Abstract
Most gastrointestinal stromal tumors (GISTs), a subgroup of mesenchymal neoplasms of the gut wall, express both Kit (CD117) and CD34 proteins. It has been suggested that GISTs originate from or differentiate into interstitial cells of Cajal (ICC), after several reports indicated that ICC are likely the only cells in the gut which express both Kit and CD34. ICC are among the few cell types resident in the gut which express Kit, together with mast cells. However, the question whether or not ICC express CD34 is currently disputed. Using single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) on cultured murine intestinal cells, single ICC were selected by morphology and tested for the expression of c-kit and CD34 mRNA. Most ICC were only c-kit-positive, however a subset (7 out of 43) were double positive for both c-kit and CD34. In the human small intestine, sequential immunohistochemical staining for Kit and CD34 proteins on the same 3-μm sections showed that some of the ICC surrounding Auerbach’s plexus and ICC within the circular muscle layer of the small intestine were positive for both Kit and CD34. In addition, CD34+Kit− cells were seen adjacent to ICC. These data from two different techniques indicate that ICC can be double positive for Kit and CD34. Thus, GISTs with the Kit+CD34+ phenotype may arise from a subpopulation of CD34+ Kit+ ICC.
The hypothesis that gastrointestinal stromal tumors (GISTs) originate from interstitial cells of Cajal (ICC) is currently disputed 1 and the controversy was highlighted by a recent editorial in the Journal Laboratory Investigation. 2 GISTs are nonepithelial mesenchymal neoplasms that arise in the wall of the gastrointestinal tract, which are commonly CD34 immunoreactive but usually lack markers for true leiomyomas and schwannomas. 3-8 The hypothesis that GISTs originate from ICC was initiated after the observation that almost all GISTs were double positive for the Kit membrane tyrosine kinase receptor (CD117) and CD34, and that ICC were the only cells in the gut which were double positive for Kit and CD34 as determined by immunohistochemistry. 5,9 ICC are mesenchymal cells in origin, and are located within the gut wall between most neural plexi and smooth muscle cells. Networks of ICC are associated with Auerbach’s plexus and with the deep muscular plexus (small intestine) and submuscular plexus (colon). ICC are also found within the muscle layers of the gut wall. Some of these ICC networks participate in the generation of the pacemaker activity of the gut 10-12 involved in the generation of peristaltic motor activity, 13 others may participate in neurotransmission. 14
ICC have been shown to be Kit-positive by immunohistochemistry, 15-17 in situ hybridization, 18 and single-cell polymerase chain reaction (PCR). 11 Whether or not ICC express CD34 is currently debated, because some authors report CD34 immunoreactivity, 5,9,19 whereas others do not find such expression in ICC. 1 This debate is important for weighing evidence for and against the hypothesis that GISTs arise from or differentiates toward ICC. ICC within the stomach and small and large intestine were found to be double positive for Kit and CD34 using immunohistochemistry and confocal laser-scanning microscopy. 9 Kindblom et al 5 identified a small subset of Kit-positive cells within the myenteric plexus of the human gastrointestinal tract that were also CD34-positive. In another study using double-fluorescence immunostaining of the external muscle layers of the human stomach and colon, most of the Kit immunoreactive cells were also positive for CD34, but some ICC-like cells were positive for CD34 and negative for Kit, and some kit-positive cells were negative for CD34. 20 In this study, 20 almost all of the Kit-positive cells in the human small intestine were CD34-negative, and CD34+Kit− ICC-like cells were much more common than in the colon or stomach. Taken together, these findings suggest that ICC within the gastrointestinal tract may be heterogeneous with respect to gene expression, and thus phenotype. In an important study, Vanderwinden and colleagues, 1 using double immunofluorescence immunohistochemistry and confocal microscopy with paraformaldehyde-fixed 15-μm-thick frozen sections, found that CD34 immunoreactivity identified previously unrecognized cells closely adjacent to, but distinct from the Kit immunoreactive cells, in the human small intestine. The CD34-positive cells also expressed a fibroblast marker prolyl 4-hydroxylase, indicating that they are likely fibroblast-like cells.
Attempts have been made to identify ultrastructural features of ICC within GISTs. Kindblom et al 5 indicated that all GISTs contain cells that exhibit ultrastructural characteristics of ICC. However, we have previously indicated that the unique combination of several distinct ultrastructural features that identify ICC was not present in GISTs, 19 suggesting that although the tumor may have originated from ICC, the tumor cells have differentiated away from the original ICC phenotype.
The hypothesis that GISTs originate from ICC is based on the fact that GISTs are CD34+Kit+, and that immunohistochemistry appears to reveal that at least some Kit-positive ICC are also positive for CD34. 5,9,19 On the other hand, the possibility is put forward that the CD34 positivity may not be in ICC but in neighboring fibroblasts 1 and that previous accounts of double positivity might have been due to staining of overlapping ICC and fibroblasts. It was our objective to resolve the current controversy. First, using single-cell PCR on isolated ICC obtained from a mouse model, evidence for the presence of CD34 mRNA was sought. Second, using sequential immunohistochemical staining on the same section from the human small intestine, possible CD34 positivity of ICC was investigated. Our data demonstrate that a subpopulation of ICC within the gastrointestinal tract is positive for CD34, confirming the possibility that GISTs originate from Kit+CD34+ ICC.
Materials and Methods
Cell Isolation
Cells used in the RT-PCR analysis were obtained from the mouse small intestine Auerbach’s plexus region with adjacent muscle layers but excluding the deep muscular plexus, according to the method described previously. 11 Cells were used after 3 to 4 days in culture.
Single-Cell RT-PCR
Single-cell RT-PCR was performed according to the method described previously. 11 In brief, one cell was harvested using a patch pipette and subjected to first-strand cDNA synthesis using oligo-dT primer and Superscript II RT (Life Technologies, Burlington, Ontario, Canada). Nested PCR was performed with a Perkin Elmer Gene Amp PCR system 2400 (Mississauga, Ontario, Canada), in a 100-μl reaction volume using Taq Polymerase (MBI, Flamborough, Ontario, Canada). The primers used to amplify cDNA for c-kit, CD34, and platelet endothelial cell adhesion molecule 1 (PECAM, a marker for endothelial cells) are listed in Table 1 ▶ , with the product size in bp. The outside (first) PCR reaction consisted of 30 cycles, whereas the nested (second) PCR reaction consisted of 35 cycles. Each cycle consisted of denaturation at 94°C for 40 seconds, annealing at 49°C for 40 seconds, and extension at 72°C for 1 minute. A final extension step at 72°C for 15 minutes was also performed. PCR products were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide staining followed by ultraviolet transillumination.
Table 1.
Primers Used for Single-cell RT-PCR
| Primer | Upstream sequence (5′-3′) | Downstream sequence (5′-3′) | Product size (bp) |
|---|---|---|---|
| c-kit outside | TGTGATGGTGCTCACCTACA | GAGTCACGCTTCCTTCTCAA | 447 |
| c-kit nested | GGAAGGTTGTCGAGGAGATA | CCTTCAGTTCCGACATTAGG | 258 |
| CD34 outside | AGACCACACCAGCCATCTCA | CCTCCACCATTCTCCGTGTA | 772 |
| CD34 nested | GTCCAGCCTGCCATCTATAA | CTCCACCATTCTCCGTGTAA | 608 |
| PECAM outside | ACTCACGCTGGTGCTCTATG | CACGGTGACGTATTCACTCC | 637 |
| PECAM nested | GAGTGCCTTGTGGACATCAG | TGCACCTTCACCTCGTACTC | 218 |
Total RNA was isolated from an enzymatically digested portion of the myenteric plexus and longitudinal muscle of the murine small intestine, using the RNeasy kit (Qiagen, Mississauga, Ontario, Canada), for use as positive controls for the PCR analysis. PCR on these samples was carried out as described above for the single cells.
Immunohistochemistry
Three-micrometer-thick sections of formaldehyde-fixed, paraffin-embedded, histologically normal, human small intestine were prepared and immunohistochemically stained with a polyclonal rabbit anti-Kit, as previously described. 19 The small intestine was chosen for study, because in the mouse model used, only for the small intestine has a reliable method been developed for the isolation of ICC. 11,12 Color photomicrographs were taken of several fields showing Kit immunoreactive ICC, both associated with the myenteric plexus and located singly within the circular muscle. The location of each photomicrograph field was recorded from the microscope slide stage Vernier scales.
Subsequently, the coverglasses were carefully removed from the sections by immersion in warm running tap water. The color, 3-amino-9-ethylcarbazole (AEC) chromagen, was removed and the peroxidase label blocked by a 30-minute exposure to methanol containing 3% hydrogen peroxide. After rehydration and washing in 0.05 mol/L Tris-buffered saline pH 7.6, the slides were checked microscopically, to ensure total removal of the signal from the initial immunostain. After proteolytic digestion with 0.05% w/v trypsin (porcine pancreas; Sigma Chemical Co., St. Louis, MO) in 0.05 mol/L Tris-buffered saline pH 7.6 containing 0.25% w/v calcium chloride for 30 minutes at 37°C, nonspecific binding sites were blocked by incubation with 5% v/v nonimmune goat serum for 20 minutes. Immunostaining for CD34 was then performed as previously described. 19
Each of the original photomicrograph fields was then relocated using the stage co-ordinates. The precise framing was checked against the original photomicrograph and the CD34 immunoreactivity photographed. Matching pairs of photomicrographs, representing the kit and CD34 immunoreactivity, respectively, of the same area of the original sections, were digitized and stored in Kodak (Rochester, NY) CD-photo format. The digital images were imported into Adobe Photoshop (Adobe Systems, San Jose, CA) and the original magenta color of the immunoreactive areas segmented and changed: Kit+ areas to yellow and CD34+ areas to red, to provide false color separation in the final image. It is important to note that low-intensity stained cells or parts of cells that had low-intensity staining were not recolored to avoid any chance of false coloring areas that were not immunostained. Three widely spaced, triangulated cell nuclei were located in each of the matched pairs and identified by arrowheads. The matched pairs were then superimposed and registered by using the three identified cell nuclei as fiducial points. In the final image, areas of Kit positivity/CD34 negativity are yellow, areas of co-localization (Kit+/CD34+) orange, and areas of Kit negativity/CD34 positivity are red.
Results
RT-PCR
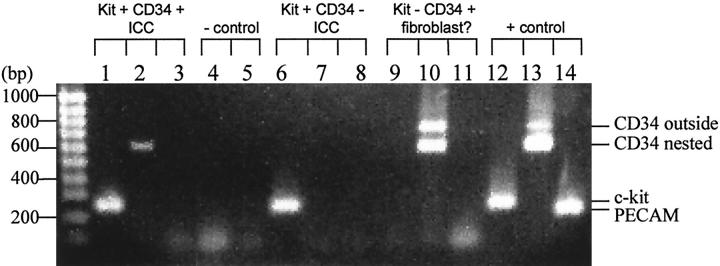
Single RT-PCR was performed on cultured cells from Auerbach’s plexus and external muscle layers of the murine small intestine to determine whether CD34 mRNA was present in c-kit mRNA-positive ICC. The majority of single ICC selected by morphology 11 were only c-kit-positive, however a small subset (7 out of 43 c-kit+ ICC tested for CD34 expression) were double positive for both c-kit and CD34. One cell, which was not considered to be an ICC by morphology, was c-kit-negative but was CD34-positive. It is possible that this cell was a fibroblast, as suggested by Vanderwinden et al. 1 None of the eight CD34-positive cells were positive for PECAM, indicating that these were not endothelial cells. Figure 1 ▶ shows an example of the various expression patterns discovered in the single cells.
Figure 1.
Two phenotypes were discovered in the morphologically identified ICC from the murine myenteric plexus: c-kit+CD34+ PECAM− (lanes 1–3) and c-kit+CD34− PECAM− (lanes 6–8). One cell that was not identified morphologically as an ICC was c-kit− CD34+, and did not express detectable levels of PECAM (lanes 9–11). Two negative controls were performed, one for c-kit (lane 4) the other for CD34 (lane 5), in which water was substituted for cell contents in the RT-PCR reactions. As a positive control, an mRNA extraction from a portion of the myenteric plexus and longitudinal muscle of the murine intestine was tested for c-kit, CD34, and PECAM (lanes 12–14). The c-kit band from the nested primers (second PCR reaction) appears at 258 bp in the morphologically identified ICC. The CD34 band from nested primers appears at 608 bp in a subset of morphologically identified ICC. Another CD34 band appears at 772 bp in the one cell tentatively labeled as a fibroblast, which is the product from the first PCR reaction (outside primers). The PECAM band from the nested primers appears at 218 bp only in the positive control.
Negative controls were performed, in which cell contents were substituted by water in the RT-PCR reactions. Negative controls demonstrated that contamination was not a problem in these experiments. Positive controls were performed by testing the primers on a mRNA extraction from the intestinal musculature. These positive controls indicated that the primers were working to detect the intended target.
Immunohistochemistry
Three-μm-thick, formaldehyde-fixed paraffin-embedded sections (n = 6 different samples) were first stained for Kit and thereafter restained for CD34. In many instances, co-localization of Kit and CD34 was observed. One example shows the circular muscle of the human small intestine where spindle-shaped Kit+ ICC were found which were positive for CD34 (Figure 2) ▶ . Another example comes from the Auerbach’s plexus region of the human small intestine (Figure 2) ▶ . In several cells showing a central nucleus, similar staining patterns surrounding the nucleus unequivocally showed that a single cell was double positive. Within the Auerbach’s plexus area, CD34-positive cells were 2 to 4 times more numerous than Kit-positive cells. Both CD34+Kit− and CD34+Kit+ cell types were located around the ganglia and into the septa. In addition, numerous CD34+Kit− endothelial cells were found. Consistent with the RT-PCR data on the mouse small intestine, in the Auerbach’s plexus region of the human small intestine, Kit+CD34− ICC were observed (Figure 2) ▶ .
Figure 2.
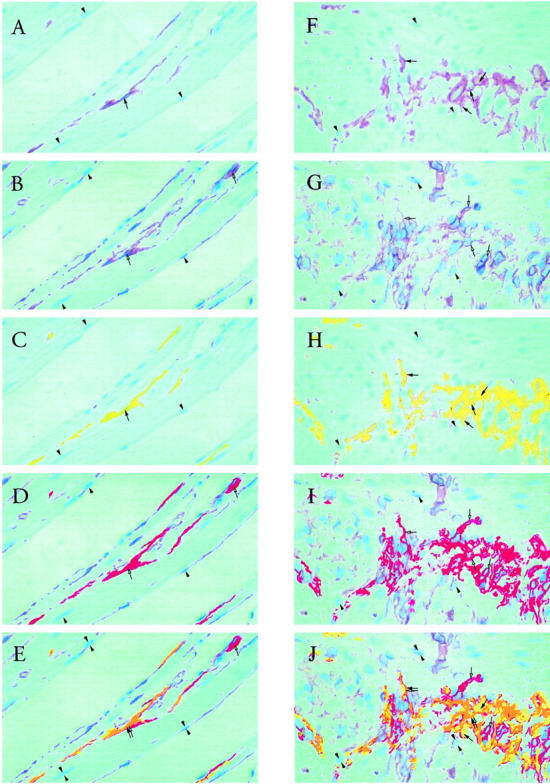
Left column: sequential immunohistochemical staining for CD117 (Kit) and CD34, using AEC chromagen, counterstained with hematoxylin, on single 3-μm sections of the circular smooth muscle region of the human small intestine. A: Kit staining of ICC in the muscle layer (brown, selected area marked by filled arrow). Arrowheads mark the tops of selected nuclei for location reference. B: CD34 staining (brown, selected areas marked by open arrows) of the same area of the section as in A. Arrowheads mark the bottoms of the same nuclei marked in A. Far more CD34 positivity is present than Kit positivity (compare B to A). C: Digital recoloring (to yellow) of selected Kit-stained areas in A. D: Digital recoloring (to red) of selected CD34-stained areas in B. Note that the recoloring produces one color intensity, compared to several color intensities in the original stain. Very low intensities of the original stain are not recolored. E: Merged fields C and D. Kit+CD34+ ICC are present in the muscle layer (orange, marked by double arrows). Also present in the muscle layer are Kit− CD34+ cells (red, single open arrow). An example of a Kit +CD34− ICC adjacent to a Kit− CD34+ fibroblast is found in the right bottom corner, confirming the recent observation from Vanderwinden et al 1 Magnification, ×200. Right column: sequential immunohistochemical staining for CD117 (Kit) and CD34, using AEC chromagen, counterstained with hematoxylin, on single 3-μm sections of the Auerbach’s plexus region of the human small intestine. F: Kit staining of ICC in the plexus (brown, selected areas marked by filled arrows). Arrowheads mark the tops of selected nuclei for location reference. G: CD34 staining (brown, selected areas marked by open arrows) of the same area of the section as in F. Arrowheads mark the bottoms of the same nuclei marked in F. Far more CD34 positivity is present than Kit positivity (compare G to F). H: Digital recoloring (to yellow) of selected Kit-stained areas in F. I: Digital recoloring (to red) of selected CD34-stained areas in G. Note that the recoloring produces one color intensity, compared to the graded color intensities in the original stain. Very low intensities of the original stain were not recolored. Hence the total staining in H and I is less than in, respectively, F and G. The staining is only much brighter for purpose of overlap identification. J: Merged fields H and I. In many instances, co-localization of Kit and CD34 was observed (orange, selected areas marked by double arrows), representing Kit+CD34+ ICC. Close to the double-positive ICC, are Kit− CD34+ cells, which may be fibroblasts (red, single open arrows). The merged fields also revealed the presence of Kit+CD34− ICC (yellow, single filled arrows). The Kit− CD34+ cells are far more numerous than Kit+CD34− or Kit+CD34+ cells. Both Kit and CD34 preferentially stained cells located around the ganglia and into the septa. Magnification, ×200.
To ensure that the observed CD34 immunoreactivity was not due to interaction with the Kit primary antibody, a digestion step with trypsin preceded the CD34 staining. As an internal control for the success of this procedure, the intensely Kit-positive mast cells were not immunoreactive in any of the CD34 stained sections.
Discussion
Single-cell RT-PCR on the mouse small intestine was combined with immunohistochemistry on the human small intestine to obtain evidence for CD34+Kit+ ICC. There is remarkable similarity in ICC morphology comparing different species. Rumessen and Thuneberg 21 listed the distinctive cytological features of the different cell types associated with Auerbach’s plexus in the human small intestine indicating that a combination of several ultrastructural features distinguishes ICC from fibroblasts and smooth muscle cells. ICC from the human small intestine compared to those of the mouse differ only quantitatively in some of those features. Using electron microscopy and Kit immunohistochemistry, in both human and mouse, networks of ICC have been revealed within the Auerbach’s plexus area, the deep muscular plexus area, and within the muscle layers connected to enteric neural structures as well as smooth muscle cells. 22-24
In the present study, the single-cell RT-PCR data unequivocally prove that a subpopulation of ICC isolated from the murine small intestine contained both c-kit and CD34 mRNA. Positive expression of both mRNA transcripts in single ICC is proof that at least a subset of ICC is programmed to synthesize both Kit and CD34 proteins. In addition, sequential immunohistochemical CD34 and Kit staining on the same 3-μm section confirmed that some ICC in the human small intestine express both Kit and CD34 proteins. These data from both RT-PCR and immunohistochemistry techniques indicate that the Kit+CD34+ GISTs may originate from Kit+CD34+ ICC, because ICC are the only cells in the intestine identified to date that are double positive for both proteins. It is obviously difficult if not impossible to proof the identity of the original cell from which the tumor developed. Further evidence for the immunophenotypic similarity of ICC and GIST came from a recent study showing that both ICC and GISTs, but not smooth muscle cells, were positive for the embryonic form of smooth muscle myosin heavy chain. 20 Interestingly, in the mouse, although adult ICC are negative, the embryonic precursor cells from which ICC develop are positive for smooth muscle myosin heavy chain. 25
Our observation of co-localization of Kit and CD34 on single 3-μm sections confirms previous observations using high resolution confocal microscopy 9,20 using formalin-fixed paraffin-embedded 3-μm sections or standard immunohistochemistry with either glutaraldehyde or formalin-fixed tissue, 5 and extends our previous experience using contiguous 3-μm thick sections. 19 Vanderwinden et al, 1 using paraformaldehyde fixed 15-μm-thick frozen sections, noted that in the Auerbach’s plexus region of the human intestine, Kit-positive ICC are often located adjacent to CD34-positive fibroblasts. In their series of experiments, no CD34-positive ICC were observed. These findings suggest that the observation of double positivity might be due to detection of overlapping cells. However, in our study on 3-μm thick sections, it is unlikely that all double-positive cells would result from CD34+ fibroblasts covering or underlying Kit+ ICC. Furthermore, the presence of central nuclei in some cells that were both Kit- and CD34-positive by sequential staining (double arrows in Figure 2 ▶ ) in these thin 3-μm sections would indicate that these are single cells and do not represent the overlapping of cytoplasms of a Kit+CD34− ICC and a Kit− CD34+ fibroblast that happen to be adjacent. In Figure 2E ▶ , in the same field, thin elongated cells are seen within the circular muscle that are clearly positive for both stains as well as CD34+Kit—cells adjacent to CD34—Kit+ ICC.
Vanderwinden et al 1 observed no difference in distribution between Kit+ and CD34+ cells. Kindblom et al 5 (compare Figure 8b with 9a in Ref. 5 ) found more CD34 immunoreactivity than Kit immunoreactivity. In our experience, based on more than 100 double-stained sections, the level of CD34 immunoreactivity in the Auerbach’s plexus area is several times greater than the level of Kit immunoreactivity. The level of CD34 immunoreactivity detected is likely proportional to the available level of CD34 epitope that is unmasked in the tissue, and thus the tissue fixation and staining pretreatments used are crucial factors. The proteolytic enzyme trypsin could potentially unmask the epitope in formaldehyde-fixed paraffin-embedded tissue. However, trypsin is unlikely to be the (only) factor explaining differences in CD34 expression, because omission of trypsin did not markedly reduce the level of CD34 immunoreactivity in 3-μm formaldehyde-fixed paraffin-embedded sections (data not shown). The higher CD34 immunoreactivity observed when using 3-μm formaldehyde-fixed paraffin-embedded sections 5,9,19,20 compared to 15-μm paraformaldehyde-fixed frozen sections 1 is likely due to differences in fixation techniques used. The technique used by Vanderwinden et al 1 may not reveal all CD34 expression, which would explain why they did not report CD34 positivity in any Kit-positive cells.
Kit and the Kit ligand (Steel factor) play a critical role in cell proliferation, cell migration, cell survival, differentiation, and postmitotic functions within several cell systems including ICC. 26,27 Because mutant forms of the Kit and other receptors in the platelet-derived growth factor subfamily have been implicated in tumorigenic development, these genes are commonly considered protooncogenes. 28 Point mutations in the c-kit gene which result in a constitutively active protein have been reported in malignant mastocytosis. 29-31 Transfection of mutant c-kit cDNAs induced malignant transformation of Ba/F3 murine lymphoid cells, suggesting that c-kit mutations contribute to tumor development. 9 GISTs may be caused by a mutation in the c-kit gene, because five out of six GISTs examined expressed a mutated form of Kit which was constitutively active without activation by the Steel factor ligand. 9 Hirota et al 9 suggested that only one allele was affected by mutation, such that Kit immunoreactivity was due to the remaining normal allele. Lasota et al 32 showed that c-kit mutations resulting in constitutively active Kit receptors occur predominantly in malignant versus benign GIST: 50% versus 5%, respectively. However, because Lasota et al 32 found that only 50% of malignant Kit+ tumors harbored c-kit mutations, they suggested c-kit may not be the only mechanism related to tumorigenesis and malignancy. Sircar et al 19 reported that loss of either Kit or CD34 immunoreactivity was more likely to occur in malignant versus benign GIST. It is possible that loss of both c-kit or both CD34 alleles results in increased metastasizing potential.
The function of CD34 remains unclear; however, it has been suggested CD34 is involved in cell adhesion. 33 Interestingly, it has been demonstrated that CD34 is down-regulated in cultured endothelial cells at the same time that known adhesion molecules, including endothelial leukocyte adhesion molecule 1 and intracellular adhesion molecule 1, are up-regulated. 34 This suggests that CD34 plays a negative role in the modulation of adhesion of endothelial cells. In this respect, it has been speculated that CD34 expression in GISTs is associated with a loss of normal adhesion capability in the neoplastic cells. 4 Loss of adhesion capability in addition to a gain of function mutation in the Kit gene 9 may make CD34-positive ICC particularly oncogenic. In our experience, and that of Kindblom, 5 co-localization of CD34 and Kit is commonly found in areas of focal diffuse hyperplasia of ICC adjacent to GISTs. This may indicate preoncogenic cells being CD34+Kit+, but this cannot be proven.
The possibility that Kit immunoreactivity in GIST is acquired and may represent a nonspecific oncogenic process unrelated to ICC differentiation has to be discussed. The somatic activating mutation of Kit identified in GIST 9 may be due to the oncogenic process rather than indicate that these tumors originate from ICC. 1 However, acquired Kit positivity in tumors is rarely seen. The strongest case for acquired Kit positivity may be the Kit immunoreactivity of subsets of small-cell carcinomas of the lung, 35,36 and papillary serous carcinomas of the ovary. 35 Even here, however, it is unclear whether Kit immunoreactivity is acquired because small-cell lung carcinomas are thought to arise from reserve or stem cells, the immunoprofile of which may be Kit-positive. Furthermore, both papillary serous carcinomas and small-cell lung carcinomas represent highly malignant tumors at an advanced stage of neoplasia, vulnerable to mutations affecting protooncogenes such as c-kit. In a recent exhaustive study, the only tumors—apart from GISTs—that showed the strong, consistent, diffuse Kit reactivity were mast cell tumors, 35 originating from Kit+ mast cells. Most tumors originating from Kit-positive cells, such as melanomas, showed loss of immunoreactivity with malignancy, not acquisition of immunoreactivity. 35 This is not to say that acquired Kit positivity is not possible. Tumors, phenotypically identical to GISTs have been reported as primary tumors in the omentum and mesentery. 37 On the other hand, mesenchymal tumors of uncertain histogenesis/differentiation arising outside the gastrointestinal tract that are CD34+, such as solitary fibrous tumors of the pleura, dermatofibrosarcoma protuberans, and inflammatory fibroid polyps seem not to acquire Kit immunoreactivity. 6,38 Interestingly, the smallest detectable gastrointestinal mesenchymal tumors have been shown to express either an ICC immunophenotype (Kit+CD34+Vim+) or a smooth muscle phenotype. 39 These data lend additional support to the distinctiveness of GISTs from other undifferentiated soft tissue tumors. It seems plausible that this distinctiveness is related to the presence of a Kit-dependent system found in the gastrointestinal tract but not at other soft tissue sites, namely the ICC.
In summary, our data indicate that there is a subpopulation of ICC that is Kit+CD34+. Thus, GISTs may originate from this subpopulation of ICC. In addition, our study confirms the discovery by Vanderwinden and colleagues 1 that CD34+ fibroblast-like cells appear companion cells to ICC both in Auerbach’s plexus as well as within the muscle layers.
Footnotes
Address reprint requests to Dr. Jan D. Huizinga, McMaster University Medical Center, HSC-3N5C, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada. E-mail: huizinga@mcmaster.ca.
Supported by grants from The Medical Research Council of Canada and a MRC scholarship (to T.L.R.).
References
- 1.Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN: CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest 1999, 79:59-65 [PubMed] [Google Scholar]
- 2.Editorial: Setting the pace for gastrointestinal stromal tumors. Lab Invest 1999, 79:1
- 3.Saul SH, Rast ML, Brooks JJ: The immunohistochemistry of gastrointestinal stromal tumors: evidence supporting an origin from smooth muscle. Am J Surg Pathol 1987, 11:464-473 [DOI] [PubMed] [Google Scholar]
- 4.Van de Rijn M, Hendrickson MR, Rouse RV: CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol 1994, 25:766-771 [DOI] [PubMed] [Google Scholar]
- 5.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM: Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998, 152:1259-1269 [PMC free article] [PubMed] [Google Scholar]
- 6.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M: CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998, 11:728-734 [PubMed] [Google Scholar]
- 7.Appleman HD, Helwig EB: Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma). Cancer 1976, 38:708-728 [DOI] [PubMed] [Google Scholar]
- 8.Miettinen M, Sarlomo-Rikala M, Lasota J: Gastrointestinal stromal tumours. Ann Chir Gynaecol 1998, 87:278-281 [PubMed] [Google Scholar]
- 9.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad TG, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 10.Thuneberg L: Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 1982, 71:1-130 [PubMed] [Google Scholar]
- 11.Thomsen L, Robinson TL, Lee JCF, Farraway L, Hughes MJG, Andrews DW, Huizinga JD: Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med 1998, 4:848-851 [DOI] [PubMed] [Google Scholar]
- 12.Koh SD, Sanders KM, Ward SM: Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol (Lond) 1998, 513:203-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Der-Silaphet T, Malysz J, Arsenault AL, Hagel S, Huizinga JD: Interstitial cells of Cajal direct normal propulsive contractile activity in the small intestine. Gastroenterology 1998, 114:724-736 [DOI] [PubMed] [Google Scholar]
- 14.Ward SM, Morris G, Reese L, Wang XY, Sanders KM: Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology 1998, 115:314-329 [DOI] [PubMed] [Google Scholar]
- 15.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K: Requirement of c-kit for development of intestinal pacemaker system. Development 1992, 116:369-375 [DOI] [PubMed] [Google Scholar]
- 16.Ward SM, Burns AJ, Torihashi S, Sanders KM: Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond) 1994, 480:91-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagon JC, Miedema BW, Kelly KA: Postgastrectomy syndromes. (Review). Surg Clin North Am 1992, 72:445-465 [DOI] [PubMed] [Google Scholar]
- 18.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A: The W/kit gene required for interstitial cells of Cajal, and for intestinal pacemaker activity. Nature 1995, 373:347-349 [DOI] [PubMed] [Google Scholar]
- 19.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH: Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 1999, 23:377-389 [DOI] [PubMed] [Google Scholar]
- 20.Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M: Embryonic form of smooth muscle myosin heavy chain (SMemb/MHC-B) in gastrointestinal stromal tumor and interstitial cells of Cajal. Am J Pathol 1999, 154:23-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rumessen JJ, Thuneberg L: Interstitial cells of Cajal in human small intestine: ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology 1991, 100:1417-1431 [PubMed] [Google Scholar]
- 22.Kenny SE, Vanderwinden JM, Rintala RJ, Connell MG, Lloyd DA, Vanderhaegen JJ, De Laet MH: Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J Pediatr Surg 1998, 33:94-98 [DOI] [PubMed] [Google Scholar]
- 23.Romert P, Mikkelsen HB: c-kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem Cell Biol 1998, 109:195-202 [DOI] [PubMed] [Google Scholar]
- 24.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM: c-kit-dependent development of interstitial cells, and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 1995, 280:97-111 [DOI] [PubMed] [Google Scholar]
- 25.Klüppel M, Huizinga JD, Malysz J, Bernstein A: Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn 1998, 211:60-71 [DOI] [PubMed] [Google Scholar]
- 26.Huang EJ, Nocka KH, Buck J, Besmer P: Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell 1992, 3:349-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein A, Chabot B, Dubreuil P, Reith A, Nocka K, Majumder S, Ray P, Besmer P: The mouse W/c-kit locus. (Review). Ciba Foundation Symposium 1990, 148:158-166 [PubMed] [Google Scholar]
- 28.Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, Jr, Brodeur D, Zuckerman EE, Hardy WD: A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature 1986, 320:415-421 [DOI] [PubMed] [Google Scholar]
- 29.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y: Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest 1993, 92:1736-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, Duffy T, Jacobs P, Tang LH, Modlin I: Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996, 12:312-314 [DOI] [PubMed] [Google Scholar]
- 31.Beghini A, Cairoli R, Morra E, Larizza L: In vivo differentiation of mast cells from acute myeloid leukemia blasts carrying a novel activating ligand-independent C-kit mutation: Blood. Cells Mol Dis 1998, 24:262-270 [DOI] [PubMed] [Google Scholar]
- 32.Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M: Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol 1999, 154:53-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu MC, Chien SL: The cytoplasmic domain of stem cell antigen CD34 is essential for cytoadhesion signaling but not sufficient for proliferation signaling. Blood 1998, 91:1152-1162 [PubMed] [Google Scholar]
- 34.Delia D, Lampugnani MG, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti MA, Greaves MF: CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood 1993, 81:1001-1008 [PubMed] [Google Scholar]
- 35.Arber DA, Tamayo R, Weiss LM: Paraffin section detection of the c-kit gene product (CD117) in human tissues: value in the diagnosis of mast cell disorders. Hum Pathol 1998, 29:498-504 [DOI] [PubMed] [Google Scholar]
- 36.Matsuda R, Takahashi T, Nakamura S, Sekido Y, Nishida K, Seto M, Seito T, Sugiura T, Ariyoshi Y: Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol 1993, 142:339-346 [PMC free article] [PubMed] [Google Scholar]
- 37.Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH: Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol 1999, 23:1109-1118 [DOI] [PubMed] [Google Scholar]
- 38.Anderson SS, Folpe AL, Bronner MP, Haggit RC: CD117 (c-kit): a sensitive and specific marker for gastrointestinal stromal tumors. Mod Pathol 1999, 12:71A [Google Scholar]
- 39.Sircar K, Hewlett BR, Chorneyko K, Riddell RH.: Differentiation of diminutive and partially myoid gut stromal tumors. Mod Pathol 1999, 12:84A