Abstract
The cellular and microvascular responses of JC Lewis rats to an intravenous injection of activated T cells specific for ovalbumin were examined with the retinal whole mount technique. The retina was examined at various times post-injection (pi) with the use of antibodies to the αβ T cell receptor (TCR) or to major histocompatibility complex class II (MHC II), the monoclonal antibody ED1, and intravascular tracers. By 12 hours pi, small numbers of TCR+, ED1+, and MHC II+ cells were present within the lumen of retinal vessels, and minor breakdown of the blood-retinal barrier (BRB) and microglial activation were evident. The intensity of these responses had increased by 1 day pi, when small numbers of TCR+ cells had also undergone extravasation. By 2 to 3 days pi, the numbers of TCR+, ED1+, and MHC II+ cells in the retinal parenchyma had increased, but the BRB breakdown and microglial activation had subsided. Thus, in the absence of target antigen, activated T cells induced limited and transient breakdown of the BRB, microglial activation, and the extravasation of ED1+, MHC II+ monocytes. In contrast, the retina of rats that received an intraocular injection of ovalbumin in addition to the intravascular injection of T cells showed massive cellular recruitment and breakdown of the BRB. These results indicate that an increase in the number of activated T cells in the circulation, such as that which occurs during viral or bacterial infection, has the potential to result in transient breakdown of the BRB and a mild local microglial response.
Endothelial barriers, including the blood-brain barrier (BBB), the blood-retinal barrier (BRB), and the blood-nerve barrier (BNB), shield the nervous system from circulating agents, such as immunoglobulins, that might prove toxic. These barriers also prevent the entry of resting leukocytes from the circulation. Activated T lymphocytes, however, are able to penetrate the barriers through the action of their surface enzymes and adhesion molecules, 1,2 and it is generally assumed that there are no implications for vascular integrity if there is no antigen recognition in the tissue. During their surveillance of a tissue such as the central nervous system (CNS), 3 if they do not encounter a relevant antigen presented appropriately by an antigen-presenting cell, activated T cells return to the circulation or die by apoptosis. 4,5
Magnetic resonance imaging of individuals with multiple sclerosis (MS), a relatively common inflammatory demyelinating disease of the CNS, has revealed that breakdown of the BBB is the earliest demonstrable abnormality in the formation of new lesions and in the extension of old lesions. 5 Given that this breakdown of the BBB is thought to play a fundamental role in the pathogenesis of MS, 6 it is important to understand the mechanism by which it occurs. Breakdown of the BBB is always associated with cellular infiltration in individuals with MS. 7 In rats with experimental allergic encephalomyelitis (EAE), an experimental model of MS, activated T cells specific for neural antigens such as myelin basic protein (MBP) or the S100 protein accumulate within the CNS and induce breakdown of the BBB. 8,9 However, there is no substantial evidence that MBP or any other neural component is a major autoantigen in MS. The demonstration of an association between MS attacks and viral infections 10,11 suggests that T cells reactive to nonneural antigens, such as those associated with viruses, also might induce CNS inflammation. Indeed, we have previously shown that activated T cells specific for the nonneural antigen ovalbumin (OVA) are able to induce breakdown of the BNB. 12
The retina is an ideal tissue in which to characterize the microvascular and cellular responses of the CNS to an intravascular injection of activated T cells of nonneural specificity, because it is possible to visualize the entire retinal vascular plexus with the normal relations among the glial, vascular, and neuronal elements intact. 13-15 In particular, with the use of intravascular barrier tracers and cell-specific reagents, it is possible to colocalize sites of cellular accumulation with sites of breakdown of the BRB. The retinal whole mount technique has the additional advantage that arteries, capillaries, and venules are readily identified, thereby allowing accurate localization of specific cellular and vascular changes to specific regions of the CNS microvasculature. Our previous application of this technique resulted in the detection of BRB breakdown and the identification of small numbers of inflammatory infiltrates in the retinas of rats with EAE 3 and of mice with experimental cerebral malaria. 13 We have now characterized the cellular and microvascular responses, in the absence or presence of target antigen, to an intravenous injection of activated T cells specific for OVA. Because the barrier properties of retinal vessels are similar to those of vessels elsewhere in the CNS, 16 the changes observed in the present study are highly relevant to those characteristic of MS and other inflammatory CNS disorders.
Materials and Methods
The Animal Model
A total of 64 adult male JC Lewis rats aged 10 to 14 weeks were used in this study. Twelve animals were used as naive controls. The first experimental group of 12 animals received only an intravenous injection of 5 × 10 6 activated OVA-specific T cells (GH2 T cell line) in 0.9 ml of RPMI medium, and they were examined 12 hours and 1, 2, and 3 days postinjection (pi). The second group of 40 animals received both the intravenous injection of activated OVA-specific T cells and intraocular injections of OVA and vehicle (see below) within 5 minutes of each other. Animals in this group were examined at 6 and 12 hours and 1, 2, 3, 5, and 7 days pi. Anesthesia for intravenous injections and terminal experiments were as previously described. 17
Intraocular Injection of OVA and Preparation of OVA-Stimulated T Cells for Intravenous Injection
Twenty to thirty microliters of an OVA (Sigma, St. Louis, MO) solution in RPMI (1 mg/ml; sterilized by passage through a low-protein binding filter) were injected at a single site in the region of the left retina with the use of a Hamilton-type syringe (SGE Scientific, Melbourne, Australia) with a Luer lock fitting (N09-S2397) for attachment to disposable 31-gauge needles. The needle was introduced into the globe at a site immediately posterior to the ora serrata and the injection was performed under direct visual guidance through a dissecting microscope, with the aim of depositing the solution adjacent to the retina. The right eye received an identical injection with RPMI alone. OVA-reactive T cells (GH-2 T cell line) were prepared as previously described. 12
Preparation of Retinal Whole Mounts, Horseradish Peroxidase (HRP), and Lectin Histochemistry and ED1, T Cell Receptor (TCR), and Major Histocompatibility Complex Class II (MHC II) Immunohistochemistry
Retinal whole mounts 18 and HRP histochemistry, 17,19 were prepared as previously described. The molecular size of HRP (40 kd) is similar to those of serum proteins, and this similarity has been taken advantage of in a widely used method for detection of BBB breakdown. 20 The Griffonia simplicifolia isolectin B4 (GS lectin), which labels α-D galactose residues, was used to visualize microglia and macrophages. GS lectin histochemistry was performed as previously described. 21 Immunohistochemistry for ED1, TCR, and MHC II was performed as previously described. 3,17 For ED1, αβTCR, and MHC II immunohistochemistry, each retina was divided equally at the optic disk into three sectors and one of the above markers was applied to each sector. Cell density was determined for each marker by counting immunoreactive cells using a 10× or 20× objective at intervals of 1 mm 2 and averaged for the entire sector.
Modified Trichrome Technique
Retinas were examined after intravascular injection of Evans blue, bisbenzimide, and Monastral blue as previously described. 3,13 Evans blue (Sigma) is an acid dye of the diazo group that binds to albumin in the blood, allowing sites of barrier breakdown to be readily detected. Bisbenzimide (or Hoechst stain, H33258; Calbiochem-Novabiochem, San Diego, CA) interacts with DNA and stains nuclei of all cell types. When the BRB is intact, retinal exposure to intravenously administered bisbenzimide is restricted to the vascular endothelial cells that line the lumen of retinal vessels. However, if the BRB is disrupted, the dye can access the retinal parenchyma, with the result that all cells, including the somas of neurons and glia, are labeled. Bisbenzimide also labels the nuclei of inflammatory cells that accumulate and adhere to the vessel wall during the progression of disease. Monastral blue is a colloidal dye, the particles of which coat, or are ingested by, activated monocytes. 22
Results
Cellular and Microvascular Responses to Activated T Cells of Nonneural Specificity
Activated T Cells, ED1+, and MHC II+ Cells Enter the CNS but Do Not Accumulate
In animals that received only an intravenous injection of OVA-specific activated T cells, few TCR+ lymphocytes were apparent within the lumen of retinal vessels at 12 hours pi (Figure 1A) ▶ , although the number was consistently higher than that apparent for naive controls; counts across the entire retina at this time point yielded values of 1.77 ± 1.29 and 0.15 ± 0.15 TCR+ cells/mm 2 for injected and naive control animals, respectively (Table 1) ▶ . At this time, no TCR+ cells were detected in the parenchyma of the retina (Table 1) ▶ . By days 1 to 2 pi, whereas the number of TCR+ cells within the lumen of vessels remained unchanged, small numbers of such cells had undergone extravasation and were apparent within the retinal parenchyma (Figure 1B) ▶ . The number of intravascular TCR+ cells also remained unchanged at days 3 to 5 pi; in contrast, the number of TCR+ cells in the parenchyma had increased, and was significantly greater than that for naive controls from day 2 pi onward (Table 1) ▶ . However, even at days 3 to 5 pi, the number of these cells in the parenchyma of injected rats was still low, 0.2 ± 0.1 cells/mm2, and they remained within ∼20 μm of the nearest vessel.
Figure 1.
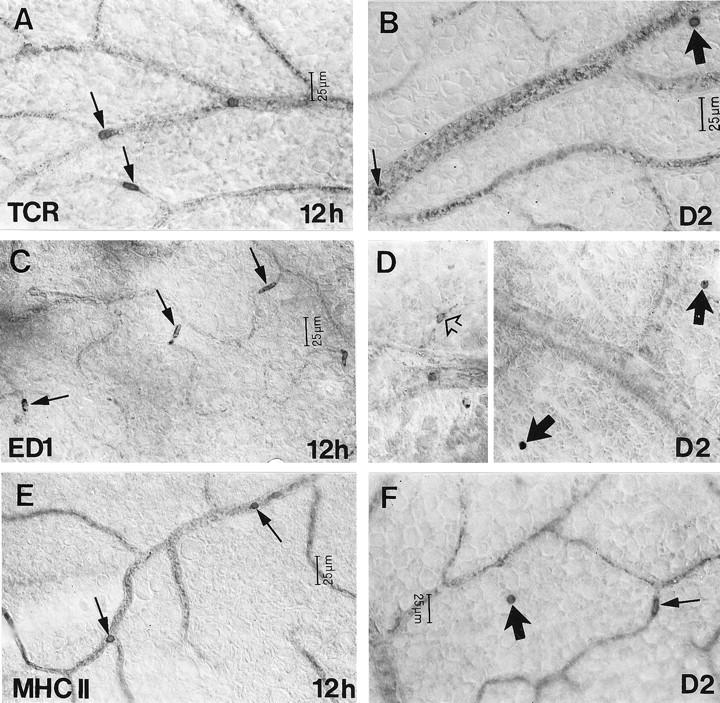
The distribution of αβTCR+ (A and B), ED1+ (C and D), and MHC II+ (E and F) cells in the retina at various times after intravenous injection of OVA-activated T cells. The number of TCR+ cells within the lumen of retinal vessels (arrows) was increased at 12 hours pi (A). Such cells were evident in vessels (small arrow) but also in the retinal parenchyma (large arrow) at day 2 pi (B). Accumulation of ED1+ cells within the lumen of retinal vessels (arrows) was apparent from 12 hours pi (C). ED1+ microglia (open arrow) and the extravasation of ED1+ monocytes (solid arrows) were evident at day 2 pi (D). Accumulation of MHC II+ cells within the lumen of retinal vessels (arrows) was apparent from 12 hours pi (E) and extravasation of these cells (large arrow) was evident by day 2 pi (F). D is shown at the same magnification as C.
Table 1.
Density of TCR+, ED1+, and MHC II+ Cells within the Lumen of Retinal Vessels and the Retinal Parenchyma of JC Lewis Rats after Intravascular Injection of OVA-Activated T Cells
| Time (pi) | n | TCR+ cell density (cells/mm2) | ED1+ cell density (cells/mm2) | MHC II+ cell density (cells/mm2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intravascular | Parenchyma | Intravascular | Parenchyma | ||||||
| Intravascular | Parenchyma | Microglial- like | Monocyte- like | Microglial-like | Monocyte-like | ||||
| Naive controls | 3 | 0.15 ± 0.15 | 0.00 ± 0.00 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 12 hr | 3 | 1.77 ± 1.29 | 0.00 ± 0.00 | 1.03 ± 0.75 | 2.33 ± 1.35* | 0.01 ± 0.02 | 0.83 ± 0.49* | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Day 1 | 3 | 1.60 ± 1.22 | 0.04 ± 0.07 | 1.33 ± 0.50* | 3.60 ± 3.14 | 0.00 ± 0.00 | 0.64 ± 0.83 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Day 2 | 3 | 1.70 ± 1.13 | 0.07 ± 0.03* | 1.87 ± 1.20 | 1.97 ± 2.00 | 0.41 ± 0.52 | 1.81 ± 1.13* | 0.00 ± 0.00 | 0.12 ± 0.62 |
| Day 3 | 3 | 1.40 ± 0.40* | 0.20 ± 0.10* | 0.67 ± 0.47 | 0.91 ± 0.10* | 0.10 ± 0.00* | 1.24 ± 0.21* | 0.00 ± 0.00 | 0.11 ± 0.10 |
Data are means ± SD. *P < 0.05 vs. control rats (Student’s one-tailed t-test). pi, postinjection.
A small increase in the number of ED1+ (Figure 1C) ▶ and MHC II+ cells (Figure 1E) ▶ within the lumen of retinal vessels were apparent in injected animals at 12 hours pi. Cell counts for ED1+ monocytes yielded values of 1.03 ± 0.75 and 0.2 ± 0.2 cells/mm 2 for injected and naive control animals, respectively. Cell counts for MHC II+ cells in naive controls were 0 ± 0 compared to 0.83 ± 0.49 cells/mm 2 at 12 hours pi (Table 1) ▶ . 3 The number of ED1+ and MHC II+ cells within the lumen of vessels of injected animals increased slightly on days 1 and 2 pi but thereafter decreased (Table 1) ▶ . From day 2 to day 3 pi, only small numbers of ED1+ (Figure 1D) ▶ and MHC II+ cells (Figure 1F) ▶ had undergone extravasation and were evident within the retinal parenchyma of injected rats.
Activated T Cells Induce a Limited Breakdown of the BRB and Limited Microglial Activation of Short Duration
The BRB is intact in naive control adult JC Lewis rats (Figure 2A) ▶ . Thus, intravascular injection of HRP and the modified trichrome technique demonstrated that the retinal vessels exhibited a sharp outline and retained tracer within the vessel lumen, with no detectable tracer leakage into the tissue parenchyma. Intravenous injection of OVA-activated T cells resulted in a limited breakdown of the BRB even at 12 hours pi, as was evident by increased HRP accumulation at junctions between endothelial cells and by a slight blurring of vessel outlines (Figure 2C) ▶ . This leakiness was increased slightly at 24 hours pi using both HRP (Figure 2E) ▶ and the modified trichrome technique (Figure 3, A ▶ −C). At day 2 pi, uneven accumulation of HRP on the venous vessel wall was evident, but no leakage of HRP was observed (Figure 2G) ▶ .
Figure 2.
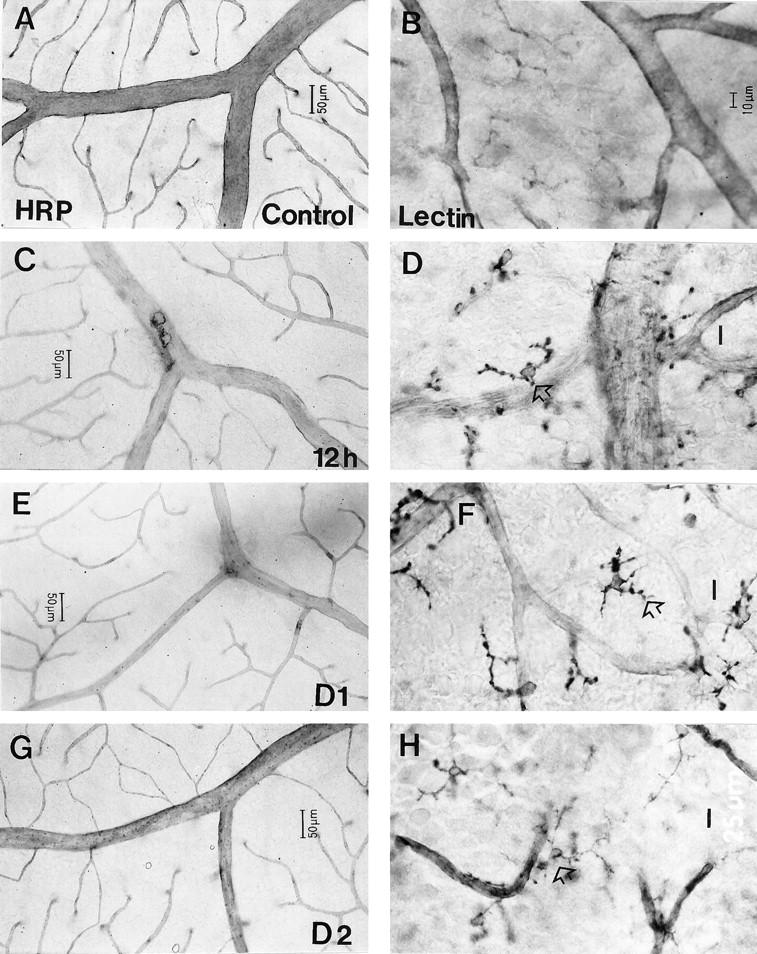
The breakdown of the BRB (C, E, and G), and microglial activation (D, F, and H) in the retina at various times after intravenous injection of OVA-activated T cells. A: Intact BRB in control adult JC Lewis rats. B: Ramified morphology and weakly GS lectin-positive microglia in control animals. Breakdown of the BRB at 12 hours pi was indicated by slight leakage of HRP from retinal veins (C). Such leakage was particularly apparent at branch points of veins at day 1 pi (E) but was no longer apparent at day 2 pi (G). Limited activation of microglia, as revealed by increased staining with GS lectin and the appearance of distensions on the cell processes, was evident at 12 hours pi (D, open arrow) and more obvious at day 1 pi. Some microglia at day 1 pi adopted an amoeboid morphology (F, open arrow). The appearance of these cells, although reduced in severity, was still activated compared to controls at day 2 pi (H, open arrow). Scale bar, 10 μm (for D, F, and H).
Figure 3.
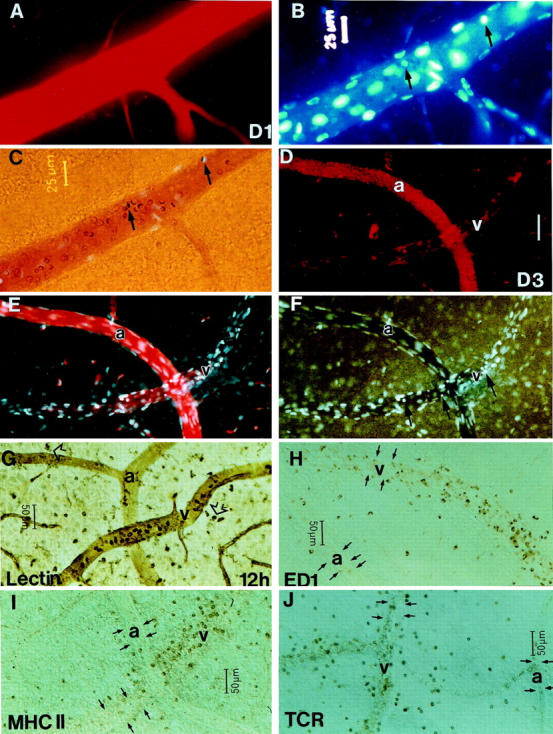
A−C: Examination by the modified trichrome technique at day 1 pi of the same venous segment in the retina of a rat that received only an intravascular injection of OVA-activated T cells. A limited breakdown of the BRB is evident as a weak halo of Evans blue (A) and Hoechst stain (B) surrounding the vessel outline. Adherent Monastral blue-positive monocytes (arrows) are apparent by Hoechst staining (B) and transmitted light microscopy (C). D−J: Difference in the microvascular and cellular responses between venous and arterial segments in OVA-injected eyes after an intravenous injection of OVA-activated T cells. D−F: Despite being in close proximity, the arteries exhibited no leakage of Evans blue, Hoechst stain, or Monastral blue, whereas the venous segments showed a marked breakdown of the BRB, which colocalized with sites of accumulation of Monastral blue-positive monocytes and other leukocytes at day 3 pi. Small arrows in F show the locations of adherent leukocytes. At 12 hours pi, the early activation of GS lectin-stained microglia (G, arrowheads) occurred adjacent to either arteries or veins. In contrast, adhesion and extravasation of leukocytes including, GS lectin-stained (G), ED1+ (H), MHC II+ (I), and TCR+ cells (J) were apparent only in the venous vasculature. Small arrows in H–J indicate the outlines of arteries (a) and venous segments (v). Scale bar, 50 μm (D−F).
In addition to causing a mild breakdown of the BRB, injection of OVA-activated T cells induced a limited activation of microglia, as indicated by changes in microglial morphology and up-regulation of αD galactose 23 and ED1 expression by resident microglia. Unlike the situation in control rats, in which microglia are stained only weakly with GS lectin and exhibit a ramified morphology 3 (Figure 2B) ▶ , resident microglia became intensely labeled with GS lectin, adopted an ameboid morphology, and exhibited prominent distensions along their processes between 12 and 24 hours after injection of OVA-activated T cells (Figure 2, D and H) ▶ . Although the severity of this peaked at day 1, the microglia still showed distensions along their processes at day 2 pi (Figure 2H) ▶ .
Topographical quantitative analysis of these microglial changes showed that by 12 hours pi, the number of ED1+ microglia-like cells in the retinal parenchyma of injected rats was 2.33 ± 1.35 cells/mm 2 (Table 1) ▶ ; such cells were absent from control retinas. The number of these cells peaked at day 1 pi (3.60 ± 3.14 cells/mm2) and decreased gradually thereafter (Table 1) ▶ . Previous investigators have reported the weakening in the intensity and reliability of GS lectin labeling in adult rat retina, making it difficult to quantify microglial numbers in naive control animals. 24 However, despite these difficulties, quantification of GS lectin-positive microglia showed a marked increase in these cells from 12 hours through to day 2 pi. GS lectin-positive microglia had an approximate density of 5 cells/mm 2 in naive controls. By 12 hours pi, this had increased to approximately 15 cells/mm2, peaking at 49 cells/mm 2 at day 1 pi. By day 2 pi, GS lectin-positive microglial density was approximately 44 cells/mm2; ED1+ microglia also persisted in the parenchyma at this time (Table 1) ▶ . However, in the absence of target antigen, no MHC II+ microglia was found throughout the observation period (Table 1) ▶ .
Cellular and Microvascular Responses to Activated T Cells in the Presence of Target Antigen
Activated T Cells Enter the CNS, Accumulate, and Induce Marked Monocyte Infiltration and Persistent Microglial Activation
The second group of animals received both an intravascular injection of OVA-activated T cells and intraocular injections of OVA (left eye) and RPMI (right eye, data not shown). Thus, the difference in responses between the two eyes can be attributed to the absence or presence of the target antigen.
In the OVA-injected eyes, TCR+ cells were first detected within the lumen of retinal vessels at 6 hours pi (data not shown). By 12 hours pi, a substantial number (74 ± 48 cells/mm2) of TCR+ cells had undergone extravasation in the vicinity of veins (Figure 4A) ▶ . From day 1 pi onward, TCR+ cells were no longer obviously aligned along retinal veins but were distributed randomly throughout the parenchyma (Figure 4B) ▶ . The density of extravasated TCR+ cells was 200 ± 288 cells/mm 2 at day 2pi. By 6 hours pi, substantial numbers of ED1+ monocytes were evident within the lumen of retinal veins in the OVA-injected eyes (data not shown). Large numbers of extravasated ED1+ cells (311 ± 89 cells/mm2) were first evident adjacent to retinal veins at 12 hours pi (Fig. C). Process-bearing cells in the parenchyma, likely to be resident microglia, also stained with ED1 at 12 hours pi (17 ± 20 cells/mm2; Figure 4C ▶ ). The number of ED1+ monocytes in the parenchyma peaked between 12 hours and 1 day pi (data not shown). Between day 1 and day 3 pi, both ED1+ monocytic infiltrates and ED1+ microglia were apparent throughout the entire retina (Figure 4D) ▶ . The monocytic nature of a significant number of ED1+ cells was confirmed by the coating of these cells by Monastral blue (Figure 4, C and D) ▶ . In the OVA-injected eyes, MHC II+ cells were first detected within the lumen of retinal vessels at 6 hours pi (data not shown). By 12 hours pi, a substantial number (97 ± 67 cells/mm2) of MHC II+ cells had undergone extravasation in the vicinity of veins (Figure 4E) ▶ . Process-bearing cells in the parenchyma, likely to be resident microglia, also stained with MHC II at 12 hours pi (4 ± 6 cells/mm2; Figure 4E ▶ ). The number of MHC II+ monocytes in the parenchyma peaked at day 1, whereas the number of MHC II+ microglia was maximal between days 3 and 7 pi (data not shown). By day 2 pi, MHC II+ cells were no longer obviously aligned along retinal veins but were distributed randomly throughout the parenchyma (Figure 4F) ▶ .
Figure 4.
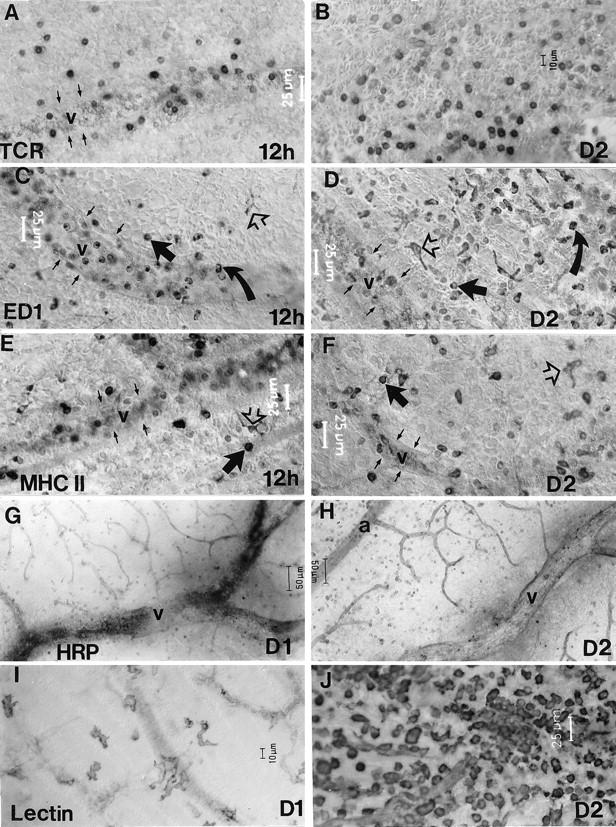
The distribution of αβTCR+ (A and B), ED1+ (C and D), and MHC II+ (E and F) cells, breakdown of the BRB (G and H), and microglial activation (I and J) in the OVA-injected eye at 12 hours, day 1, and day 2 after intravenous injection of OVA-activated T cells. In the presence of target antigen, a substantial number of TCR+ cells accumulated within the venous circulation, with marked extravasation from the venous vasculature apparent by 12 hours pi (A). These cells were distributed throughout the parenchyma on day 2 pi (B). Substantial numbers of ED1+ (C) and MHC II+ (E) cells accumulated within the venous circulation of the retina by 12 hours pi (C) and marked extravasation from the venous vasculature was apparent by 12 hours pi (C and E, solid arrow). The extravasated cells were distributed throughout the parenchyma on day 2 pi (D and F). ED1+ and MHC II+ microglia were also observed within the parenchyma from 6 or 12 hours pi (C−F, open arrows). A substantial proportion of ED1+ cells were also positive for Monastral blue, present either on their surface or internally, confirming their monocytic origin; these cells are evident as the darker particles in (C and D, curved arrow). Breakdown of the BRB at days 1 and 2 pi as revealed by leakage of intravascularly perfused HRP (G and H). In the presence of OVA, the extent of BRB breakdown was marked surrounding veins at day 1 pi (G). By day 2 pi, the extent of HRP leakage was increased even further (H). Activation of resident microglia was apparent at day 1 pi, (I) from a marked increase in GS lectin staining and the adoption of an amoeboid morphology. By day 2 pi, a large population of round monocyte-like cells was also apparent in the retinal parenchyma (J). a, artery; v, vein. Small arrows in A−F indicate the outlines of venous segments.
Microglial activation, as revealed by the adoption of an amoeboid morphology and an increased staining with GS lectin, was apparent throughout the observation period. Resident microglia adopted an amoeboid morphology and displayed a marked increase in reactivity for GS lectin shown from 12 hours (Figure 3G) ▶ through to day 1 (Figure 4I) ▶ . A substantial number of GS lectin-stained monocyte-like cells had undergone extravasation in the OVA-injected eyes by day 2 pi (Figure 4J) ▶ .
Activated T Cells Induce a Persistent and Marked Breakdown of the BRB
In the presence of the specific T cell antigen, a marked breakdown of the BRB was evident from day 1 and persisted throughout the observation period. Leakage of HRP from the vessel lumen resulted in a pronounced blurring of the vessel outline shown here for day 1 pi (Figure 4G) ▶ . At day 2 pi, the extent of BRB breakdown was so great that virtually all of the enzyme had been lost from the vessel lumen and had diffused over a large region of the parenchyma (Figure 4H) ▶ . Hemorrhaging was observed using the colloidal intravascular tracer Monastral blue from day 1 pi persisting until day 7 pi. Figure 2, D ▶ −F, shows marked leakage of Evans blue, Hoechst stain, and Monastral blue colocalized to a venous segment with numerous adherent leukocytes in the presence of target antigen at day 3 pi.
Microvascular and Cellular Response Are More Pronounced in the Venous Circulation
Irrespective of the technique used to examine the extent of T cell and monocyte accumulation and extravasation and the breakdown of the BRB, the severity of the responses was consistently greater in venous segments than in arteries (Figure 3, D ▶ -J). Given the soluble nature of OVA, it would be expected that the target antigen would be equally accessible in the region of arteries and in that of veins. Figure 3, D ▶ −F, shows the same arterial and venous segments in an OVA-injected eye at 3 days pi examined under three different types of illumination according to the modified trichrome technique. The arterial segment, clearly evident from the characteristic elongated morphology of the endothelial cell nuclei, had retained the Evans blue as shown by the distinct vessel outline (Figure 3, D and E) ▶ . In contrast, the venous segment exhibits a marked breakdown of the BRB, with the loss of most of the Evans blue-bound plasma albumin from the vessel lumen (Figure 3D) ▶ . Accumulation of leukocytes is also greatly enhanced in the venous segment as evidenced by the large number of nuclei labeled with Hoechst stain within the lumen. Several of these adherent leukocytes were either coated by Monastral blue or had internalized the dye, indicative of their monocytic nature (Figure 3, D and E) ▶ . The adherent leukocytes also had a marked effect on blood rheology, as was apparent under transmitted illumination (Figure 3F) ▶ . The pronounced leakiness of the venous segment is also evident from the accumulation of Monastral blue particles in the parenchyma surrounding the vein; such particles were not detected in the parenchyma surrounding the artery (Figure 3F) ▶ .
Despite these marked differences between the arterial and venous responses, the activation of resident microglia did not appear to be restricted to the region of veins. Even in OVA-injected eyes at 12 hours pi, activation of microglia, as revealed by increased staining with GS lectin and adoption of an amoeboid morphology, was equally evident in regions surrounding arteries and in those surrounding veins (Figure 3G) ▶ . However, extravasation of GS lectin-stained (Figure 3G) ▶ , ED1+ monocytes (Figure 3H) ▶ in OVA-injected eyes was limited to venous segments. Similarly, extravasation of MHC II+ (Figure 3I) ▶ and TCR+ cells (Figure 3J) ▶ were also restricted to venous segments. These differences in cellular infiltration in arterial and venous segments could be due to a greater up-regulation of cell adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) on venous segments, as shown previously in an experimental model of cerebral malaria.14 A further possible explanation for the increased cellular infiltration from venous segments could be due to the lower flow rates and resultant sheer forces in venous segments.
Discussion
Breakdown of the BRB Induced by Activated T cells of Nonneural Specificity: Functional Implications
We have shown that OVA-activated T cells can enter the CNS and induce a limited and transient breakdown of the BRB as well as transient and mild activation of microglia. OVA is an egg protein and is not normally present in the rat CNS. Therefore, our data constitute direct in vivo evidence that activated T cells of nonneural specificity can induce breakdown of the BRB. Because the retina is an extension of the CNS and its vessels possess barrier properties identical to those of vessels elsewhere in the CNS, our results also suggest that such activated T cells would similarly induce breakdown of the BBB. The segments of vessels in which inflammatory cells accumulate might contain high concentrations of cytokines and chemokines including interleukin-1 and tumor necrosis factor-α and may therefore exhibit localized changes in permeability. 3 Our data support an earlier speculation from EAE studies 25 that intravascular injection of T cells alone may have the capacity to cause CNS vascular damage directly.
Under normal circumstances, the BBB protects the CNS parenchyma from plasma proteins as a result both of the tight junctions between adjacent vascular endothelial cells and of the low pinocytotic activity of these cells. 26 However, our observations suggest that, even in healthy individuals, the CNS is subjected to the consequences of minor breakdown of the BBB whenever a population of activated T cells is present within the circulation. The number of such cells in the circulation can increase as a result of viral or bacterial infections 27,28 or, possibly, in response to psychological stress.
Evidence to date for activated T cell of nonneural specificity to induce breakdown of the BBB has been limited because of the infrequent nature in which the BBB is studied. In the present study, BRB breakdown was limited in extent and of short duration, peaking 1 day after injection of activated T cells and being almost resolved by the second day. Thus, such changes would be unlikely to be detected in a clinical setting without specially designed studies. It would appear that such mild repeated bouts of minor breakdown of the BBB are tolerated physiologically by the majority of the population.
Recruitment of ED1+/MHC II+ Leukocytes and Activation of Microglia by Activated T Cells of Nonneural Specificity
In the present study, we injected activated T cells directly into the circulation of rats. By 6 hours pi, a marked increase in the number of ED1+ and MHC II+ leukocytes were apparent within retinal blood vessels, and, throughout the experimental period, extravasation of ED1+ and MHC II+ leukocytes, though low in number, were closely associated with that of TCR+ T cells. These observations suggest that activated T cells in the circulation recruit other leukocytes, including monocytes. Given that monocytes express the costimulatory molecule B7 on their surface, 29 their recruitment is possibly an additional pathway for the enhancement of the cell-mediated immune response. T cells also contribute to the activation of resident microglia. Whether this effect is mediated directly through the release of cytokines or indirectly through breakdown of the BBB remains to be clearly elucidated.
Initiation of the Immune Response in Neural Tissue
Two mechanisms have been proposed for the initiation of an immune response in the CNS. The first hypothesis, based on accidental encounter, is supported by the fact that a small number of circulating lymphocytes enter the CNS as part of their role in immune surveillance. 2,3 After activation of lymphocytes in the periphery, a small proportion of the activated cells will enter the CNS. 1,30,31 In the presence of target antigen as well as of antigen-presenting cells expressing MHC II molecules, 32-34 infiltrating lymphocytes will initiate an immune response, resulting in further recruitment of circulating lymphocytes. 35-38 The second hypothesis is based on the concept that lymphocytes are recruited into the CNS by chemotaxis induced by lesioned neural parenchyma. Thus, as in infection or neurodegeneration, a primary selective injury to the neural parenchyma is thought to result in local production of proinflammatory cytokines and chemotactic molecules, 39-43 followed by secondary changes in the adhesion properties of the surrounding vascular endothelium and site-specific chemotaxis of circulating lymphocytes.
Our observations provide support for random encounter as the mechanism by which an immune response is initiated in the normal CNS. Our data show that, as a result of an intravascular injection of activated T cells of nonneural specificity, T lymphocytes, ED1+, and MHC II+ leukocytes accumulate within the venous circulation and small numbers of these cells enter the parenchyma. This limited influx of leukocytes is accompanied by a mild, transient breakdown of the BRB and microglial activation. Thus, in the absence of target antigen, extravasated activated T cells do not encounter MHC II+ perivascular cells and microglia. Thus, further recruitment of circulating lymphocytes into the CNS parenchyma was not triggered, and the BRB and microglia returned rapidly to their normal states. However, in the animals that received both the intravenous injection of OVA-activated T cells and an intraocular injection of OVA, MHC II+ perivascular cells and microglia were encountered by the activated T cells, resulting in an exponential increase in the influx of T cells and monocytes, activation of microglia, and a massive and persistent breakdown of the BRB.
These results confirm our previous observation in EAE, 3,17 showing that expression of MHC II on resident microglia occurs only when antigen recognition has taken place within the tissue parenchyma. They further demonstrate that, in the absence of target antigen, activated T cells are able only to initiate a mild inflammatory response by cells such as macrophages; the T cells are not capable of inducing MHC II expression by resident microglia. Taken together, these observations lead us to suggest that microglia are not the cells that induce the immune response even when the target antigen is encountered; rather, other cells, such as the perivascular cells that constitutively express MHC II or circulating monocytes, are responsible for initiation of the immune response.
Acknowledgments
We thank J. Bonner for culture of OVA-activated T cells, B. Hall and J. Sedgwick for antibody OX6 and R73, C. Jeffrey and R. Smith for assistance with photography, and P. Kent for general technical assistance.
Footnotes
Address reprint requests to Dr. T. Chan-Ling, Department of Anatomy and Histology F13, Institute of Biomedical Research, University of Sydney, Sydney, NSW 2006, Australia. E-mail: tailoi@anatomy.usyd edu.au.
Supported by grants from the National Health and Medical Research Council of Australia and the National Multiple Sclerosis Society of Australia.
References
- 1.Hickey WF, Hsu BL, Kimura H: T-lymphocyte entry into the central nervous system. J Neurosci Res 1991, 28:254-260 [DOI] [PubMed] [Google Scholar]
- 2.Wekerle H, Linington C, Lassmann H, Meyermann R: Cellular immune reactivity within the CNS. Trends Neurosci 1986, 9:271-277 [Google Scholar]
- 3.Hu P, Pollard J, Hunt N, Chan-Ling T: Microvascular and cellular responses in the retina of rats with acute experimental allergic encephalomyelitis (EAE). Brain Pathol 1998, 8:487-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickey WF, Lassmann H, Cross AH: Lymphocyte entry and the initiation of inflammation in the central nervous system. Keane RW Hickey WF eds. Immunology of the Nervous System. 1997, :pp 200-225 Oxford University Press, New York [Google Scholar]
- 5.Miller DH, Rudge P, Johnson G, Kendall BE, Macmanus DG, Moseley IF, Barnes D, McDonald W: Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain 1988, 111:927-939 [DOI] [PubMed] [Google Scholar]
- 6.Poser CM: The pathogenesis of multiple sclerosis: additional considerations. J Neurol Sci 1993, 115:S3-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassmann H, Suchanek G, Ozawa K: Histopathology and the blood-cerebrospinal fluid barrier in multiple sclerosis. Ann Neurol 1994, 36:S42-S46 [DOI] [PubMed] [Google Scholar]
- 8.Linington C, Bradl M, Lassmann H, Brunner C, Vass K: Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 1988, 130:443-454 [PMC free article] [PubMed] [Google Scholar]
- 9.Wekerle H, Kojima K, Lannes VJ, Lassmann H, Linington C: Animal models. Ann Neurol 1994, 36:S47-S53 [DOI] [PubMed] [Google Scholar]
- 10.Andersen O, Lygner P, Bergstrom T, Andersson M, Vahlne A: Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol 1993, 240:417-422 [DOI] [PubMed] [Google Scholar]
- 11.Sibley W, Bamford C, Clark K: Clinical viral infections and multiple sclerosis. Lancet 1985, 1:1313-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard JD, Westland KW, Harvey GK, Jung S, Bonner J, Spies JM, Toyka KV, Hartung HP: Activated T cells of nonneural specificity open the blood-nerve barrier to circulating antibody. Ann Neurol 1995, 37:467-475 [DOI] [PubMed] [Google Scholar]
- 13.Chan-Ling T, Neill AL, Hunt NH: Early microvascular changes in murine cerebral malaria detected in retinal wholemounts. Am J Pathol 1992, 140:1121-1130 [PMC free article] [PubMed] [Google Scholar]
- 14.Ma N, Hunt NH, Madigan MC, Chan-Ling T: Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. Am J Pathol 1996, 149:1745-1762 [PMC free article] [PubMed] [Google Scholar]
- 15.Medana IM, Hunt NH, Chaudhri G: Tumor necrosis factor-α expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes. Am J Pathol 1997, 150:1473-1486 [PMC free article] [PubMed] [Google Scholar]
- 16.Chan-Ling T: Glial, neuronal and vascular interactions in the mammalian retina. Prog Retinal Res 1994, 13:357-389 [Google Scholar]
- 17.Hu P, Pollard J, Hunt N, Taylor J, Chan-Ling T: Microvascular and cellular responses in the optic nerve of rats with acute experimental allergic encephalomyelitis (EAE). Brain Pathol 1998, 8:475-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan-Ling T: Glial, vascular, and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Techniq 1997, 36:1-16 [DOI] [PubMed] [Google Scholar]
- 19.Chan-Ling T, Tout S, Hollander H, Stone J: Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1992, 33:2128-2147 [PubMed] [Google Scholar]
- 20.Stewart PA, Farrell CR, Farrell CL, Hayakawa E: Horseradish peroxidase retention and washout in blood-brain barrier lesions. J Neurosci Meth 1992, 41:75-84 [DOI] [PubMed] [Google Scholar]
- 21.Chan-Ling T, Halasz P, Stone J: Development of retinal vasculature in the cat: stages, topography and mechanisms. Curr Eye Res 1990, 9:459-478 [DOI] [PubMed] [Google Scholar]
- 22.Neill AL, Hunt NH: Pathology of fatal and resolving cerebral malaria in mice. Parasitology 1992, 105:165-175 [DOI] [PubMed] [Google Scholar]
- 23.Maddox DE, Shibata S, Goldstein IJ: Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci USA 1982, 79:166-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashwell KWS, Hollander H, Streit W, Stone J: The appearance and distribution of micoglia in the developing retina of the rat. Vis Neurosci 1989, 2:437-448 [DOI] [PubMed] [Google Scholar]
- 25.Sedgwick J, Brostoff S, Mason D: Experimental allergic encephalomyelitis in the absence of a classical delayed-type hypersensitivity reaction: severe paralytic disease correlates with the presence of interleukin 2 receptor-positive cell infiltrating the central nervous system. J Exp Med 1987, 165:1058-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese TS, Karnovsky MJ: Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 1967, 34:207-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin R, McFarland H: Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci 1995, 32:121-182 [DOI] [PubMed] [Google Scholar]
- 28.Wekerle H, Lassmann H: Contra: evidence against a primary lesion in the target organ in autoimmune disease. Int Arch Allergy Immunol 1994, 103:328-331 [DOI] [PubMed] [Google Scholar]
- 29.Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM: B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA 1991, 88:6575-6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeine R, Owens T: Direct demonstration of the infiltration of murine central nervous system by Pgp-1/CD44high CD45RB(low) CD4+ T cells that induce experimental allergic encephalomyelitis. J Neuroimmunol 1992, 40:57-69 [DOI] [PubMed] [Google Scholar]
- 31.Ludowyk PA, Willenborg DO, Parish CS: Selective localisation of neuro-specific T lymphocytes in the central nervous system. J Neuroimmunol 1992, 37:237-250 [DOI] [PubMed] [Google Scholar]
- 32.Maehlen J, Olsson T, Zachau A, Klareskog L, Kristensson K: Local enhancement of major histocompatibility complex (MHC) class I and II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neurons. J Neuroimmunol 1989, 23:125-132 [DOI] [PubMed] [Google Scholar]
- 33.Molleston MC, Thomas ML, Hickey WF: Novel major histocompatibility complex expression by microglia and site-specific experimental allergic encephalomyelitis lesions in the rat central nervous system after optic nerve transection. Adv Neurol 1993, 59:337-348 [PubMed] [Google Scholar]
- 34.Konno H, Yamamoto T, Suzuki H, Yamamoto H, Iwasaki Y, Ohara Y, Terunuma H, Harata N: Targeting of adoptively transferred experimental allergic encephalitis lesion at the sites of wallerian degeneration. Acta Neuropathol 1990, 80:521-526 [DOI] [PubMed] [Google Scholar]
- 35.Cross AH, Cannella B, Brosnan CF, Raine CS: Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest 1990, 63:162-170 [PubMed] [Google Scholar]
- 36.Olsson T, Diener P, Ljungdahl A, Hojeberg B, van der Meide PH, Kristensson K: Facial nerve transection causes expansion of myelin autoreactive T cells in regional lymph nodes and T cell homing to the facial nucleus. Autoimmunity 1992, 13:117-126 [DOI] [PubMed] [Google Scholar]
- 37.Kawai K, Ito K, Imamura K, Hickey WF, Zweiman B, Takahashi A: Enhancing effects of irrelevant lymphocytes on adoptive transferred experimental allergic encephalomyelitis. J Neuroimmunol 1993, 42:39-45 [DOI] [PubMed] [Google Scholar]
- 38.Schnell L, Schneider R, Berman MA, Perry VH, Schwab ME: Lymphocyte recruitment following spinal cord injury in mice is altered by prior viral exposure. Eur J Neurosci 1997, 9:1000-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesselingh SL, Levine B, Fox RJ, Choi S, Griffin DE: Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol 1994, 152:1289-1297 [PubMed] [Google Scholar]
- 40.Calvo CF, Yoshimura T, Gelman M, Mallat M: Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci 1996, 8:1725-1734 [DOI] [PubMed] [Google Scholar]
- 41.McGeer PL, McGeer EG: Anti-inflammatory drugs in the fight against Alzheimer’s disease. Ann NY Acad Sci 1996, 777:213-220 [DOI] [PubMed] [Google Scholar]
- 42.Schluesener HJ, Seid K, Kretzschmar J, Meyermann R: Leukocyte chemotactic factor, a natural ligand to CD4, is expressed by lymphocytes and microglial cells of the MS plaque. J Neurosci Res 1996, 44:606-611 [DOI] [PubMed] [Google Scholar]
- 43.Klein MA, Moller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich G: Impaired neuroglial activation in interleukin-6 deficient mice. Glia 1997, 19:227-233 [DOI] [PubMed] [Google Scholar]


