rats (male, body weight 350–400 g) were deeply anesthetized with pentobarbital-Na given i.p. (100 mg/kg body weight). After local anesthesia with 2% xylocaine and a median incision, the trachea was dissected and a tracheal cannula was immediately inserted. Subsequently, mechanical ventilation was started with 4% CO2, 17% O2, and 79% N2 (tidal volume 4 ml, frequency 65/minute, endexpiratory pressure 3 cm H2O) using a small animal respirator KTR-4 (Hugo Sachs Elektronik, March, Germany). A median laparatomy was performed, and then the rats were anticoagulated with 1000 units of heparin. After midsternal thoracotomy the right ventricle was incised, a cannula was fixed in the pulmonary artery, and the apex of the heart was cut off to allow pulmonary venous outflow. Simultaneously, pulsatile perfusion with buffer solution was started. The buffer contained 2.4 mmol/L CaCl2, 1.3 mmol/L MgCl2, 4.3 mmol/L KCl, 1.1 mmol/L KH2PO4, 125.0 mmol/L NaCl, 25 mmol/L NaHCO3, and 13.32 mmol/L glucose (pH 7.35–7.40).
The lungs were excised carefully to avoid any damage while being perfused with buffer solution, and suspended in an upright position. Next, a cannula was inserted through the left ventricle and fixed in the left atrium to obtain a closed perfusion circuit without leakage. Subsequently, lungs were placed in a temperature-equilibrated housing chamber (37°C) freely suspended from a force transducer.
After extensive rinsing of the vascular bed, the lungs were perfused with a pulsatile flow of 13 ml/minute. The alternate use of two separate perfusion circuits, each containing 100 ml, allowed the repetitive exchange of perfusion fluid. Perfusion pressure, ventilation pressure, and the weight of the isolated organ were registered continuously. The left atrial pressure was set at 2 mmHg under baseline conditions (0 referenced at the hilum) to guarantee zone III conditions at endexpiration throughout the lung.
Lungs that (i) did not have a homogeneous white appearance without signs of hemostasis or edema formation, (ii) did not have pulmonary artery or ventilation pressures in the normal range, or (iii) were not isogravimetric during a steady state period of 30 minutes were excluded from the study.
Experimental Protocol
Following a 30-minute steady state period, time was set at zero, and the lungs were subjected to different experimental protocols. A total of 120 experiments with isolated rat lung perfusion were undertaken (60 lungs for fixation with paraformaldehyde for paraffin embedding and 60 lungs for snap-freezing for Northern blot and cryomicrotomy), including two control groups, and challenged with three different LPS doses for 1 or 2 hours.
Buffer-perfused control lungs (n = 10) were perfused with buffer fluid for 2 hours without further experimental procedures.
Buffer/plasma-perfused control lungs (n = 10) were perfused for 2 hours with buffer fluid to which 1.5% rat plasma was admixed.
For challenge with 50 ng/ml LPS, lungs were perfused with sole buffer fluid (n = 10) or buffer/plasma (1.5%; n = 10), to which 50 ng/ml LPS was admixed, for 2 hours.
For challenge with 1000 ng/ml LPS/1 hour, lungs were perfused with sole buffer fluid (n = 10) or buffer/plasma (1.5%; n = 10), to which 1000 ng/ml LPS was admixed, for 1 hour.
For challenge with 1000 ng/ml LPS/2 hours, lungs were perfused with sole buffer fluid (n = 10) or buffer/plasma (1.5%; n = 10), to which 1000 ng/ml LPS was admixed, for 2 hours.
For challenge with 10,000 ng/ml LPS/1 hour, lungs were perfused with sole buffer fluid (n = 10) or buffer/plasma (1.5%; n = 10), to which 10,000 ng/ml LPS was admixed, for 1 hour.
For challenge with 10,000 ng/ml LPS/2 hours, lungs were perfused with sole buffer fluid (n = 10) or buffer/plasma (1.5%; n = 10), to which 10,000 ng/ml LPS was admixed, for 2 hours.
Samples for perfusate analysis were taken at the onset of the experimental protocol and after 30 and 60 minutes and, in the 2-hour protocols, also after 90 and 120 minutes.
Tissue Preparation
Immediately after termination of perfusion, fixation was performed by tracheal instillation of a 3% paraformaldehyde solution at a pressure of 20 cm H2O and immersion of the lungs in the same fixation solution for 1 hour (60 lungs, n = 5 each group). In addition, the non-paraformaldehyde-instilled lungs (60 lungs, n = 5 each group) were snap-frozen in liquid nitrogen and stored at −80°C until they were prepared for RNA isolation and cryomicrotomy. For paraffin embedding, all lobes of the paraformaldehyde-fixed lungs were dissected in tissue blocks, and tissue blocks from each lobe were then embedded in low-temperature paraffin with a melting temperature of 40 to 42°C. Dissection into tissue blocks was also performed for all lobes from the snap-frozen lungs. Sectioning at 10 μm thickness was performed on all paraffin-embedded and frozen tissue blocks.
Perfusate Analysis
Perfusate samples for analysis of thromboxane A2 (TxA2) and prostacyclin (PGI2), detected as their stable endproducts 6-keto PGF1α and TxB2, were collected at times 0, 30, and 60 minutes, as well as 90 and 120 minutes from 2-hour perfusion tissues.
Immunohistochemistry
The paraffin sections were dewaxed, rehydrated, and washed in phosphate-buffered saline (PBS: 0.01 mol/L, 150 mmol/L NaCl, pH 7.6) for 3× 5 minutes. They were treated for 15 minutes with a 1% Triton solution. The sections were pre-incubated in PBS containing 5% goat serum, 0.025% acetylated bovine serum albumin (BSA-C), 0.05% Tween-20, and 0.02 mol/L glycine to block nonspecific binding. Overnight incubation with the polyclonal primary antibody rabbit-anti Cox-2 diluted 1:50 in PBS containing 0.025% BSA-C and 0.05% Tween-20 was carried out at 4°C. Cox-1 was correspondingly detected with the polyclonal primary antibody goat-anti Cox-1 diluted 1:200 in PBS dilution buffer. The sections were then washed in PBS and incubated with secondary gold-conjugated antibodies diluted 1:400 overnight at 4°C. Next, the sections were washed in PBS again and fixed for 5 minutes in 2% phosphate-buffered glutaraldehyde. After several washes in glass-double-distilled water, the sections were incubated in silver enhancer solution for 50 minutes. Counterstaining of the sections was performed with nuclear fast red. Control staining was performed by omission of the primary antibody or substitution with nonspecific preimmune serum at the same dilution.
Counterstaining with Alcian blue was performed with some of the Cox-2 sections for identification of a special type of macrophage-like cell, which was intensely stained by anti-Cox-2. After silver enhancement these sections were washed for 3 minutes in 3% acetic acid and afterward stained for 30 minutes with 1% Alcian blue solution. Counterstaining was performed with nuclear fast red as described above.
For CD14 immunostaining, cryosections were fixed in a 3% paraformaldehyde solution for 5 minutes. Afterward they were washed in 0.01 mol/L PBS for 3× 5 minutes and preincubated in PBS containing 5% rabbit serum, 1% BSA and 0.05% Tween-20 to block nonspecific binding. The primary anti-CD14 antibody was incubated overnight at 4°C diluted 1:50 in PBS containing 5% rabbit serum, 1% BSA, and 0.05% Tween-20. The sections were then washed in PBS and incubated with rabbit anti-goat f(ab)2 alkaline phosphatase conjugate diluted 1: 4000 in the same dilution buffer overnight at 4°C, followed by 3× 5-minute washes in PBS. Subsequently, the sections were developed with a VectorRed Substrate Kit. Levamisol 2.5 mmol/L was added to inhibit endogenous alkaline phosphatase activity. Counterstaining of the sections was performed with Mayer’s hematoxylin. Control staining was performed using either nonimmune serum or by omission of the primary antibody. Complete inhibition of endogenous phosphatase activity was evaluated by omission of both antibodies.
Image Analysis
The technique used for image analysis has been described previously. 33 However, a 12-bit cooled CCD camera (4096 gray scales; Sensys KAF 1400, Photometrics, Tucson, AZ) instead of the previously used 8bit system (256 gray scales) was used, which markedly enhanced the sensitivity for the detection of changes in staining intensity. The camera was mounted on a fully automated Leica DM RXA microscope (Leica, Wetzlar, Germany) to digitize epipolarization images to a Pentium 200MHz host computer. Microscope settings were kept constant throughout all measurements (objective: 25× oil, Leica PL Fluotar 25×/0.75, Leica IGS epipolarization filter block, illumination: stabilized 12V high pressure mercury vapor lamp, 100W). Adjustment of all microscope settings was stored and recalled before measurement. The measurement system was calibrated with a reference slide before data collection. Epipolarization depiction of the immunogold-silver-stained structures created a complete segmentation between positive stained and nonstained tissue. Gray scale images were digitized to 12-bit accuracy, resulting in an intensity scale ranging from 0 to 4095. Image analysis was performed by means of the image analysis program ImagePro 3.0 (Media Cybernetics, Silver Spring, MD). Three randomly selected complete cross-sections from different lung lobes per animal were measured. Structures and cell types were classified in the brightfield image and the corresponding epipolarization image. A measurement of the mean gray values of the selected structures was performed, and then the data were transferred into the spreadsheet program Excel (Microsoft, Redmond, WA). CD14-stained sections were digitized as fluorescent images (Leica Texas Red filter block) in 12-bit accuracy. For direct visualization of staining intensity a pseudocolor scale with 11 colors was chosen, each representing an equal sector of the intensity scale, and applied to the images. Background measurement was performed to evaluate the influence of nonspecific antibody binding.
Detection of Cyclooxygenase mRNA by Northern Blot Technique
Separation of total RNA (10 μg/lane) and hybridization were performed by standard procedures. The specific Cox-1 and Cox-2 probes were 2.767- and 1.156-kb EcoRI fragments from the 5′ end of mouse cDNA, respectively. 43 The GAPDH probe was obtained with a 500-bp reverse-transcribed fragment. 44 Hybridization of DNA/RNA hybrids was detected by autoradiography using Kodak X-OMAT AR film. Quantitative analysis was performed by densitometric scanning of the autoradiographs (Froebel, Wasserburg, Germany). All values were corrected for differences in RNA loading by calculating the ratio of Cox-2 to GAPDH or 18S rRNA expression.
Statistical Analysis
Analysis of variance was used to evaluate differences among different groups. A value of P < 0.05 was considered significant. Data are given as mean ± SE.
Results
In buffer- or buffer/plasma-perfused control lungs, no lung weight gain or change in vascular or ventilation pressure was registered over the entire observation period of 2 hours. Similarly, administration of the different LPS doses did not result in lung edema formation or alterations of perfusion or ventilation pressure.
For all groups of lungs, immunostaining performed on cryosections from snap-frozen tissue revealed no differences to paraformaldehyde-fixed and paraffin-embedded tissue sections. Control sections randomly obtained from the different experimental groups, which were treated with preimmune serum or with omission of the primary antibody, did not show nonspecific staining. Background staining was low, with a mean gray value of 264 ± 13.8. Both buffer- and buffer/plasma-perfused control lungs expressed mRNA levels of Cox-1 and Cox-2 that were detectable by Northern blot analysis (Figure 1A) ▶ .
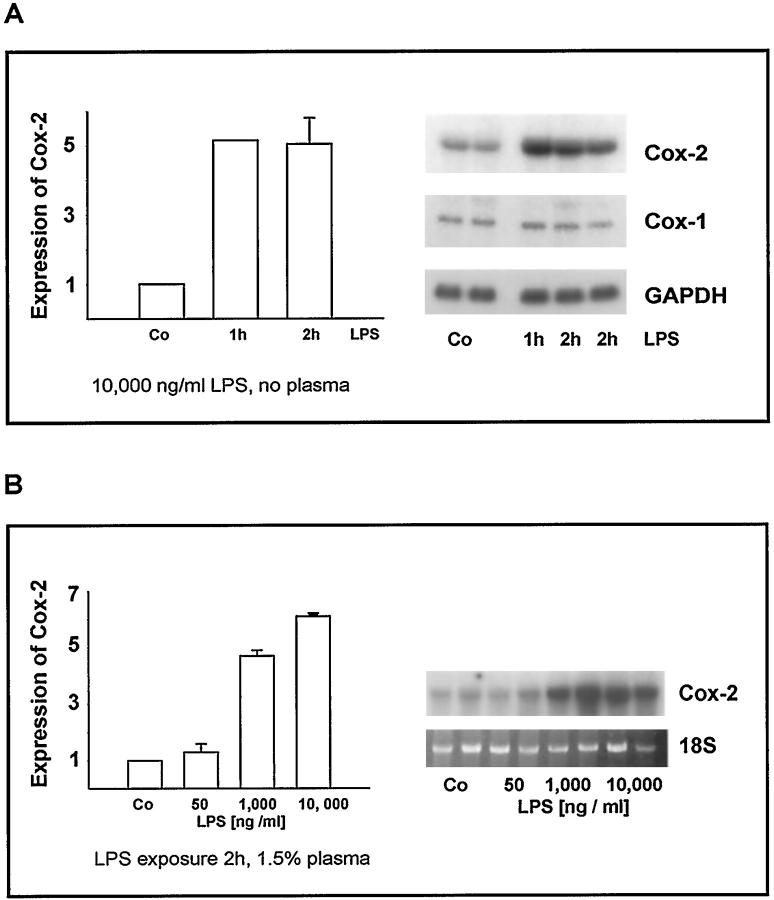
Figure 1.
A: Time-dependent induction of Cox-2 mRNA by LPS. Lungs undergoing buffer perfusion for 2 hours in the absence of LPS (control; Co) and lungs perfused in the presence of 10,000 ng/ml LPS for 1 or 2 hours were snap-frozen, tissue was homogenized, and expression of Cox-1 and Cox-2 mRNA was detected by Northern blot analysis (examples given in right panel). To compare different blots, expression of Cox-2 mRNA in control lungs was set at 1. After 1 and 2 hours, Cox-2 mRNA expression was increased to approximately fivefold values (means ± SE for n = 3 lungs per group given in the left panel). B: Concentration-dependent increase in Cox-2 mRNA. Lungs undergoing buffer/plasma perfusion for 2 hours in the absence of LPS (control; Co) and lungs perfused in the presence of 1.5% plasma and 50, 1000, and 10,000 ng/ml LPS for 2 hours were snap-frozen, homogenization of tissue was performed, and expression of Cox-2 mRNA was detected by Northern blot analysis (examples given in right panel). To compare different blots, expression of Cox-2 mRNA in control lungs was set at 1 (left panel; means ± SE of n = 3 blots each).
Cox-1
In buffer- or buffer/plasma-perfused control lungs, strong Cox-1 immunostaining was detected in the bronchial epithelial cells of all bronchi and in myocytes located in the large veins of the hilum, which originate from cardiac myocytes. In addition, alveolar macrophages, endothelial cells, and bronchial smooth muscle cells (BSMC) exhibited positive immunostaining as described previously. 33 LPS treatment for either 1 or 2 hours, under both buffer and buffer/plasma perfusion conditions, did not change the pattern and intensity of Cox-1 immunostaining as compared to the control groups (data not shown in detail). In accordance, the Cox-1 mRNA expression remained essentially unaltered after LPS stimulation (Figure 1A) ▶ .
Cox-2
Control Lungs
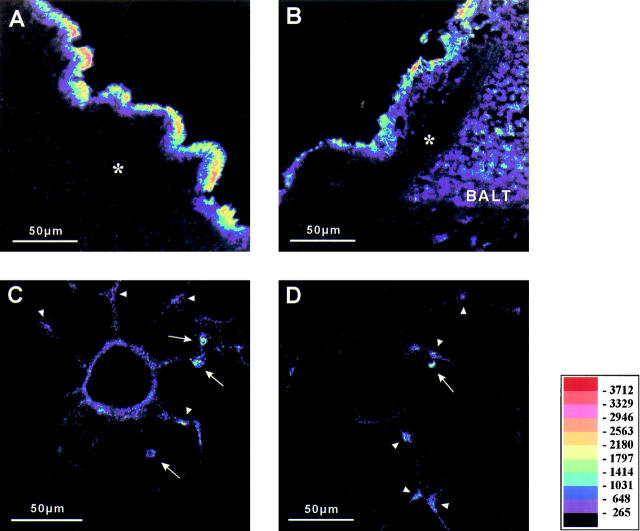
Control rat lungs, which were buffer- or buffer/plasma-perfused for 2 hours without LPS administration, showed a profile of Cox-2 immunostaining corresponding to that recently described for normal rat lung tissue (Tables 1 and 2) ▶ ▶ . 33 Strong Cox-2 staining was localized to smooth muscle cells of partially muscular vessels (Figure 2A) ▶ , myocytes of large hilar veins (Figure 2C) ▶ , and macrophage-like cells in the perivascular and peribronchial connective tissue and subpleural tissue (Figure 2E) ▶ . In addition, bronchial epithelial cells, cells of the bronchus-associated lymphoid tissue (BALT), BSMC, single cells within the alveolar septum, and smooth muscle cells of large arteries showed positive immunoreactivity. Only a few alveolar macrophages were positive for Cox-2. No difference between controls with buffer perfusion and those with buffer/plasma perfusion was noted.
Table 1.
Quantification of Cox-2 Immunogold-Silver Staining Intensity: Experiments in the Absence of Plasma (Mean Gray Values)
| Structures | Control | 50 ng/ml LPS (2 h) | 1000 ng/ml LPS | 10,000 ng/ml LPS | ||
|---|---|---|---|---|---|---|
| 1 hour | 2 hours | 1 hour | 2 hours | |||
| Bronchial epithelial cells of bronchi (1st and 2nd generation) | 641 ± 17.8 | 654 ± 50.4 | 795 ± 14.6* | 929 ± 34.0*** | 780 ± 47.4* | 852 ± 16.1*** |
| Bronchial epithelial cells of bronchi (3rd generation) and bronchioli | 595 ± 17.2 | 624 ± 45.7 | 646 ± 22.4 | 659 ± 9.8 | 644 ± 16.8 | 613 ± 25.0 |
| Bronchial smooth muscle cells | 690 ± 49.5 | 785 ± 108.8 | 799 ± 7.3 | 780 ± 28.8 | 689 ± 12.9 | 804 ± 33.1 |
| Cells of the BALT | 523 ± 1.3 | 660 ± 41.0*** | 751 ± 9.7*** | 736 ± 27.5*** | 640 ± 1.9*** | 583 ± 1.6*/### |
| Single cells in the alveolar septum | 486 ± 3.7 | 434 ± 4.1 | 460 ± 3.2 | 713 ± 6.5*** | 557 ± 3.2** | 629 ± 30.6***/### |
| Alveolar macrophages | 466 ± 12.9 | 429 ± 6.1 | 509 ± 22.7 | 495 ± 18.9 | 474 ± 22.5 | 493 ± 25.5 |
| Macrophage-like cells | 1378 ± 101.5 | 1500 ± 199.0 | 2045 ± 94.1*** | 797 ± 70.9** | 946 ± 111.4* | 802 ± 64.2** |
| Endothelial cells | n.d. | n.d. | 455 ± 10.1 | 445 ± 15.2 | 499 ± 19.6 | 532 ± 11.3*/# |
| Vascular smooth muscle cells of large arteries at the hilum | 706 ± 16.8 | 705 ± 35.5 | 929 ± 31.9 | 621 ± 58.2 | 806 ± 53.1 | 835 ± 34.0 |
| Vascular smooth muscle cells of partially muscular vessels | 1066 ± 21.7 | 1207 ± 72.6 | 1584 ± 53.7 | 2090 ± 158.9*** | 1717 ± 204.4* | 1400 ± 27.2* |
| Myocytes of large veins at the hilum | 860 ± 54.1 | 1109 ± 26.7* | 1410 ± 78.5*** | 2098 ± 41.9*** | 1282 ± 49.8*** | 1207 ± 28.8**/### |
Mean ± SE gray values for each group of buffer-perfused lungs is given. Each group comprised 5 independent lung experiments, and cross-sections from three randomly selected tissue blocks originating from different lung lobes were investigated per lung. Statistical significance: P < 0.05*, < 0.01**, < 0.001*** versus control; endothelial cells versus 1000 ng/ml, 1 hour; P < 0.05#, < 0.01##, <0.001### versus 1000 ng/ml, 2 hours.
n.d., staining not detected.
Table 2.
Quantification of Cox-2 Immunogold-Silver-Staining Intensity: Experiments in the Presence of 1.5% Plasma (Mean Gray Values)
| Structures | Control | 50 ng/ml LPS (2 h) | 1000 ng/ml LPS | 10,000 ng/ml LPS | ||
|---|---|---|---|---|---|---|
| 1 hour | 2 hours | 1 hour | 2 hours | |||
| Bronchial epithelial cells of bronchi (1st and 2nd generation) | 462 ± 14.4 | 659 ± 3.4*** | 651 ± 19.9*** | 907 ± 28.3*** | 741 ± 54.6*** | 575 ± 24.9*/### |
| Bronchial epithelial cells of bronchi (3rd generation) and bronchioli | 450 ± 13.7 | 634 ± 27.2*** | 588 ± 17.6*** | 748 ± 20.0*** | 679 ± 12.3*** | 610 ± 18.0***/## |
| Bronchial smooth muscle cells | 605 ± 9.8 | 832 ± 27.4* | 622 ± 7.9 | 949 ± 45.5*** | 762 ± 6.6 | 604 ± 38.6### |
| Cells of the BALT | 415 ± 0.3 | 656 ± 1.5*** | 534 ± 10.9*** | 869 ± 4.3*** | 757 ± 3.2*** | 467 ± 1.2*/### |
| Single cells in the alveolar septum | 419 ± 3.1 | 444 ± 4.9 | 430 ± 4.4 | 680 ± 6.7*** | 623 ± 24.8*** | 561 ± 27.7***/### |
| Alveolar macrophages | 424 ± 7.7 | 482 ± 28.3 | 487 ± 18.9 | 620 ± 47.4*** | 553 ± 4.5* | 506 ± 26.2## |
| Macrophage-like cells | 1413 ± 144.6 | 1578 ± 94.1 | 1008 ± 115.9* | 960 ± 74.1* | 720 ± 73.8*** | 447 ± 6.6***/# |
| Endothelial cells | n.d. | n.d. | 436 ± 16.4 | 577 ± 27.8*** | 639 ± 22.5*** | 464 ± 38.8### |
| Vascular smooth muscle cells of large arteries at the hilum | 450 ± 14.0 | 883 ± 33.3* | 793 ± 63.8* | 959 ± 45.2** | 1057 ± 26.5*** | 1121 ± 250.2*** |
| Vascular smooth muscle cells of partially muscular vessels | 1190 ± 11.7 | 1750 ± 154.3 | 1623 ± 223.7 | 2435 ± 110.2*** | 2207 ± 229.4*** | 1831 ± 69.3*/# |
| Myocytes of large veins at the hilum | 1073 ± 72.0 | 1671 ± 20.7*** | 1462 ± 80.6*** | 1674 ± 101.1*** | 1530 ± 65.0*** | 872 ± 3.2*/### |
Mean ± SE gray values for each group of buffer-perfused lungs is given. Each group comprised 5 independent lung experiments, and cross-sections from three randomly selected tissue blocks originating from different lung lobes were investigated per lung. Statistical significance: P < 0.05*, < 0.01**, < 0.001*** versus control; endothelial cells versus 1000 ng/ml, 1 hour; P < 0.05#, < 0.01##, <0.001### versus 1000 ng/ml, 2 hours.
n.d., staining not detected.
Figure 2.
Immunostaining of Cox-2, epipolarization. A, C, and E show different structures of control lungs. B, D, and F show comparable structures from LPS-treated groups; differences of staining intensity are visualized by pseudocolor conversion of the epipolarization image. Vascular smooth muscle cells of partially muscular vessels express Cox-2 in buffer-perfused control lungs (A). Up-regulation of Cox-2 in the smooth muscle layer of partially muscular vessels in LPS-treated lungs is visible by the shift of pseudocolors representing the staining intensity (B, 1,000 ng/ml, 2 hours perfusion time). Figure 1, C and D ▶ , shows myocytes of large hilar veins under control conditions (C, 1 hour buffer perfusion) and after LPS-exposure (D, 10,000 ng/ml, 1 hour perfusion time). Perivascular macrophage-like cells exhibit strongest Cox-2 staining intensity in control lungs (E, control with 2 hours buffer/plasma perfusion), but are hardly detectable in lung tissue after LPS-treatment (F, arrows, 10,000 ng/ml LPS, 2 hours buffer/plasma perfusion). Scale bar, 50 μm.
Expression of Cox-2 Message after LPS
In response to LPS, Cox-2 mRNA expression was enhanced in a time- and concentration-dependent manner when analyzed for total lung homogenate. As an example, the time course of Cox-2 mRNA expression in lungs perfused without plasma and the dose dependency of Cox-2 mRNA expression in buffer/plasma-perfused lungs are given in Figure 1A and B ▶ . Quantification of these data indicated an approximately fivefold increase in Cox-2 message in the homogenized tissue in response to 1000 and 10,000 ng/ml LPS, both for 1- and 2-hour perfusion periods in the absence and presence of plasma.
Cox-2 Immunostaining in Response to 50 ng/ml LPS, Perfusion Period 2 Hours
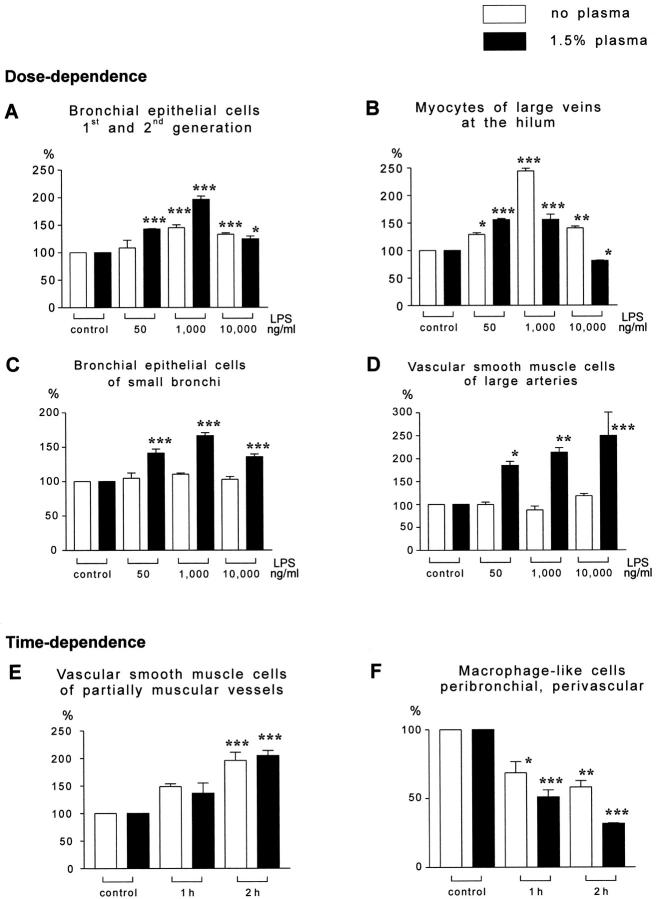
A significant increase in Cox-2 staining intensity was detected in bronchial epithelial cells, BSMC, and VSMC of large arteries only in the presence of plasma (Table 2 ▶ and Figure 3 ▶ ), whereas cells of the BALT and myocytes of large hilar veins showed significantly higher staining intensity in both the absence and presence of plasma constituents (Tables 1 and 2 ▶ ▶ and Figure 3 ▶ ). Cox-2 reactivity was also increased in VSMC of partially muscular vessels (± plasma), macrophage-like cells (± plasma), alveolar macrophages (+ plasma), and single cells within the alveolar septum (+ plasma); however, data were not statistically different from controls. Cox-2 staining of endothelial cells was not detected.
Figure 3.
Quantitative evaluation of Cox-2 staining intensity relative changes in response to LPS exposure. Mean ± SE for buffer-perfused (open bars) or buffer/plasma-perfused (closed bars) lungs are given (each group n = 5 lungs); all data refer to the respective controls, which were set at 100%. Each group comprised 5 independent lung experiments with cross-sections from three randomly selected tissue blocks originating from different lobes of each lung. Dose and time dependencies are indicated. Note the different response patterns: i) Cox-2 up-regulation in the absence and presence of plasma, eg, bronchial epithelial cells of large bronchi (A), myocytes of large hilum veins (B), and VSMC of partially muscular vessels (E). ii) Cox-2 up-regulation only in the presence of plasma, eg, bronchial epithelial cells of small bronchi (C), vascular smooth muscle cells of large arteries (D). iii) Cox-2 down-regulation in macrophage-like cells in the peribronchial and perivascular tissue (F). P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), compared to the respective control groups.
Cox-2 Immunostaining in Response to 1000 ng/ml LPS, Perfusion Period 1 Hour or 2 Hours
In both groups, with and without plasma, bronchial epithelial cells of large bronchi, cells of the BALT, myocytes of large veins, and VSMC of partially muscular vessels exhibited strong increase in Cox-2 staining intensity after 1 hour of perfusion, which was even further enhanced after 2 hours (Figures 2B and 3 ▶ ▶ ; Tables 1 and 2 ▶ ▶ ). Single cells within the alveolar septum (± plasma) and alveolar macrophages (+ plasma) showed a significant increase in Cox-2 staining intensity under 1000 ng/ml LPS only after 2 hours of perfusion. Bronchial epithelial cells of small bronchi, BSMC, VSMC of large arteries, and alveolar macrophages were not significantly different from control lungs in groups perfused in the absence of plasma, whereas Cox-2 reactivity was clearly elevated under conditions of buffer/plasma perfusion. Staining of endothelial cells was detected in all groups receiving 1000 ng/ml LPS. Macrophage-like cells exhibited increased staining intensity after 1 hour in the group lacking plasma, but showed significantly reduced Cox-2 reactivity in the presence of plasma and after a 2-hour perfusion time in both groups with and without plasma (Tables 1 and 2 ▶ ▶ , Figure 2F ▶ ).
Cox-2 Immunostaining in Response to 10,000 ng/ml LPS, Perfusion Period 1 Hour or 2 Hours
The pattern of changes largely corresponded to that on challenge with 1000 ng/ml LPS (Tables1 and 2 and Figure 3 ▶ ). A time-dependent decrease of staining intensity was observed between 1 and 2 hours in the presence of plasma in bronchial epithelial cells, BSMC, cells of the BALT, single cells within the alveolar septum, alveolar macrophages, VSMC of partially muscular vessels, and large hilar vein myocytes. The down-regulation of Cox-2 immunostaining in macrophage-like cells was even more prominent in response to 10,000 ng/ml LPS as compared to the 1000 ng/ml dosage.
CD14
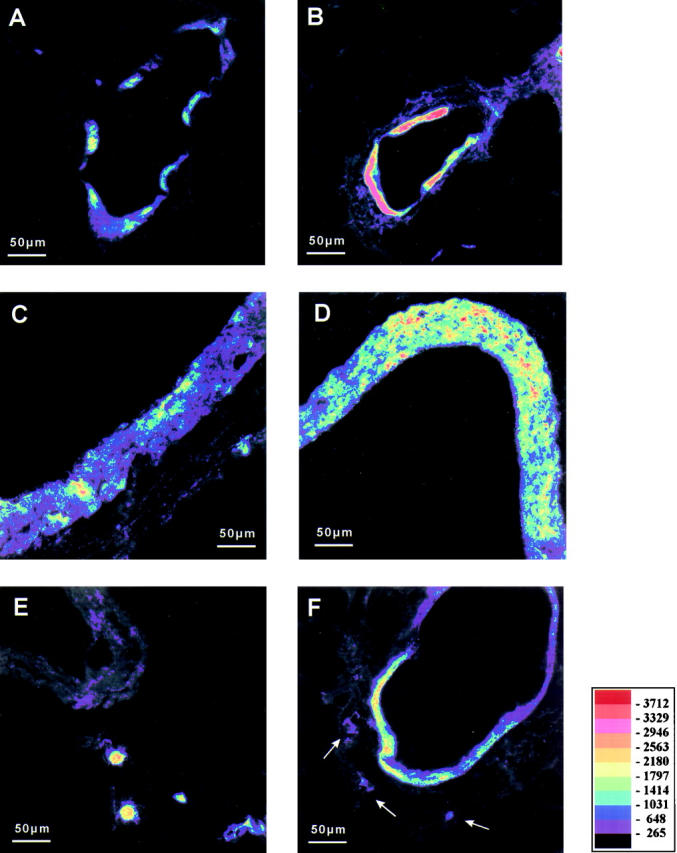
In control lungs, strong staining was observed in bronchial epithelial cells of large bronchi of first and second generation (Figure 4, A and B) ▶ . The BALT showed moderate positive immunostaining (Figure 4B) ▶ . In addition, alveolar macrophages and perivascularly and peribronchially located leukocytes were markedly reactive (Table 3 ▶ and Figure 4C ▶ ). Within the lung parenchyma, single cells located in the alveolar septum showed extensive staining. In contrast, bronchial epithelial cells of small bronchi were only found to be weakly positive for CD14 in a few cross-sections at few locations. BSMC were not positive for CD14 (Figure 4, A and B) ▶ . VSMC of small vessels exhibited a marked positive immunostaining and this was mainly in the partially muscular vessels, which may be pre- or postcapillary (Figure 4C) ▶ . VSMC of large arteries, myocytes of veins near the hilum, and endothelial cells were not stained. The alveolar septum in general and peribronchial nerve fibers did not show any staining for CD14. In addition, macrophage-like cells, which were positive for Cox-2, did not express CD14.
Figure 4.
Immunostaining of CD14, fluorescence. Positive immunostaining is visualized by pseudocolor conversion of the fluorescence image. Bronchial epithelial cells are heavily stained (bronchus of first or second generation A and B), whereas bronchial smooth muscle cells (*, A and B) exhibit no staining for CD14. Cells of the BALT express CD14 (B). Vascular smooth muscle cells of a small partially muscular vessel within the lung parenchyma are positive for CD14 (C). Note that alveolar macrophages (arrows) express CD14 (C and D). In the lung parenchyma, single cells (arrowheads) within the alveolar septum show positive immunostaining for CD14 (D). Scale bar, 50 μm.
Table 3.
Immunolocalization of CD14 in Normal Rat Lung Tissue
| CD14-positive | CD14-negative |
|---|---|
| Bronchial epithelial cells (large bronchi) | Bronchial epithelial cells (small bronchi) |
| Alveolar macrophages | Bronchial smooth muscle cells |
| Perivascular leukocytes | Macrophage-like cells |
| Single cells within the alveolar septum | Endothelial cells |
| Cells of the BALT | Alveolar septum |
| VSMC of partially muscular vessels | VSMC of large arteries |
| Myocytes of large veins |
Prostanoid Release into the Perfusate
In control lungs without LPS administration, only minor amounts of TxB2 and 6-keto PGF1α accumulated in the recirculating perfusate over the 2-hour perfusion period (Figure 5, A ▶ -D). After LPS administration, a moderate (50 ng/ml LPS) to marked (1000 or 10,000 ng/ml LPS) increase in prostanoid liberation became apparent in both buffer- and buffer/plasma-perfused lungs, significantly different from control perfusion conditions.
Figure 5.
Release of arachidonic acid metabolites after LPS challenge. The time course of TxB2 and 6-keto PGF1α liberation in buffer or buffer/plasma-perfused lungs under control conditions and in response to different LPS doses is given (2-hour perfusion experiments only; each group n = 5 lungs). P < 0.05 (*), P < 0.01 (**) compared to the respective controls.
Discussion
In the present study, control lungs perfused with buffer fluid in the absence or presence of plasma constituents for up to 2 hours displayed the same cellular pattern of Cox-1 and Cox-2 expression as recently described for nonperfused rat lungs. 33 Incubation with LPS at doses which did not induce functional changes in the perfused organs per se resulted in enhanced Cox-2 mRNA, but unchanged Cox-1 mRNA expression, on Northern blot analysis of the whole lung homogenate. Accordingly, immunostaining revealed unaltered cellular distribution of Cox-1 protein, whereas marked changes of Cox-2 expression occurred, with cell-specific reaction patterns to the endotoxin: in addition to LPS-induced up-regulation of Cox-2 in various cell types expressing this enzyme already under baseline conditions, induction of Cox-2 in cells negative for this enzyme in control lungs was noted, and even down-regulation of Cox-2 occurred. Moreover, differential impact of the presence or absence of plasma components on the various reaction patterns was noted, and this was largely but not fully consistently correlated with the surface expression of CD14 of the various cell types. Quantitative image analysis on the cellular level thus revealed a hitherto unrecognized complex lung reaction pattern to endotoxin exposure.
As previously described for nonperfused rat lungs, 33 strong Cox-1 immunostaining was detected in the bronchial epithelial cells of all bronchi and in cardiac myocyte-derived muscle cells located in the large veins of the hilum. Less prominent Cox-1 expression was noted for alveolar macrophages, endothelial cells, and BSMC. The present finding that this pattern of immunostaining as well as the Cox-1 mRNA quantification was unchanged in response to LPS is in line with the recent observation of unaltered Cox-1 message in the homogenate of rabbit lungs stimulated with endotoxin for 2 hours. 42 These observations thus further support the view of Cox-1 as a constitutive enzyme, not rapidly responding to inflammatory conditions and most probably linked with the regulation of physiological functions. 21,43
In contrast to Cox-1, rapid and dose-dependent up-regulation of Cox-2 mRNA, as analyzed in the whole organ homogenate, was noted in LPS-exposed rat lungs. This observation is in accordance with previous studies demonstrating enhanced Cox-2 message in homogenized tissue of lungs undergoing endotoxin stimulation 40,42 and with studies in several cultured cell types responding with Cox-2 up-regulation to LPS stimulation. 22-24,35-37,45,46 Immunohistochemical staining of the lung tissue revealed different cellular patterns of reactivity to the pulmonary endotoxin challenge.
First, LPS resulted in up-regulation of Cox-2 in cells expressing this enzyme under baseline conditions. This profile of reactivity was noted in several cell types, including bronchial epithelial cells, BSMC, cells within the BALT, single cells within the alveolar septum, VSMC of partially muscular vessels, and large arteries and myocytes of the large hilum veins. Positive immunostaining for Cox-2 was noted in only a small percentage of alveolar macrophages. With respect to BSMC, this type of response to endotoxin was already suggested from in vitro studies demonstrating basal levels of Cox-2 and LPS-induced rapid up-regulation of this enzyme. 45 Single cells within the alveolar septum may well represent intracapillary neutrophils and monocytes, as the lung vasculature is known to harbor a large pool of intravascular leukocytes even under noninflamed conditions. 47-49 This view is further supported by the fact that these cells were consistently positive for CD14, a typical feature of the myelomonocytic lineage. 50-52
Second, LPS induced Cox-2 in cells with negative immunostaining under control conditions. This response pattern was noted in endothelial cells and in the majority of alveolar macrophages. 33 The appearance of Cox-2 in the endothelial cells of LPS-treated lungs is in accordance with studies in cultured endothelial cells (of nonpulmonary origin) demonstrating Cox-2 to be an inducible enzyme in this cell type in vitro. 36,37 Concerning the alveolar macrophages, it must be kept in mind that in noninflamed lungs, only minute amounts of intravascularly applied LPS spill over into the alveolar compartment, 53 but these amounts apparently suffice to up-regulate Cox-2 in this cell type. It may be speculated that the responsiveness of the alveolar macrophage Cox-2 system to pulmonary LPS challenge might be even more prominent in the case of intra-alveolar endotoxin deposition, as suggested by the compartmentalized cytokine release evoked by lung alveolar versus intravascular LPS administration. 53
Third, LPS down-regulated Cox-2. To the best of our knowledge, this type of reactivity of Cox-2 has hitherto never been recognized in cell culture experiments employing LPS stimulation, but occurred unambiguously in a time- and dose-dependent fashion in macrophage-like cells located in the perivascular and peribronchial tissue. As recently described, these cells exhibit strong Cox-2 staining intensity in noninflamed control lungs. 33 The physiological role of this Cox-2 expression is currently unresolved, but may be of interest in view of the recent finding that Cox-2 may even possess anti-inflammatory properties at distinct sites, assumed to be related to baseline generation of prostanoids acting to suppress inflammatory events. 54-57
In addition to the fact that different types of regulation of Cox-2 were noted in response to the endotoxin exposure of the intact lungs, the absence or presence of plasma constituents exerted differential impact on these regulatory responses. This experimental approach was chosen against the background that some cellular effects of LPS are known to demand the presence of plasma components such as soluble CD14 and/or LBP, 3-7 but LPS signaling pathways independent of the LBP/CD14-axis were noted to exist. 11-16 In the present study, the presence of plasma components was found to be a prerequisite for the LPS-induced Cox-2 response of epithelial cells of small bronchi, BSMC, and VSMC of large arteries. In addition, in the presence of plasma components more prominent/rapid LPS-induced Cox-2 up-regulation in endothelial cells and more prominent Cox-2 down-regulation in macrophage-like cells in the peribronchial and perivascular tissue were noted. Interestingly, all these cell types did not display CD14 immunostaining (absence of membrane CD14), which might suggest that soluble CD14 is demanded or at least largely supportive for the responsiveness of these cells to LPS, as known from several in vitro studies in cultured endothelial cells undergoing endotoxin challenge. 4,7,58,59 In line with this view, the LPS responsiveness of bronchial epithelial cells of the large bronchi, cells of the BALT, single cells of the alveolar septum and VSMC of partially muscular vessels, all of which displayed positive CD14 immunostaining and did not differ in their response whether plasma components were absent or present. The correlation between plasma dependence versus independence of the LPS-induced Cox-2 responses and absence versus presence of cell-bound CD14 was, however, not fully consistent. Alveolar macrophages, clearly displaying immunolocalization of CD14 as expected for cells of the myelomonocytic lineage, 50,51 responded with significant Cox-2 up-regulation only in the presence of plasma components. This might be explained by the fact that only low quantities of LPS reached the alveolar compartment, as discussed above, and plasma-derived LBP might facilitate the responsiveness of alveolar macrophages to low amounts of endotoxin despite the presence of membrane CD14. Moreover, muscle cells of the large veins, which resemble cardiac myocytes, did not display CD14 immunostaining but nevertheless responded to LPS with Cox-2 up-regulation in both the presence and absence of plasma constituents. Additional LPS signal transduction pathways thus appear to be involved in this phenomenon; they are clearly beyond the scope of the present study. Future investigations are mandatory to address in a more detailed fashion the intracellular signaling events in the various lung cells responding with changes in Cox-2 regulation to LPS exposure, and these studies should obviously include the recently discovered family of toll-like receptors. 8,9
Different physiological and pathophysiological functions of the Cox-isoenzymes are apparently linked with the cellular equipment of downstream enzymes. Not surprisingly, both enhanced prostacyclin release and thromboxane liberation were noted in the LPS-exposed lungs, which may well be explained with the de novo Cox-2 synthesis in endothelial cells, harboring prostacyclin synthase as a predominant downstream enzyme, and Cox-2 up-regulation in vascular smooth muscle cells, now known to be strongly positive for thromboxane synthase. 34,60 We speculate that as a net result of the effects of enhanced vasodilator and vasoconstrictor generation, the vascular tone in the LPS-challenged lungs did not change, and the same was true for vascular permeability and bronchial tone. It is, however, known that a second proinflammatory agent, such as bacterial exotoxins 18,38,39,41 or platelet-activating factor, 42 provokes dramatically enhanced thromboxane generation and vasoconstrictor responses in such LPS-primed lungs. This phenomenon may well be explained by the currently described finding of LPS-induced Cox-2 up-regulation in the VSMC of large and in particular partially muscularized small vessels, the latter playing a predominant role in lung vasotone regulation, 61 which is supported by a prominent expression of thromboxane synthase. 34,60
In conclusion, the present study significantly extends current knowledge on pulmonary endotoxin effects by analyzing cell-specific reaction patterns of Cox-1 and Cox-2 to LPS exposure in intact lungs. Whereas Cox-1 expression remained constant, dynamic changes of Cox-2 included up-regulation of this enzyme in various cell types, induction of Cox-2 in cells negative for this enzyme in control lungs, and even down-regulation of Cox-2 in a distinct cell population. In addition, the LPS responsiveness of the different cells was found to be dependent or independent of the presence of plasma components, which largely but not exclusively correlated with negative or positive CD14 immunostaining of the respective cells. Immunohistochemical staining of the lung tissue with quantitative analysis of epipolarization images thus proved to be a potent tool to discover a differential cellular response pattern to endotoxin in an organ such as the lung, being composed of a large variety of different cell types. These findings may help to elucidate the complex mechanisms of lung injury encountered in sepsis and adult respiratory distress syndrome.
Acknowledgments
We thank G. Müller and M. Rehm for excellent technical assistance and Dr. R. L. Snipes, Department of Anatomy, Giessen, for linguistically reviewing the manuscript.
Footnotes
Address reprint requests to Leander Ermert, Institut fuer Anatomie und Zellbiologie, Aulweg 123, 35385 Giessen, Germany. leander. ermert@anatomie.med.uni-giessen.de.
Supported by the Deutsche Forschungsgemeinschaft (SFB 547).
References
- 1.Brigham KL, Meyrick B: Endotoxin and lung injury. Am Rev Respir Dis 1986, 133:913-927 [PubMed] [Google Scholar]
- 2.Ulevitch RJ: Recognition of bacterial endotoxin in biologic systems. Lab Invest 1991, 65:121-122 [PubMed] [Google Scholar]
- 3.Ulevitch RJ: Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol 1993, 53:267-289 [DOI] [PubMed] [Google Scholar]
- 4.Pugin J, Schürer-Maly C-C, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS: Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 1993, 90:2744-2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias PS, Soldau K, Ulevitch RJ: Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem 1989, 264:10867-10871 [PubMed] [Google Scholar]
- 6.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC: CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249:1431-1433 [DOI] [PubMed] [Google Scholar]
- 7.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V: Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med 1992, 176:1665-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R-B, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ: Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998, 395:284-288 [DOI] [PubMed] [Google Scholar]
- 9.Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M: Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 1998, 188:2091–2097 [DOI] [PMC free article] [PubMed]
- 10.Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D: Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 1999, 189:615-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynn WA, Liu Y, Golenbock DT: Neither CD14 nor serum is absolutely necessary for activation of mononuclear phagocytes by bacterial lipopolysaccharide. Infect Immun 1993, 61:4452-4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen L, Haziot A, Shen DR, Lin X-Y, Sia C, Harper R, Silver J, Goyert SM: CD14-independent responses to LPS require a serum factor that is absent from neonates. J Immunol 1995, 155:5337-5342 [PubMed] [Google Scholar]
- 13.Wright SD: Multiple receptors for endotoxin. Curr Opin Immunol 1991, 3:83-90 [DOI] [PubMed] [Google Scholar]
- 14.Ingalls RR, Golenbock DT: CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med 1995, 181:1473-1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright SD, Ramos RA, Patel M, Miller DS: Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med 1992, 176:719-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Samalouti VT, Schletter J, Chyla I, Lentschat A, Mamat U, Brade L, Flat HD, Ulmer AJ, Hamann L: Identification of the 80-kDa LPS-binding protein (LMP80) as decay-accelerating factor (DAF, CD55). FEMS Immunol Med Microbiol 1999, 23:259-269 [DOI] [PubMed] [Google Scholar]
- 17.Chang S-W, Westcott JY, Pickett WC, Murphy RC, Voelkel NF: Endotoxin-induced lung injury in rats: role of eicosanoids. J Appl Physiol 1989, 66:2407-2418 [DOI] [PubMed] [Google Scholar]
- 18.Steudel W, Krämer H-J, Degner D, Rosseau S, Schütte H, Walmrath D, Seeger W: Endotoxin priming of thromboxane-related vasoconstrictor responses in perfused rabbit lungs. J Appl Physiol 1997, 83:18-24 [DOI] [PubMed] [Google Scholar]
- 19.Uhlig S, Nüsing R, Bethmann Av, Featherstone RL, Klein T, Brasch F, Müller K-M, Ullrich V, Wendel A: Cyclooxygenase-2-dependent bronchoconstriction in perfused rat lungs exposed to endotoxin. Mol Med 1996, 2:373–383 [PMC free article] [PubMed]
- 20.Seeger W, Menger M, Walmrath D, Becker G, Grimminger F, Neuhof H: Arachidonic acid lipoxygenase pathways and increased vascular permeability in isolated rabbit lungs. Am Rev Respir Dis 1987, 136:964-972 [DOI] [PubMed] [Google Scholar]
- 21.Goppelt-Struebe M: Regulation of prostaglandin endoperoxide synthase (cyclooxygenase) isozyme expression. Prostaglandins Leukot Essent Fatty Acids 1995, 52:213-222 [DOI] [PubMed] [Google Scholar]
- 22.Hempel SL, Monick MM, Hunninghake GW: Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest 1994, 93:391-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D: Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem 1992, 267:25934-25938 [PubMed] [Google Scholar]
- 24.Mitchell JA, Belvisi MG, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall J, Barnes PJ, Vane JR: Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol 1994, 113:1008-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD: Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 1994, 94:2504-2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O: Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 1995, 83:473-482 [DOI] [PubMed] [Google Scholar]
- 27.Iseki S: Immunocytochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the rat stomach. Histochem J 1995, 27:323-328 [DOI] [PubMed] [Google Scholar]
- 28.Sorli CH, Zhang H-J, Armstrong MB, Rajotte RV, Maclouf J, Robertson RP: Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci USA 1998, 95:1788-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirois J: Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology 1994, 135:841-848 [DOI] [PubMed] [Google Scholar]
- 30.Lim H, Paria BC, Dey SK: Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91:197-208 [DOI] [PubMed] [Google Scholar]
- 31.Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M: Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett 1996, 390:165-169 [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P: Cox-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA 1996, 93:2317-2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ermert L, Ermert M, Goppelt-Struebe M, Walmrath D, Grimminger F, Steudel W, Ghofrani HA, Homberger C, Duncker H-R, Seeger W: Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol 1998, 18:479-488 [DOI] [PubMed] [Google Scholar]
- 34.Ermert L, Ermert M, Althoff A, Merkle M, Grimminger F, Seeger W: Vasoregulatory prostanoid generation proceeds via cyclooxygenase-2 in noninflamed rat lungs. J Pharmacol Exp Ther 1998, 286:1309-1314 [PubMed] [Google Scholar]
- 35.Arias-Negrete S, Keller K, Chadee K: Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun 1995, 208:582-589 [DOI] [PubMed] [Google Scholar]
- 36.Habib A, Créminon C, Frobert Y, Grassi J, Pradelles P, Maclouf J: Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem 1993, 268:23448-23454 [PubMed] [Google Scholar]
- 37.Akarasereenont P, Bakhle YS, Thiemermann C, Vane JR: Cytokine-mediated induction of cyclo-oxygenase-2 by activation of tyrosin kinase in bovine endothelial cells stimulated by bacterial lipopolysaccharide. Br J Pharmacol 1995, 115:401-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walmrath D, Ghofrani HA, Rosseau S, Schütte H, Cramer A, Kaddus W, Grimminger F, Bhakdi S, Seeger W: Endotoxin “priming” potentiates lung vascular abnormalities in response to Escherichia coli hemolysin: an example of synergism between endo- and exotoxin. J Exp Med 1994, 180:1437-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schütte H, Rosseau S, Czymek R, Ermert L, Walmrath D, Krämer H-J, Seeger W, Grimminger F: Synergism between endotoxin priming and exotoxin challenge in provoking severe vascular leakage in rabbit lungs. Am J Respir Crit Care Med 1997, 156:819-824 [DOI] [PubMed] [Google Scholar]
- 40.Liu SF, Newton R, Evans TW, Barnes PJ: Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci 1996, 90:301-306 [DOI] [PubMed] [Google Scholar]
- 41.Walmrath D, Ghofrani H-A, Grimminger F, Seeger W: Synergism of alveolar endotoxin “priming” and intravascular exotoxin challenge in lung injury. Am J Respir Crit Care Med 1996, 154:460-468 [DOI] [PubMed] [Google Scholar]
- 42.Delong P, O’Sullivan MG, Huggins E, Hubbard CL, McCall C: Bacterial lipopolysaccharide induction of the prostaglandin G/H synthase 2 gene causes thromboxane-dependent pulmonary hypertension in rabbits. Am J Respir Cell Mol Biol 1999, 20:493-499 [DOI] [PubMed] [Google Scholar]
- 43.DeWitt DL, Meade EA: Serum and glucocorticoid regulation of gene transcription and expression of the prostaglandin H synthase-1 and prostaglandin H-synthase-2 isozymes. Arch Biochem Biophys 1993, 306:94-102 [DOI] [PubMed] [Google Scholar]
- 44.Stroebel M, Goppelt-Struebe M: Signal transduction pathways responsible for serotonin-mediated prostaglandin G/H synthase expression in rat mesangial cells. J Biol Chem 1994, 269:22952-22957 [PubMed] [Google Scholar]
- 45.Vigano T, Habib A, Hernandez A, Bonazzi A, Boraschi D, Lebret M, Cassina E, Maclouf J, Sala A, Folco G: Cyclooxygenase-2 and synthesis of PGE2 in human bronchial smooth muscle cells. Am J Respir Crit Care Med 1997, 155:864-868 [DOI] [PubMed] [Google Scholar]
- 46.Endo T, Ogushi F, Sone S, Ogura T, Taketani Y, Hayashi Y, Ueda N, Yamamoto S: Induction of cyclooxygenase-2 is responsible for interleukin-1 beta-dependent prostaglandin E2 synthesis by human lung fibroblasts. Am J Respir Cell Mol Biol 1995, 12:358-365 [DOI] [PubMed] [Google Scholar]
- 47.Doerschuk CM, Allard MF, Martin BA, MacKenzie A, Autor AP, Hogg JC: Marginated pool of neutrophils in rabbit lungs. J Appl Physiol 1987, 63:1806-1815 [DOI] [PubMed] [Google Scholar]
- 48.Ermert L, Seeger W, Duncker H-R: Computer-assisted morphometry of the intracapillary leukocyte pool in the rabbit lung. Cell Tissue Res 1993, 271:469-476 [DOI] [PubMed] [Google Scholar]
- 49.Ermert L, Duncker H-R, Rosseau S, Schütte H, Seeger W: Morphometric analysis of pulmonary intracapillary leukocyte pools in ex vivo-perfused rabbit lungs. Am J Physiol 1994, 267:L64-L70 [DOI] [PubMed] [Google Scholar]
- 50.Martin TR, Mongovin SM, Tobias PS, Mathison JC, Moriarty AM, Leturcq DJ, Ulevitch RJ: The CD14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J Leukocyte Biol 1994, 56:1-9 [DOI] [PubMed] [Google Scholar]
- 51.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM: The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol 1988, 141:547-552 [PubMed] [Google Scholar]
- 52.Wright SD, Ramos RA, Hermanowski-Vosatka A, Rockwell P, Detmers PA: Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med 1991, 173:1281-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghofrani HA, Rosseau S, Walmrath D, Kaddus W, Kramer A, Grimminger F, Lohmeyer J, Seeger W: Compartmentalized lung cytokine release in response to intravascular and alveolar endotoxin challenge. Am J Physiol 1996, 270:L62-L68 [DOI] [PubMed] [Google Scholar]
- 54.Picado C, Fernandez-Morata JC, Juan M, Roca-Ferrer J, Fuentes M, Xaubet A, Mullol J: Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med 1999, 160:291-296 [DOI] [PubMed] [Google Scholar]
- 55.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA: Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999, 5:698-701 [DOI] [PubMed] [Google Scholar]
- 56.Seibert K, Lefkowith J, Tripp C, Isakson P, Needleman P: Cox-2 inhibitors - is there cause for concern? Nat Med 1999, 5:621-622 [DOI] [PubMed] [Google Scholar]
- 57.Newberry RD, Stenson WF, Lorenz RG: Cyclooxygenase-2 dependent arachidonic acid metabolites are critical modulators of the intestinal immune response to dietary antigen. Nat Med 1999, 5:900-906 [DOI] [PubMed] [Google Scholar]
- 58.Haziot A, Rong G-W, Silver J, Goyert SM: Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol 1993, 151:1500-1507 [PubMed] [Google Scholar]
- 59.Goldblum SE, Brann TW, Ding X, Pugin J, Tobias PS: Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J Clin Invest 1994, 93:692-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ermert L, Ermert M, Duncker HR, Grimminger F, Seeger W: In-situ localization and regulation of thromboxane A2-synthase in normal and LPS-primed lungs. Am J Physiol, in press [DOI] [PubMed]
- 61.Reid LM: Vascular remodeling. Fishman AP eds. The Pulmonary Circulation. 1990, :pp 259-282 University of Pennsylvania Press, Philadelphia [Google Scholar]