Abstract
We have examined the expression of p75, a member of the TNF receptor superfamily in hepatic stellate cells (HSC) and pancreatic stellate cells (PSC). Activated HSC and PSC were demonstrated by Western blot analysis to express p75. p75 was immunolocalized to cells with a myofibroblast-like morphology in the fibrotic bands of six fibrotic and cirrhotic liver biopsies and three biopsies of fibrotic human pancreas. Immunostaining of parallel sections indicated that these cells were α-smooth muscle actin-positive, identifying them as activated HSC and PSC, respectively. HSC apoptosis in tissue culture in the presence of serum was quantified after addition of 0.1 to 100 ng/ml of nerve growth factor (NGF) a ligand for p75, by in situ counting of apoptotic bodies after addition of acridine orange. HSC demonstrated a significant increase in apoptosis in response to 100 ng/ml NGF (0.05 > P by Wilcoxon’s rank; n = 7) after 24 hours. NGF 100 ng/ml had no effect on HSC proliferation, but reduced total HSC DNA by 19% relative to control after 24 hours (n = 3). These data demonstrate that activated HSC express p75 and respond to NGF stimulation by undergoing apoptosis. We therefore report p75 as a novel marker of activated HSC and suggest that signaling via ligand binding to p75 may provide a mechanism for selective apoptosis of HSC.
In response to fibrotic injury, the hepatic stellate cell (HSC, previously termed Ito cell, lipocyte, or fat storing cell) undergoes transformation from a retinoid-rich pericyte-like cell to a myofibroblast-like cell, a process termed activation. 1,2 Studies of the morphology and gene expression of these cells have been facilitated by the observation that freshly isolated HSC plated onto uncoated tissue culture plastic undergo activation in a manner that parallels the changes in vivo during injury. 1 Data from this model, together with observations of fibrotic human liver samples and tissue derived from experimentally injured rat liver, have demonstrated that activated HSC are the major source of fibrillar collagens and other matrix proteins that characterize liver fibrosis. In addition to secretion of matrix, HSC regulate matrix degradation by the expression of matrix-degrading metalloproteinase enzymes and specific matrix-degrading metalloproteinase inhibitors, the tissue inhibitors of metalloproteinases 1 and 2. 2-9 Dysregulation of matrix degradation resulting from tissue inhibitor-mediated matrix-degrading metalloproteinase inhibition is postulated to contribute to the progression of fibrosis. 10-12 Activation of HSC is also associated with the cells entering the cell cycle, resulting in a significant increase in the overall numbers of HSC in fibrotic liver. 1,13,14 Thus, the quantitative changes in matrix observed in fibrotic liver are the result of an increase in the activated HSC pool, in addition to specific changes in HSC gene expression.
We and others have recently demonstrated that during progressive fibrosis, apoptosis of HSCs occurs. 15,16 This suggests that at any time, the total number of activated HSC within an injured liver represents the net result of proliferation and apoptosis. Moreover, recent data from our laboratory have demonstrated that spontaneous recovery from comparatively advanced fibrosis can occur and that sustained apoptosis of HSC contributes critically to that recovery. 16
For this reason, the mechanisms regulating HSC apoptosis have become an active area of investigation. Ramadori and coworkers have identified that HSC express Fas, a member of the tumor necrosis factor (TNF) receptor superfamily that responds to stimulation with the appropriate ligand (Fas-L) by triggering apoptosis via an intracytoplasmic death domain. 15,17-20 Fas expression is widespread; hepatocytes in vivo that express Fas and other myofibroblast-like cells such as mesangial cells have been demonstrated to respond to Fas-L stimulation in tissue culture. 15,18,19,21 Fas is, however, only one of a series of related TNF receptors which function to mediate cell survival, apoptosis or intercellular signaling. 20,22,23
Low affinity nerve growth factor receptor (LANGFR or p75) is a death domain-bearing member of the TNF receptor family. p75 is a receptor for the neurotrophin peptide family, of which nerve growth factor (NGF) is the paradigm member. 24 Recent work has established a model in which p75 can mediate cell survival or death depending on whether tyrosine kinase-A (Trk-A) is coexpressed. It has been proposed that costimulation of Trk-A and p75 results in cell survival, whereas when p75 is expressed in isolation, ligand binding triggers cellular apoptosis. 25-29 The role of p75 in nonneuronal tissues is not known.
Following the observations by Cattoretti et al and Wilkins and Jones that antibodies reactive with p75 identified dendritic fibroblast-like cells in bone marrow stroma, 30,31 we determined to examine p75 expression in HSC with the aim of examining the hypothesis that HSC express p75 and that stimulation of p75 by NGF is associated with HSC apoptosis. We have also examined the recently described pancreatic stellate cells (PSC) 31-33 for expression of p75, to determine whether expression of this cell receptor is potentially a more general feature of fibrogenic cells.
Materials and Methods
Cell Extraction
Hepatic stellate cells were isolated as previously described 3 and activated in primary culture on plastic in the presence of 16% fetal calf serum (FCS). Highly activated cells were obtained by passage of primary cultures of HSC and used for experiments between the first and fourth passage. Human HSC were isolated from the margin of an hepatic resection and cultured as described previously. 7 Human HSC were used after the fourth passage. Pancreatic stellate cells were extracted and cultured as described. 32,33 Cells were cultured in Dulbecco’s modified Eagle’s medium in the presence of 16% FCS and subjected to serial passage. PSC were used for experiments after the fourth passage.
Samples of Diseased Human Liver and Experimentally Induced Rat Liver Fibrosis
Six formalin-fixed, wax-embedded biopsies of fibrotic and cirrhotic human liver tissue were immunostained for p75. These consisted of three examples of early and three of advanced micronodular cirrhosis. In all six the pathology was alcoholic liver disease. Normal liver biopsy samples were obtained from the margins of two resection specimens (one for colonic cancer metastasis and the second for a simple vascular cyst.) and from four samples of unused normal donor liver that had been perfused with University of Wisconsin solution. In addition, three examples of fibrotic human pancreas were obtained at resection for chronic pancreatitis. These were formalin-fixed and wax-embedded for p75 immunostaining. Finally, fibrotic rat liver obtained after 4 weeks of CCl4 intoxication (as previously described 16 ) was also used for immunohistochemistry studies.
Antibodies
Immunostaining of rat HSC and liver tissue and Western blot analysis of rat HSC were undertaken using a monoclonal antibody reactive with rat p75 (Boehringer Mannheim, Lewes, UK). Human liver and pancreatic biopsies were immunostained using a monoclonal antibody reactive with human p75 (Dako, Kidlington, UK). The same antibody was used for Western blot analysis of human HSC. Monoclonal anti α-smooth muscle action (α-SMA, Sigma, Poole, UK) was used to identify activated HSC in cultured HSC and tissue sections. Polyclonal rabbit anti-Fas was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunodetection of p75
Immunostaining of histological sections of liver and pancreas was undertaken using a streptavidin-biotin complex immunoperoxidase technique. Briefly, sections were deparaffinized, treated to inhibit endogenous peroxidase, and subjected to wet-heating antigen retrieval as previously described. 16 Sections were then washed in Tris-buffered saline (TBS), pH 7.6, before addition of the primary antibodies at optimal dilutions as determined by prior titration for 18 to 24 hours at 4°C. For each liver or pancreas sample negative controls were performed on adjacent sections, replacing the primary antibody with nonimmune IgG, omitting the primary antibody and replacing the primary antibody with TBS. For each biopsy, sequential adjacent sections were stained for p75 and α-SMA.
After incubation with the primary antibody, sections were allowed to warm to room temperature before washing in TBS (3 × 5 minutes) and incubation with the biotinylated anti-mouse antiserum (Boehringer Mannheim), diluted in TBS for 30 minutes at room temperature. Sections were then washed in TBS (3 × 5 minutes) before addition of streptavidin complexed with biotinylated horseradish peroxidase (Dako) for 30 minutes. After 30 minutes slides were washed in TBS (3 × 5 minutes) before adding 3′- 3′-diaminobenzidine (DAB, Sigma) for 8 minutes, then rinsed in TBS followed by running water. Finally, the sections were counterstained in Harris’ hematoxylin, dehydrated through graded alcohols, and mounted.
In a further experiment, colocalization of α-SMA and p75 was undertaken by incubating a representative cirrhotic biopsy sequentially with the antibodies reactive with α-SMA and p75, detected using immunoperoxidase, DAB, immunoalkaline phosphatase, and fast blue B, respectively. Appropriate parallel single immunostains and negative controls were undertaken concurrently.
Representative sections of the cirrhotic liver biopsies were also immunostained to detect Fas expression as previously described. 34
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blot Analysis of p75 Expression
Human and rat HSC and rat PSC were harvested and the extracted protein subjected to electrophoresis on a 9% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel as described 35 after normalization for protein content. After resolution, the protein samples were electrotransferred onto Polyvinylidene Fluoride using the semidry method as described. 35
The membrane was blocked for 1 hour in 5% nonfat dry milk in TBS. Membranes were incubated overnight at room temperature with the primary antibody or with nonimmune IgG (as negative control) in TBS. Membranes were washed twice for 5 minutes in TBS before the addition of the secondary antibody (rabbit anti-mouse IgG HRP in a final dilution of 1:1000) in TBS containing 5% nonfat dry milk for 1 hour. The membranes were then washed in TBS for 5 minutes followed by water for 5 minutes. Reactive bands were identified using enhanced chemiluminescence (ECL, Amersham, Poole, UK) and autoradiography according to the manufacturer’s instructions. Parallel Western blot analysis for α-SMA was undertaken in an identical manner.
Characterizing the HSC Response to NGF
Following the observation that p75 is expressed by the HSC in vivo, we determined to characterize further the HSC response to a ligand known to stimulate the receptor. The pro-apoptotic effect of p75 stimulation by NGF in a number of neural cell types has now been clearly defined. We therefore investigated the potential effect of NGF stimulation on HSC.
HSC apoptosis in response to recombinant NGF was quantified by in vitro counting of HSC under fluorescent illumination in tissue culture wells after addition of acridine orange. Using this method, apoptotic cells can be identified by virtue of their enhanced fluorescence and characteristic nuclear morphology (bright green fluorescence, condensed chromatin, cytoplasmic shrinkage, nuclear and cytoplasmic blebbing, and detachment from the monolayer) and counted in situ together with cells of the attached monolayer.
Quantification of HSC apoptosis in response to NGF was carried out using 80% confluent passaged cells (passage 2–4) on 24-well plates. Cells were washed twice and incubated for 1 hour in serum-free media. Thereafter cells were returned to serum-containing media together with varying concentrations of recombinant human NGF (Cambridge Bioscience, Cambridge, UK). Parallel control wells containing serum were also prepared. NGF was added to final concentrations of 0.1, 1, 10, and 100 ng/ml. Each concentration was added to 2 parallel wells and incubated for 6 or 24 hours. After this time, 0.5 μl of acridine orange (final concentration 1 μg/ml) was added per well and left for 15 minutes before viewing the wells with an inverted fluorescent microscope. Normal and apoptotic cells were counted by an observer blind to the treatment conditions in 3 high power fields per well and 2 wells per condition. Cells that had detached from the monolayer and were floating in the media supernatant were also counted by racking the objective lens up after assessing the monolayer.
To confirm that condensed cells on the surface of the monolayer were apoptotic, loosely adherent and detached cells were harvested from representative HSC cultures by gentle washing followed by centrifugation. Total DNA was extracted from these cells, subjected to electrophoresis on an agarose gel containing ethidium bromide, and visualized under UV light to detect 200 bp laddering as previously described. 36
To determine whether NGF affects the proliferation rate of HSC and to estimate the comparative cell number after incubation with NGF, a series of experiments to analyze cell proliferation (by [3H]-thymidine incorporation) and DNA content (by PicoGreen binding, Molecular Probes, Eugene, OR) was undertaken on three cell preparations in parallel.
For each series of three experiments, three preparations of passaged cells were washed three times in serum-free media then incubated in serum-free media or serum-containing media with or without 100 ng/ml NGF. For the last 6 hours of incubation with NGF, 0.5 μCi of [3H]-thymidine were added to triplicate wells at a concentration of 1:1000 (0.5 μl/500 μl/well). Thymidine incorporation was then determined as previously described. 37 For DNA analysis, the supernatant was discarded and 150μl of 1× TE (10 mmol/L Tris-HCl, 1 mmol/EDTA, pH 8.0) was added to duplicate wells. Adherent cells were removed with a cell scraper, transferred to a microcentrifuge tube, and sonicated for 15 minutes. Each sample (50 μl) was diluted with 50 μl TE and added to individual wells of a 96-well plate. Standard concentrations (0 to 2 ng/ml) of DNA were diluted from herring sperm DNA stock. PicoGreen (1:200 dilution with 1× TE to a final volume of 100 μl) was added to all samples and left to incubate in light-free conditions at room temperature for 5 minutes. Fluorescence was measured using a Cytofluor II Microwell Fluorescence reader (Perseptive Biosystems, Framingham, MA) at standard fluorescein wavelengths (excitation 485 nm, emission 530 nm). A standard curve was generated using known concentrations of herring sperm DNA. Concentrations of double-stranded DNA in the samples were subsequently calculated.
Results
Cultured Hepatic and Pancreatic Stellate Cells Express p75
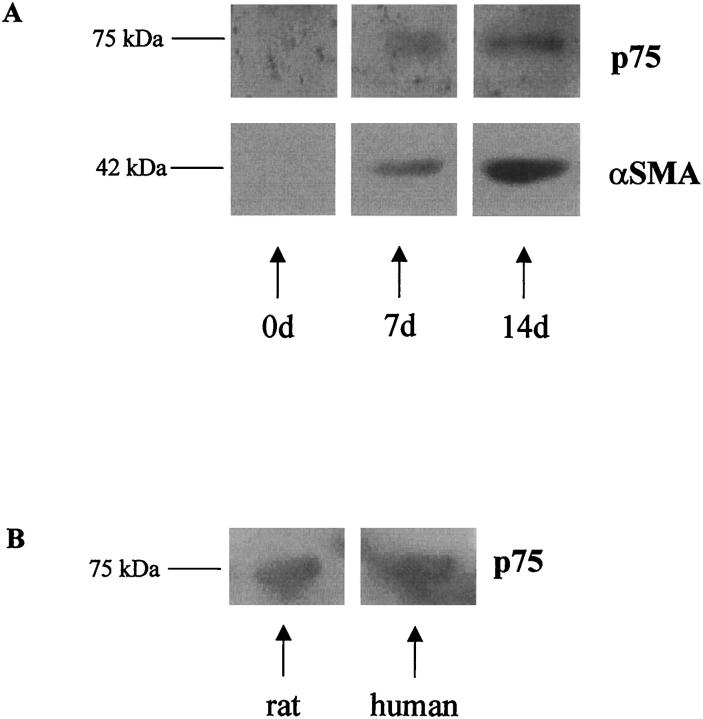
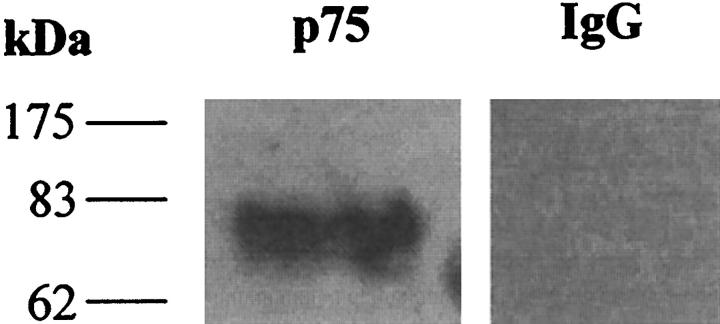
Western blot analysis of total HSC protein derived from rat HSC during activation in culture on plastic using an antibody to rat p75 demonstrated that at 14 days (highly activated, α-SMA-expressing HSC) a single band of appropriate molecular weight was detected, indicating that activated HSC express p75 (Figure 1A) ▶ . In contrast, quiescent (freshly isolated) HSC did not express p75; indeed, expression of this protein first became detectable in activated HSC after 7 days of culture (Figure 1A) ▶ . In a further experiment, 14 day activated human HSC protein extracts were subjected to Western blot analysis using the anti-human p75 antibody. Comparable immunoreactivity was demonstrated in human HSC (Figure 1B) ▶ . Protein extracts from PSC were also demonstrated to contain p75 by Western blot analysis (Figure 2) ▶ .
Figure 1.
A: Protein extracts from freshly isolated rat HSC (0d, quiescent), 7-day cultures (7d, activated), and 14-day cultures (14d, highly activated) were subjected to Western analysis for p75 and α-SMA as described in Materials and Methods. p75 protein became progressively more abundant with increasing activation of HSC as defined by α-SMA expression. B: Protein extracts from 14-day (highly activated) cultures of rat and passaged human HSC were subjected to Western blot analysis for p75. Immunoreactivity (75 kd) was present in both extracts.
Figure 2.
Protein extract from passaged (fourth passage) pancreatic stellate cells was subjected to Western blot analysis for p75 as described in Materials and Methods (left panel). Parallel negative control incubated with nonimmune IgG is presented in the right panel. The results indicate that PSC express p75.
Hepatic Stellate Cells in Normal and Fibrotic Liver Express p75
p75 was detected in perisinusoidal cells in sections of normal donor livers (one child and three adults) and normal liver tissue from the margins of hepatic resections (n = 2). Representative examples are shown in Figures 3A ▶ (low power) and 3C (high power). Staining was also visible in the cells with a fibroblastic phenotype within portal tracts. Staining of these normal liver samples demonstrated perisinusoidal α-SMA positivity in an identical distribution (Figure 3B) ▶ .
Figure 3.
Normal donor liver was stained for p75 as described in Materials and Methods. Perisinusoidal cells with morphology consistent with HSC were found to be positive (A). Analysis of a parallel section for α-SMA indicated that these cells were also positive for this marker (B; original magnification, ×10). C: A high power view of a p75-positive perisinusoidal cell (original magnification, ×40).
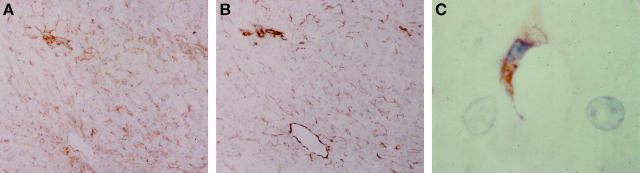
In samples of fibrotic and cirrhotic liver (n = 6) intense staining for p75 was observed in cells with a myofibroblastic appearance within and surrounding areas of fibrosis and fibrotic bands. In addition, p75-positive cells could be observed in a perisinusoidal position extending from fibrotic bands into regenerative nodular parenchyma. No p75 expression was observed in hepatocytes (Figure 4A) ▶ . Positive immunostaining of parallel sections of the same fibrotic liver samples for α-SMA demonstrated that the myofibroblast-like cells in this distribution were activated HSC (Figure 4B) ▶ .
Figure 4.
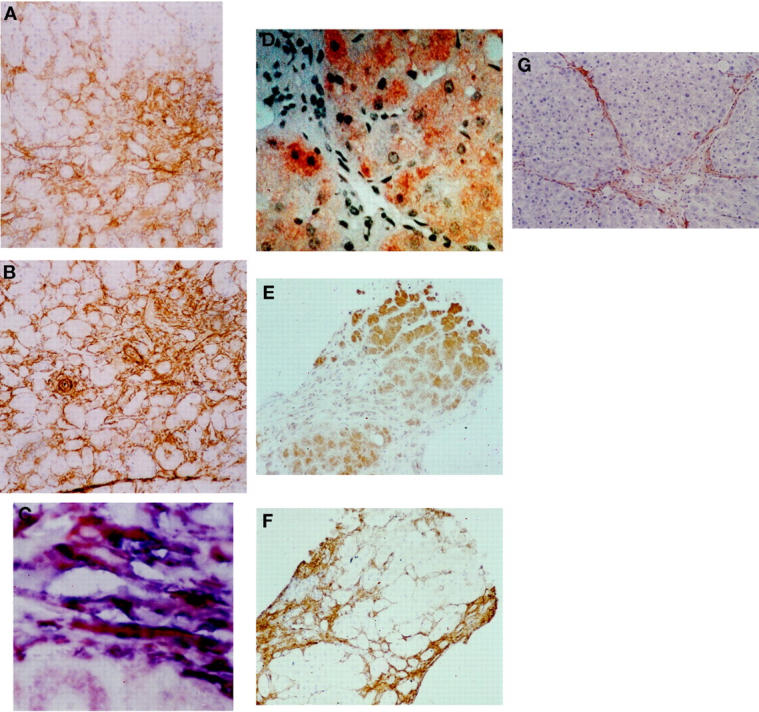
Cirrhotic liver biopsies were immunostained for p75 as described in Materials and Methods. Clear staining was observed in the areas of fibrosis in a distribution consistent with activated HSC. A representative example of this is demonstrated in A. Analysis of a sequential section for α-SMA confirms that the cells in the fibrotic areas are positive and therefore activated HSC (B; original magnification, ×10). Colocalization for p75 and α-SMA was determined as described in Materials and Methods (C), the majority of the activated HSC in the fibrotic septae are stained by both antibodies resulting in a deep purple color (original magnification, ×40). Staining of cirrhotic biopsies for Fas demonstrates a dramatically different pattern with intense staining of hepatocytes (D; original magnification, ×20) and little or no staining of activated HSC within the fibrotic bands (E), which were demonstrated to be α-SMA-positive (F; original magnification, ×10). Fibrotic rat liver was also stained for p75. Positivity was observed in the myofibroblast-like cells within and adjacent to the fibrotic bands in a distribution consistent with activated HSC (G; original magnification, ×10).
Dual staining of α-SMA together with p75 demonstrated a superimposition of blue and brown chromogens in the majority of myofibroblast-like cells, indicating coexpression of the two proteins in activated HSC. Of note, the blood vessel walls and a minority of perisinusoidal cells extending from the fibrotic bands into the regenerative parenchyma expressed only α-SMA-positive staining (Figure 4C) ▶ .
When biopsies of cirrhotic liver were immunostained for Fas, a dramatically different pattern of expression was observed. Strong Fas positivity was present in the hepatocytes present as isolated clusters or in regenerative nodules (Figure 4, D and E) ▶ . In contrast, there was only weak or no staining of activated HSC in the fibrotic bands defined by α-SMA positivity (Figure 4, D ▶ −F).
Immunostaining of fibrotic rat liver for p75 demonstrated strong positivity in myofibroblast-like cells within the fibrotic bands (Figure 4G) ▶ .
Stellate Cells in Fibrotic Pancreas Express p75
Immunostaining for p75 in samples of fibrotic human pancreas (n = 3) demonstrated expression as expected in neural tissue. In addition, staining was observed in cells with a myofibroblast-like phenotype within and around fibrotic septa (Figure 5A) ▶ . Staining of parallel sections demonstrated that the cells in these regions were α-SMA-positive.
Figure 5.
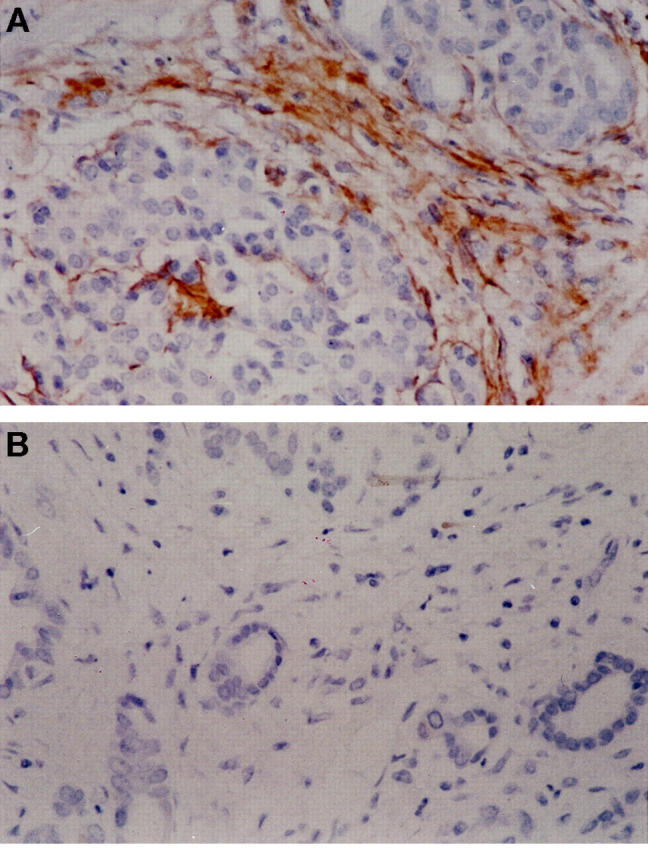
Sections of fibrotic pancreas were immunostained for p75 (A), as described in Materials and Methods. Staining was evident in nerve bundles. In addition, there was clear staining of the fibrotic bands within the pancreas in a distribution entirely consistent with activated PSC and in cells with a myofibroblast-like morphology (original magnification, ×20). A representative negative control for the immunostaining is given in B.
In each immunostaining experiment for liver and pancreatic sections staining in the negative control samples analyzed in parallel was uniformly negative, a representative example is given in Figure 5B ▶ .
Culture Activated HSC Undergo Apoptosis in Response to Recombinant NGF
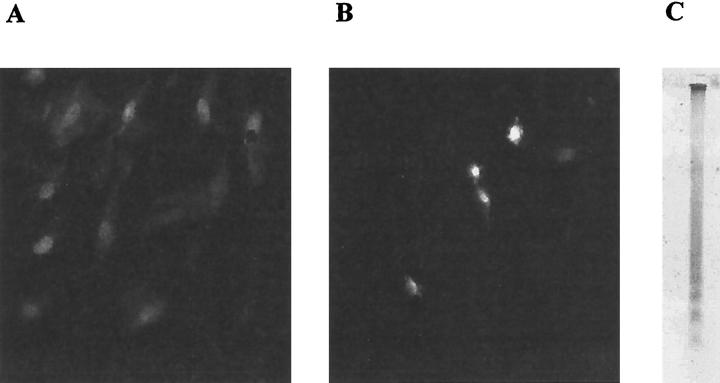
Hepatic stellate cells, activated by culture on uncoated tissue culture plastic in the presence of serum and passaged (passages 1–3) were exposed to a series of concentrations of NGF for periods of up to 24 hours in the presence of serum. Parallel control wells were incubated in serum-containing media only. In each condition, apoptosis was quantified in situ by the counting the apoptotic bodies after acridine orange staining as previously described. 16 In comparison with serum-containing media (Figure 6A) ▶ , cells cultured in serum-containing media plus 100 ng/ml NGF for up to 24 hours showed an increase in number of rounded, condensed cells on the monolayer surface. After acridine orange staining these cells were seen to have condensed, crescentic, and blebbed chromatin, indicating that they were undergoing apoptosis (Figure 6B) ▶ . DNA analysis of representative samples of these condensed, morphologically apoptotic cells demonstrated that oligonucleosomal fragmentation had occurred, leading to the characteristic DNA laddering pattern of apoptosis (Figure 6C) ▶ .
Figure 6.
Passaged cultures of activated rat HSC were washed in serum-free media, then incubated in media containing 16% serum alone (A) or in the presence of 100 ng/ml NGF (B). After 24 hours, apoptotic cells were identified by acridine orange staining and photographed under blue fluorescence. The condensed and blebbed nuclear morphology in combination with the increased intensity of fluorescence of the chromatin is consistent with HSC that have undergone apoptosis. Direct counting of these cells was used to quantify the rate of apoptosis after culture manipulation. To confirm that the condensed cells that were being counted were apoptotic, they were harvested from a representative culture and subjected to DNA extraction and analysis as described in Materials and Methods. C shows the DNA extracted from the loosely adherent, condensed cells identified and quantified by acridine orange staining. The DNA demonstrates the laddering characteristic of oligonucleosomal fragmentation that occurs in apoptotic cells.
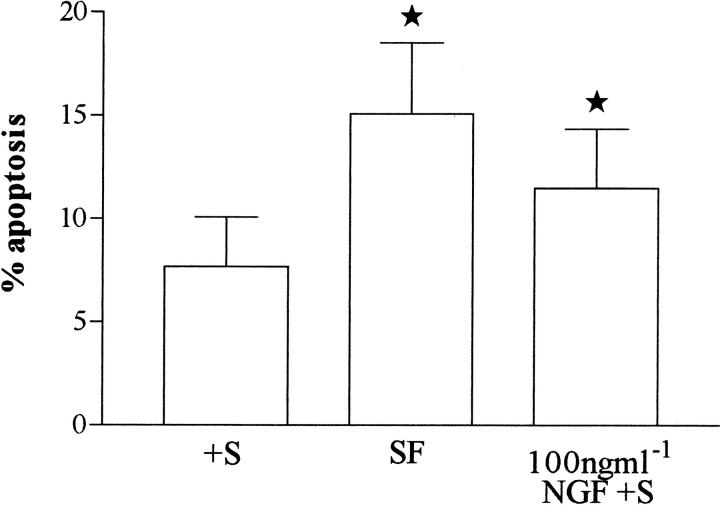
Incubation of HSC in serum plus NGF was associated with a concentration-dependent increase in the rate of apoptosis, which had maximal effect at 10 to 100 ng/ml. When expressed as a percentage change from control (incubated in serum-only cultures given the arbitrary value of 100%), incubation of cells with 100 ng/ml of NGF in the presence of serum was associated with an increase in the percentage of cells undergoing apoptosis at 6 and 24 hours. This effect was most profound at 24 hours, when incubation of HSC with 100 ng/ml NGF was associated with a significant 1.5-fold increase (0.05 > P > 0.01 by Wilcoxon’s matched pairs test, n = 7) in apoptosis relative to control cultures (Figure 7) ▶ .
Figure 7.
Passaged rat HSCs were washed in serum-free medium, then incubated in serum-containing medium in the presence of increasing quantities of NGF. After 24 hours, the number of cells demonstrating an apoptotic morphology (as demonstrated in Figure 6B ▶ ) were determined by acridine orange staining and counted in triplicate high power fields of two parallel tissue culture wells. The figure is representative of the mean (± SE) of 7 separate HSC preparations. Incubation of HSC with 100 ng/ml of NGF in the presence of serum (+S NGF) was associated with a significant increase in the rate of HSC apoptosis when compared with cells exposed to serum alone (+S). *, 0.05 > P > 0.01 by Wilcoxon’s rank sum test.
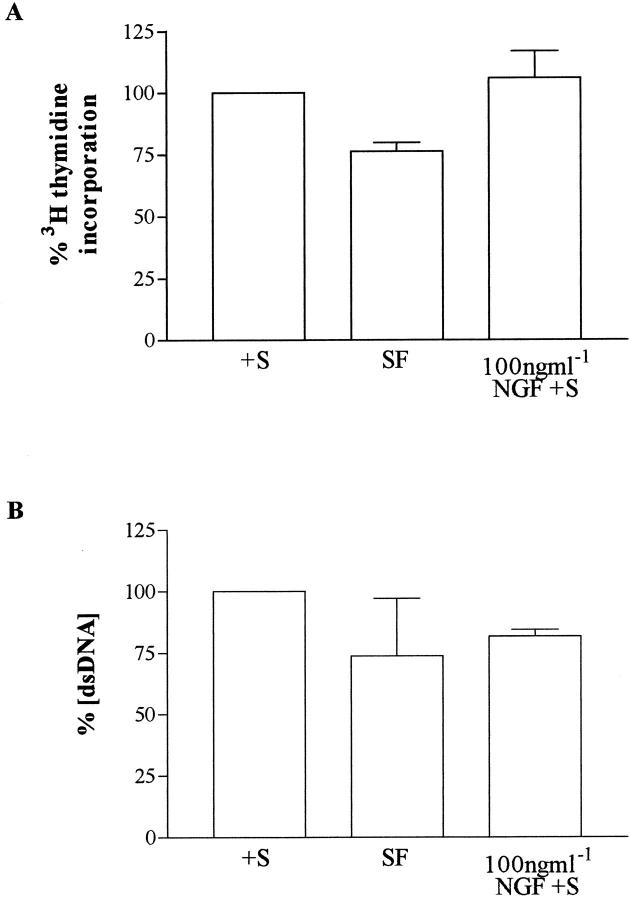
The rate of HSC proliferation in response to NGF in the presence of serum was not different from that with serum-containing medium alone (Figure 8) ▶ . In contrast, 3H-thymidine incorporation in parallel wells of HSC incubated in serum-free conditions was greatly reduced. To determine the relative changes in cell numbers in each condition, total DNA concentration was determined in three parallel HSC cultures, each incubated with serum-containing medium with or without NGF and serum-free medium. Addition of NGF to serum-containing medium resulted in a reduction in DNA concentration, reflecting a decrease in overall cell numbers when compared to control cultures. Intriguingly, the DNA concentration was very close to that observed in parallel cultures incubated in serum-free conditions (Figure 8) ▶ .
Figure 8.
To determine whether incubation of cells in the presence of 100 ng/ml NGF in the presence of serum altered proliferation and to determine changes in overall cell numbers, 3H thymidine incorporation and DNA concentration, respectively, were measured as described in Materials and Methods. Passaged HSC were washed in serum-free medium before being returned to serum-containing medium (+S) in the presence or absence of 100 ng/ml of NGF. In addition, other cells were returned to serum-free medium (SF). After 24 hours, the rate of proliferation and overall DNA concentration was measured in parallel (A and B, respectively). The results indicate that, as described above, NGF increases apoptosis above the level observed in serum-exposed cultures but does not alter proliferation. Accordingly, there is a concomitant decrease in cell number as determined by DNA concentration after exposure to NGF. Absolute serum deprivation for 24 hours is associated with a decrease in both cellular proliferation and cellular number as determined by DNA concentration. The data presented in these figures represent the mean (± SE) of three independent observations.
Discussion
We have presented consistent data demonstrating that rat and human hepatic stellate cells and rat pancreatic stellate cells in tissue culture express the low affinity nerve growth factor receptor, p75. We have immunolocalized p75 to activated HSC (defined by α-SMA expression) in biopsy material representing early and advanced fibrotic liver disease, and to pancreatic stellate cells in examples of chronic pancreatitis. Our results provide strong evidence that this receptor is expressed by the major fibrogenic cell type in fibrotic disease of the liver and pancreas. Taken together with previous reports of p75 expression in bone marrow fibroblasts, 30 these data suggest that p75 may represent a novel marker of stromal fibrogenic cells in a variety of tissues. The pattern of expression of p75 contrasts markedly with that of another member of the TNF receptor superfamily, Fas. This highly discriminant differential expression of Fas and p75 may provide a mechanism for selective targeting of hepatocytes or HSC or ultimately provide a mechanism for selective induction of apoptosis in HSC to manipulate the fibrotic process.
Western blot analysis of protein extracts from rat and human HSC were found to give single bands of the appropriate molecular weight after probing with antibodies to p75. Expression of protein was not observed in freshly isolated (quiescent) HSC but became detectable in 7-day (activated) and 14-day (highly activated) cultures of HSC. Parallel analysis for α-SMA confirmed that p75 expression increased with HSC activation, as defined by this marker. Further evidence for the consistency of our observations is provided by the data obtained using human hepatic stellate cells and fibrotic human tissue. By Western blot analysis of protein extract from passaged human HSC, using a monoclonal antibody directed exclusively against human p75, a single band of appropriate molecular weight was observed. We proceeded to use this antibody to immunostain a series of fibrotic human liver biopsies and biopsies of normal liver tissue. In each example of diseased liver there was staining of cells within and surrounding fibrotic bands and in a perisinusoidal distribution extending into the residual and regenerative parenchyma. This distribution is entirely consistent with that of activated HSC in fibrotic liver. To demonstrate further that these cells expressed markers for activated HSC, parallel sections were stained for α-SMA, 38 and p75 was colocalized to α-SMA-positive cells in a distribution consistent with activated HSC in a representative cirrhotic biopsy. The resulting data confirmed that p75-positive cells were α-SMA-positive and therefore consistent with activated HSC.
In normal liver, p75-positive myofibroblast-like cells were observed in the stromal capsule at the margin of the hepatic cysts and metastases. In addition, in the parenchyma at the resection margin and in the parenchyma of the normal donor liver, perisinusoidal cells with morphology consistent with HSC were observed and found to be p75-positive. In this context it is important to review the limitations of assuming that resected liver tissue (that had been clamped and ischemic) and donor liver (that had been cooled and University of Wisconsin solution-perfused) are entirely normal. Indeed, parallel α-SMA staining of these liver samples indicated positivity in an identical distribution. Nevertheless, these data suggest that HSC in normal human liver may express p75.
The hepatic stellate cell is normally considered to be mesenchymally derived. 31,39 Recent work by Niki et al, who have demonstrated that HSC also express the glial fibrillary acidic protein and the neural protein nestin, 40,41 has rekindled debate over whether HSC cells may be derived from the neural crest. Our demonstration of p75, a receptor previously associated with neural tissue, 26,27,41-43 will strengthen the case for a more detailed study of the embryological origin of the stellate cell. Of interest in this context are our data relating to the recently described pancreatic stellate cell. 31-33 These cells share common morphological features with HSC and, during culture in the presence of serum, become activated to a myofibroblast-like phenotype. We have established in vitro techniques for PSC activation and have established cultures of activated PSC after passage. In this study, we have demonstrated that PSC express p75 in a manner identical to the HSC in culture. In addition, we have clearly demonstrated evidence of PSC expression of p75 in fibrotic human pancreas. Our results illustrate a further similarity between the two cell types and reinforce the hypothesis that p75 expression may be a general feature of the wound healing myofibroblast.
The paradigm ligand for p75 is NGF, although other neurotrophins may stimulate the receptor. 24 For this reason, and to determine whether ligand stimulation of p75 can induce HSC apoptosis, we incubated activated HSC in the presence of increasing concentrations of recombinant NGF. Apoptosis was quantified at defined time points by counting the total number of cells with an apoptotic morphology and expressing this as a percentage of the total number of cells in the field. This method has the advantage that apoptosis can rapidly be quantified in situ in tissue culture wells. 36 Moreover, apoptosis can be observed readily by distinct changes in nuclear morphology associated with increased fluorescence and quantified simultaneously. We and others have demonstrated that this method can be applied to the study of fibroblasts and myofibroblasts in culture. 16,36 Cells counted on the adherent monolayer surface and within the supernatant demonstrate morphological features of apoptosis by other methods and are positive by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling. 16,36,44 In this study, we have also demonstrated that the condensed, detached cells demonstrate the characteristic pattern of DNA laddering that results from oligonucleosomal DNA fragmentation during apoptosis. In response to 100 ng/ml NGF there was a significant increase in the rate of apoptosis even at 24 hours in the presence of serum. The observed increase in apoptosis was similar in magnitude to that observed in parallel cultures incubated in conditions of absolute serum deprivation.
These data strongly support the hypothesis that, when p75 is stimulated in HSC, apoptosis results and that the apoptotic response is not attenuated by growth factors present in serum, although the response is not as complete as that seen with certain Fas-expressing cells when stimulated with Fas-L. We have shown that addition of NGF has no effect on HSC proliferation in the presence of serum. By contrast, there is a 19% decrease in overall cell number, determined by total DNA concentration, in the presence of NGF compared with cells cultured in serum alone. These data reinforce the evidence for a significant apoptotic effect being mediated by NGF, which shifts the balance of cell proliferation and cell death to favor a net decrease in HSC number.
Our observations relating to the expression and function of p75 make an interesting contrast with that of another paradigm pro-apoptotic member of the TNF receptor superfamily, Fas. The expression of Fas has been demonstrated in HSC in culture and in fibrotic rat liver septae and is associated with HSC apoptosis after stimulation by Fas ligand. 15,45 Fas is highly expressed by hepatocytes 18,46 and, although local and cell surface expression of Fas-L may limit apoptotic effects, 47 the profound hepatocellular damage that is a consequence of Fas-L hepatocyte stimulation may limit the application of Fas manipulation for potential therapeutic benefit in the context of fibrosis. We have recently demonstrated that HSC apoptosis is a vital stage in recovery from hepatic fibrosis. 16 The identification of a cell surface apoptotic trigger, not expressed by other parenchymal and nonparenchymal liver cells, may provide an avenue for selective therapeutic targeting of HSC for apoptosis in vivo during injury.
The role of p75 in vivo during fibrogenic injury has not been determined. Cellular sources of NGF within the liver have been identified and include mast cells. 48 These are recruited to the liver during fibrotic injury, and hepatic fibrosis has recently been demonstrated to be exacerbated in livers depleted of mast cells. 49 The presence of NGF in the degranulation products of mast cells provides a direct mechanism whereby these cells can influence HSC numbers and, through this mechanism, the progression of fibrosis. A further consideration is that there may be other ligands expressed by parenchymal and nonparenchymal liver cells that interact with p75.
In summary, we have made the novel observation that HSC express p75, the low affinity NGF receptor, and respond to NGF stimulation by undergoing apoptosis. We suggest that p75 activation represents a potential mechanism to target HSC and PSC for apoptosis and may provide a potential mechanism for the selective depletion of HSC.
Footnotes
Address reprint requests to John P. Iredale, MRC Senior Clinical Fellow, Mailpoint (811), Level D, South Block, Southampton General Hospital, Southampton SO16 6YD, UK. E-mail: jpi@soton.ac.uk.
J. I. is a Medical Research Council UK Senior Clinical Fellow and this funding supports N. T. also. J. I. and R. I. gratefully acknowledge the support of the Wessex Medical Trust and Research Grant funding from Bayer AG. D. F. is in receipt of a National Health Service Research and Development Fellowship.
References
- 1.Friedman SL: The cellular basis of hepatic fibrosis: mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 2.Alcolado R, Arthur MJP, Iredale JP: Pathogenesis of liver fibrosis. Clin Sci 1997, 92:103-112 [DOI] [PubMed] [Google Scholar]
- 3.Arthur MJP, Friedman SL, Roll FJ, Bissell DM: Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest 1989, 84:1076-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur MJP, Jackson CL, Friedman SL: Release of type IV collagenase by human lipocytes. Wisse E Knook DL McCuskey RS eds. Cells of the Hepatic Sinusoid, 1991, vol 3.:pp 161-163 The Kupffer Cell Foundation, Leiden [Google Scholar]
- 5.Vyas SK, Leyland H, Gentry J, Arthur MJP: Transin (rat stromelysin) expression by hepatic lipocytes in early primary culture: analysis of gene transcription, protein activity and immunolocalization. Edited by E Wisse, K Wake, DL Knook. Cells of the Hepatic Sinusoid, vol 5. Leiden, The Kupffer Cell Foundation, 1995, pp 396–399
- 6.Benyon RC, Iredale JP, Ferris WF, Arthur MJP: Increased expression of mRNA for gelatinase A and TIMP-2 in human fibrotic liver disease. Hepatology 1993, 18:198A8325611 [Google Scholar]
- 7.Iredale JP, Goddard S, Murphy G, Benyon RC, Arthur MJP: Tissue inhibitor of metalloproteinase-1 and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci 1995, 89:75-81 [DOI] [PubMed] [Google Scholar]
- 8.Iredale JP, Benyon RC, Ferris WF, Cottrell B, Alcolado R, Murphy G, Arthur MJP: Tissue inhibitor of metalloproteinase-1 expression is up-regulated relative to interstitial collagenase in CCl4 induced liver fibrosis and activated human hepatic lipocytes. Edited by E Wisse, DL Knook, K Wake. Cells of the Hepatic Sinusoid, vol 5. Leiden, The Kupffer Cell Foundation, 1995, pp 418–419
- 9.Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJP: Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1 (TIMP-1): implications for regulation of matrix degradation in liver. J Clin Invest 1992, 90:282–287 [DOI] [PMC free article] [PubMed]
- 10.Iredale JP: Matrix turnover in fibrogenesis. Hepatogastroenterology 1996, 43:56-71 [PubMed] [Google Scholar]
- 11.Iredale JP: Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol 1997, 29:43-54 [DOI] [PubMed] [Google Scholar]
- 12.Arthur MJP, Iredale JP: Hepatic lipocytes, TIMP-1 and liver fibrosis. J R Coll Physicians Lond 1994, 28:200-208 [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman SL, Roll FJ, Boyles J, Bissell DM: Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA 1985, 82:8681-8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher JJ, McGuire RF: Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 1990, 86:1641-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saile B, Knittel T, Matthes N, Schott P, Ramadori G: CD95/CD95L-mediated apoptosis of the hepatic stellate cell. Am J Pathol 1997, 151:1265-1272 [PMC free article] [PubMed] [Google Scholar]
- 16.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJP: Mechanisms of spontaneous resolution of rat liver fibrosis: hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998, 102:538-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaux DL, Strasser A: The molecular biology of apoptosis. Proc Natl Acad Sci USA 1996, 93:2239-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L: Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med 1995, 182:1223-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser A, Evan G: A license to kill. Cell 1996, 85:781-784 [DOI] [PubMed] [Google Scholar]
- 20.Cleveland JL, Ihle JN: Contenders in FasL/TNF death signaling. Cell 1995, 81:479-482 [DOI] [PubMed] [Google Scholar]
- 21.Mooney A, Jobson T, Bacon R, Kitamura M, Savill J: Cytokines promote glomerular mesangial cell survival in vitro by stimulus-dependent inhibition of apoptosis. J Immunol 1997, 159:3949-3960 [PubMed] [Google Scholar]
- 22.Smith C, Farrah T, Goodwin RG: The TNF receptor superfamily of cellular and viral proteins: activation, co-stimulation and death. Cell 1994, 76:959-962 [DOI] [PubMed] [Google Scholar]
- 23.Dechant G, Barde YA: Signalling through the neurotrophin receptor p75NTR. Curr Opin Neurobiol 1997, 7:413-418 [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA: Binding of neurotrophin-3 to its neuronal receptors and interactions with the nerve growth factor and brain derived neurotrophic factor. EMBO J 1992, 11:912-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker P, Shooter E: Disruption of NGF binding to the low affinity neurotrophin receptor p75NTR reduces NGF binding to TrkA on PC12 cells. Neuron 1994, 13:203-215 [DOI] [PubMed] [Google Scholar]
- 26.Bamji SX, Majdan M, Pozniak C, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD: The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 1998, 140:911-923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KF, Bachman K, Landis S, Jaenisch R: Dependence on p75 for innervation of some sympathetic targets. Science 1994, 263:1447-1449 [DOI] [PubMed] [Google Scholar]
- 28.Van der Zee CEEM: Survival of cholinergic forebrain neurons in the developing p75 (NGRF) deficient mice. Science 1996, 274:1729-1732 [DOI] [PubMed] [Google Scholar]
- 29.Yeo TT, Chua-Couzens J, Butcher LL, Redesen DE, Cooper JD, Valletta JS, Mobley MC, Longo FM: Absence of p75 causes increased basal forebrain cholinergic neuron size, cholinergic neuron size and target innervation. J Neurosci 1997, 17:7594-7605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattoretti G, Schiro R, Orazi O, Soligo D, Columbo MP: Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood 1993, 81:1726-1735 [PubMed] [Google Scholar]
- 31.Wilkins B, Jones D: Immunohistochemical characterization of intact stromal layers in long term cultures of human bone marrow. Br J Haematol 1995, 90:757-766 [DOI] [PubMed] [Google Scholar]
- 32.Pinzani M: New kids on the block: pancreatic stellate cells enter the fibrogenesis world. Gut 1999, 44:451-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G: Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115:421-432 [DOI] [PubMed] [Google Scholar]
- 34.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS: Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 1999, 44:534-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afford SC, Randham S, Eliopoulis AG, Hubscher SG, Young LS, Adams DH: CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface Fas ligand expresion and amplifies Fas-mediated hepatocyte death during allograft rejection. J Exp Med 1999, 189:441-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaca MDA, Pickering JA, Arthur MJP, Benyon RC: Human and rat hepatic stellate cells produce stem cell factor: a possible mechanism for mast cell recruitment in liver fibrosis. J Hepatol 1999, 30:850-858 [DOI] [PubMed] [Google Scholar]
- 37.Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J: Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest 1994, 94:2105-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vyas SK, Leyland H, Gentry J, Arthur MJP: Transin (stromelysin) is expressed in early rat lipocyte primary culture. Gastroenterology 1995, 109:889-898 [DOI] [PubMed] [Google Scholar]
- 39.Rockey DC, Boyles JK, Gabbiani G, Friedman SL: Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol 1992, 24:193-203 [PubMed] [Google Scholar]
- 40.Niki T, Pekny M, Hellemans K, Blesser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A: Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology 1999, 29:520-527 [DOI] [PubMed] [Google Scholar]
- 41.Niki T, Debleser PJ, Xu GX, Vandenberg K, Wisse E, Geerts A: Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatology 1996, 23:1538-1545 [DOI] [PubMed] [Google Scholar]
- 42.Carter BD, Lewin GL: Neurotrophins live or let die: does p75 decide? Neuron 1997, 18:187-190 [DOI] [PubMed] [Google Scholar]
- 43.Cassaccia-Bonnedil P, Carter BD, Dobrowsky RT, Chao MV: Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature 1996, 383:716-719 [DOI] [PubMed] [Google Scholar]
- 44.Chao MV: The p75 neurotrophin receptor. J Neurobiol 1994, 25:1373-1385 [DOI] [PubMed] [Google Scholar]
- 45.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC: Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992, 69:119-128 [DOI] [PubMed] [Google Scholar]
- 46.Gong W, Pecci A, Roth S, Lahme B, Beato M, Gressner AM: Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology 1998, 28:492-502 [DOI] [PubMed] [Google Scholar]
- 47.Patel T, Gores GJ: Apoptosis and hepatobiliary disease. Hepatology 1995, 21:1725-1741 [DOI] [PubMed] [Google Scholar]
- 48.Schneider P, Holler N, Hahne M, Frei K, Fontana A, Tschopp J: Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med 1998, 187:1205-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leon A, Burniani A, Dal Toso R, Fabkis M, Romanello S, Aloe L, Levi-Motalcini: Mast cells synthesize, store and release nerve growth factor. Proc Natl Acad Sci USA 1994, 91:3739–3743 [DOI] [PMC free article] [PubMed]