Abstract
Primary effusion lymphoma (PEL) develops in immunodeficient patients, selectively localizes to the serous body cavities, and harbors infection by human herpesvirus type-8 (HHV-8), also known as Kaposi’s sarcoma-associated herpesvirus. HHV-8 encodes a viral (v)-cyclin homologous to cellular D-type cyclins, a class of positive cell-cycle regulators that are physiologically modulated by the p27Kip1 cell cycle inhibitor. The aims of the present study were: 1) to establish the expression pattern of p27Kip1 in PEL; and 2) to address the relationship between p27Kip1 expression, proliferation index, and expression of cellular cyclin D1 and v-cyclin in PEL. Expression of p27Kip1 was detected in all (n = 18) PEL samples analyzed by both immunocytochemistry and Western blot. All PELs displayed a high proliferation index as assessed by Ki-67 staining. Expression of cellular cyclin D1 was absent in all PELs tested, which conversely expressed (14 out of 14 samples) v-cyclin by immunocytochemistry and/or Western blot. In contrast to PELs, HHV-8-negative lymphomatous effusions secondary to a tissue-based lymphoma generally failed to express p27Kip1. Overall, these data show that PELs consistently express p27Kip1 protein despite the high proliferative rate of the lymphoma clone, suggesting that p27Kip1 may be unable to drive cell-cycle arrest in PEL cells. The co-existence of p27Kip1 expression and high proliferative index is a selective feature of PEL among lymphomas involving the serous body cavities, because lymphomatous effusions secondary to a tissue-based lymphoma generally display the inverse relationship between p27Kip1 positivity and growth fraction observed in normal lymphoid tissues and in most other lymphomas. Expression of p27Kip1 in PEL associates with expression of HHV-8 v-cyclin, but not of cellular cyclin D1. The fact that HHV-8 v-cyclin is resistant to p27Kip1-modulated inhibition, whereas cellular cyclin D1 is sensitive, may explain, at least in part, the co-existence of p27Kip1 expression and high proliferative index observed in PEL.
Primary effusion lymphoma (PEL) represents a unique non-Hodgkin’s lymphoma (NHL) entity characterized by consistent infection by human herpesvirus type-8 (HHV-8), also known as Kaposi’s sarcoma-associated herpesvirus. PEL preferentially develops in immunodeficient patients and selectively localizes to the serous body cavities. 1,2 The basic pathological feature of PEL is a net predilection for diffuse spreading along the serous membranes without infiltrative or destructive growth patterns. 2 Although immunogenotypic studies have confirmed that PEL belongs in all cases to the B-cell lineage, the overwhelming majority of cases exhibit a non-B, non-T (ie, indeterminate) phenotype, lacking expression of surface immunoglobulins and common B-cell associated antigens. 1,2
The molecular pathogenesis of PEL is understood only in part. Most genetic lesions commonly seen in mature B-cell malignancies, eg, rearrangements of MYC, BCL-2, and BCL-6 as well as mutations of p53 1 (see Reference 2 for a review), are not involved in PEL pathogenesis. Conversely, it is conceivable that HHV-8 plays a pathogenetic role, because the virus encodes several genes which are homologous to cellular loci implicated in B-cell growth and survival 3 (see review in Reference 4). One such gene is represented by the HHV-8 viral- (v-)cyclin that displays highest sequence similarity to the cellular D-type cyclins, 4 a group of positive cell-cycle regulators that favor G1 progression. Although the precise function of v-cyclin is not fully known, it is conceivable that it shares several properties with cellular D-type cyclins. The expression of HHV-8 v-cyclin in latently infected PEL cells 5 suggests a possible role of this molecule in growth control of the lymphoma.
The cellular D-type cyclins contribute to cell cycle control by forming complexes with catalytic subunits termed cyclin-dependent kinases (CDK). 6,7 The activation of the CDK/cyclin complex is controlled by CDK inhibitors, which regulate cell cycle. 7 A major inhibitor of the CDK/cyclins complex is represented by p27Kip1, a nuclear phosphoprotein belonging to the Kip family of CDK inhibitors. 8-12 In physiological conditions, expression of p27Kip1 is highest in quiescent cells and declines as cells reenter the cell cycle. In lymphoid tissues, p27Kip1 is expressed in nonproliferating lymphocytes, whereas activated lymphocytes, eg, germinal center cells, score consistently negative for p27Kip1 expression. 13 The inverse relationship between p27Kip1 expression and proliferation physiologically observed in normal lymphoid tissues is also encountered in most subtypes of NHL. 13-15
The aims of the present study were: 1) to establish the expression pattern of p27Kip1 in PELs; and 2) to address the relationship between p27Kip1 expression, proliferation index, and expression of cellular cyclin D1 and v-cyclin of the lymphoma. We report that PELs consistently express p27Kip1 protein despite the high proliferative rate of the lymphoma clone, suggesting that p27Kip1 may be unable to drive cell-cycle arrest in PEL cells. Expression of p27Kip1 in PEL associates with expression of HHV-8 v-cyclin, but not of cellular cyclin D1, suggesting implications for PEL pathogenesis and growth. The co-existence of p27Kip1 expression and high proliferative index is a selective feature of PEL among lymphomas involving the serous body cavities, because secondary lymphomatous effusions generally display the inverse relationship between p27Kip1 positivity and growth fraction observed in most other types of NHL.
Materials and Methods
Tumor Panel
The present study was based on 18 samples of PEL and, for comparative purposes, 10 samples of HHV-8-negative lymphomatous effusions secondary to systemic B-cell NHL.
Samples of PEL included nine clinical tumor samples and nine cell lines. Clinical samples were collected under sterile conditions during routine diagnostic procedures at diagnosis; after centrifugation, the effusion sediments were used to prepare cytospins and cell blocks. For morphological study, cytospins were routinely fixed and stained by the Papanicolaou method. For cell-block preparation, cell pellets were Bouin- or formalin-fixed and treated according to a standard regimen used at the Division of Pathology at Centro di Riferimento Oncologico. The sectioned material from cell blocks was stained with hematoxylin and eosin. In all cases the cytospins and cell blocks prepared from fluid samples showed a tumor cell population ≥95% as evaluated by morphological and immunophenotypic analysis. Clinical tumor samples were classified as PELs based on HHV-8 infection of the tumor clone, and on specific morphological, immunophenotypic, and molecular features, according to previously reported criteria. 1,2 Tumor cells usually exhibited an indeterminate (non-B, non-T) phenotype (8 out of 9), lacked expression of surface immunoglobulins and common B-cell associated antigens, were devoid of rearrangements of the c-MYC gene, and clinically displayed exclusive or predominant involvement of the serous body cavities. Tumor cells from six PEL clinical samples carried Epstein-Barr virus (EBV) infection, whereas tumor cells from the remaining cases were EBV-negative. All, but one, PEL clinical samples were obtained from patients infected with the human immunodeficiency virus (HIV); the remaining sample was derived from an HIV-negative individual.
The PEL cell lines included in this study were HBL-6, BC-1, BC-2, CRO-AP/2, CRO-AP/3, CRO-AP/5, BCBL-1, BC-3, and BCP-1. 16-22 The detailed characterization of these cell lines has been reported previously 16-22 (see review in Ref. 23 ). BCBL-1 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Five PEL cell lines (HBL-6, BC-1, BC-2, CRO-AP/2, and CRO-AP/5) carry EBV infection, whereas four PEL cell lines (BC-3, CRO-AP/3, BCBL-1, and BCP-1) are EBV-negative. All but two (BC-3 and BCP-1) PEL cell lines were derived from HIV-infected patients; BC-3 and BCP-1 were derived from HIV-negative individuals. All cell lines were cultured in RPMI 1640 (Life Technologies, Inc., Paisley, Scotland), supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Inc.), 2 mmol/L L-glutamine (Life Technologies, Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin (Irvine Scientific, Santa Ana, CA) at 37°C in the presence of 5% CO2.
Secondary lymphomatous effusions, which were included in the study for comparative purposes, were collected and subjected to diagnostic procedures as described above. All secondary lymphomatous effusions scored negative for HHV-8 sequences, expressed B-cell markers and were assigned to the B-cell lineage. Clinically, all these effusions were documented at diagnosis. They were consistently associated with solid lymphomatous masses and were considered to be secondary to a tissue-based lymphoma (eight diffuse large B-cell lymphomas –B-DLCL - and two Burkitt’s lymphomas -BL). Tumor cells from two secondary lymphomatous effusions carried EBV infection, whereas tumor cells from the remaining cases were EBV-negative. All cases were included in a previous study. 24 Three samples of secondary lymphomatous effusions were derived from HIV-infected individuals and seven were derived from HIV-negative individuals.
Immunocytochemistry
All cases had been immunophenotyped as previously described. 20,22-24 Immunostaining was performed by using the avidin biotin peroxidase complex (ABC) method 25 or by the alkaline phosphatase–anti-alkaline phosphatase method. 26
The expression of p27Kip1 was investigated with the monoclonal antibody (mAb) Kip-1 (Transduction Laboratories, Lexington, KY). The proliferation index was assessed using the MIB-1 mAb (Immunotech, Marseille, France) directed against the Ki-67 nuclear proliferation antigen. Cellular cyclin D1 was assessed by using mAb AM29 (Zymed Laboratories, San Francisco, CA). All these antigens were tested on paraffin-embedded sections from cell blocks with a previous step of antigen retrieval. For p27Kip1 assessment, sections were treated twice in a microwave oven for 5 minutes in citrate buffer (pH 6); for Ki-67, sections were first treated with trypsin (Sigma Chemical Co., St. Louis, MO) (0.33 mg/ml) for 1 minute and then twice for 5 minutes in citrate buffer (pH 6) in a microwave oven at 650 W; for cellular cyclin D1, Bouin-fixed sections were treated with trypsin (Sigma Chemical Co.) (0.2 mg/ml) for 5 minutes whereas formalin-fixed sections were treated for 30 minutes in citrate buffer (pH 7) in a microwave oven at 250 W.
Immunocytochemical staining for p27Kip1and Ki-67 was performed by using the ABC method 25 (ABC-Elite kit, Vector, Burlingame, California), whereas immunocytochemical staining for cellular cyclin D1 was performed on an automated immunostainer (Ventana Medical Systems, Inc, Tucson, AZ) according to a modified version of the company’s protocols. Positive controls for p27Kip1, Ki-67 and cellular cyclin D1 were used to confirm the adequacy of the staining.
For comparative purposes cyclin D3 was tested on cytospin preparations from all PEL cell lines. Cyclin D3 was detected with the mAb DCS-22 (NeoMarkers, Inc., Fremont, California) and the ABC method. 25
HHV-8 v-cyclin was assessed in cytospin preparations using sheep polyclonal v-cyclin-specific antibodies (Bionostics Inc., Toronto, Canada). Cytospins were fixed in acetone-chloroform (1:1) solution for 5 minutes and stored at −80°C until use. Slides were then brought to room temperature; fixed in acetone for 5 minutes at room temperature; air dried; fixed in buffered 10% formalin for 10 minutes at room temperature; rinsed in PBS, pH 7.4; fixed in cold methanol at −20°C for 10 minutes; and then incubated overnight at 4°C with sheep anti-HHV-8 cyclin. After washing, cytospins were incubated with rabbit anti-sheep IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and then immunostained by using the ABC method 25 (ABC-Elite kit, Vector).
The percentage of cells showing positive nuclear staining for the different antigens was assessed in each case.
Western Blot Analysis
Cells were washed twice in ice-cold phosphate-buffered saline (PBS, pH 7.4), and lysed on ice for 30 minutes in a buffer containing 10 mmol/L Tris-HCl, pH 8.0; 1% Triton X-100; 150 mmol/L NaCl; 1.0 mmol/L EDTA; 1.0 mmol/L phenylmethylsulfonyl fluoride; and 10% glycerol. After centrifugation for 10 minutes at 13,000 rpm, the supernatant was collected and samples were diluted in Laemmli sample buffer containing final concentrations of 125 mmol/L Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 5% 2-mercaptoethanol, and 0.001% bromphenol blue. Furthermore, total cell lysates were also prepared by direct lysis of cell pellets with Laemmli sample buffer. After boiling for 5 minutes, total cell lysates and/or protein extracts were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose filters (Sartorius, Göttingen, Germany) which were then blocked by incubation with 5% non-fat dried milk dissolved in Tris-buffered saline–Tween (50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.5, 0.1% Tween-20) for 2 hours under shaking. Membranes were then incubated overnight at room temperature with p27Kip1 or cellular cyclin D1 antibodies or at 4°C with v-cyclin antibodies. After washing in Tris-buffered saline–Tween, filters were incubated with the appropriate secondary antibody, ie, peroxidase-conjugated goat anti-mouse immunoglobulin (Dako EnVision; Dako Corporation, Carpinteria, CA) or peroxidase-conjugated rabbit anti-sheep IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hour at room temperature and then developed using the enhanced chemiluminescence system (ECL; Amersham Pharmacia Biotech), following the manufacturer’s instructions.
Results
Immunocytochemical Evaluation of p27Kip1 and Ki-67 Expression in PEL
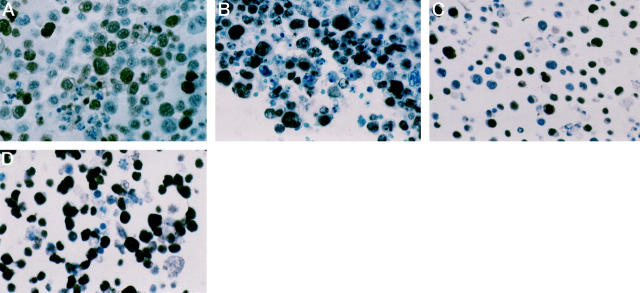
A total of 18 cases of PEL were immunostained for p27Kip1 and Ki-67. The results are summarized in Table 1 ▶ . Eighteen out of 18 cases (100%) showed expression of p27Kip1 (Table 1 ▶ , Figure 1 ▶ ). The cases consistently showed nuclear p27Kip1 staining of moderate-to-strong intensity in a large number of tumor cells (median, 55%; range, 30% to 90%). p27Kip1 expression in PELs occurred regardless of the generally high proliferative index of the neoplastic population, as assessed with the proliferation marker Ki-67 (median, 90%; range, 60% to 100%) (Table 1 ▶ , Figure 1 ▶ ).
Table 1.
Expression of p27Kip1, Ki-67, HHV-8 v-Cyclin, and Cellular Cyclin D1 in HHV-8 Positive Primary Effusion Lymphomas (PELs) and HHV-8 Negative Secondary Lymphomatous Effusions (SLE)
| Cell line/case no. | Diagnosis | p27Kip1 | Ki-67 | HHV-8 v-cyclin | Cellular cyclin D1 | |||
|---|---|---|---|---|---|---|---|---|
| ICC* | WB† | ICC* | ICC* | WB† | ICC† | WB† | ||
| CRO-AP/2 | PEL | 30 | + | 90 | 0 | + | − | − |
| CRO-AP/3 | PEL | 60 | + | 95 | 60 | + | − | − |
| CRO-AP/5 | PEL | 40 | + | 80 | 0 | + | − | − |
| HBL-6 | PEL | 60 | + | 90 | 0 | + | − | − |
| BC-1 | PEL | 30 | + | 100 | 60 | + | − | − |
| BC-2 | PEL | 35 | + | 90 | 70 | + | − | − |
| BC-3 | PEL | 35 | + | 90 | 80 | + | − | − |
| BCBL-1 | PEL | 60 | + | 90 | 0 | + | − | − |
| BCP-1 | PEL | 50 | + | 90 | 60 | + | − | − |
| 1 | PEL | 90 | ND | 90 | ND | ND | − | ND |
| 2 | PEL | 80 | ND | 80 | 90 | ND | − | ND |
| 3 | PEL | 80 | ND | 60 | 80 | ND | − | ND |
| 4 | PEL | 60 | ND | 90 | 90 | ND | − | ND |
| 5 | PEL | 90 | ND | 90 | 90 | ND | − | ND |
| 6 | PEL | 40 | ND | 60 | 90 | ND | − | ND |
| 7 | PEL | 80 | ND | 90 | ND | ND | − | ND |
| 8 | PEL | 30 | ND | 90 | ND | ND | − | ND |
| 9 | PEL | 40 | ND | 60 | ND | ND | − | ND |
| 10 | SLE/Burkitt | 1 | ND | 80 | ND | ND | − | ND |
| 11 | SLE/B-DLCL | 1 | ND | 30 | ND | ND | − | ND |
| 12 | SLE/B-DLCL | 1 | ND | 90 | ND | ND | − | ND |
| 13 | SLE/Burkitt | 0 | ND | 90 | ND | ND | − | ND |
| 14 | SLE/B-DLCL | 10 | ND | 90 | ND | ND | − | ND |
| 15 | SLE/B-DLCL | 0 | ND | 10 | ND | ND | − | ND |
| 16 | SLE/B-DLCL | 90 | ND | 1 | ND | ND | − | ND |
| 17 | SLE/B-DLCL | 0 | ND | 90 | ND | ND | − | ND |
| 18 | SLE/B-DLCL | 0 | ND | 0 | ND | ND | − | ND |
| 19 | SLE/B-DLCL | 30 | ND | 50 | ND | ND | − | ND |
ICC, immunocytochemistry; WB, Western blot; PEL, primary effusion lymphoma; B-DLCL, B-lineage diffuse large cell lymphoma; ND, not done. All but two (BC-3 and BCP-1) PEL cell lines were derived from HIV-1 seropositive patients; cases 1 to 8 and cases 10 to 12 were from HIV-1 seropositive patients.
* Percentage of positive tumor cells. †+, positive; −, negative.
Figure 1.
p27Kip1 and Ki-67 expression in PEL cells. In a case of PEL (case 3) p27Kip1 is expressed by a large number of tumor cells (A), despite their high proliferation rate, as revealed by Ki-67 expression (B). In the BCP-1 PEL cell line, p27Kip1 (C) and Ki-67 (D) positively-stained tumor cells are numerous. Sections from cell blocks, avidin-biotin-peroxidase complex immunostaining, hematoxylin counterstain, ×250.
Immunocytochemical Evaluation of Cellular Cyclin D1 and HHV-8 v-Cyclin in PEL
All 18 cases of PEL were immunostained for cellular cyclin D1, whereas immunostaining for HHV-8 v-cyclin was performed in 14 PEL samples for which cytospin preparations were available.
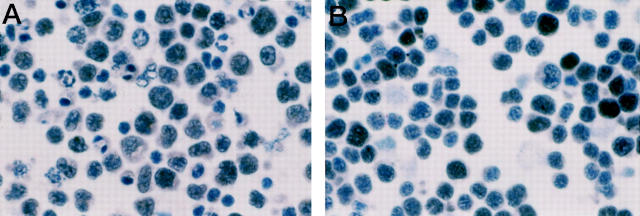
Cellular cyclin D1 scored consistently negative in all PEL samples investigated. In contrast, cyclin D3 scored positive in all PEL cell lines. Nuclear staining for cyclin D3 was observed in a large number of tumor cells (median, 70%; range, 20% to 90%). The intensity of staining was moderate to strong among the cells. HHV-8 v-cyclin was found to be expressed in 10 out of 14 samples of PEL (five clinical samples and five cell lines) (Figure 2) ▶ . Nuclear staining for HHV-8 v-cyclin was detected in a large number of tumor cells (median, 80%; range, 60% to 90%); the intensity of the staining was moderate to weak among the cells.
Figure 2.
HHV-8 v-cyclin expression in PEL cells. HHV-8 v-cyclin is expressed by the majority of tumor cells in a case of PEL (case 4) (A) and in the BC-3 PEL cell line (B). Cytospin preparations, avidin-biotin-peroxidase complex immunostaining, hematoxylin counterstain, ×250.
Western Blot Analysis of p27Kip1, Cellular Cyclin D1, and HHV-8 v-Cyclin in PEL Cell Lines
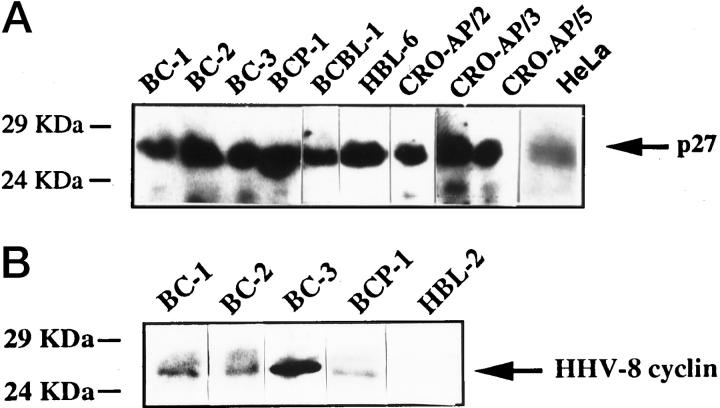
Western blot analysis of p27Kip1, cellular cyclin D1, and HHV-8 v-cyclin was performed in the PEL cell lines (Table 1) ▶ . All PEL cell lines investigated by this technique scored positive for the presence of p27Kip1 (Table 1 ▶ and Figure 3 ▶ ). Conversely, cellular cyclin D1 was not detected in any of the samples tested (Table 1) ▶ . Nine out of nine PEL cell lines expressed HHV-8 v-cyclin (Table 1 ▶ and see Figure 3 ▶ for representative results). The signal intensity of HHV-8 v-cyclin was variable among different PEL cell lines, and was higher in BC-1, BC-2, BC-3, BCP-1, and BCBL-1, whereas was weaker in the remaining four cell lines.
Figure 3.
Western blot analysis of p27Kip1 and HHV-8 v-cyclin in PEL cell lines. A: All PEL cell lines investigated by Western blot analysis score positive for the presence of a p27Kip1-specific signal indicated by the arrow. HeLa cell lysate was used as positive control. B: Western blot analysis of HHV-8 v-cyclin in representative PEL cell lines. All PEL samples included in the figure display a positive signal for HHV-8 v-cyclin indicated by the arrow. The signal is between 24 and 29 kd. The HHV-8-negative HBL-2 cell line derived from Burkitt’s lymphoma was used as negative control.
Immunocytochemical Evaluation of p27Kip1, Ki-67, and Cellular Cyclin D1 Expression in HHV-8-Negative Secondary Lymphomatous Effusions
A total of ten cases of HHV-8-negative secondary lymphomatous effusions were immunostained for p27Kip1, Ki-67, and cellular cyclin D1. The results are summarized in Table 1 ▶ . Overall, secondary lymphomatous effusions displayed an inverse correlation between the percentage of tumor cells expressing p27Kip1 and that of tumor cells expressing the proliferation marker Ki-67 (Table 1) ▶ . In all cases, reactive small lymphocytes admixed with the tumor cells exhibited strong nuclear positivity for p27Kip1. Cellular cyclin D1 expression was completely negative in all 10 cases tested (Table 1) ▶ .
Discussion
In this study we analyzed a relatively large panel of PEL clinical samples and cell lines for the expression status of p27Kip1 as well as for the relationship between p27Kip1 positivity, the lymphoma proliferation index and the expression of cellular cyclin D1 and HHV-8 v-cyclin. We report that PELs consistently show expression of p27Kip1 despite a high proliferation rate, thus suggesting a failure of p27Kip1 to inhibit the cell cycle. The abrogation of the inverse relationship between p27Kip1 expression and proliferation in PELs is associated with tumor cell expression of HHV-8 v-cyclin, whereas cellular cyclin D1 is absent in this lymphoma. The co-existence of p27Kip1 expression and high proliferative index is a selective feature of PEL among lymphomas involving the serous body cavities.
Our results unequivocally define that PEL is characterized by the co-existence of p27Kip1 overexpression and high proliferation index, as detected by the Ki-67 nuclear antigen. The consistent association between p27Kip1 overexpression and high proliferation index may be considered a peculiarity of PEL and is otherwise exceptional in other lymphoid tissues. In fact, consistent with the role of p27Kip1 as a negative regulator of cell cycle progression, reactive lymphoid tissues and most tissue-based B-cell NHL analyzed to date, with the exception of a small subset of B-DLCL and BL cases, 13,27 display an inverse relation between p27Kip1 expression and growth fraction measured by Ki-67 staining. 13-15 Co-expression of p27Kip1 and Ki-67 is a selective feature of PEL also when considering lymphomas involving the serous body cavities, because HHV-8-negative secondary lymphomatous effusions generally show very weak p27Kip1 expression and high proliferative rate.
The precise mechanism for the co-existence of p27Kip1 expression and high proliferation index in PEL is not formally clarified. However, a potential explanation may lie in differences in the biochemical properties of HHV-8 v-cyclin as opposed to cellular D-type cyclins. In fact, the interaction between HHV-8 v-cyclin and p27Kip1 in PEL seems to differ in some respects from that observed between cellular D-type cyclins and p27Kip1 in other B-cell lymphomas. 15,27 Experimental studies indicate that the HHV-8 v-cyclin forms complexes with CDK 28,29 which are able to exert functional activity on downstream targets also shared by CDK/cyclin D complexes. 28,30 Unlike CDK/cyclin D complexes, however, CDK/v-cyclin activity is resistant to inhibition by CDK inhibitors, including p27Kip1. 31 Overall, these observations suggest a model in which PEL cells express high levels of p27Kip1which, however, is unable to inactivate HHV-8 v-cyclin and, therefore, is unable to exert its physiological function of negative regulator of cell cycle progression.
The model of p27Kip1/cyclin interactions proposed for PEL differs significantly from that of other B-cell NHL in which cyclin D plays a major pathogenetic role. 15,27 In mantle cell lymphoma, the t(11;14) translocation leads to overexpression of cyclin D1, which in turn sequesters p27Kip1, thus causing its functional inactivation. 15,32 Conversely, PEL escape from p27Kip1 mediated control through substitution of the p27Kip1 physiological target cyclin D with the HHV-8 encoded v-cyclin that is resistant to p27Kip1 activity. 31 The differences in p27Kip1/cyclin interactions may also explain the different expression pattern of p27Kip1 in PEL as opposed to mantle cell lymphoma. In fact, the p27Kip1 protein is immunologically undetectable when bound to cyclin D1, 15,32 thus explaining the abrogation of its expression in mantle cell lymphoma but not in PEL.
Several issues remain to be clarified with respect to the interactions between p27Kip1 and HHV-8 v-cyclin. For example, in PEL cell lines, the CDK/v-cyclin complexes phosphorylate p27Kip1 on a C-terminal threonine that is implicated in destabilization of this CDK inhibitor. 33 Because our results show that p27Kip1 is expressed at high levels in PEL cells in vivo, future studies need to address the detailed balance between p27Kip1 production and degradation in HHV-8-positive lymphomas, resulting in p27Kip1 overexpression despite destabilization of the molecule.
Footnotes
Address reprint requests to Prof. Antonino Carbone, Division of Pathology, Centro di Riferimento Oncologico, IRCCS, Istituto Nazionale Tumori, Via Pedemontana Occidentale, Aviano I-33081, Italy. E-mail: acarbone@ets.it.
Supported in part by ISS, II Programma nazionale di ricerca sull’AIDS —Progetto Patologia clinica e terapia dell’AIDS, Rome, Italy (to A. C. and G. G.). P. J. B. was supported in part by the Public Health Service grant R01CA75535 from the National Institutes of Health, Department of Health and Human Services. D. B. was supported by a fellowship from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy.
References
- 1.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM: Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body cavity-based lymphomas. N Engl J Med 1995, 332:1186-1191 [DOI] [PubMed] [Google Scholar]
- 2.Carbone A, Gaidano G: HHV-8 positive body cavity-based lymphoma: a novel lymphoma entity. Br J Haematol 1997, 97:515-522 [DOI] [PubMed] [Google Scholar]
- 3.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS: Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci USA 1996, 93:14862-14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshoff C: Kaposi’s sarcoma associated herpesvirus. Cancer Surv 1998, 33:157-190 [DOI] [PubMed] [Google Scholar]
- 5.Davis MA, Stürzl M, Blasig C, Schreier A, Guo H-G, Reitz M, Opalenik SR, Browning PJ: Expression of human herpesvirus 8-encoded cyclin D in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst 1997, 24:1868-1874 [DOI] [PubMed] [Google Scholar]
- 6.Grana X, Reddy EP: Cell cycle control in mammalian cells: role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11:211-219 [PubMed] [Google Scholar]
- 7.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 10.Reynisdóttir I, Polyak K, Iavarone A, Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 1995, 9:1831-1845 [DOI] [PubMed] [Google Scholar]
- 11.Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A: Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ 1996, 7:135-146 [PubMed] [Google Scholar]
- 12.Cheng M, Sexl V, Sherr CJ, Roussel MF: Assembly of cyclin D-dependent kinase and titration of p27/KIP1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc Natl Acad Sci USA 1998, 95:1091-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-Beato M, Sáez AI, Martínez-Montero JC, Mateo MS, Sánchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin dependent kinase inhibitor p27KIP1 in lymphoid tissue. p27KIP1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 14.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G: Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas. Prognostic implications. Blood 1998, 92:770-777 [PubMed] [Google Scholar]
- 15.Quintanilla-Martinez L, Thieblemont C, Fend F, Kumar S, Pinyol M, Campo E, Jaffe ES, Raffeld M: Mantle cell lymphomas lack expression of p27 Kip1 a cyclin-dependent kinase inhibitor. Am J Pathol 1998, 153:175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y: In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 1995, 86:2708-2714 [PubMed] [Google Scholar]
- 17.Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E: Establishment and characterization of primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 1996, 88:2648-2654 [PubMed] [Google Scholar]
- 18.Gaidano G, Cechova K, Chang Y, Moore PS, Knowles DM, Dalla-Favera R: Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia 1996, 10:1237-1240 [PubMed] [Google Scholar]
- 19.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D: Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nature Med 1996, 2:342-346 [DOI] [PubMed] [Google Scholar]
- 20.Carbone A, Cilia AM, Gloghini A, Canzonieri V, Pastore C, Todesco M, Cozzi M, Perin T, Volpe R, Pinto A, Gaidano G: Establishment of HHV-8 positive and HHV-8 negative lymphoma cell lines from primary lymphomatous effusions. Int J Cancer 1997, 73:562-569 [DOI] [PubMed] [Google Scholar]
- 21.Boshoff C, Gao S-J, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, Chang Y: Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 1998, 91:1671-1679 [PubMed] [Google Scholar]
- 22.Carbone A, Carbone A, Cilia AM, Gloghini A, Capello D, Todesco M, Quattrone S, Volpe R, Gaidano G: Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harbouring human herpesvirus type-8. Br J Haematol 1998, 102:1081-1089 [DOI] [PubMed] [Google Scholar]
- 23.Drexler HG, Uphoff CC, Gaidano G, Carbone A: Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas). Leukemia 1998, 12:1507-1517 [DOI] [PubMed] [Google Scholar]
- 24.Carbone A, Gloghini A, Vaccher E, Zagonel V, Pastore C, Dalla Palma P, Branz F, Saglio G, Volpe R, Tirelli U, Gaidano G: Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br J Haematol 1996, 94:533-543 [DOI] [PubMed] [Google Scholar]
- 25.Hsu S-M, Raine L, Fanger H: A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol 1981, 75:734-738 [DOI] [PubMed] [Google Scholar]
- 26.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, Macdonald S, Pulford KAF, Stein H, Mason DY: Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal antialkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Beato M, Camacho FI, Martínez-Montero JC, Sáez AI, Villuendas R, Sánchez-Verde L, García JF, Piris MA: Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 1999, 94:765-772 [PubMed] [Google Scholar]
- 28.Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S: The cyclin encoded by Kaposi’s sarcoma associated herpesvirus (KSHV) stimulates cdk6 to phosphorilate the retinoblastoma protein and histone H1. J Virol 1997, 71:4193-4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU: Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol 1997, 1991, 71:1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y, Moore PS, Talbot SJ, Boshoff C, Zarkowska T, Godden-Kent D, Paterson H, Weiss RA, Mittnacht S: Cyclin encoded by KS herpesvirus. Nature 1996, 382:410. [DOI] [PubMed] [Google Scholar]
- 31.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N: Herpes viral cyclin/cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 1997, 390:184-187 [DOI] [PubMed] [Google Scholar]
- 32.Quintanilla-Martinez L, Davies-Hill T, Sorbara L, Jaffe ES, Raffeld M: Loss of detectable p27 protein expression in mantle cell lymphomas (MCL) is a result of its sequestration by cyclin D1: implication for pathogenesis. Blood 1999, 92(suppl 1):314a [Google Scholar]
- 33.Ellis M, Chew YP, Fallis L, Freddersdorf S, Boshoff C, Weiss RA, Lu X, Mittnacht S: Degradation of p27Kip1 cdk inhibitor triggered by Kaposi’s sarcoma virus cyclin-cdk6 complex. EMBO J 1999, 18:644-653 [DOI] [PMC free article] [PubMed] [Google Scholar]