Abstract
Monocyte chemoattractant protein-1 is one of the major C-C chemokines that has been implicated in liver injury. The C-C chemokine receptor, CCR2, has been identified as the primary receptor that mediates monocyte chemoattractant protein-1 (MCP-1) responses in the mouse. Accordingly, the present study addressed the role of CCR2 in mice acutely challenged with acetaminophen (APAP). Mice genetically deficient in CCR2 (CCR2−/−) and their wild-type counterparts (CCR2+/+) were fasted for 10 hours before receiving an intraperitoneal injection of APAP (300 mg/kg). Liver and serum samples were removed from both groups of mice before and at 24 and 48 hours post APAP. Significantly elevated levels of MCP-1 were detected in liver samples from CCR2+/+ and CCR2−/− mice at 24 hours post-APAP. Although CCR2+/+ mice exhibited no liver injury at any time after receiving APAP, CCR2−/− mice exhibited marked evidence of necrotic and TUNEL-positive cells in the liver, particularly at 24 hours post-APAP. Enzyme-linked immunosorbent assay analysis of liver homogenates from both groups of mice at the 24 hours time point revealed that liver tissue from CCR2−/− mice contained significantly greater amounts of immunoreactive IFN-γ and TNF-α. The in vivo immunoneutralization of IFN-γ or TNF-α significantly attenuated APAP-induced liver injury in CCR2−/− mice and increased hepatic IL-13 levels. Taken together, these findings demonstrate that CCR2 expression in the liver provides a hepatoprotective effect through its regulation of cytokine generation during APAP challenge.
Following acute hepatic injury or trauma, the repair process in the liver requires a complex sequence of events that are orchestrated by cytokines. 1 For example, liver regeneration involves cytokines such as tumor necrosis factor-α (TNF-α) 2,3 and interleukin (IL)-6, 4 and chemokines such as macrophage inflammatory protein-2, epithelial neutrophil activating factor-78 (ENA-78), and IL-8. 5-7 The presence of anti-inflammatory cytokines such as IL-10, IL-4, and IL-13 has also been shown to facilitate the recovery of the liver from acute injury 8 Nevertheless, cytokines may also exert deleterious effects in the traumatized liver. Numerous reports have shown that interferon-γ (IFN-γ) is the injurious factor in a number of acute liver injury settings. 9-12 In addition, acute liver injury due to an infectious 12 or chemical 13 insult may result from excessive TNF-α synthesis. Increased chemokine synthesis in the liver following ischemic injury 14 or viral infection 15 has also been shown to contribute to hepatic injury. Thus, liver injury and recovery appears to depend on the balanced generation of cytokines that promote hepatocyte mitogenesis but limit hepatocyte necrosis 16 or apoptosis. 17
At present, little is known about what factor or factors are responsible for maintaining the balance between regenerative or protective and destructive cytokines in the liver. We hypothesized that monocyte chemoattractant protein-1 (MCP-1) via its receptor, C-C chemokine receptor-2 (CCR2), 18 may be an important regulator of cytokine homeostasis within the liver. This hypothesis was based on previous findings that MCP-1 directly enhances the synthesis of IL-4 by T cells 19,20 and inhibits the ability of these cells to generate IFN-γ. 20 In other recent studies, the immunoneutralization of MCP-1 in endotoxin-challenged mice significantly augmented circulating and hepatic levels of IL-12 and TNF-α. In contrast, the administration of recombinant murine MCP-1 protected endotoxin-challenged mice, and this effect was associated with decreased serum levels of IL-12 and TNF-α. 21 Further support for an immunoregulatory role for MCP-1 in the liver was derived from other observations that MCP-1 and CCR2 are constitutively expressed by immune and nonimmune cells 22-24 in the liver. 25 Hepatic levels of MCP-1 are also greatly increased during acute liver inflammation. 26-28 In the present study, experiments performed in mice expressing CCR2 (ie, CCR2+/+) and mice lacking CCR2 due to gene targeting (ie, knockout or CCR2−/− mice) 29 revealed that CCR2−/− mice were markedly more sensitive to the hepatotoxic effects of acetaminophen (APAP) compared with CCR2+/+ mice. The exacerbated apoptotic and necrotic liver injury observed in CCR2−/− mice was a consequence of hepatic production of IFN-γ and TNF-α that followed APAP challenge.
Materials and Methods
APAP Model
Breeding pairs of CCR2+/+ and CCR2−/− mice were kindly provided by Dr. Israel Charo (Gladstone Institute, University of California San Francisco). A breeding colony containing both mouse genotypes was maintained under specific-pathogen-free conditions in the University Laboratory Animal Medicine facility (University of Michigan Medical School), and prior approval for mouse usage was obtained from University Laboratory Animal Medicine. In all experiments, female CCR2+/+ and CCR2−/− mice (C57Bl/6 × 129sv/J; 6–8 weeks of age) were allowed free access to water alone for 10 hours before an intraperitoneal challenge with 300 mg/kg of APAP. 6 Fresh suspensions of APAP (Sigma Chemical Company, St. Louis, MO) were made immediately before each experiment by dissolving a powdered preparation of APAP in phosphate-buffered saline (PBS) warmed to 40°C.
Generation of Neutralizing Antibodies
Antisera containing polyclonal antibodies against IFN-γ and TNF-α were generated in multiple-site-immunized New Zealand rabbits using a well-established protocol. 30 The specificity of each antibody was screened before their use in an experiment or enzyme-linked immunosorbent assay (ELISA), and all antibodies were found to lack cross-reactivity with other chemokines and cytokines. In passive immunoneutralization experiments, each mouse was injected with either 0.5 ml of pre-immune rabbit serum or an equivalent amount of anti-IFN-γ or anti-TNF-α immune serum approximately 2 hours before APAP challenge. This volume of immune serum has been previously shown to significantly attenuate systemic levels of the targeted cytokine or chemokine for approximately 36 hours. 31
Cytokine and Chemokine ELISA
Immunoreactive levels of MCP-1, IFN-γ, TNF-α, and IL-13 were measured in cultured cell supernatants and liver homogenates using a modified double-ligand ELISA procedure as described in detail elsewhere. 30 Before each ELISA, snap-frozen liver samples were thawed on ice, weighed, and homogenized in a solution containing 2 mg of protease inhibitor (Complete; Boehringer Mannheim, Indianapolis, IN) per milliliter of normal saline. Previous studies in this laboratory have shown that Complete does not interfere with any of the chemokine ELISAs. 30 Cell-free supernatants from the liver homogenates were loaded in duplicate into 96-well microtiter plates coated with the appropriate capture antibody and blocked with 2% bovine serum albumin in PBS. Each ELISA consistently detected concentrations of cytokines or chemokines below 10 pg/ml, and the specificity of the polyclonal detection and capture antibodies was confirmed before its use in an ELISA. Cytokine and chemokines levels in liver homogenates were normalized to the weight of the liver sample.
Serum Alanine Aminotransferase (ALT) Measurement
Acute hepatocellular injury results in elevated levels of circulating ALT. Serum levels of ALT were determined before and at 24 and 48 hours after mice were challenged with APAP by Clinical Pathology at the University of Michigan Medical School (Ann Arbor, MI) using standardized techniques.
Histological and TUNEL Analysis
Liver tissues were fixed in 4% paraformaldehyde for 24 hours before routine histological processing. Five-micron liver sections were placed on standard microscope slides, and slides were stained with hematoxylin and eosin (H&E) or subjected to the TUNEL method. Detailed histological grading of liver necrosis and hemorrhage was determined from H&E-stained slides before and at 24 hours after APAP challenge. An ApopTag Apoptosis Detection kit (Intergen Co., Purchase, NY) was used according to the manufacturer’s specifications to selectively detect apoptotic cells within liver sections before and at 24 hours after APAP challenge.
Statistical Analysis
Results are expressed as means ± SE of 5 to 8 mice per group, and analysis of variance and Dunnett’s test were used to detect significant differences between means. All statistical calculations were performed using GraphPad Prism 2.0 computer software (San Diego, CA), and a P value ≤0.05 was considered significant.
Results
Hepatic Levels of MCP-1 Were Significantly Increased in CCR2+/+ and CCR2−/− Mice Challenged with 300 mg/kg APAP
Liver samples were removed from CCR2+/+ and CCR2−/− mice before and at 24 hours after APAP challenge for the measurement of immunoreactive levels of MCP-1 by specific ELISA. Constitutive MCP-1 levels were measured in both groups of mice before APAP (9.0 ± 2.3 vs. 7.5 ± 2.8 ng/g liver tissue; CCR2+/+ vs. CCR2−/−). At 24 hours post-APAP challenge, hepatic levels of MCP-1 were significantly increased in CCR2+/+ and CCR2−/− mice challenged with APAP. However, immunoreactive levels of MCP-1 in CCR2+/+ were 47 ± 12 ng/g liver tissue, whereas MCP-1 levels in CCR2−/− mice were 341 ± 69 ng/g liver tissue. These findings suggested that the hepatic generation of MCP-1 was significantly increased after an in vivo APAP challenge in both groups of mice. The observation that immunoreactive MCP-1 levels were markedly greater in liver tissues from CCR2−/− mice compared with CCR2+/+ mice was consistent with previous studies showing similar disparities in MCP-1 levels. 32,33 Nevertheless, CCR2−/− mice completely lack all of the MCP-1-induced effects observed in CCR2+/+ mice. 29,34
Enhanced Hepatotoxic Effects of APAP in CCR2−/− Mice
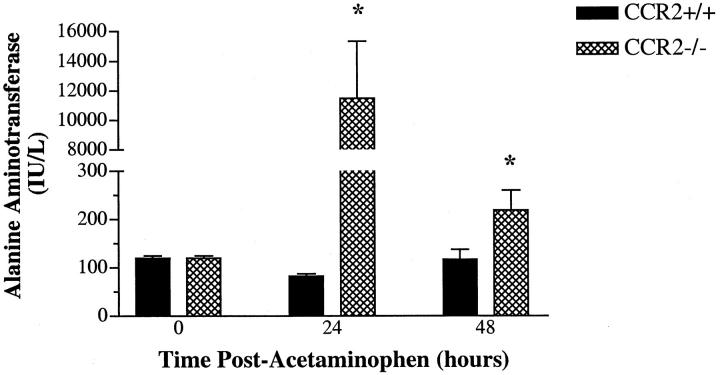
Changes in serum ALT levels before and at 24 and 48 hours after APAP challenge in CCR2+/+ and CCR2−/− mice are illustrated in Figure 1 ▶ . The intraperitoneal injection of 300 mg/kg of APAP into fasted CCR2+/+ mice did not augment circulating ALT above levels measured in CCR2+/+ mice before the APAP challenge. In contrast, the CCR2−/− mice exhibited a significant increase in serum ALT levels at 24 and 48 hours post-APAP challenge. Specifically, a 120-fold increase in ALT levels above baseline was observed in CCR2−/− mice at 24 hours post-APAP (Figure 1) ▶ . These data demonstrated that CCR2−/− mice were markedly more susceptible to the hepatotoxic effects of APAP than CCR2+/+ mice and subsequent experiments were designed to address the mechanism(s) responsible for this difference.
Figure 1.
Changes in serum levels of alanine aminotransferase (ALT) in C-C chemokine receptor-2 wild-type (CCR2+/+) and knockout (CCR2−/−) mice before and at 24 and 48 hours after acetaminophen (APAP) challenge. CCR2+/+ and CCR2−/− mice were fasted for 10 hours before an intraperitoneal injection with 300 mg/kg of APAP. Data show mean ± SE from 5 mice at each time point. *P ≤ 0.05 compared with control mice.
Increased Necrosis and/or Apoptosis in CCR2−/− Mice
The histological appearance of liver tissues from CCR2+/+ and CCR2−/− mice was similar immediately before APAP challenge (Figure 2A ▶ , CCR2+/+ liver shown). At 24 hours after challenge with 300 mg/kg of APAP, liver samples from CCR2+/+ showed little evidence of hepatic injury (Figure 2B) ▶ . In contrast, CCR2−/− clearly showed profound centrilobular hepatic necrosis and hemorrhagic injury (Figure 2C) ▶ . Although widely recognized that APAP induces hepatic necrosis, this drug also promotes the apoptosis of hepatocytes. 35 Recent studies have demonstrated that the apoptosis of hepatocytes contributes to the overall hepatic injury associated with APAP toxicity. 36 In the present study, whole liver sections from CCR2+/+ and CCR2−/− mice challenged with 300 mg/kg of APAP were subjected to histological analysis for the presence of TUNEL-positive liver cells. As shown in Figure 3C ▶ , no TUNEL-positive liver cells were observed in liver sections from CCR2+/+ mice at 24 hours after APAP challenge. In contrast, TUNEL-positive liver cells were abundant in CCR2−/− mice at this time (Figure 3D) ▶ . The TUNEL-positive cells observed in CCR2−/− mice were localized predominantly around central veins, corresponding to the zone of the liver that is most susceptible to the toxic effects of APAP. 37 Thus, these histological findings demonstrated that the lack of CCR2 predisposed the liver to APAP-induced liver cell necrosis and/or apoptosis.
Figure 2.
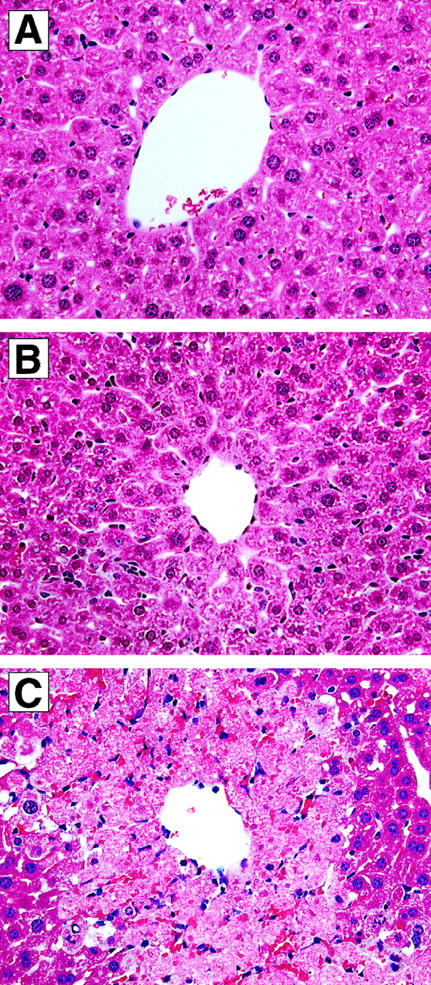
Histological appearance of liver samples from untreated (A) and APAP-challenged (300 mg/kg) mice (B and C). The liver sample in A was removed from a CCR2+/+ mice immediately before APAP challenge, and no evidence of centrilobular hepatic injury was evident in any liver section examined. Similarly, liver sections from CCR2−/− mice lacked any evidence of liver injury at this time (not shown). At 24 hours after APAP challenge, liver sections from CCR2+/+ mice (B) exhibited a minor amount of centrilobular injury, whereas liver sections from CCR2−/− mice contained markedly greater necrotic injury around central veins (C). Original magnifications, ×400.
Figure 3.
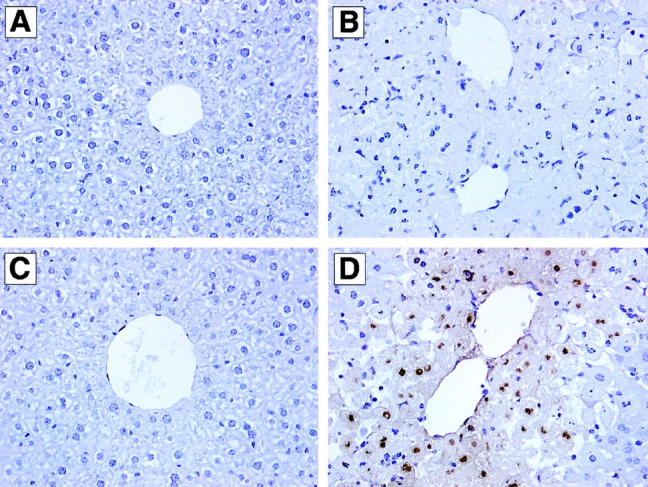
TUNEL analysis in liver samples from CCR2+/+ (A and C) and CCR2−/− (B and D) at 24 hours after APAP challenge. CCR2+/+ and CCR2−/− mice were fasted for 10 hours before an intraperitoneal injection with 300 mg/kg of APAP. Whereas no TUNEL-positive cells were observed in CCR2+/+ at 24 hours after APAP challenge (C), TUNEL-positive cells were prevalent around central veins in liver samples from CCR2−/− mice (D) at this time. The appropriate negative staining control for CCR2+/+ and CCR2−/− mice are shown in A and B, respectively. Original magnifications, ×400.
Hepatic Levels of IFN-γ and TNF-α, but Not IL-13, Were Significantly Increased in CCR2−/− Mice after APAP Challenge
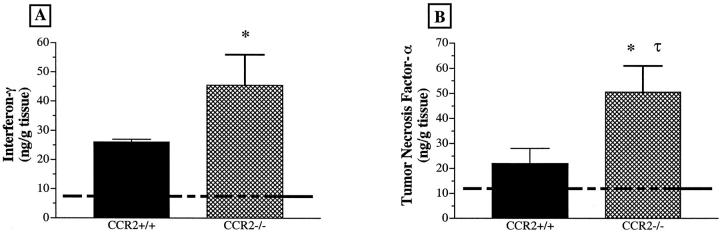
To examine the role of CCR2 during APAP challenge, we next examined the hepatic cytokine response in CCR2+/+ and CCR2−/− mice challenged with 300 mg/kg of APAP. Accordingly, we focused on changes in hepatic IFN-γ, TNF-α, and IL-13 in both groups of mice, as numerous studies have demonstrated that the IFN-γ and TNF-α are potent mediators of acute liver injury. 9,12 IL-13 has recently been shown to reduce the hepatic inflammatory response during endotoxemia, 8 and this cytokine is produced by hepatocytes in response to inflammatory stimuli. 22 In the present experiment, we examined whether the levels of these two cytokines were altered during APAP challenge in CCR2+/+ and CCR2−/− mice. Immunoreactive levels of IFN-γ and TNF-α in liver samples are illustrated in Figure 4, A and B ▶ , respectively. Cytokine levels in CCR2+/+ and CCR2−/− mice were similar before APAP challenge (dashed line). Twenty-four hours after a challenge with 300 mg/kg of APAP, significantly more IFN-γ and TNF-α was detected in liver samples from CCR2−/− mice compared with the CCR2+/+ group (Figure 4B) ▶ .
Figure 4.
Hepatic levels of immunoreactive IFN-γ (A) and TNF-α (B) before and at 24 hours after APAP challenge in CCR2+/+ and CCR2−/− mice. CCR2+/+ and CCR2−/− mice were fasted for 10 hours before an intraperitoneal injection with 300 mg/kg of APAP. The dashed lines represent the mean IFN-γ and TNF-α levels in hepatic samples from unchallenged CCR2+/+ mice, and the baseline cytokine levels in CCR2+/+ mice did not differ from cytokine levels in unchallenged CCR2−/− (not shown). IFN-γ and TNF-α levels were determined using specific ELISAs. Data show mean ± SE from 5 mice at each time point. *P ≤ 0.05 compared with unchallenged mice. τ, P ≤ 0.05 compared with CCR2+/+ mice challenged with 300 mg/kg of APAP.
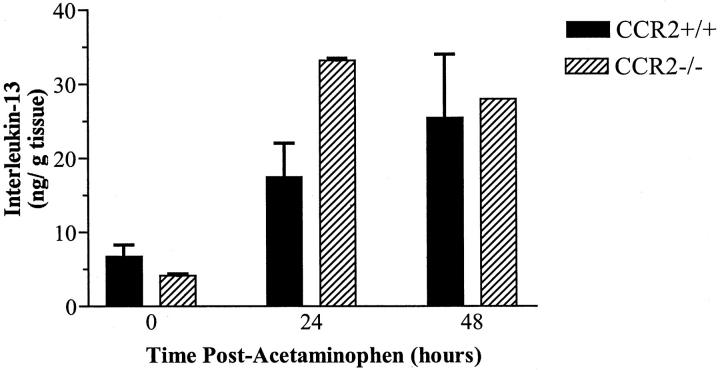
Changes in hepatic IL-13 levels are summarized in Figure 5 ▶ . Both groups of mice expressed similar amounts of IL-13 in the liver before APAP challenge. At 24 and 48 hours post-APAP, CCR2+/+ and CCR2−/− mice exhibited a similar four- to sixfold increases in hepatic IL-13 synthesis. No differences in IL-13 levels were observed between the two groups of mice at any time after APAP challenge. Thus, in contrast to CCR2+/+ mice, APAP challenge in CCR2−/− mice was associated with significant increases in hepatic levels of IFN-γ and TNF-α, but a concomitant significant increase in hepatic IL-13 was not observed.
Figure 5.
Hepatic levels of immunoreactive interleukin-13 before and at 24 and 48 hours after APAP challenge in C-C chemokine receptor-2 (CCR2) wild-type (+/+) and knockout (−/−) mice. CCR2+/+ and CCR2−/− mice were fasted for 10 hours before an intraperitoneal injection with 300 mg/kg of APAP. IL-13 levels were determined using specific ELISAs. Data show mean ± SE from 5 mice at each time point.
Immunoneutralization of IFN-γ or TNF-α Attenuated the Hepatotoxicity of APAP and Increased Hepatic IL-13 in CCR2−/− Mice
The impact of increased IFN-γ and TNF-α during APAP challenge in CCR2−/− mice was examined next. CCR2−/− mice received either polyclonal anti-IFN-γ or anti-TNF-α antiserum approximately 2 hours before an intraperitoneal challenge with 300 mg/kg of APAP. Immunoneutralization of systemic IFN-γ levels significantly decreased serum levels of ALT in CCR2−/− mice (Figure 6A) ▶ . Similarly, the attenuation of TNF-α levels in CCR2−/− mice also significantly reduced serum ALT levels. The immunoneutralization of IFN-γ or TNF-α during APAP challenge in CCR2−/− mice was also associated with marked increases in IL-13 in the liver (Figure 6B) ▶ . Anti-IFN-γ-treated CCR2−/− mice exhibited hepatic levels of IL-13 that were significantly greater than levels measured in normal CCR2−/− mice. In CCR2−/− mice that received anti-TNF-α antiserum, the hepatic levels of IL-13 were significantly greater than levels measured in normal CCR2−/− mice and control APAP-challenged CCR2−/− mice. These findings confirmed that the hepatic injury in CCR2−/− mice was related, in part, to the increased presence of IFN-γ and TNF-α in the liver.
Figure 6.
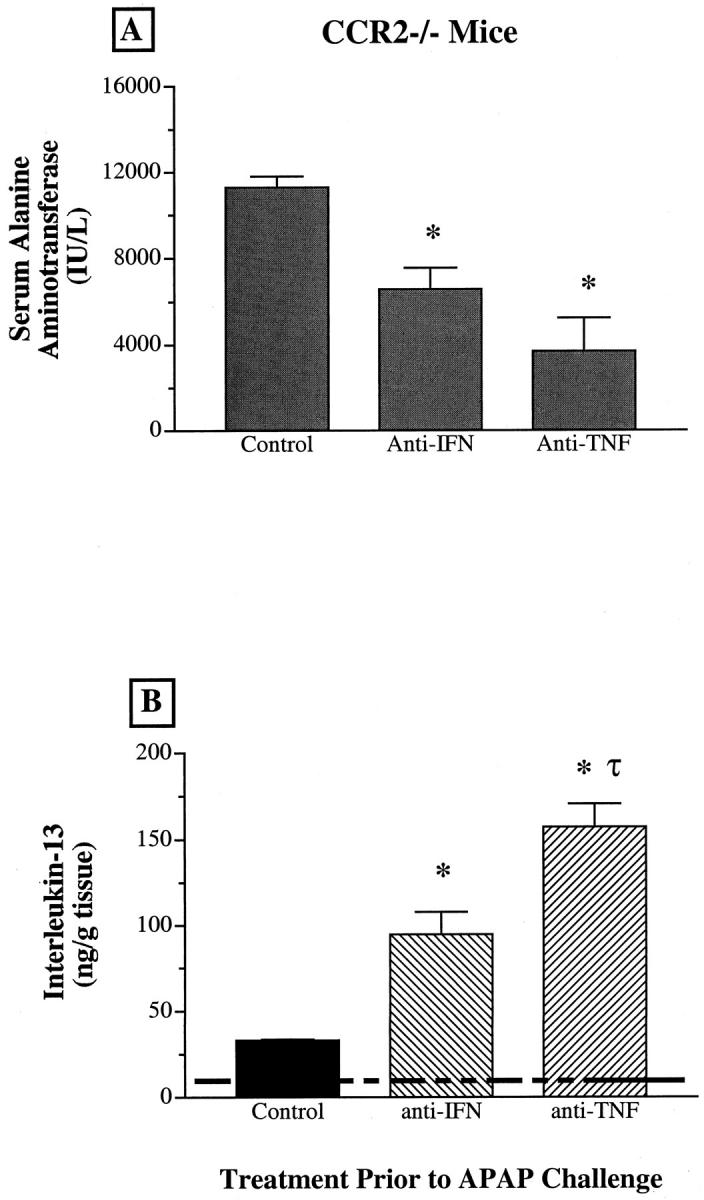
A: Changes in serum levels of alanine aminotransferase (ALT) in CCR2−/− mice before and at 24 hours after APAP challenge. B: Hepatic levels of immunoreactive IL-13 before and at 24 hours after APAP challenge in CCR2+/+ and CCR2−/− mice. CCR2−/− mice were fasted for 8 hours before receiving an intraperitoneal injection of normal rabbit serum, anti-IFN-γ, or anti-TNF-α antiserum. Two hours after the serum treatment, CCR2−/− were challenged with 300 mg/kg of APAP. Data show mean ± SE from 5 mice at each time point. *P ≤ 0.05 compared with APAP-challenged CCR2−/− mice that received preimmune serum. τ, P ≤ 0.05 compared with anti-IFN-γ-pretreated CCR2−/− mice challenged with 300 mg/kg of APAP.
Discussion
In the present study, we observed that CCR2, the receptor for MCP-1 in the mouse, 18 was necessary for the balanced production of pro- and anti-inflammatory cytokines in the APAP-challenged liver. Significant liver necrosis and/or apoptosis was observed in APAP-challenged CCR2−/− mice, whereas similarly challenged CCR2+/+ mice showed no evidence of liver injury. In addition, significant elevations in proinflammatory cytokines such as IFN-γ and TNF-α were observed in liver samples from CCR2−/− mice compared with CCR2+/+ mice. However, a concomitant significant increase in hepatic levels of IL-13 to compensate for increased levels of IFN-γ and TNF-α was not observed in CCR2−/− mice following APAP challenge. The toxic effect of acetaminophen in CCR2−/− mice was abolished when these mice received either anti-IFN-γ or anti-TNF-α antiserum before APAP challenge. In addition, CCR2−/− mice that received either antiserum exhibited significantly greater hepatic levels of IL-13. Taken together, these studies suggest that MCP-1 and CCR2 interactions in the liver are necessary for maintaining a balance in the cytokine production during acute injury by APAP.
Clinical 24,26 and experimental 38 studies have demonstrated that hepatic levels of MCP-1 are markedly enhanced during various types of liver injury. MCP-1 is a constitutive and induced byproduct of a number of liver resident cells including bile duct epithelial cells, stellate cells, 27 and hepatocytes. 23 At present, the role of MCP-1 in the liver has been examined only in terms of its ability to promote and maintain the leukocyte infiltrate during liver disease. 24,28,39 However, in the present study, we examined whether MCP-1 and CCR2 were also involved in the regulation of cytokine production during the hepatic response to acetaminophen challenge. Our impetus to examine the cytokine modulatory role of MCP-1 and CCR2 in the liver was derived from previous in vitro 19,20 and in vivo 21 studies demonstrating that MCP-1 possesses immunomodulatory and anti-inflammatory properties. The best example of this novel role for MCP-1 was demonstrated in an experimental model of sepsis characterized by overproduction of proinflammatory cytokines such as IL-12 and TNF-α, resulting in shock, multiorgan dysfunction, and death. Furthermore, it was shown in these previous studies that MCP-1 was a protective cytokine in endotoxin-challenged mice because of its ability to shift the systemic and organ-specific cytokine balance away from IL-12 and TNF-α in favor of IL-10 expression. 21 Recent studies suggest that a balance between pro- and anti-inflammatory cytokines is also necessary in the liver to prevent excessive injury during alcohol-induced, 40 lipopolysaccharide- and galactosamine-induced, 41 or Concanavalin A-induced 42 liver injury. Thus, the presence of MCP-1/CCR2 in the liver is necessary to maintain a balance between pro- and anti-inflammatory cytokines, thereby limiting the damaging effects of hepatotoxins such as APAP.
The injurious effect of IFN-γ 9,12 in the liver is well documented. In contrast, the role of TNF-α during acute liver injury is more controversial since studies have shown that this cytokine has no effect 43 or a deleterious effect 16,37,44 following an APAP challenge. In other models of toxic or surgically induced liver injury, the role of TNF-α appears to depend on its relative abundance in the liver 3,45 and on the TNF-α receptor that binds it. 46 In addition, it is known that chemical hepatotoxins promote the production of TNF-α, which in turn promotes the apoptosis of hepatocytes. 17 Conversely, IL-13 is an anti-inflammatory cytokine that exhibits hepatoprotective effects that are related to its inhibitory effect on the synthesis of pro-inflammatory cytokines. 8 Interestingly, it has been previously demonstrated that IL-13 selectively induces MCP-1 synthesis and secretion by human endothelial cells, 47 and it is conceivable that a similar IL-13-dependent effect is observed in the liver. However, the mechanisms through which MCP-1 and CCR2 balance the production of cytokines in the liver are currently unknown, but studies are underway to examine whether this is a direct or indirect effect.
In conclusion, the present study demonstrates that the presence of CCR2 in the liver is necessary to maintain a balance of pro- and anti-inflammatory cytokines following APAP challenge in the liver. Given the major cytokine regulatory role for MCP-1/CCR2 during APAP challenge, further investigation is warranted to determine the role of this ligand and its receptor in other types of chemical or infectious hepatic injury models.
Footnotes
Address reprint requests to Dr. Cory M. Hogaboam, Ph.D., Department of Pathology, 5214 Med. Sci. I, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor MI, 48109-0602. E-mail: hogaboam@path.med.umich.edu.
Supported by National Institutes of Health grants 1P50HL56402, 1P50HL60289, CA66180, HL35276, HL31963, and AI36302. K. J. S. was supported in part by the Medical Research Council of the United Kingdom and by Scottish Hospital Endowment Research Trust (1512).
References
- 1.Fausto N, Laird AD, Webber EM: Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995, 9:1527-1536 [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Kirillova I, Peschon JJ, Fausto N: Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 1997, 94:1441-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruccoleri A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI: Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatoxicity. Hepatology 1997, 25:133-141 [DOI] [PubMed] [Google Scholar]
- 4.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R: Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996, 274:1379-1383 [DOI] [PubMed] [Google Scholar]
- 5.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM: Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock 1998, 10:248-257 [DOI] [PubMed] [Google Scholar]
- 6.Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL: Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated liver injury. Gene Ther 1999, 6:573-584 [DOI] [PubMed] [Google Scholar]
- 7.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, Kunkel SL: Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J 1999, 13:1565-1574 [DOI] [PubMed] [Google Scholar]
- 8.Baumhofer JM, Beinhauer BG, Wang JE, Brandmeier H, Geissler K, Losert U, Philip R, Aversa G, Rogy MA: Gene transfer with IL-4 and IL-13 improves survival in lethal endotoxemia in the mouse and ameliorates peritoneal macrophages immune competence. Eur J Immunol 1998, 28:610-615 [DOI] [PubMed] [Google Scholar]
- 9.Cao Q, Batey R, Pang G, Russell A, Clancy R: IL-6, IFN-gamma and TNF-alpha production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol 1998, 76:542-549 [DOI] [PubMed] [Google Scholar]
- 10.Yokochi S, Ishiwata Y, Hashimoto H, Ninomiya F, Suzuki T: Hepatoprotective effect of propagermanium on Corynebacterium parvum and lipopolysaccharide-induced liver injury in mice. Scand J Immunol 1998, 48:183-191 [DOI] [PubMed] [Google Scholar]
- 11.Mihm S, Hutschenreiter A, Fayyazi A, Pingel S, Ramadori G: High inflammatory activity is associated with an increased amount of IFN-gamma transcripts in peripheral blood cells of patients with chronic hepatitis C virus infection. Med Microbiol Immunol (Berl) 1996, 185:95-102 [DOI] [PubMed] [Google Scholar]
- 12.Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, Kaneda K, Fujii T, Senoh H, Fujiwara H: Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 1996, 23:1608-1615 [DOI] [PubMed] [Google Scholar]
- 13.Czaja MJ, Xu J, Alt E: Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 1995, 108:1849-1854 [DOI] [PubMed] [Google Scholar]
- 14.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ: Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC (corrected and republished article originally printed in Hepatology 1998, 27:507–512). Hepatology 1998, 27:1172-11779537464 [Google Scholar]
- 15.Muruve DA, Barnes MJ, Stillman IE, Libermann TA: Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther 1999, 10:965-976 [DOI] [PubMed] [Google Scholar]
- 16.Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI: Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 1995, 133:43-52 [DOI] [PubMed] [Google Scholar]
- 17.Leist M, Gantner F, Bluethmann H, Vogt K, Brigelius-Flohe R, Nicotera P, Volk HD, Wendel A: Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology 1997, 112:923-934 [DOI] [PubMed] [Google Scholar]
- 18.Kurihara T, Bravo R: Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem 1996, 271:11603-11607 [DOI] [PubMed] [Google Scholar]
- 19.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA: Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol 1997, 158:4129-4136 [PubMed] [Google Scholar]
- 20.Hogaboam CM, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL: Monocyte chemoattractant protein-1 synthesis by murine lung fibroblasts modulates CD4+ T cell activation. J Immunol 1998, 160:4606-4614 [PubMed] [Google Scholar]
- 21.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ: MCP-1 protects mice in lethal endotoxemia. J Clin Invest 1997, 99:2832-2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowell DL, Eckmann L, Dwinell MB, Carpenter SP, Raucy JL, Yang SK, Kagnoff MF: Human hepatocytes express an array of proinflammatory cytokines after agonist stimulation or bacterial invasion. Am J Physiol 1997, 273:G322-G332 [DOI] [PubMed] [Google Scholar]
- 23.Dong W, Simeonova PP, Gallucci R, Matheson J, Fannin R, Montuschi P, Flood L, Luster MI: Cytokine expression in hepatocytes: role of oxidant stress. J Interferon Cytokine Res 1998, 18:629-638 [DOI] [PubMed] [Google Scholar]
- 24.Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, Laffi G, Gentilini P: Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol 1998, 152:423-430 [PMC free article] [PubMed] [Google Scholar]
- 25.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N: Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 1997, 94:12053-12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, Adams DH: Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol 1998, 186:82-89 [DOI] [PubMed] [Google Scholar]
- 27.Sprenger H, Kaufmann A, Garn H, Lahme B, Gemsa D, Gressner AM: Differential expression of monocyte chemotactic protein-1 (MCP-1) in transforming rat hepatic stellate cells. J Hepatol 1999, 30:88-94 [DOI] [PubMed] [Google Scholar]
- 28.Marra F, DeFranco R, Grappone C, Parola M, Milani S, Leonarduzzi G, Pastacaldi S, Wenzel UO, Pinzani M, Dianzani MU, Laffi G, Gentilini P: Expression of monocyte chemotactic protein-1 precedes monocyte recruitment in a rat model of acute liver injury, and is modulated by vitamin E. J Invest Med 1999, 47:66-75 [PubMed] [Google Scholar]
- 29.Boring L, Gosling J, Cleary M, Charo IF: Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394:894-897 [DOI] [PubMed] [Google Scholar]
- 30.Evanoff H, Burdick MD, Moore SA, Kunkel SL, Strieter RM: A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest 1992, 21:39-49 [DOI] [PubMed] [Google Scholar]
- 31.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL: A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol 1995, 57:782-787 [DOI] [PubMed] [Google Scholar]
- 32.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997, 100:2552-2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warmington KS, Boring L, Ruth JH, Hogaboam CM, Kunkel SL, Charo IR, Chensue SW: Effect of CCR2 knockout on type-2 (Schistosomal antigen-elicited) pulmonary granuloma formation: analysis of cellular recruitment and cytokine responses. Am J Pathol 1998, 154:1407-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara T, Warr G, Loy J, Bravo R: Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997, 186:1757-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen W, Kamendulis LM, Ray SD, Corcoran GB: Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: correlation of nuclear Ca2+ accumulation and early DNA fragmentation with cell death. Toxicol Appl Pharmacol 1991, 111:242-254 [DOI] [PubMed] [Google Scholar]
- 36.Ray SD, Mumaw VR, Raje RR, Fariss MW: Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp Ther 1996, 279:1470-1483 [PubMed] [Google Scholar]
- 37.Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI: Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 1996, 24:181-189 [DOI] [PubMed] [Google Scholar]
- 38.Czaja MJ, Geerts A, Xu J, Schmiedeberg P, Ju Y: Monocyte chemoattractant protein-1 (MCP-1) expression occurs in toxic rat liver injury and human liver disease. J Leukoc Biol 1994, 55:120-126 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Matsumura F, Takeya M, Ichiguchi O, Kuratsu JI, Horiuchi T, Akizuki E, Matsuda T, Okabe K, Ohshiro H, Liang J, Mori K, Yamada S, Takahashi K, Ogawa M: Monocyte chemoattractant protein-1 enhances expression of intercellular adhesion molecule-1 following ischemia-reperfusion of the liver in rats. Hepatology 1998, 27:727-734 [DOI] [PubMed] [Google Scholar]
- 40.Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL: Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology 1999, 30:934-943 [DOI] [PubMed] [Google Scholar]
- 41.Louis H, Le Moine O, Peny MO, Gulbis B, Nisol F, Goldman M, Deviere J: Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology 1997, 112:935-942 [DOI] [PubMed] [Google Scholar]
- 42.Louis H, Le Moine O, Peny MO, Quertinmont E, Fokan D, Goldman M, Deviere J: Production and role of interleukin-10 in concanavalin-A-induced hepatitis in mice. Hepatology 1997, 25:1382-1389 [DOI] [PubMed] [Google Scholar]
- 43.Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA: Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology 1998, 27:1021-1029 [DOI] [PubMed] [Google Scholar]
- 44.Goldin RD, Ratnayaka ID, Breach CS, Brown IN, Wickramasinghe SN: Role of macrophages in acetaminophen (paracetamol)-induced hepatotoxicity. J Pathol 1996, 179:432-435 [DOI] [PubMed] [Google Scholar]
- 45.Nastevska C, Gerber E, Horbach M, Rohrdanz E, Kahl R: Impairment of TNF-alpha expression and secretion in primary rat liver cell cultures by acetaminophen treatment. Toxicology 1999, 133:85-92 [DOI] [PubMed] [Google Scholar]
- 46.Yamada Y, Fausto N: Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 and not type 2 tumor necrosis factor receptor. Am J Pathol 1998, 152:1577-1589 [PMC free article] [PubMed] [Google Scholar]
- 47.Goebeler M, Schnarr B, Toksoy A, Kunz M, Brocker EB, Duschl A, Gillitzer R: Interleukin-13 selectively induces monocyte chemoattractant protein-1 synthesis and secretion by human endothelial cells. Involvement of IL-4R alpha and Stat6 phosphorylation. Immunology 1997, 91:450–457 [DOI] [PMC free article] [PubMed]