Abstract
Sporadic inclusion-body myositis (s-IBM) is the most common progressive muscle disease of older persons. Pathologically, the muscle biopsy manifests various degrees of inflammation and specific vacuolar degeneration of muscle fibers characterized by paired helical filaments (PHFs) composed of phosphorylated tau. IBM vacuolated fibers also contain accumulations of several other Alzheimer-characteristic proteins. Molecular mechanisms leading to formation of the PHFs and accumulations of proteins in IBM muscle are not known. We report that the abnormal muscle fibers of IBM contained (i) acridine-orange-positive RNA inclusions that colocalized with the immunoreactivity of phosphorylated tau and (ii) survival motor neuron protein immunoreactive inclusions, which by immuno-electron microscopy were confined to paired helical filaments. This study demonstrates two novel components of the IBM paired helical filaments, which may lead to better understanding of their pathogenesis.
Sporadic inclusion-body myositis (s-IBM), the most common muscle disease of older persons, leads to severe disability. 1,2 The morphological features of s-IBM are vacuolar degeneration of muscle fibers, various degrees of lymphocytic inflammation, and intracellular congophilia. 3,4 An intriguing aspect is that the pathological phenotypes of muscle in s-IBM and of brain in Alzheimer’s disease (AD) share many similarities. 1,2 Those include intramuscle fiber clusters (tangles) of paired helical filaments (PHFs) containing phosphorylated tau 5,6 and abnormal accumulations of several proteins, such as amyloid-β precursor protein (AβPP), including its epitope amyloid-β, apolipoprotein E, ubiquitin, presenilin, and others. 1,2 Similarly to AD brain, 7-9 IBM abnormal muscle fibers express markers of oxidative stress. 10-12
Unknown in both IBM and AD are the factors leading to the abnormal accumulation of various proteins in the respective tissues, including the yet unidentified molecular pathways responsible for PHF formation. Recently it was demonstrated that AD neurofibrillary tangles (composed of PHFs) contain RNA 13 and that RNA in vitro promotes assembly of tau into PHFs. 14
We have now determined that clusters of IBM-PHFs contain RNA and a known RNA-binding protein, survival motor neuron (SMN). 15 We previously demonstrated that in human muscle biopsies SMN is expressed at normal neuromuscular junctions postsynaptically 16 in regenerating muscle fibers and in denervated, very atrophic, apoptotic-like fibers.
Materials and Methods
Patients
Studies were performed on 10-μm transverse sections of fresh-frozen diagnostic muscle biopsies of 19 patients with these diagnoses: 11 s-IBM, 2 non-IBM vacuolar myopathies of unknown cause, 3 morphologically nonspecific myopathies, 1 polymyositis, 2 dermatomyositis, and 4 normal muscle biopsies. All IBM biopsies had muscle fibers containing tau-positive PHFs and congophilia. 1
Acridine Orange (AO) Staining
This was performed as described by Miike et al. 17 Sections were fixed in ether:95% ethanol (1:1) for 1 hour, rehydrated, rinsed, incubated in 1% acetic acid, rinsed, and stained for 3 minutes in 0.01% AO in 0.1 mol/L phosphate buffer, pH 6.0. The sections were then rinsed, incubated 1 minute in 0.1 mol/L calcium chloride, rinsed, and mounted in phosphate buffer. Fluorescence microscopy used a Zeiss Axiophot microscope with a BP 450–490 exciter filter, LP 520 barrier filter, and FT 510 chromatic beam-splitter.
Double Localization of AO and SMI-31 Immunoreactivity on the Same Section
Sections were incubated for 1 hour in SMI-31 mouse monoclonal antibody (Sternberger Monoclonals Inc., Baltimore, MD, diluted 1:1000), which recognizes phosphorylated tau in IBM PHFs, 6,18 followed by incubation in a rabbit anti-mouse serum conjugated to 7-amino-4-methylcoumarin-3-acetic acid (AMCA) (DAKO, Carpinteria, CA). Subsequently the sections were rinsed, fixed in ether: 95% ethanol (1:1), and processed for AO staining as above. The blue color of AMCA was visualized with a G 365 exciter filter, LP 420 barrier filter and FT 395 chromatic beam-splitter, and the orange color of AO histofluorescence was visualized as above.
SMN Immunocytochemistry
For light microscopy, SMN was immunolocalized using two mouse monoclonal anti-SMN antibodies: 2B1, a gift from Dr. Gideon Dreyfuss, diluted 1:50, and an antibody directed against residues 14–174 of human SMN (Transduction Laboratories, Lexington, KY), diluted 1:20. We have previously described immunocytochemistry of SMN and its specificity in non-IBM human muscle biopsies. 16 Electronmicroscopic immunocytochemistry of SMN was performed according to our previously established technique. 4-6,18,19
Control Experiments
To evaluate whether orange AO histofluorescence was specific to RNA, before AO staining the sections were pretreated for 4 hours at 37°C with either 200 U/ml RNase-A solution (Ambion Inc., Austin, TX), containing 20 mmol/L of the protease-inhibitor phenylmethylsulfonyl fluoride (PMSF; Sigma, St. Louis, MO), or 10,000 U/ml RNase-free DNase solution (Roche Molecular Biochemicals, Indianapolis, IN). To determine whether SMN immunoreactivity reflects binding of the antigen to RNA, SMN immunohistochemistry was performed after the sections were pretreated with RNase solution as above. To block nonspecific binding of antibodies to Fc receptors, the sections were preincubated in normal goat serum diluted 1:10, as described. 4-6,16,18,19 Omission of the primary antibody or replacement of the primary antibody with a nonrelevant serum controlled for specificity of the immunohistochemical reactions, as described. 16,18,19
Results
AO Staining
AO positivity in this study refers only to the orange fluorescence of RNA. In s-IBM, 70 to 80% of the vacuolated muscle fibers (VMFs), and some of the non-VMFs, had AO positivity in the form of focal, well-demarcated squiggly, rod-like, and dot-like cytoplasmic inclusions (Figure 1, A ▶ −D, F, H, J, and L). Pretreatment with RNase-A, but not with DNase, completely abolished the AO positivity of more than 95% of the inclusions (Figure 1, K ▶ −M); rare inclusions retained weakly positive AO staining.
Figure 1.
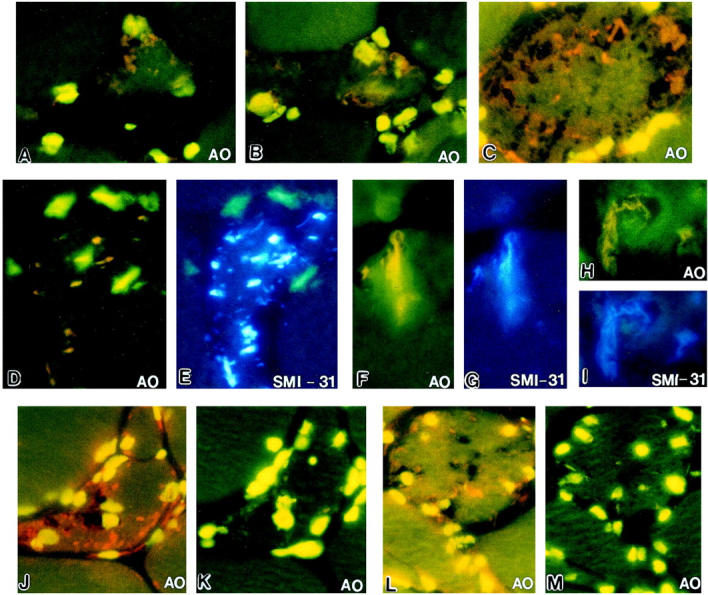
Fluorescence microscopy of acridine orange (AO) staining (RNA is orange, DNA bright yellow-green) and SMI-31 immunocytochemistry (bluish color), localizing IBM PHFs. A−D, F, H, J, and L illustrate orange squiggly, rod-like and dot-like inclusions in IBM-abnormal muscle fibers. Pairs D and E (low-power microscopy), F and G, H and I (high-power microscopy) show double localization of AO and SMI-31. There is close colocalization of AO-positive orange RNA and bluish SMI-31 immunoreactive PHFs. K and M: Serial sections to J and L. AO-positive orange PHF inclusions (J and L) are not present after RNase pretreatment (K and M). J-M were photographed at the same exposure time; the darker background of K and M results from the elimination of the slight diffuse non-PHF RNA species within the muscle fibers. Original magnifications, ×1250 (A, B, D, E, J−M), ×2000 (C), and ×7000 (F−I).
Normal muscle biopsies did not have any AO-positive muscle fibers. In regenerating muscle fibers of all biopsies that contained them, AO positivity was present in a diffuse pattern, and pretreatment with RNase-A completely abolished their AO positivity. Non-IBM vacuolar myopathies did not have AO-positive inclusions.
Double Localization of AO Positivity and SMI-31 Immunoreactivity
In all IBM muscle biopsies, AO positivity of the cytoplasmic inclusions colocalized with SMI-31 immunoreactivity (Figure 1, D ▶ −I). Pretreatment with RNase-A did not influence the SMI-31 immunoreactivity.
SMN Immunoreactivity
By light microscopy, 70 to 80% of the IBM-VMFs, and some of the non-VMFs, had strongly SMN-immunoreactive squiggly, rod-like, and dot-like inclusions (Figure 2, A ▶ −C and E). RNase pretreatment performed on serial sections either abolished or prominently diminished SMN immunoreactivity (Figure 2, D and F) ▶ . Ultrastructural immunocytochemistry of the IBM-VMFs revealed that SMN was immunolocalized only to the clusters of PHFs (Figure 3, A and B) ▶ . PHFs appear as tubulofilaments when horseradish peroxidase is used as the immunohistochemical marker. 5,6,18,19
Figure 2.
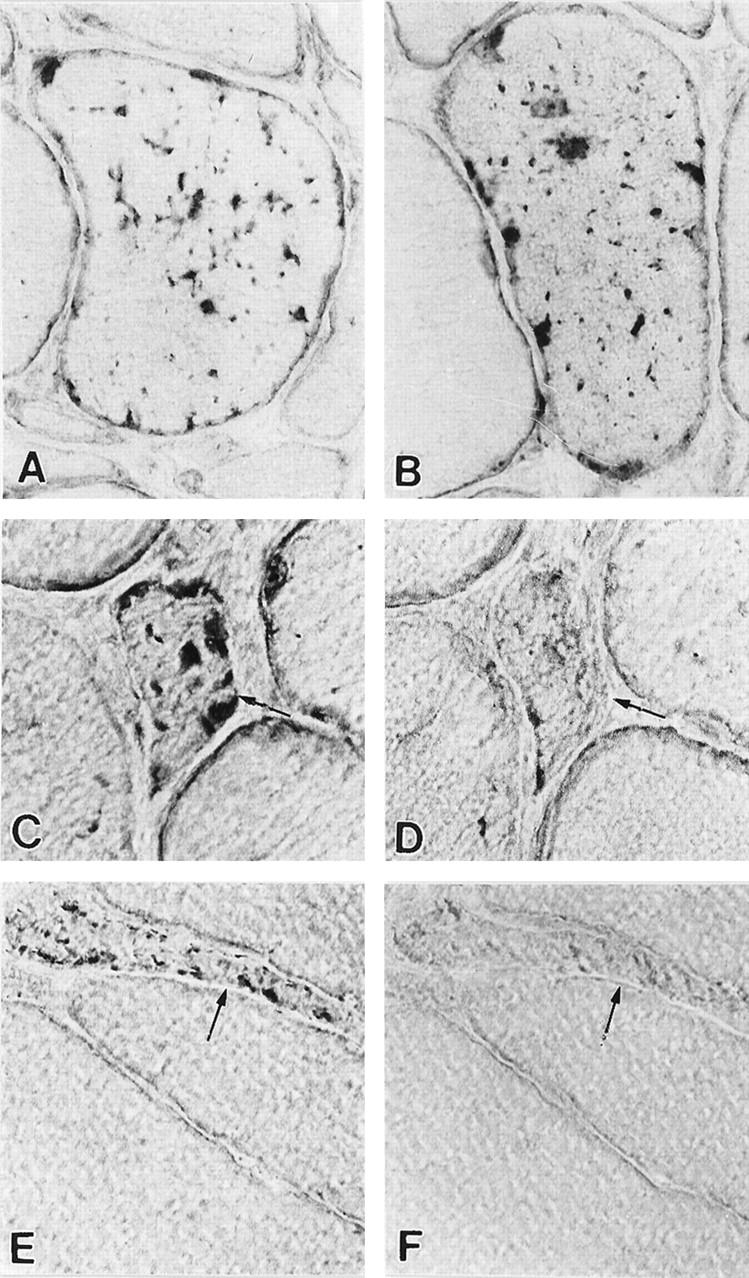
SMN immunoreactivity. A−C, E: Darkly immunoreactive squiggly, rod-like, and dot-like inclusions in IBM abnormal muscle fibers. D and F: Serial sections to C and E; after pretreatment with RNase, SMN immunoreactivity is greatly diminished (D) or virtually eliminated (F). Arrows identify abnormal muscle fibers in D−F. Original magnifications, ×1250.
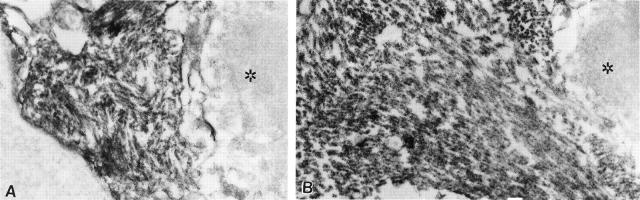
Figure 3.
Immunoelectronmicroscopy of SMN in two IBM-vacuolated muscle fibers. A and B: The peroxidase reaction demonstrates dark reaction-product covering PHFs exclusively and entirely, whereas adjacent portions of the myofiber (right side of each picture, identified by asterisk) are not immunostained. Original magnifications, ×28,000.
Discussion
Our study demonstrates that clusters of IBM-PHFs contain AO positivity and SMN immunoreactivity.
Under ultraviolet excitation, AO that is intercalated into RNA gives a bright orange fluorescence, whereas AO staining of DNA gives a yellow fluorescence. 20 Cytoplasm lacking abundant RNA and DNA gives a pale green background. Individual subtypes of accumulated RNA (ie, mRNA, rRNA, tRNA) cannot be distinguished by this method. The IBM muscle biopsies emitted orange AO fluorescence of squiggly, rod-like, and dot-like inclusions against a pale green background of nonstained cytoplasm. RNase, but not DNase, pretreatment abolished the AO labeling of more than 95% of the inclusions, indicating that their AO positivity was specific for RNA. (The RNase pretreatment did not totally abolish orange fluorescence of the occasional inclusions, perhaps because the RNase solution did not completely penetrate some tight clusters of PHFs, or because, in them, a fraction of RNA was more tightly complexed with PHF proteins.) The close colocalization of AO positivity with the immunoreactivity achieved with SMI-31 antibody recognizing IBM-PHFs suggests that the AO positivity reflects RNA on or within clusters of PHFs.
The mechanism of RNA accumulation within the clusters of IBM-PHFs is not known. In normal cells, some RNA associates with proteins of the cellular cytoskeleton. 21 For example, the microtubule-associated protein MAP 1A is one component of the RNA-cytoskeleton complex. 22 Normal tau is a microtubule-associated protein. When associated with tau, tubulin polymerizes into microtubules and by doing so prevents tau aggregation. 14,23 Phosphorylated tau (P-tau) has a decreased ability to interact with tubulin, a phenomenon proposed to be important in causing P-tau aggregation into PHFs in AD brain. 23 Free RNA can compete with tubulin for the cellular pool of tau, and in vitro RNA stimulates aggregation of tau into PHFs. 14 In IBM muscle fibers, possibilities include: i) that phosphorylation of tau diminishes its interaction with tubulin and increases its association with RNA, and this process facilitates formation of PHFs; ii) that RNA has an abnormal propensity to associate with tau, resulting in the pathological phosphorylation of tau and aggregation of it into PHFs; or iii) that RNA is being sequestered in the PHFs due to the presence there of a non-tau RNA-binding protein, eg, SMN.
SMN is a recently discovered protein, encoded by a gene on chromosome 5q13, mutations of which are associated with, and putatively responsible for, more than 98% of the autosomal-recessive spinal muscular atrophy patients. 24 Normal biological functions of SMN are largely unknown, but SMN is known to bind directly to RNA and to RNA-binding proteins. 15,25 A role of SMN has also been proposed in RNA processing, in the biogenesis of small nuclear ribonucleic proteins, and in pre-mRNA splicing. 25 In human muscle, SMN is developmentally regulated and, based on its immunolocalization, it probably plays a yet unknown role at normal nerve-muscle postsynaptic regions, in regenerating muscle fibers, and in post-denervation apoptosis-like processes of human muscle fibers. 16 In IBM muscle the immunoreactivity of SMN was localized to PHFs, and it was abolished or greatly diminished after pretreatment with RNase. We therefore postulate that on PHFs the SMN is present, at least partially, through its association with RNA, and/or another protein already bound to PHF-linked RNA. For example, it would be interesting to determine whether small nuclear ribonucleoproteins (snRNPs) involved in spliceosome assembly are also accumulated within the clusters of PHFs, which might represent presumably inactive foci of these splicing elements.
PHFs in both AD brain and IBM muscle contain accumulation of several proteins, including markers of oxidative stress. 12,26 Molecular mechanisms leading to accumulation of those proteins and their role in the pathogenic cascade of both IBM and AD remain unknown. Several mechanisms, including RNA binding and oxidative damage, have been proposed in relation to PHF formation in AD brain. 14 RNA has been shown to be oxidized in AD brain. 27 Whether RNA is oxidized in IBM muscle fibers is not yet known.
Our present study demonstrates two novel components of IBM PHFs, and it raises the possibility that RNA and possibly the RNA-binding protein SMN participate in PHF formation in IBM.
Acknowledgments
We thank Dr. Gideon Dreyfuss for his gift of 2B1 anti-SMN antibody. Aldobrando Broccolini is a postdoctoral fellow in Dr. Askanas’ laboratory.
Footnotes
Address reprint requests to Dr. Valerie Askanas, USC Neuromuscular Center, 637 South Lucas Avenue, Los Angeles, CA 90017-1912. E-mail: askanas@hsc.usc.edu.
Supported in part by grants from the National Institutes of Health (NS34103 and AG16768) and the Muscular Dystrophy Association (all to V. A.).
References
- 1.Askanas V, Engel WK: Sporadic inclusion-body myositis and its similarities to Alzheimer disease brain: recent approaches to diagnosis and pathogenesis, and relation to aging. Scand J Rheumatol 1998, 27:389-405 [DOI] [PubMed] [Google Scholar]
- 2.Askanas V, Engel WK: Sporadic inclusion-body myositis and its similarities to Alzheimer disease brain. Recent approaches to diagnosis and pathogenesis, and relation to aging. Scand J Rheumatol 1998, 27:389-405 [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Sahenk Z, Gales T, Paul L: Amyloid filaments in inclusion body myositis. Arch Neurol 1991, 48:1229-1234 [DOI] [PubMed] [Google Scholar]
- 4.Askanas V, Engel WK, Alvarez RB: Light- and electronmicroscopic localization of β-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol 1992, 141:31-36 [PMC free article] [PubMed] [Google Scholar]
- 5.Askanas V, Engel WK, Bilak M, Alvarez RB, Selkoe DJ: Twisted tubulofilaments of inclusion-body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol 1994, 144:177-187 [PMC free article] [PubMed] [Google Scholar]
- 6.Mirabella M, Alvarez RB, Bilak M, Engel WK, Askanas V: Difference in expression of phosphorylated tau epitopes between sporadic inclusion-body myositis and hereditary inclusion-body myopathies. J Neuropathol Exp Neurol 1996, 55:774-786 [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G: Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci 1997, 17:2653-2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MA, Sayre LM, Monnier VM, Perry G: Oxidative posttranslational modifications in Alzheimer disease. Mol Chem Neuropathol 1996, 28:41-48 [DOI] [PubMed] [Google Scholar]
- 9.Butterfield DA: β-amyloid-associated free radical oxidative stress, and neurotoxicity: implications for Alzheimer’s disease. Chem Res Toxicol 1997, 10:495-506 [DOI] [PubMed] [Google Scholar]
- 10.Yang C-C, Alvarez RB, Engel WK, Askanas V: Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. NeuroReport 1996, 8:153-158 [DOI] [PubMed] [Google Scholar]
- 11.Yang C-C, Askanas V, Engel WK, Alvarez RB: Immunolocalization of transcription factor NF-κB in inclusion-body myositis muscle and at normal human neuromuscular junctions. Neurosci Lett 1998, 254:77-80 [DOI] [PubMed] [Google Scholar]
- 12.Askanas V, Engel WK: Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: diseases of oxidative stress and aging? Arch Neurol 1998, 55:915-920 [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg SD, Crino PB, Lee VM-Y, Eberwine JH, Trojanowski JQ: Sequestration of RNA in Alzheimer’s disease neurofibrillary tangles and senile plaques. Ann Neurol 1997, 41:200-209 [DOI] [PubMed] [Google Scholar]
- 14.Kampers T, Friedhoff P, Biernat J, Mandelkow E-M, Mandelkow E: RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett 1996, 399:344-349 [DOI] [PubMed] [Google Scholar]
- 15.Lorson CL, Androphy EJ: The domain encoded by exon 2 of the survival motor neuron protein mediates nucleic acid binding. Hum Mol Genet 1998, 7:1269-1275 [DOI] [PubMed] [Google Scholar]
- 16.Broccolini A, Engel WK, Askanas V: Localization of survival motor neuron protein in human apoptotic-like and regenerating muscle fibers, and neuromuscular junctions. NeuroReport 1999, 10:1637-1641 [DOI] [PubMed] [Google Scholar]
- 17.Miike T, Tamari H, Ohtani Y, Nakamura H, Matsuda I, Miyoshino S: A fluorescent microscopy study of biopsied muscles from infantile neuromuscular disorders. Acta Neuropathol (Berl) 1983, 59:48-52 [DOI] [PubMed] [Google Scholar]
- 18.Askanas V, Alvarez RB, Mirabella M, Engel WK: Use of anti-neurofilament antibody to identify paired-helical filaments in inclusion-body myositis. Ann Neurol 1996, 39:389-391 [DOI] [PubMed] [Google Scholar]
- 19.Askanas V, Engel WK, Yang C-C, Alvarez RB, Lee VM-Y, Wisniewski T: Light and electron microscopic immunolocalization of presenilin 1 in abnormal muscle fibers of patients with sporadic inclusion-body myositis and autosomal-recessive inclusion-body myopathy. Am J Pathol 1998, 152:889-895 [PMC free article] [PubMed] [Google Scholar]
- 20.Schümmelfeder N: Histochemical significance of the polychromatic fluorescence induced in tissue stained with acridine orange. J Histochem Cytochem 1958, 6:392-393 [Google Scholar]
- 21.Bassell G, Singer RH: mRNA and cytoskeletal filaments. Curr Opin Cell Biol 1997, 9:109-115 [DOI] [PubMed] [Google Scholar]
- 22.DeFranco C, Chicurel ME, Potter H: A general RNA-binding protein complex that includes the cytoskeleton-associated protein MAP 1A. Mol Biol Cell 1998, 9:1695-1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow E-M, Mandelkow E: Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). J Biol Chem 1995, 270:7679-7688 [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J: Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80:155-165 [DOI] [PubMed] [Google Scholar]
- 25.Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G: A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 1998, 95:615-624 [DOI] [PubMed] [Google Scholar]
- 26.Markesbery WR: Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 1997, 23:134-147 [DOI] [PubMed] [Google Scholar]
- 27.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA: RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci 1999, 19:1959-1964 [DOI] [PMC free article] [PubMed] [Google Scholar]