Abstract
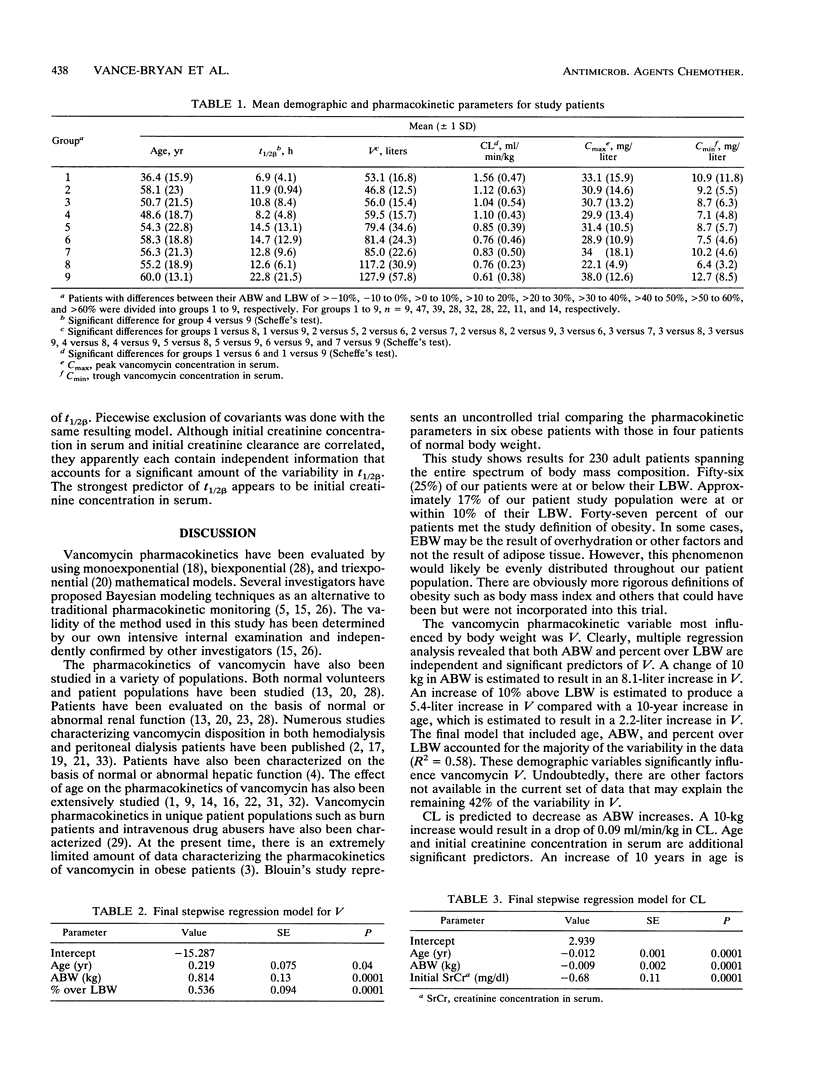
Few data exist concerning the effect of obesity on the pharmacokinetic parameters of vancomycin. The purpose of this investigation was to assess the effect of obesity on vancomycin pharmacokinetic parameters in 95 nonobese and 135 obese adult patients (age range, 18 to 92 years) receiving vancomycin. All subjects had normal renal function as defined by a creatinine concentration in serum of < or = 1.5 mg/dl (mean estimated creatinine clearance +/- 1 standard deviation, 76 +/- 34; range, 23 to 215 ml/min). Vancomycin concentrations in serum were determined by the fluorescence polarization immunoassay. All data for vancomycin concentration in serum versus time for each course of therapy were fitted by using a two-compartment Bayesian forecasting program. Subjects were stratified into nine groups on the basis of the percent difference between actual body weight (ABW) and lean body weight (LBW) (> -10%, -10 to 0%, > 0 to 10%, > 10 to 20%, > 20 to 30%, > 30 to 40%, > 40 to 50%, > 50 to 60%, > 60%). Analysis of variance with post hoc Scheffe's testing revealed that statistically significant differences occurred in terminal disposition half-life (t1/2 beta) between the extremes of modestly obese (group 4) and morbidly obese (group 9, P < 0.05) patients. Similar analysis with distribution volume (V) identified significant differences in patients at or near their LBW (groups 2 to 4) and patients who were morbidly obese (groups 8 and 9, P < 0.05). Multiple regression models for the pharmacokinetic parameters V, t1/2beta, and vancomycin total body clearance were developed to assess the joint predictive power of LBW, ABW, and percent over LBW, controlling for the effects of age, initial creatinine concentration in serum, initial creatinine clearance, and gender. In the final model for V, both ABW and percent over LBW were independent and significant predictors. For total body clearance, only ABW was significant and predictive. Percent over LBW was a significant and independent predictor of t1/2beta. LBW is not predictive of these pharmacokinetic parameters and should not be used for initial dosing. On the basis of these data, ABW appears to be superior to LBW for calculating initial dose requirements for vancomycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert G., Campos J. M., Harris M. C., Preblud S. R., Plotkin S. A. Vancomycin dosage in pediatrics reconsidered. Am J Dis Child. 1984 Jan;138(1):20–22. doi: 10.1001/archpedi.1984.02140390012005. [DOI] [PubMed] [Google Scholar]
- Blevins R. D., Halstenson C. E., Salem N. G., Matzke G. R. Pharmacokinetics of vancomycin in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1984 May;25(5):603–606. doi: 10.1128/aac.25.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin R. A., Bauer L. A., Miller D. D., Record K. E., Griffen W. O., Jr Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982 Apr;21(4):575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N., Ho D. H., Fong K. L., Bogerd L., Maksymiuk A., Bolivar R., Fainstein V., Bodey G. P. Effects of hepatic function on vancomycin clinical pharmacology. Antimicrob Agents Chemother. 1983 Apr;23(4):603–609. doi: 10.1128/aac.23.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M. E., Gentle D. L., Vasko M. R. Evaluation of a Bayesian method for predicting vancomycin dosing. DICP. 1989 Apr;23(4):294–300. doi: 10.1177/106002808902300404. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Cook F. V., Farrar W. E., Jr Vancomycin revisited. Ann Intern Med. 1978 Jun;88(6):813–818. doi: 10.7326/0003-4819-88-6-813. [DOI] [PubMed] [Google Scholar]
- Cunha B. A., Ristuccia A. M. Clinical usefulness of vancomycin. Clin Pharm. 1983 Sep-Oct;2(5):417–424. [PubMed] [Google Scholar]
- Cutler N. R., Narang P. K., Lesko L. J., Ninos M., Power M. Vancomycin disposition: the importance of age. Clin Pharmacol Ther. 1984 Dec;36(6):803–810. doi: 10.1038/clpt.1984.260. [DOI] [PubMed] [Google Scholar]
- Edwards D. J., Pancorbo S. Routine monitoring of serum vancomycin concentrations: waiting for proof of its value. Clin Pharm. 1987 Aug;6(8):652–654. [PubMed] [Google Scholar]
- Geraci J. E., Hermans P. E. Vancomycin. Mayo Clin Proc. 1983 Feb;58(2):88–91. [PubMed] [Google Scholar]
- Golper T. A., Noonan H. M., Elzinga L., Gilbert D., Brummett R., Anderson J. L., Bennett W. M. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther. 1988 May;43(5):565–570. doi: 10.1038/clpt.1988.74. [DOI] [PubMed] [Google Scholar]
- Gross J. R., Kaplan S. L., Kramer W. G., Mason E. O., Jr Vancomycin pharmacokinetics in premature infants. Pediatr Pharmacol (New York) 1985;5(1):17–22. [PubMed] [Google Scholar]
- Hurst A. K., Yoshinaga M. A., Mitani G. H., Foo K. A., Jelliffe R. W., Harrison E. C. Application of a Bayesian method to monitor and adjust vancomycin dosage regimens. Antimicrob Agents Chemother. 1990 Jun;34(6):1165–1171. doi: 10.1128/aac.34.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A., Koren G., Milliken J., Soldin S., Prober C. Vancomycin pharmacokinetics and dose recommendations for preterm infants. Antimicrob Agents Chemother. 1987 Jan;31(1):52–54. doi: 10.1128/aac.31.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanese D. M., Alfrey P. S., Molitoris B. A. Markedly increased clearance of vancomycin during hemodialysis using polysulfone dialyzers. Kidney Int. 1989 Jun;35(6):1409–1412. doi: 10.1038/ki.1989.141. [DOI] [PubMed] [Google Scholar]
- Matzke G. R., McGory R. W., Halstenson C. E., Keane W. F. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984 Apr;25(4):433–437. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke G. R., O'Connell M. B., Collins A. J., Keshaviah P. R. Disposition of vancomycin during hemofiltration. Clin Pharmacol Ther. 1986 Oct;40(4):425–430. doi: 10.1038/clpt.1986.201. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Krogstad D. J., Greenblatt D. J. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med. 1981 Mar;94(3):343–346. doi: 10.7326/0003-4819-94-3-343. [DOI] [PubMed] [Google Scholar]
- Morse G. D., Farolino D. F., Apicella M. A., Walshe J. J. Comparative study of intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987 Feb;31(2):173–177. doi: 10.1128/aac.31.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi S. H., Keenan W. J., Reichley R. M., Fortune K. P. Vancomycin pharmacokinetics in small, seriously ill infants. Am J Dis Child. 1986 Feb;140(2):107–110. doi: 10.1001/archpedi.1986.02140160025021. [DOI] [PubMed] [Google Scholar]
- Nielsen H. E., Hansen H. E., Korsager B., Skov P. E. Renal excretion of vancomycinin in kidney disease. Acta Med Scand. 1975 Apr;197(4):261–264. doi: 10.1111/j.0954-6820.1975.tb04914.x. [DOI] [PubMed] [Google Scholar]
- Pryka R. D., Rodvold K. A., Erdman S. M. An updated comparison of drug dosing methods. Part IV: Vancomycin. Clin Pharmacokinet. 1991 Jun;20(6):463–476. doi: 10.2165/00003088-199120060-00003. [DOI] [PubMed] [Google Scholar]
- Rodvold K. A., Blum R. A., Fischer J. H., Zokufa H. Z., Rotschafer J. C., Crossley K. B., Riff L. J. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988 Jun;32(6):848–852. doi: 10.1128/aac.32.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvold K. A., Zokufa H., Rotschafer J. C. Routine monitoring of serum vancomycin concentrations: can waiting be justified? Clin Pharm. 1987 Aug;6(8):655–658. [PubMed] [Google Scholar]
- Rotschafer J. C., Crossley K., Zaske D. E., Mead K., Sawchuk R. J., Solem L. D. Pharmacokinetics of vancomycin: observations in 28 patients and dosage recommendations. Antimicrob Agents Chemother. 1982 Sep;22(3):391–394. doi: 10.1128/aac.22.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak M. J., Albrecht L. M., Berman J. R., Warbasse L. H., Svensson C. K. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agents Chemother. 1990 May;34(5):792–795. doi: 10.1128/aac.34.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak M. J., Boike S. C. Individualized adjustment of vancomycin dosage: comparison with two dosage nomograms. Drug Intell Clin Pharm. 1986 Jan;20(1):64–68. doi: 10.1177/106002808602000112. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Nelson J. D. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980 Jan;96(1):119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- Schaible D. H., Rocci M. L., Jr, Alpert G. A., Campos J. M., Paul M. H., Polin R. A., Plotkin S. A. Vancomycin pharmacokinetics in infants: relationships to indices of maturation. Pediatr Infect Dis. 1986 May-Jun;5(3):304–308. doi: 10.1097/00006454-198605000-00006. [DOI] [PubMed] [Google Scholar]
- Torras J., Cao C., Rivas M. C., Cano M., Fernandez E., Montoliu J. Pharmacokinetics of vancomycin in patients undergoing hemodialysis with polyacrylonitrile. Clin Nephrol. 1991 Jul;36(1):35–41. [PubMed] [Google Scholar]
- Zokufa H. Z., Rodvold K. A., Blum R. A., Riff L. J., Fischer J. H., Crossley K. B., Rotschafer J. C. Simulation of vancomycin peak and trough concentrations using five dosing methods in 37 patients. Pharmacotherapy. 1989;9(1):10–16. doi: 10.1002/j.1875-9114.1989.tb04097.x. [DOI] [PubMed] [Google Scholar]


