Abstract
Angiogenesis depends on the cooperation of growth factors and cell adhesion events. Although αv integrins have been shown to play critical roles in angiogenesis, recent studies in αv-null mice suggest that other adhesion receptors and their ligands also regulate this process. Evidence is now provided that the integrin α5β1 and its ligand fibronectin are coordinately up-regulated on blood vessels in human tumor biopsies and play critical roles in angiogenesis, resulting in tumor growth in vivo. Angiogenesis induced by multiple growth factors in chick embryos was blocked by monoclonal antibodies to the cell-binding domain of fibronectin. Furthermore, application of fibronectin or a proteolytic fragment of fibronectin containing the central cell-binding domain to the chick chorioallantoic membrane enhanced angiogenesis in an integrin α5β1-dependent manner. Importantly, antibody, peptide, and novel nonpeptide antagonists of integrin α5β1 blocked angiogenesis induced by several growth factors but had little effect on angiogenesis induced by vascular endothelial growth factor (VEGF) in both chick embryo and murine models. In fact, these α5β1 antagonists inhibited tumor angiogenesis, thereby causing regression of human tumors in animal models. Thus, fibronectin and integrin α5β1, like integrin αvβ3, contribute to an angiogenesis pathway that is distinct from VEGF-mediated angiogenesis, yet important for the growth of tumors.
The development of vascular networks during embryogenesis or normal and pathological angiogenesis depends on growth factors1-4 and cellular interactions with the extracellular matrix.5,6 Genetic and functional analyses indicate that extracellular components and cell surface receptors regulate endothelial cell growth, survival or differentiation in vasculogenesis and/or angiogenesis.5-10
Blood vessels arise during embryogenesis by two processes: vasculogenesis and angiogenesis.5 The roles of growth factors in both processes are well established. For example, vascular endothelial growth factor (VEGF)11 and its receptors12-15 and basic fibroblast growth factor (bFGF)16,17 promote not only the initial development of the embryonic vascular network but also the formation of new blood vessels from pre-existing vessels during development, wound healing and the female reproductive cycle. VEGF,18-20 bFGF,19,21-23 interleukin-8 (IL-8),20,24-31 and tumor necrosis factor-α (TNF-α)20 are some of the growth factors with roles in the pathological angiogenesis that is associated with solid tumors, diabetic retinopathy, and rheumatoid arthritis.
Although growth factors stimulate new blood vessel growth, adhesion to the extracellular matrix (ECM) regulates endothelial cell survival, proliferation, and motility during new blood vessel growth.6,7 Recent studies suggest that specific integrins or their ligands influence vascular development and angiogenesis. For example, the αv integrins participate in angiogenesis by providing survival signals to activated endothelial cells.10,11,32-37 However, recent studies demonstrate that, in the absence of αv integrins, some aspects of angiogenesis can proceed normally,11 suggesting that other molecules may compensate for the absence of αv integrins during development. In fact, the β1 integrin family has recently been shown to play a role in angiogenesis.10,38
Although these studies identify active roles for integrins in the promotion of angiogenesis, the cognate ECM ligands for integrins during in vivo angiogenesis have rarely been identified. One extracellular matrix protein, fibronectin, is expressed in provisional vascular matrices and provides proliferative signals to vascular cells during wound healing, atherosclerosis, and hypertension.39 Fibronectin expression is up-regulated on blood vessels in granulation tissues during wound healing.40 In fact, one isoform of fibronectin, the ED-B splice variant, is preferentially expressed on blood vessels in fetal and tumor tissues, but not on normal quiescent adult blood vessels.41-43 As fibronectin has been shown to regulate cell proliferation,44 these observations suggest a possible role for fibronectin in angiogenesis. Animals lacking fibronectin die early in development from a collection of defects, including missing notochord and somites as well as an improperly formed vasculature.7 However, a functional role for fibronectin in vasculogenesis or in angiogenesis has never been directly established. As fibronectin may have a direct role in promoting angiogenesis, we sought to evaluate its functional role in angiogenesis and to identify the integrin receptor(s) with which it interacts.
One candidate receptor for some of the biological roles of fibronectin is the integrin α5β1. Although several integrins bind to fibronectin,45 integrin α5β1 is generally selective for fibronectin46 as it requires peptide sequences on the ninth (PHSRN) and tenth (RGDS) type III repeats of fibronectin for ligand recognition.47 Loss of the gene encoding the integrin α5 subunit is embryonic lethal and is associated with a complete absence of the posterior somites, as well as some vascular and cardiac defects.8,48 From these studies, however, it is unclear whether integrin α5β1 directly plays a role in the regulation of vascular development or of angiogenesis in particular.
Evidence is provided in this report that both fibronectin and its receptor integrin α5β1 directly regulate angiogenesis. Moreover, interaction of fibronectin and α5β1 is central to the contribution of these two molecules to angiogenesis. In addition, evidence is provided that integrin α5β1 and integrin αvβ3 participate in the same pathways of angiogenesis, which are distinct from those involving integrin αvβ5. Finally, these studies reveal that antagonists of the interaction between vascular cell integrin α5β1 and fibronectin may be useful for the therapy of solid tumor cancers.
Materials and Methods
Antibodies and Reagents
Culture media and reagents were from Irvine Scientific (Irvine, CA). HT29 integrin α5β1-positive and integrin α5β1-negative colon carcinoma cells,49 as well as chick embryo fibroblasts, were maintained in DMEM high glucose supplemented with 10% fetal bovine serum and gentamicin. Human umbilical vein endothelial cells (HUVECs) were maintained in M199 medium containing sodium bicarbonate, HEPES, heparin, endothelial cell growth supplement, 20% fetal bovine serum, and gentamicin. Vitronectin, LM609, and P1F6 were the kind gifts of Dr. David Cheresh. Fibronectin and collagen were from Collaborative Biomedical Products (Bedford, MA). Human 120-kd and 40-kd chymotryptic fragments were purchased from Chemicon, Inc. (Temecula, CA). Murine anti-human CD31 (PECAM; MA-3100) was purchased from Endogen (Woburn, MA). Rabbit anti-von Willebrand factor (vWF; 016P) was purchased from Biogenex (San Ramon, CA). Anti-α5β1 cytoplasmic tail polyclonal antibody (AB1928P), anti-α5β1 function-blocking antibodies (NKI-SAM-1 and JBS5), anti-α5β1 non-function-blocking antibody (HA5), anti-fibronectin cell-binding peptide monoclonal antibody (784A2A6), and anti-fibronectin N-terminal peptide monoclonal antibody were the kind gifts of Chemicon. Anti-α5β1 function-blocking antibody (IIA1) and anti-α5β1 non-function-blocking antibody (VC5) were purchased from Pharmingen (San Diego, CA). Cross-absorbed secondary antibodies were purchased from Biosource International (Camarillo, CA). OCT embedding medium was obtained from Baxter (McGraw Park, IL). Fluoromount-G was purchased from Southern Biotechnology Associates (Birmingham, AL). Six-week-old CB17 female SCID mice were purchased from Charles River (Wilmington, MA). Fresh human neonatal foreskins were obtained from the Cooperative Human Tissue Network of the National Institutes of Health and were stored in RPMI-1640 medium (Irvine Scientific, Irvine, CA) supplemented with 2% fetal bovine serum and 1% gentamicin. Growth factor-depleted matrigel was purchased from Becton Dickinson (Bedford, MA). Ten-day-old chicken eggs were purchased from McIntyre Poultry (Ramona, CA). bFGF, vascular endothelial growth factor, IL-8, and TNF-α were purchased from Genzyme, Inc. (Cambridge, MA). Cyclic peptides were synthesized as described.50,51 Integrin α5β1 nonpeptide small molecule antagonist SJ749 had the following structure: (S)−2-[(2,4,6-trimethylphenyl) sulfonyl] amino-3-[7-benzyloxycarbonyl-8-(2-pyridinylaminomethyl)−1-oxa-2,7-diazaspiro-(4,4)-non-2-en-3-yl] car-bonylamino] propionic acid. Control nonpeptide small molecule XU065 had the following structure: 3-[[3-[(4-Amidinophenyl)oxy]isoxazol-5-yl]carboxamido]-2(S)(butoxycarbonylamino) propionic acid methyl ester.
Immunohistochemical Analysis of Blood Vessels
Five-micron frozen sections of human normal breast and colon, colon carcinoma, breast carcinoma, human tumor xenotransplants in SCID mice, and breast tumors from transgenic mice expressing the polyoma virus (PyV) middle T antigen under control of the mouse mammary tumor virus (Mtag) were fixed for 1 minute in acetone, air dried, and rehydrated for 5 minutes in phosphate buffered saline (PBS). Sections were then blocked for 2 hours in 8% normal goat serum in PBS and incubated with 5 μg/ml anti-α5β1 cytoplasmic tail polyclonal antibody and 5 μg/ml anti-CD31 monoclonal antibody, with 5 μg/ml anti-α5β1 monoclonal antibody and 5 μg/ml anti-vWF antibody, or with 5 μg/ml anti-fibronectin cell-binding peptide monoclonal antibody and 5 μg/ml anti-vWF antibody in 2% bovine serum albumin in PBS for 2 hours at room temperature. Sections were washed by dipping in six fresh changes of PBS and incubated in 1:400–1:600 dilutions of goat anti-rabbit-fluorescein isothiocyanate (FITC) and in 1:400–1:600 goat anti-mouse-rhodamine for 1 hour at room temperature. Slides were well washed, and coverslips were mounted in one drop of Fluoromount before digital image analysis under fluorescent illumination using a supercooled CCD camera.
Cell Adhesion Assays
The wells of 48-well culture dishes (Costar, Inc., Cambridge, MA) were coated with 1 μg/ml vitronectin, 2 μg/ml fibronectin (chick embryo fibroblasts and human umbilical vein endothelial cells), or 10 μg/ml fibronectin (HT29-α5-positive cells) for 1 hour at 37°C and blocked with 2% heat denatured bovine serum albumin in PBS for 1 hour. Fifty thousand cells in 250 μl of adhesion buffer were added to triplicate wells containing 250 μl of a solution of 50 μg/ml of an anti-α5β1 function-blocking antibody (NKI-SAM-1, JBS5, or IIA1), 50 μg/ml of an anti-α5β1 non-function-blocking antibody (HA5 or VC5), 10 μmol/L cyclic peptides, 0–10 μmol/L SJ749, 50 μg/ml of LM609, an anti-αvβ3 function-blocking antibody, 50 μg/ml P4C10, an anti-β1 function-blocking antibody, 50 μg/ml of an anti-fibronectin cell-binding domain monoclonal antibody, or 50 μg/ml of an anti-fibronectin N-terminus monoclonal antibody in adhesion buffer (HEPES-buffered Hanks’ balanced salt solution containing 1% bovine serum albumin, 2 mmol/L MgCl2, 2 mmol/L CaCl2, and 0.2 mmol/L MnCl2). Cells were allowed to adhere to dishes for 20 minutes at 37°C. Nonadherent cells were removed by washing each well four times with 500 μl of warm adhesion buffer. Adherent cells were then fixed for 15 minutes with 3.7% paraformaldehyde in PBS and stained with a 2% crystal violet solution. After extensive water washing to remove excess crystal violet, plates were dried overnight. Crystal violet was extracted by incubation for 15 minutes in 10% acetic acid and absorbance at 562 nm determined as an indicator of number of cells bound. Each experiment was performed in triplicate, with triplicate samples per condition. The data are presented as percentage of adhesion exhibited by the positive control (adhesion medium alone) ± SEM.
Migration Assays
The lower side of 8-μm pore transwell inserts (Costar, Inc.) were coated with 2 μg/ml of fibronectin, collagen, or no protein for 1 hour and were blocked with 2% bovine serum albumin in PBS for 1 hour. The inserts were then placed into 24-well culture dishes containing 500 μl migration buffer in the lower chamber. Twenty-five thousand HUVECs in 50 μl of migration buffer were added to the upper chamber of duplicate inserts containing 50 μl of a solution of 50 μg/ml of an anti-α5β1 function-blocking antibody (NKI-SAM-1, JBS5 or IIA1), 50 μg/ml of an anti-α5β1 non-function-blocking antibody (HA5 or VC5), or 50 μg/ml of LM609, an anti-αvβ3 function-blocking antibody in migration buffer (Hepes-buffered M199 medium containing 1% BSA, 2 mmol/L MgCl2, 2 mmol/L CaCl2, and 0.2 mmol/L MnCl2) or migration buffer alone. Cells were allowed to migrate from the upper to the lower chamber for 4 hours at 37°C. Nonmigratory cells were removed from the upper surface by wiping the upper side with an absorbant tip. Cells that had migrated to the lower side of the transwell insert were then fixed for 15 minutes with 3.7% paraformaldehyde in PBS and stained with a 2% crystal violet solution. After extensive water washing to remove excess crystal violet, the number of cells that had migrated were counted in three representative high power (200×) fields per insert. The data are presented as number of cells migrating ± SEM.
Integrin Receptor Ligand Binding Assays
Integrin αvβ3 and α5β1 receptors purified from human placenta were obtained from Chemicon International. Platelet integrin αIIbβ3 was purified from platelets according to established procedures. Receptors were coated (100 μl/well) on Costar (3590) high capacity binding plates overnight at 4°C. Coating solution was discarded and plates were washed once with blocking/binding (B/B) buffer (50 mmol/L Tris-HCl, pH 7.4, 100 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mmol/L MnCl2, and 1% BSA). One hundred ten microliters of B/B buffer was applied for 60 minutes at room temperature. Thirty microliters of biotinylated extracellular matrix protein ligand (fibronectin for integrin α5β1, vitronectin for integrin αvβ3, and fibrinogen for integrin αIIbβ3) plus 50 μl of either SJ749 in B/B buffer or B/B buffer alone were added to each well, and incubated for 25 minutes at room temperature. Plates were washed twice with B/B buffer and incubated 1 hour at room temperature, with anti-biotin alkaline phosphatase (100 μl/well) in B/B buffer. Finally, plates were washed twice with B/B followed by the addition of 100 μl of phosphatase substrate (1.5 mg/ml). Reaction was stopped by adding 2 N NaOH (25 μl/well), and developed color was read at 405 nm.
In Ovo Chick Chorioallantoic Membrane Angiogenesis Assays
Ten-day-old embryonated chicken eggs were candled to illuminate blood vessels under the shell and an area with a minimum of small blood vessels is identified. The CAM was dropped away from the eggshell in this area by grinding a small hole in the mineralized shell and applying pressure to the underlying inner shell membrane. This caused an air pocket to shift from the wide end of the egg to the identified area and forced a circular region of the CAM approximately 2 cm in diameter to drop away from the shell. A window was cut in the egg shell and a cortisone acetate pretreated filter disk 5 mm in diameter that had been saturated in 1 μg/ml bFGF, VEGF, TNF-α, IL-8, or saline was placed on the CAM. The window in the shell was sealed with adhesive tape and the egg was incubated for 4 days. A range of 0 to 25 μg in 25 μl of function-blocking anti-α5β1 or a control non-function-blocking anti-α5β1, 0 to 25 μmol/L in 25 μl cyclic peptide (CRRETAWAC) or scrambled control peptide (CATAERWRC), 0 to 25 μmol/L in 25 μl of a small molecule antagonist of integrin α5β1 (SJ749), an inactive control small molecule (XU065), or 25 μl of saline were applied to the growth factor-saturated filter 24 hours later. Anti-fibronectin antibodies (25 μg in 25 μl) were also applied topically to the CAM. Fibronectin, vitronectin, and fibronectin fragments (59 pmoles in a final volume of 25 μl) were applied to stimulated or unstimulated CAMs. Peptide or small molecule antagonists of α5β1 (at a final serum concentration of 0 to 25 μmol/L) were also injected intravenously into the chick circulation 24 hours later. CAMs were harvested on the fourth day of stimulation by fixation with a drop of 3% paraformaldehyde in PBS before excision of the stimulated area. Blood vessel branch points in the 5-mm filter disk area were counted at 30× magnification under fiber optic illumination in a blinded fashion as a size-independent quantitative indicator of vascular sprouting in response to growth factors. As angiogenesis is characterized by the sprouting of new vessels in response to growth factors, counting blood vessel branch points is a useful quantitative means of obtaining an angiogenic index.52 At least 10 embryos were used per treatment group. Each experiment was performed a minimum of three times. Data were evaluated in terms of average number of blood vessel branch points per treatment group ± SEM. Statistical analyses were performed using Student’s t-test. Representative CAMS from each treatment group were photographed at 10× magnification.
In some cases, CAM tissue excised from the egg was frozen in OCT in liquid nitrogen, cut into 5-μm sections, air dried, and processed as described in immunohistochemistry methods, without fixation.
Chick Chorioallantoic Membrane Tumor Assays
Ten million tumor cells were placed on the surface of each CAM and cultured for 1 week. The resulting tumors were excised and cut into 50-mg fragments. These fragments were placed on additional CAMs and treated topically the following day with 25 μg in 25 μl of anti-α5β1 or a control non-function-blocking anti-α5β1, or systemically by intravenous injection with a final serum concentration of 25 μmol/L cyclic peptide CRRETAWAC or 25 μmol/L small molecule antagonist of integrin α5β1 (SJ749) and 25 μmol/L scrambled control peptide CATAERWRC or 25 μmol/L inactive small molecule (XU065) or 25 μl of saline. Forty-eight hours later, CAMs were excised from the egg and the number of blood vessels entering the tumors were counted (as vessel branch points). The data are presented as mean blood vessel number per treatment group (± SEM). Each treatment group incorporated at least 10 tumors per experiment. Representative tumors were photographed at 10× magnification. Tumors were then excised from the egg and weighed. The data are presented as mean tumor weight per treatment group (± SEM). Statistical analyses were performed using Student’s t-test. In some cases, excised tumors were fixed in 3% paraformaldehyde, embedded in paraffin, and sectioned before immunohistochemical analysis for the presence of blood vessels.
SCID Mouse Model of Human Angiogenesis
Engraftment of SCID mice with human skins was performed as previously described.53 SCID mice were engrafted with an 8 mm × 13 mm piece of human neonatal foreskin. Four weeks later, after the skin had completely healed, 50 μl of growth factor depleted matrigel reconstituted with 1 μg/ml bFGF, with 1 μg/ml bFGF containing 25 μg/ml anti-α5β1 function-blocking monoclonal antibody or with 1 μg/ml bFGF containing 25 μg/ml non-function-blocking anti-α5β1 monoclonal antibody was injected intradermally in the center of each engrafted skin. Three days later, the human skin was excised from the mouse. Boundaries were easily observed because the human skin was pink and hairless; the mouse skin was covered with white fur. The human skin was embedded in freezing medium, frozen, and sectioned. Sections were stained for the presence of human blood vessels with human specific anti-CD31, as described in immunohistochemical analyses of blood vessel densities. The data are presented as mean CD31-positive blood vessel numbers per 100× microscopic field, ± SEM. Statistical analyses were performed using Student’s t-test.
Results
Enhanced Expression of Fibronectin and Its Receptor Integrin α5β1 on Tumor-Associated Blood Vessels
Although previous reports have implicated αv integrins in angiogenesis,9,32-37 recent studies suggest that alternative adhesion proteins regulate angiogenesis in the absence of αv expression.10 In addition, studies of integrin α5β1 null mice8,48 and fibronectin null mice7 suggest that the integrin α5β1 and its ligand fibronectin may be required for the proper formation of the vasculature during development. However, it is unclear from these studies whether fibronectin and integrin α5β1 play direct roles in angiogenesis. To determine whether fibronectin and its receptor, integrin α5β1, are expressed during angiogenesis, we evaluated their expression patterns on the vasculature in human normal and tumor tissues (Figure 1)▶ and in response to growth factor stimulation in animal models of angiogenesis (Figure 2)▶ .
Figure 1.
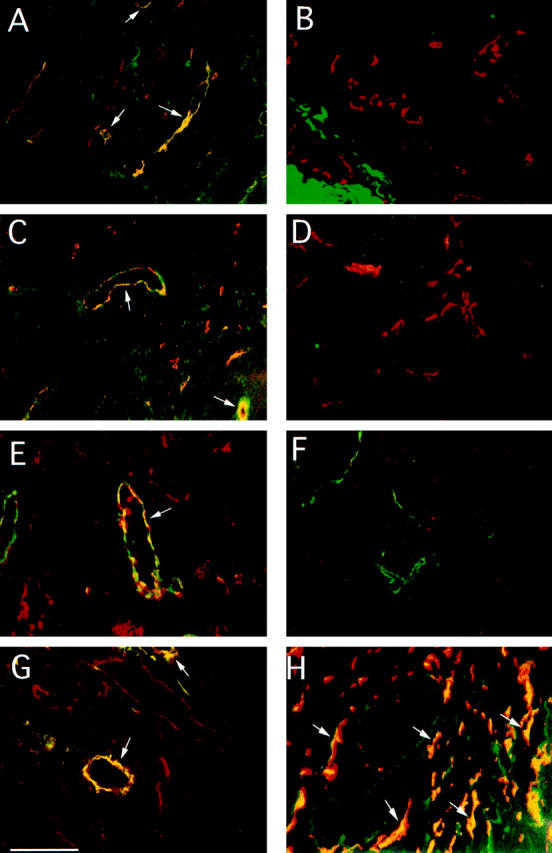
Expression of integrin α5β1 and fibronectin on human and murine tumor blood vessels. Cryostat sections 5 μm in width of human colon carcinoma (A), normal colon (B), breast carcinoma (C, E, and G), normal breast (D and F), and subcutaneous human tumor xenotransplants in SCID mice (H) were analyzed by fluorescence microscopy at 200× magnification for expression of integrin α5β1, fibronectin, CD31, or vWF, as described in Materials and Methods. A−D, H: Tissue sections stained for integrin α5β1 (FITC) and CD31 (rhodamine) expression. E and F: Tissue sections stained for fibronectin (rhodamine) and vWF (FITC) expression. G: Tissue sections stained for integrin α5β1 (FITC) and fibronectin (rhodamine) expression. Merged images of these tissues stained with both antibodies indicate where colocalization (yellow) occurs. Representative costaining vessels are indicated by arrows. Scale bar, 10 μm.
Figure 2.

Enhanced expression of integrin α5β1 and fibronectin on blood vessels after growth factor stimulation. Cryostat sections of normal unstimulated (A and B), bFGF-stimulated (C and D), or VEGF-stimulated CAMs (E and F) were stained with anti-integrin α5β1 (rhodamine) and anti-vWF (FITC) antibodies (A, C, and E), anti-fibronectin (rhodamine) and anti-vWF (FITC) antibodies (B, D, and F). Merged images of these tissues stained with both antibodies indicate where colocalization (yellow) occurs. Scale bar, 10 μm.
Analysis of frozen sections of human colon carcinoma and breast carcinoma for expression of the endothelial cell marker CD31 (PECAM) and integrin α5β1 by two-color immunohistochemistry indicated that CD31-positive tumor vessels (red) were also positive for integrin α5β1 expression (green). Vessels positive for both molecules are shown in yellow (Figure 1, A and C)▶ . Large vessels with lumens, as well as large and small vessels without apparent lumens, stain positively for integrin α5β1 and CD31. Sections of ovarian and pancreatic carcinoma showed similar patterns of integrin α5β1 expression on blood vessels (not shown).
In contrast, CD31-positive blood vessels (red) routinely present in sections of normal human colon and breast were negative for integrin α5β1 (Figure 1, B and D)▶ . Blood vessels in other normal adult tissues, including skin, were also negative for integrin α5β1 (data not shown). These results indicate that integrin α5β1 expres- sion is up-regulated on tumor vasculature and that the majority of blood vessels in these tumor sections are integrin α5β1 positive. Furthermore, these studies indicate that integrin α5β1 is not significantly expressed on blood vessels in normal adult tissues.
We next stained tumor tissues with antibodies directed against fibronectin (red) and vWF (green), another marker of blood vessels. Examination of frozen sections of breast carcinoma (Figure 1E)▶ and colon carcinoma (data not shown) as well as normal human breast (Figure 1F)▶ and colon (data not shown) indicated that the extracellular matrix surrounding tumor vessels was positive for fibronectin expression (arrows). In contrast, blood vessels in normal tissues expressed little, if any, fibronectin. Sections of ovarian and pancreatic carcinoma showed similar patterns of fibronectin expression on blood vessels (not shown).
Notably, the expression of integrin α5β1 (green) and its ligand, fibronectin (red), were coordinately up-regulated on many of the same blood vessels (yellow) within human tumor sections (Figure 1G)▶ , suggesting a possible functional interaction between these two proteins. Expression of integrin α5β1 and fibronectin were also observed on tumor vasculature in animal models of neoplasia, including human M21L melanoma tumor xenotransplants in SCID mice (Figure 1H)▶ and spontaneous mammary tumors (data not shown) in Mtag transgenic mice expressing the polyoma virus (PyV) middle T antigen under control of the mouse mammary tumor virus.54 Thus, significantly elevated expression of integrin α5β1 and fibronectin is associated with the vasculature in spontaneous as well as experimentally induced human and murine tumors compared to normal tissues.
In Vivo Up-Regulation of Fibronectin and Integrin α5β1 Expression in Response to Angiogenic Growth Factors
To determine whether fibronectin and integrin α5β1 are functionally involved in angiogenesis, chick chorioallantoic membranes (CAMs) were stimulated with angiogenic growth factors and cryostat sections of these tissues were stained with antibodies to fibronectin and α5β1. Integrin α5β1 expression on the pre-existing vasculature of unstimulated CAMs was minimal (Figure 2A)▶ but was significantly up-regulated 24 hours after exposure to bFGF (Figure 2C)▶ , TNF-α, or IL-8 (data not shown). In contrast, VEGF did not induce α5β1 expression (Figure 2E)▶ . Integrin α5β1 was not noticeably expressed on other cell types in the CAM.
Fibronectin expression in the extracellular matrix surrounding blood vessels was also minimal on unstimulated CAM tissue (Figure 2B)▶ and, like α5β1, was significantly enhanced after bFGF (Figure 2D)▶ , TNF-α, and IL-8 (data not shown) stimulation. Fibronectin expression in the extracellular matrix surrounding blood vessels was also up-regulated after VEGF stimulation (Figure 2F)▶ . Fibronectin expression was principally found in association with blood vessels and minimally on other cell types in these tissues. These results indicate that integrin α5β1 and fibronectin expression are both up-regulated during angiogenesis and that α5β1 and fibronectin are found closely associated with each other on growth factor-stimulated blood vessels.
Inhibition of Angiogenesis by Antibody Antagonists of Fibronectin
Since fibronectin was localized to α5β1-expressing blood vessels in tumors and growth factor-treated tissues, the effects of function-blocking anti-fibronectin antibodies on angiogenesis were evaluated. An antibody directed against the central cell-binding domain peptide (CBP) of human and chicken fibronectin was first tested for its ability to inhibit cell adhesion to fibronectin in vitro. This antibody significantly inhibited the adhesion to fibronectin of integrin α5β1-positive cells, including α5β1-positive HT29 colon carcinoma cells, chick embryo fibroblasts (CEF), and HUVECs. HUVEC adhesion was blocked 70 ± 3% by the anti-CBP antibody (Figure 3A)▶ . In contrast, antibodies directed against the N-terminal domain (NT) of fibronectin were ineffective in blocking cell adhesion to fibronectin (Figure 3A)▶ .
Figure 3.

Role of fibronectin in angiogenesis. A: Adhesion of HUVECs to fibronectin in the presence of adhesion medium (medium) or 25 μg/ml antibodies directed to the cell-binding peptide region of fibronectin (Anti-CBP) or to the N-terminal heparin binding region of fibronectin (Anti-NT). B: Angiogenesis induced on the CAM by bFGF or VEGF in the presence of saline, 25 μg of an antibody directed against the cell-binding peptide of fibronectin (Anti-CBP) or 25 μg of an antibody directed against the fibronectin N-terminus (Anti-NT). The number of blood vessel branch points within a standard 5-mm area are shown. C: bFGF-induced angiogenesis on the CAM in the presence of saline or equimolar amounts of full length fibronectin, the 120-kd cell-binding fibronectin fragment, the 40-kd fibronectin fragment, or full length fibronectin plus 10 μg anti-integrin α5β1. The data are presented as blood vessel branch points above background; * indicates treatments that resulted in significantly different numbers of vessel branch points than bFGF treatment alone, fibronectin (P = 0.05), and 120-kd fibronectin fragments (P = 0.05).
To assess the role of fibronectin in angiogenesis in vivo, CAMs from ten-day-old embryos were stimulated with bFGF or VEGF. Twenty-four hours later, anti-fibronectin antibodies were directly applied to the CAMs (Figure 3, B and C)▶ . Two days later, CAMs were excised and blood vessels were quantified by counting vessel branch points, as described.52 The counting of blood vessel branch points provides a size-independent measure of the sprouting of new vessels that occurs during angiogenesis. The anti-CBP antibody was able to inhibit the growth of new blood vessels induced by bFGF by 75 ± 10% (P = 0.002), whereas the anti-NT antibody had a minimal effect on angiogenesis (34 ± 15% inhibition, P = 0.02) as shown in Figure 3B▶ . The anti-CBP antibody also inhibited VEGF angiogenesis by 71 ± 7% (P = 0.02), as did the anti-NT antibody (89 ± 17% inhibition, P = 0.035; Figure 3C▶ ). In contrast to anti-fibronectin antibodies, function-blocking antibodies directed against vitronectin failed to block angiogenesis significantly (not shown). These results indicate that the cell-binding domain of fibronectin plays a critical role in angiogenesis. The N-terminal domain of fibronectin may also contribute to some angiogenesis.
Enhancement of Growth Factor-Induced Angiogenesis by Fibronectin or Its 120-kd Cell-Binding Domain
To demonstrate further if there is a specific functional association between fibronectin and angiogenesis stimulation, fibronectin and vitronectin were directly applied to the CAMs of 10-day-old embryos in the presence or absence of growth factors. Neither fibronectin nor vitronectin applied to CAMs in the absence of growth factors promoted angiogenesis, as we have previously documented.55 Equimolar amounts of intact human fibronectin, a 120-kd fragment of fibronectin with the RGD containing cell-binding domain or a 40-kd C-terminal chymotryptic fibronectin fragment, which lacks the RGD containing cell-binding domain56,57 were applied to bFGF-stimulated CAMs. As shown in Figure 3C▶ , intact fibronectin enhanced growth factor-stimulated angiogenesis at least 46 ± 11% (P = 0.04). The 120-kd cell-binding fragment of fibronectin also significantly enhanced angiogenesis (65 ± 20%; P = 0.05), whereas the 40-kd fragment of fibronectin had no significant effect. This fibronectin-enhanced angiogenesis was dependent on integrin α5β1 activity, since anti-integrin α5β1 antibodies reversed this process (Figure 3C)▶ . Application of vitronectin to bFGF stimulated CAMs had no effect on vessel number (data not shown). Application of either fibronectin or vitronectin to VEGF-stimulated CAMs did not potentiate the angiogenic effect of VEGF (data not shown). These results suggest that fibronectin and the endothelial cell integrin α5β1 play critical functional roles in growth factor-induced angiogenesis.
Antibody, Peptide, and Nonpeptide Antagonists of Integrin α5β1 Selectively Block Adhesion and Migration on Fibronectin
Because integrin α5β1 is one of the primary receptors for fibronectin on endothelial cells and colocalizes with fibronectin on blood vessels in tumors and in growth factor-stimulated tissues, experiments were designed to evaluate the effects of monoclonal antibody, peptide, and nonpeptide antagonists of integrin α5β1 on angiogenesis in vivo. To demonstrate the efficacy of these three classes of inhibitors, we first tested these α5β1 antagonists for their abilities to interfere with the attachment and migration of three types of integrin α5β1-positive cells: HT29 colon carcinoma integrin α5 transfectants, CEF, and HUVEC. Function-blocking monoclonal antibody antagonists of integrin α5β1, but not control (non-function-blocking) anti-integrin α5β1 monoclonal antibodies, selectively inhibited HT29 α5+ (100 ± 6%), CEF (89.7 ± 3.4%), and HUVEC (72 ± 2.5%) adhesion to fibronectin (Figure 4A)▶ . Function-blocking monoclonal antibody antagonists of integrin α5β1, did not block attachment of HUVECs (Figure 4A)▶ , HT29 or CEF cells to vitronectin, although LM609, an anti-αvβ3 specific antibody, did (Figure 4A)▶ . These results demonstrate that α5β1 antagonists selectively block human and chick α5β1-mediated cell adhesion to fibronectin, as well as endothelial cell α5β1-mediated adhesion to fibronectin.
Figure 4.
Inhibition of cell adhesion and migration by integrin α5β1 antagonists. A: The adhesion of HT29 colon carcinoma cells transfected with the integrin α5 cDNA (HT29 α5-positive), chick embryo fibroblasts (CEF) and human umbilical vein endothelial cells (HUVEC) to fibronectin in the presence of 25 μg/ml function-blocking (▪) or non-function-blocking anti-integrin α5β1 antibodies (▨) expressed as percent of cells adhering in adhesion buffer alone. The adhesion of HUVECs to vitronectin in the presence of 25 μg/ml function-blocking anti-α5β1 antibodies (▪) or LM609, a function-blocking anti-integrin αvβ3 antibody (▩) B: The adhesion of HT29 α5-positive cells, CEFs, and HUVECs to fibronectin in the presence of 10 μmol/L cyclic peptide CRRETAWAC (▪) or the control scrambled peptide, CATAERWRC (▨). C: Adhesion of HT29 α5-positive cells to fibronectin in the presence of dilutions of the small molecule integrin α5β1 antagonist SJ749. D: Migration of HUVECs on fibronectin or collagen in the presence of migration medium (▪), 25 μg/ml function-blocking (▨), and 25 μg/ml non-function-blocking (▩) antibodies to integrin α5β1 or 25 μg/ml antibodies to integrin β1 ( ).
Non-antibody antagonists of integrin α5β1 also potently inhibit cell attachment to fibronectin. A selective cyclic peptide antagonist of integrin α5β1, CRRETAWAC,56,57 also significantly inhibited α5+ HT29 colon carcinoma, CEF and HUVEC cell adhesion to fibronectin (Figure 4B)▶ but not to vitronectin (not shown). A scrambled control peptide (CATAERWRC) had little impact on cell adhesion to either fibronectin (Figure 4B)▶ or vitronectin (not shown). Furthermore, a selective nonpeptide antagonist of integrin α5β1, SJ749 {(S)-2-[(2,4,6-trimethylphenyl) sulfonyl]amino-3-[7-benzyloxycarbonyl-8-(2-pyridinylaminomethyl)−1-oxa-2,7]-diazaspiro-(4,4)-non-2-en-3-yl] carbonylamino] propionic acid}, blocked the adhesion of each of these cell types to fibronectin in a concentration-dependent manner with a half maximal inhibitory concentration of 0.8 μM for α5+ HT29 cells (Figure 4C)▶ . SJ749 was ineffective in blocking cell attachment to vitronectin or other extracellular matrix ligands (Table 1)▶ . This compound selectively inhibited ligand binding to integrin α5β1 and was substantially less effective in blocking ligand binding to integrin αvβ3 and other integrins (Table 1)▶ . These results demonstrate that all three classes of α5β1 antagonists significantly and selectively inhibit human and chick α5β1 functions.
Table 1.
Integrin Selectivity of SJ749
| Assay type | IC50 | ||||
|---|---|---|---|---|---|
| α5β1/FN | αvβ3/VN | αIIbβ3/FBG | αvβ5/VN | α2β1/COL | |
| Purified receptor | 1.8 nmol/L | 1 μmol/L | >10 μmol/L | − | − |
| Cell adhesion | 340 nmol/L* | >10 μmol/L | − | >10 μmol/L† | >10 μmol/L‡ |
| Cell migration | 2.9 μmol/L§ | − | − | − | − |
*IC50 determined for α5β1-positive Jurkat cell; IC50 for HT29 α5-positive transfectants was slightly higher (800 nmol/L).
†IC50 determined for SK-BR-3 as well as HT29 tumor cells.
‡IC50 determined for HUVEC as well as HT29 adhesion to collagen.
§IC50 determined for HUVEC cell migration on fibronectin.
As angiogenesis depends in part on endothelial cell migration and invasion, the ability of these selective inhibitors of integrin α5β1 to block HUVEC migration was evaluated. Migration on fibronectin was significantly inhibited (87 ± 2%) by function-blocking antibodies directed against integrin α5β1 (Figure 4D)▶ . In contrast, this antibody did not affect endothelial cell migration on other matrix proteins, including collagen (Figure 4D)▶ . Peptide (not shown) and nonpeptide (Table 1)▶ inhibitors of integrin α5β1 were also highly effective in blocking endothelial cell migration on fibronectin but not on other matrix protein such as collagen.
Antagonists of Integrin α5β1 Block Angiogenesis in Vivo
To establish whether integrin α5β1 might contribute to angiogenesis, we evaluated the abilities of these same integrin α5β1 antagonists to impact growth factor-induced angiogenesis on the chick CAM. Twenty-four hours after stimulating angiogenesis on the CAM with bFGF, antagonists of integrin α5β1 were applied directly to the growth factor-saturated filter disk or were injected intravenously into the embryonic circulation.
As shown in Figure 5, A, B, and E▶ , antibody antagonists of integrin α5β1 applied topically (Figure 5, A and B)▶ or intravenously (Figure 5E)▶ blocked bFGF-induced angiogenesis on the CAM by at least 88 ± 6% (P = 0.01) whereas control non-function-blocking anti-α5β1 antibodies had no significant effect. Applications of function-blocking or control anti-α5β1 antibodies to unstimulated CAMs had no effect on the number or integrity of blood vessels present within the application area (data not shown). Similar to antibody antagonists of α5β1 and as predicted by our previous studies,36 antibody antagonists of αvβ3 also blocked angiogenesis induced by bFGF by 65 ± 10% (P = 0.008).
Figure 5.

Inhibition of angiogenesis by anti-integrin α5β1 antibody, peptide, and nonpeptide small molecule antagonists. A: Chick chorioallantoic membranes stimulated by bFGF were treated with either saline, 10 μg anti-α5β1 monoclonal (Anti-α5β1), 10 μg non-function-blocking anti-α5β1 antibodies (control IgG), or 10 μg of anti-αvβ3 antibodies. Forty-eight hours after administering the antagonists, CAMs were excised. Selected representative CAMS were photographed at 10× magnification. B: Blood vessel branch points within the 5-mm treatment area were counted under 30× magnification using a stereo dissecting microscope for CAMs treated as in A. C: Blood vessel branch points within the 5-mm treatment area of CAMs stimulated by saline (PBS) or by bFGF and treated with saline (bFGF), 750 pmoles cyclic peptide CRRETAWAC, or 750 pmoles control peptide CATAERWRC. were counted at 30× magnification. D: Blood vessel branch points within the 5-mm treatment area of CAMs stimulated by bFGF and treated with dilutions of the nonpeptide integrin α5β1 antagonist SJ749 were counted at 30× magnification and are expressed as a percentage of bFGF-induced branch points. E and F: Blood vessel branch points on bFGF-stimulated CAMs treated by intravenous injections of saline, anti-α5β1 or control antibody (P1F6), cyclic peptide CRRETAWAC, or control peptide CATAERWRC (25 μmol/L, final serum concentration), nonpeptide integrin α5β1 antagonist SJ749 or control nonpeptide XU065 were counted at 30× magnification. At least 10 embryos were used per treatment group. Each experiment was performed a minimum of three times. Data were evaluated in terms of average number of blood vessel branch points per treatment group ± SEM. Statistical analyses were performed using Student’s t-test.
Cyclic peptide (Figure 5C)▶ antagonists of integrin α5β1 also significantly blocked bFGF-induced angiogenesis by 90 ± 6% (P < 0.0001), whereas control peptides did not inhibit angiogenesis. Nonpeptide antagonists blocked bFGF-induced angiogenesis (Figure 5D)▶ in a dose-dependent manner when applied either topically or systemically; control nonpeptide molecules did not inhibit angiogenesis, even at the highest dose. Cyclic peptide and SJ749 antagonists of integrin α5β1 were equally effective in inhibiting angiogenesis when applied systemically by intravenous injection (Figure 5F)▶ . The cyclic peptide CRRETAWAC inhibited angiogenesis by 86 ± 13%, whereas SJ749 inhibited angiogenesis by 81 ± 3%.
In summary, antibody, peptide, and nonpeptide small molecule antagonists inhibited growth factor-induced angiogenesis with IC50 values of approximately 5 μg, 120 pmoles, and 15 pmoles, respectively. These results indicate that the fibronectin receptor integrin α5β1 contributes to growth factor-induced angiogenesis on the CAM.
To extend these findings, we evaluated the ability of these integrin α5β1 antagonists to block angiogenesis in an animal model of human angiogenesis. Human neonatal foreskin engrafted onto SCID mice was injected intradermally with growth factor depleted basement membrane impregnated with bFGF in the presence or absence of the function-blocking and control anti-α5β1 antibodies. Analysis of the human skin after 3 days for the presence of human CD31-positive blood vessels revealed that the function-blocking α5β1 antibody selectively blocked angiogenesis induced by the growth factor (Figure 6, A and B)▶ , reducing the number of CD31-positive blood vessels per high power field by 94 ± 4.7% (P = 0.006). These results indicate that integrin α5β1 has a functional role in the angiogenic response to growth factors of human blood vessels.
Figure 6.

Inhibition of angiogenesis by anti-integrin α5β1 in the SCID mouse/human skin chimera. Angiogenesis was induced by intradermal injection of growth factor depleted matrigel supplemented with 1 μg/ml bFGF and 25 μg/ml function-blocking or control anti-integrin α5β1 antibodies into human skin transplanted onto SCID mice. A: Anti-human CD31 immunohistochemical analysis of frozen sections of function-blocking or control treated human skin at 100× magnification. Arrows indicate human CD31-positive blood vessels. Scale bar, 10 μm. B: Quantification of CD31-positive blood vessels per 100× microscopic field in bFGF-stimulated human skin treated with function-blocking or control antibodies. The data are presented as mean CD31-positive blood vessel numbers per 100× microscopic field, ± SEM. Statistical analyses were performed using Student’s t-test.
Integrin α5β1 and αvβ3 Regulate the Same Pathways of Angiogenesis
Distinct growth factors can induce selective pathways of angiogenesis that activate and/or use distinct integrins.36 For example, integrin αvβ3 participates in the bFGF and TNF-α pathways of angiogenesis, whereas αvβ5 participates in the VEGF and transforming growth factor-α (TGF-α) pathways.36 Therefore, the effects of antagonists of integrin α5β1 on angiogenesis induced by additional growth factors were examined. When angiogenesis was stimulated with TNF-α or IL-8, antibody antagonists of integrin α5β1 blocked angiogenesis by up to 70.4 ± 12% (P = 0.04) and 85 ± 4.8% (P < 0.0001), respectively (Figure 7, A and B)▶ . In some experiments anti-α5β1 inhibited TNF-α and IL-8 angiogenesis by up to 99 ± 5% (P = 0.005). Similarly, antibody antagonists of integrin αvβ3 also blocked TNFα and IL-8 angiogenesis by 93.6 ± 6.2% (P = 0.004) and 77 ± 5.2% (P = 0.0001), respectively. However, when angiogenesis was induced with VEGF (Figure 7C)▶ , antibody antagonists of integrin α5β1 failed to block angiogenesis, although an antibody antagonists of integrin αvβ5 did block angiogenesis by 99 ± 0.1% (P = 0.004), as we previously reported.36 Peptide and nonpeptide antagonists of integrin α5β1 also failed to block VEGF angiogenesis. These results suggest that integrin α5β1 regulates the same pathway of angiogenesis as does integrin αvβ3 and that this pathway is distinct from that regulated by integrin αvβ5. When anti-integrin α5β1 and anti-integrin αvβ3 antibodies were applied to bFGF-stimulated CAMs either alone or together, no additive or synergistic inhibitory effects were observed (data not shown). These results suggest that these integrins αvβ3 and α5β1 participate in the same angiogenic pathway.
Figure 7.
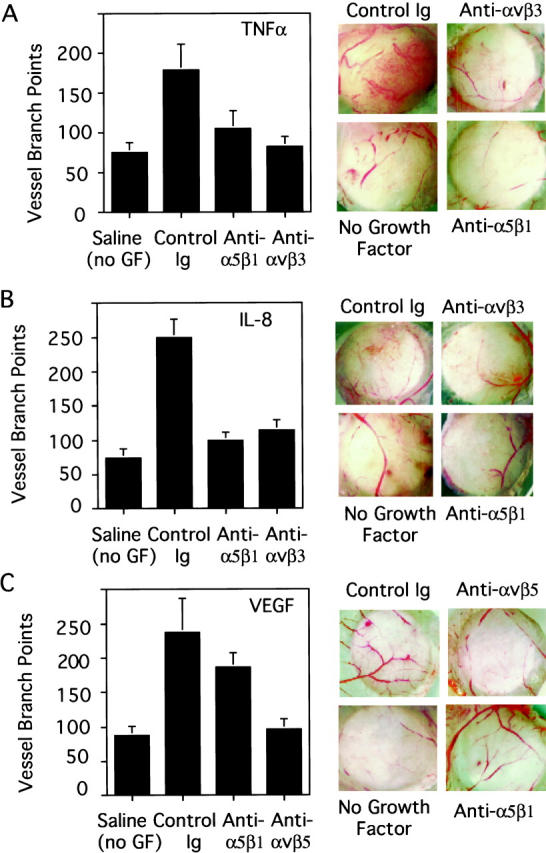
Inhibition of TNF-α and IL-8 but not VEGF angiogenesis by integrin α5β1 antagonists. Chick chorioallantoic membranes stimulated by TNFα (A), IL-8 (B), or VEGF (C) were treated with either saline, 25 μg anti-α5β1 monoclonal (Anti-α5β1), 25μg non-function-blocking anti-α5β1 antibodies (control IgG), or 25 μg of anti-αvβ3 antibodies (TNF-α- or IL-8-stimulated CAMs) or 25 μg of anti-αvβ5 antibodies (VEGF-stimulated CAMs). Forty-eight hours after administering the antagonists, CAMs were excised. Blood vessel branch points within the 5-mm treatment area were counted under 30× magnification using a stereo dissecting microscope (Left panels). At least 10 embryos were used per treatment group. Each experiment was performed a minimum of three times. Data were evaluated in terms of average number of blood vessel branch points per treatment group ± SEM. Statistical analyses were performed using Student’s t-test. Right panels: Selected representative CAMS were photographed at 10× magnification.
Integrin α5β1 Is Required for Human Tumor Angiogenesis
The enhanced expression of α5β1 on tumor-associated blood vessels and its functional role in growth factor-stimulated angiogenesis prompted us to examine its role in tumor angiogenesis and growth. HT29 colon carcinoma cells, which lack α5β1 expression, were grown on the CAMs of 10-day-old embryos. These tumor cells have been shown to secrete several angiogenic growth factors that include VEGF, TGF-α, TGF-β, TNF-α, and IL-8.49,58,59 Integrin α5β1-negative tumor cells were used to distinguish the potential anti-tumor effects from anti-vasculature effects of integrin α5β1 antagonists. The tumor-bearing embryos were treated with doses of either function-blocking or non-function-blocking antibodies directed to integrin α5β1. Tumors were excised after several days of treatment and the number of tumor-associated blood vessels was assessed under a stereo microscope.
Treatment with anti-α5β1 function-blocking, but not control, antibodies resulted in significant reduction (70 ± 10%, P = 0.02) of the number of tumor-associated blood vessels as measured by quantification of blood vessels entering tumors (Figure 8, A and B)▶ or blood vessel density present in tumors (Figure 8, F and G)▶ . No significant differences were observed between saline and control antibody treated tumors or their associated blood vessels. Importantly, treatment with function-blocking anti-α5β1 antibodies resulted in tumor regression. Anti-α5β1 treated tumors were 32% smaller than control treated tumors (P = 0.02; Figure 8C▶ ). Control antibody treated tumors increased in size by 25% whereas anti-α5β1 antibody treated tumors decreased in size by 15%. In support of these findings, systemic (intravenous) administration of cyclic peptide inhibitors of integrin α5β1 (Figure 8D)▶ and nonpeptide small molecule inhibitors of integrin α5β1 (Figure 8E)▶ also induced tumor regression on the CAM while control peptide and control nonpeptide treated tumors continued to increase in size. Tumors treated with peptide inhibitors were 31% smaller than control treated tumors (P = 0.003). Control peptide treated tumors increased in size by 20% whereas the α5β1 peptide antagonist treated tumors decreased in size by 17%. Tumors treated with nonpeptide (SJ749) inhibitors were 51% smaller than control-treated tumors (P = 0.003). Control organic molecule treated tumors increased in size by 78% whereas the α5β1 organic antagonist treated tumors decreased in size by 13%. Tumor cells remained integrin α5β1 negative throughout the course of the experiment (data not shown), suggesting the anti-tumor effects were based on the targeting of the tumor associated blood vessels.
Figure 8.


Inhibition of tumor angiogenesis by antagonists of integrin α5β1. Tumor fragments (50 mg) cultured on CAMs were treated topically with function-blocking or control anti-α5β1 or systemically with active (CRRETAWAC) and control (CATAERWRC) peptides and active (SJ749) and control small molecule inhibitors of integrin α5β1. Forty-eight hours later, CAMs were excised from the egg and representative tumors from antibody treatment groups were photographed under 10× magnification (A). Tumor-associated blood vessels were quantified by counting blood vessel branch points. The data are presented as mean blood vessel number per treatment group (± SEM). Each treatment group incorporated at least 10 tumors per experiment (B). Tumors excised from the egg and tumor weights were determined for the antibody (C), peptide (D), and nonpeptide antagonist (E) treatment groups. The data are presented as mean tumor weight per treatment group (± SEM). Each treatment group incorporated at least 10 tumors per experiment. Immunohistochemical analysis of frozen sections from representative tumors for expression of vWF, a marker of blood vessels. Representative 200× fields were photographed (F). Arrows indicate individual vWF-positive vessels. vWF-positive blood vessels were counted in random 200× fields from each of 6 tumors per treatment group (G). The data are presented as mean blood vessel number per treatment group (± SEM). Hematoxylin-and-eosin-stained, paraffin-embedded sections of control and anti-α5β1-treated tumors were photographed at 100× magnification (H) and at 400× magnification (I). Arrows indicate blood vessels at the tumor periphery. All statistical analyses were performed using Student’s t-test.
Anti-α5β1 treated tumors were mostly necrotic (Figure 8H)▶ with few mitotic bodies visible (Figure 8I)▶ . In contrast, control antibody-treated tumors were invasive, actively proliferating tumors (Figure 8H)▶ with robust mitotic bodies visible (Figure 8I)▶ . Many microvessels were associated with the invasive edges of control-treated tumors, whereas only a few large vessels were present in anti-α5β1-treated tumors (Figure 8H)▶ . These results demonstrate that targeting vascular cell integrin α5β1 can lead to inhibition of tumor growth and tumor angiogenesis and that antagonists of integrin α5β1 are potent inhibitors of tumor growth and tumor-induced angiogenesis.
Discussion
Vascular development during embryogenesis as well as during normal and pathological angiogenesis is regulated by growth factors and by integrins.3,4,6 While the roles of integrins αvβ3 and αvβ5 in angiogenesis have been well described,9,10,32-37 recent evidence suggests that certain β1 integrins may play roles in angiogenesis.9,10,38,60 In contrast to cell surface molecules, little is known about the extracellular matrix requirements for angiogenesis. Interestingly, genetic analyses of fibronectin- and integrin α5β1-deficient mice implicate fibronectin and integrin α5β1 in vascular development and in a number of nonvascular events.7,8,47 However, a direct functional role for either of these molecules in angiogenesis has not been previously established.
In this report, several lines of evidence demonstrate the participation of the central cell-binding domain of fibronectin and its receptor α5β1 in angiogenesis. First, expression of both integrin α5β1 and fibronectin were significantly enhanced on blood vessels of human tumors and in growth factor stimulated tissues, whereas these molecules were minimally expressed on normal human vessels and on unstimulated tissues. Second, antibody antagonists of the central cell-binding domain of fibronectin as well as three classes of integrin α5β1 antagonists (antibody, peptide, and a novel nonpeptide antagonist) blocked growth factor-stimulated angiogenesis. Antagonists of integrin α5β1 blocked bFGF-, TNF-α-, and IL-8-stimulated angiogenesis, but had a minimal effect on VEGF-induced angiogenesis. Interestingly, antagonists of fibronectin function blocked both bFGF and VEGF angiogenesis, suggesting that other fibronectin receptors may be critical for VEGF-mediated angiogenesis. Evidence was also provided that all three types of integrin α5β1 antagonists inhibit tumor angiogenesis and result in tumor regression in animal models.
Our results demonstrate that the roles of integrin α5β1 and fibronectin in angiogenesis are coordinated. When the expression of each molecule is minimal (on unstimulated, quiescent blood vessels), antagonists of each molecule and addition of fibronectin to CAMs have little effect on quiescent blood vessels. In contrast, after stimulation with growth factors, integrin α5β1 and fibronectin expression is enhanced and blood vessels become sensitive to antagonists of either molecule and to the effects of extraneous fibronectin. Importantly, VEGF stimulation does not increase α5β1 expression, supporting our observation that VEGF angiogenesis is refractory to antagonists of α5β1. This is further substantiated by Collo and Pepper,61 who found that in vitro expression of integrin α5β1 on endothelial cells was up-regulated in response to bFGF, whereas Senger and colleagues60,62 found that VEGF failed to up-regulate α5β1 expression. Thus, the functional roles of integrin α5β1 and fibronectin in angiogenesis appear to be the direct consequence of their growth factor-induced expression.
Antibodies directed against the central cell-binding fragment of fibronectin, which contains the PHSRN and RGDS integrin-binding sites, inhibited angiogenesis, suggesting that these antibodies inhibit integrin ligation by fibronectin and possible downstream signal transduction events in vivo. Stimulation of bFGF angiogenesis by fibronectin and its cell-binding domain in an α5β1-dependent manner suggests that integrin α5β1 is the integrin receptor for fibronectin during angiogenesis. In fact, the absence of integrin α5β1 expression in VEGF-stimulated angiogenesis may account for the failure of fibronectin to enhance VEGF angiogenesis even though antibodies directed against the cell-binding peptide of fibronectin blocked VEGF angiogenesis. It is possible that other fibronectin binding integrins, such as αvβ1 or α3β1,63 support VEGF-induced angiogenesis. Thus, it is possible that fibronectin may bind to or activate distinct integrin receptors during VEGF versus bFGF angiogenesis. Our results are the first demonstration of a direct in vivo role for fibronectin in angiogenesis.
Our results are also the first to identify clearly a role for an extracellular matrix protein in the promotion of angiogenesis. Although collagens have been suggested to have roles in vascular development,64,65 intact collagens do not support endothelial cell outgrowth, survival, or proliferation.66,67 In fact, inhibition of the collagen receptor integrins α2β1 and α1β1 was shown to prevent the formation of large blood vessels and to promote the formation of small vessels.60 These results suggest that α2β1, α1β1, and their ligand collagen play roles in blood vessel maturation rather than the promotion of new blood vessel sprouts.
A functional role for integrin α5β1 in angiogenesis similar to that of integrin αvβ3 was clearly established when antagonists of integrin α5β1 blocked angiogenesis induced by growth factors and tumor fragments. Interestingly, integrin αvβ3, like integrin α5β1,68 can serve as a fibronectin receptor, although endothelial cells use α5β1 as the major fibronectin receptor when both integrins are expressed (data not shown). The expression of both integrins is regulated by similar growth factors. Both integrin α5β1 and αvβ3 play significant roles in bFGF-, TNF-α-, IL-8-, and tumor-induced angiogenesis, but not in VEGF-induced angiogenesis.32,36,51 These two integrins appear to influence the same angiogenesis pathways, in that combinations of their antagonists in angiogenesis animal models are neither additive nor synergistic. Such results suggest the possibility that one of these integrins functions downstream of the other. Although integrin α5β1 is clearly required for angiogenesis, it may interact with more than one ligand during angiogenesis. Integrin α5β1 can also serve as a receptor for fibrinogen on endothelial cells in vitro, though no such associations have been demonstrated in vivo.69
Ligation of integrins by extracellular matrix proteins has been shown to promote cell attachment, migration, invasion, survival, and proliferation6 via integrin signal transduction.70 Antagonists of integrin αvβ3 induce apoptosis of proliferating endothelial cells in vitro and in vivo by interrupting integrin signal transduction.32,36,37 We have also observed that antagonists of integrin α5β1 induce apoptosis of growth factor stimulated endothelial cells in vitro and in vivo (unpublished data). Interestingly, some in vitro studies suggest that integrins αvβ3 and α5β1 influence each other through cross-talk signaling events.71,72 Thus it is possible that one of these integrins regulates the actions of the other through signal transduction mechanisms during angiogenesis.
Antagonists of integrin α5β1 blocked tumor angiogenesis and growth as did antagonists of integrin αvβ3.32,51 The tumor cell lines chosen for in vivo tumorigenicity and angiogenesis studies were integrin α5β1 negative to discount any effect of the integrin antagonists on the tumor cells. The tumor cells remained integrin α5β1 negative through the course of their culture on CAMs. HT29 tumors express a variety of growth factors, including VEGF, TNF-α, TGF-α, TGF-β, PDGF, and IL-8.49,58,59 It is not known if these cells also express bFGF. Most, if not all, tumors, use multiple growth factors for angiogenesis, including IL-8, bFGF, VEGF, and others of the 15 to 20 known angiogenic growth factors. In fact, VEGF is most commonly associated with the hypoxic core of the tumor as it is transcriptionally regulated by hypoxia, whereas bFGF and other factors are associated with the growing edge of the tumor.72-74 Thus, it is not unexpected that angiogenesis induced by the complex mixture of growth factors expressed by the HT29 colon carcinoma can be inhibited by antagonists that do not impact VEGF angiogenesis. As observed for growth factor-stimulated CAMs, antagonists of integrin α5β1 did not impact large pre-existing vessels on the CAM that underlay the tumors. These results suggest that inhibitors of integrin α5β1, like inhibitors of integrin αvβ3, may provide clinical benefits to patients with certain solid tumors.
Acknowledgments
We thank Dave Beckman and Traci Libby of Chemicon International for generous gifts of anti-fibronectin and anti-integrin α5β1 monoclonal antibodies.
Footnotes
Address reprint requests to Judith A. Varner, Department of Medicine/Cancer Center, Cellular and Molecular Medicine East, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0684. E-mail: jvarner@ucsd.edu.
Supported by a grant from the Charles Stern and Anna Stern Foundation and grant R01 CA71619 from the National Cancer Institute to J. A. V.
References
- 1.Breier G, Risau W: The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol 1996, 6:454-456 [DOI] [PubMed] [Google Scholar]
- 2.Breier G, Damert A, Plate KH, Risau W: Angiogenesis in embryos and ischemic diseases. Thromb Haemost 1997, 8:678-683 [PubMed] [Google Scholar]
- 3.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 4.Risau W: Mechanism of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 5.Stromblad S, Cheresh DA: Integrins, angiogenesis and vascular cell survival. Chem Biol 1996, 3:881-885 [DOI] [PubMed] [Google Scholar]
- 6.Varner JA: The role of vascular cell integrin αvβ3 and αvβ5 in angiogenesis. Exs 1997, 79:361-390 [DOI] [PubMed] [Google Scholar]
- 7.George EL, Georges EN, Patel-King RS, Rayburn H, Hynes RO: Defects in mesodermal migration and vascular development in fibronectin-deficient mice. Development 1993, 119:1079-1091 [DOI] [PubMed] [Google Scholar]
- 8.Yang JT, Rayburn H, Hynes RO: Embryonic mesodermal defects in α5 integrin-deficient mice. Development 1993, 119:1093-1105 [DOI] [PubMed] [Google Scholar]
- 9.Bloch W, Forsberg E, Lentini S, Brakebusch C, Martin K, Krell HW, Weidle UH, Addicks K, Fassler R: β1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol 1997, 139:265-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bader BL, Rayburn H, Crowley D, Hynes RO: Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 1998, 95:507-519 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KL, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of VEGF gene. Nature 1996, 380:439-442 [DOI] [PubMed] [Google Scholar]
- 12.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 13.Fong GH, Rossant J, Gersenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 14.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NPH, Risau W, Ullrich A: High affinity VEGF binding and development expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 15.Shalaby F, Ho J, Stanford WL, Fischer K-D, Schuh AC, Schwartz L, Bernstein A, Rossant J: A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 1997, 89:981-990 [DOI] [PubMed] [Google Scholar]
- 16.Basilico C, Moscatelli D: The FGF family of growth factors and oncogenes. Adv Cancer Res 1992, 59:115-165 [DOI] [PubMed] [Google Scholar]
- 17.Klein S, Roghani M, Rifkin DB: Fibroblast growth factors as angiogenesis factors. Exs 1997, 79:159-192 [DOI] [PubMed] [Google Scholar]
- 18.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N: Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995, 95:1789-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M: Involvement of interleukin-8, vascular endothelial growth factor and basic fibroblast growth factor in tumor necrosis growth alpha dependent angiogenesis. Mol Cell Biol 1997, 17:14015-4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong HL, Hecht D, Song W, Kovesdi I, Hackett NR, Yayon A, Crystal RG: Regional suppression of the extracellular domain of the flt-1 vascular endothelial growth factor receptor. Hum Gene Ther 1998, 9:823-833 [DOI] [PubMed] [Google Scholar]
- 21.Stan AC, Nemati MN, Pietsch T, Walter GF, Dietz H: In vivo inhibition of angiogenesis and growth of the human U-87 malignant tumor by treatment with an antibody against bFGF. J Neurosurg 1995, 82:1044-1052 [DOI] [PubMed] [Google Scholar]
- 22.Chopra V, Dinh TV, Hannigan EV: Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J Cancer Res Clin Oncol 1997, 123:167-172 [DOI] [PubMed] [Google Scholar]
- 23.Czubayko F, Liaudet-Coopman ED, Aigner A, Tuveson AT, Berchem GJ, Wellstein A: A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med 1997, 3:1137-1140 [DOI] [PubMed] [Google Scholar]
- 24.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, Pietra LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 25.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM: Inhibition of interleukin-8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994, 179:1409-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strieter RM, Kunkel SL, Elner VM, Martoryi CL: Interleukin-8: a corneal factor that induces neovascularization. Am J Pathol 1992, 141:1279-1284 [PMC free article] [PubMed] [Google Scholar]
- 27.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM: Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in mice. J Clin Invest 1996, 97:2792-2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M: Expression of interleukin-8 by human melanoma cells upregulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997, 151:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 29.Keane MP, Arenberg DA, Lynch JP, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGivine B, Kunkel SL, Strieter RM: The CXC chemokines, IL-8 and IL-10, regulate angiogeneic activity in idiopathic pulmonary fibrosis. J Immunol 1997, 159:1437-1443 [PubMed] [Google Scholar]
- 30.Yatsunami J, Tsuruta N, Ogata K, Wakamatsu K, Takyama K, Kawasaki M, Nakanishi Y, Hara N, Hayashi S: Interleukin-8 participates in angiogenesis in non-small cell, but not small cell carcinoma of the lung. Cancer Lett 1997, 120:101-108 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida A, Yoshida S, Khalil AK, Ishibashi T, Inomata H: Role of NF-kappa B mediated interleukin-8 expression in intraocular neovascularization. Invest Ophthalmol Vis Sci 1998, 39:1097-1106 [PubMed] [Google Scholar]
- 32.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 33.Carron CP, Meyer DM, Pegg JA, Engleman VW, Nickols MA, Settle SL, Westlin WF, Ruminski PG, Nichols GA: A peptidomimetic antagonist of the integrin αvβ3 inhibits Leydig cell tumor growth and development of hypercalcemia of malignancy. Cancer Res 1998, 58:1930-1955 [PubMed] [Google Scholar]
- 34.Clark RAF, Tonneson MG, Gailit J, Cheresh DA: Transient functional expression of αvβ3 on vascular cells during wound repair. Am J Pathol 1996, 148:1407-1421 [PMC free article] [PubMed] [Google Scholar]
- 35.Drake CJ, Cheresh DA, Little CD: An antagonist of integrin αvβ3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 1995, 108:2655-2661 [DOI] [PubMed] [Google Scholar]
- 36.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA: Definition of two angiogenic pathways by distinct alpha v integrins. Science 1995, 270:1500-1502 [DOI] [PubMed] [Google Scholar]
- 37.Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA: Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest 1996, 98:426-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake CJ, Davis LA, Little CD: Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn 1992, 193:83-91 [DOI] [PubMed] [Google Scholar]
- 39.Magnusson MK, Mosher DF: Fibronectin: Structure, assemby, and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998, 18:1363-1370 [DOI] [PubMed] [Google Scholar]
- 40.Clark RAF, DellaPelle P, Manseua E, Lanigan JM, Dvorak HF, Colvin RB: Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol 1982, 79:269-276 [DOI] [PubMed] [Google Scholar]
- 41.Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L: The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer 1994, 59:612-618 [DOI] [PubMed] [Google Scholar]
- 42.Kaczmarek J, Castellani P, Nicolo G, Spina B, Allemanni G, Zardi L: Distribution of oncofetal fibronectin isoforms in normal hyperplastic and neoplastic human breast tissues. Int J Cancer 1994, 58:11-16 [DOI] [PubMed] [Google Scholar]
- 43.Neri D, Carnemolla B, Nissim A, Blaza E, Leprini A, Querze G, Pina A, Tarli L, Halin C, Neri P, Zardi L, Winter G: Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat Biotechnol 1997, 15:1271-1275 [DOI] [PubMed] [Google Scholar]
- 44.Sechler JL, Schwartzbauer JE: Control of cell cycle progression by fibronectin matrix architechture. J Biol Chem 1998, 273:25533-25536 [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO: Integrins: versatility, modulation and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 46.Pytela R, Pierschbacher MD, Ruoslahti E: Identification and isolation of a 140 kD cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 1985, 40:191-198 [DOI] [PubMed] [Google Scholar]
- 47.Aota S, Nomizu M, Yamada KM: The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem 1994, 269:24755-24761 [PubMed] [Google Scholar]
- 48.Goh KL, Yang JT, Hynes RO: Mesodermal defects and cranial neural crest apoptosis in α5 integrin-null embryos. Development 1997, 124:4309-4319 [DOI] [PubMed] [Google Scholar]
- 49.Varner JA, Emerson DA, Juliano RL: Integrin α5β1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell 1995, 6:725-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koivunen E, Gay DA, Ruoslahti E: Selection of peptides binding to the α5β1 integrin from a phage display library. J Biol Chem 1993, 268:20205-20210 [PubMed] [Google Scholar]
- 51.Koivunen E, Wang B, Ruoslahti E: Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J Cell Biol 1994, 124:373-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooks PC, Montgomery AMP, Cheresh DA: Use of the ten day old chick embryo model for studying angiogenesis. Methods in Molecular Biology 1999, 129:257-269 [DOI] [PubMed] [Google Scholar]
- 53.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA: Anti-integrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995, 96:1815-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guy CT, Cardiff RD, Muller WJ: Induction of mammary tumors by expression of polyoma middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992, 12:954-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T: Del-1 induces integrin signaling and angiogenesis by ligation of αvβ3. J Biol Chem 1999, 274:11101-11109 [DOI] [PubMed] [Google Scholar]
- 56.Pierchbacher MD, Hayman EG, Ruoslahti E: Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell 1981, 26:259-67 [DOI] [PubMed] [Google Scholar]
- 57.Ruoslahti E, Hayman EG, Engvall E, Cothran WC, Butler WT: Alignment of biologically active domains in the fibronectin molecule. J Biol Chem 1981, 256:7277-81 [PubMed] [Google Scholar]
- 58.Anzano MA, Rieman D, Prichett W, Bowen-Pope DF, Grieg R: Growth factor production by human colon carcinoma cell lines. Cancer Res 1989, 49:2898-2904 [PubMed] [Google Scholar]
- 59.Ellis LM, Staley CA, Liu W, Flemming RY, Parikh NU, Bucana CD, Gallich GE: Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem 1998, 273:1052-1057 [DOI] [PubMed] [Google Scholar]
- 60.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M: Angiogenesis promoted by vascular endothelial growth factors: Regulation through α1β1 and α2β1 integrins. Proc Natl Acad Sci USA 1997, 94:13612-13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collo G, Pepper MS: Endothelial cell integrin α5β1 expression is modulated by cytokines and during migration in vitro. J Cell Sci 1999, 112:569-578 [DOI] [PubMed] [Google Scholar]
- 62.Senger DR, Ledbetter SR, Caffey KP, Papadopoulos-Sergiou A, Perruzzi CA, Detmar M: Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphav beta3 integrin, osteopontin, and thrombin. Am J Pathol 1996, 149:1-7 [PMC free article] [PubMed] [Google Scholar]
- 63.Akiyama SK, Aota S, Yamada KM: Function and receptor specificty of a minimal 20 kilodalton cell adhesive fragment of fibronectin. Cell Adhes Commun 1995, 3:13-25 [DOI] [PubMed] [Google Scholar]
- 64.Löhler J, Timpl R, Jaenisch R: Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell 1984, 38:597-607 [DOI] [PubMed] [Google Scholar]
- 65.Ingber D: Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem 1991, 47:236-241 [DOI] [PubMed] [Google Scholar]
- 66.Ilan N, Mahooti S, Madri JA: Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci 1998, 111:3621-3631 [DOI] [PubMed] [Google Scholar]
- 67.Isik FF, Gibran NS, Jang YC, Sandell L, Schwartz SM: Vitronectin decreases microvascular endothelial cell apoptosis. J Cell Phys 1999, 175:149-155 [DOI] [PubMed] [Google Scholar]
- 68.Charo IF, Nannizzi L, Smith JW, Cheresh DA: The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol 1990, 111:2795-2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suehiro K, Gailit J, Plow EF: Fibrinogen is a ligand for integrin α5β1 on endothelial cells. J Biol Chem 1997, 272:5360-5366 [DOI] [PubMed] [Google Scholar]
- 70.Keely P, Parise L, Juliano R: Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol 1998, 8:101-106 [DOI] [PubMed] [Google Scholar]
- 71.Blystone SD, Graham IL, Lindberg FP, Brown EJ: Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol 1994, 127:1129-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon KO, Nutt EM, Abraham DG, Rodan GA, Duong L: The αvβ3 integrin regulates α5β1-mediated cell migration toward fibronectin. J Biol Chem 1997, 272:29380-29389 [DOI] [PubMed] [Google Scholar]
- 73.Shweiki D, Itin A, Sotter D, Keshet E: Vascular endothelial growth factor induced by hyoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 74.Kumar R, Kuniyasu H, Bucana CD, Wilson MR, Fidler IJ: Spatial and temporal expression ofangiogenesis molecules during tumor growth and angiogenesis. Oncol Res 1998, 10:301-311 [PubMed] [Google Scholar]



