Abstract
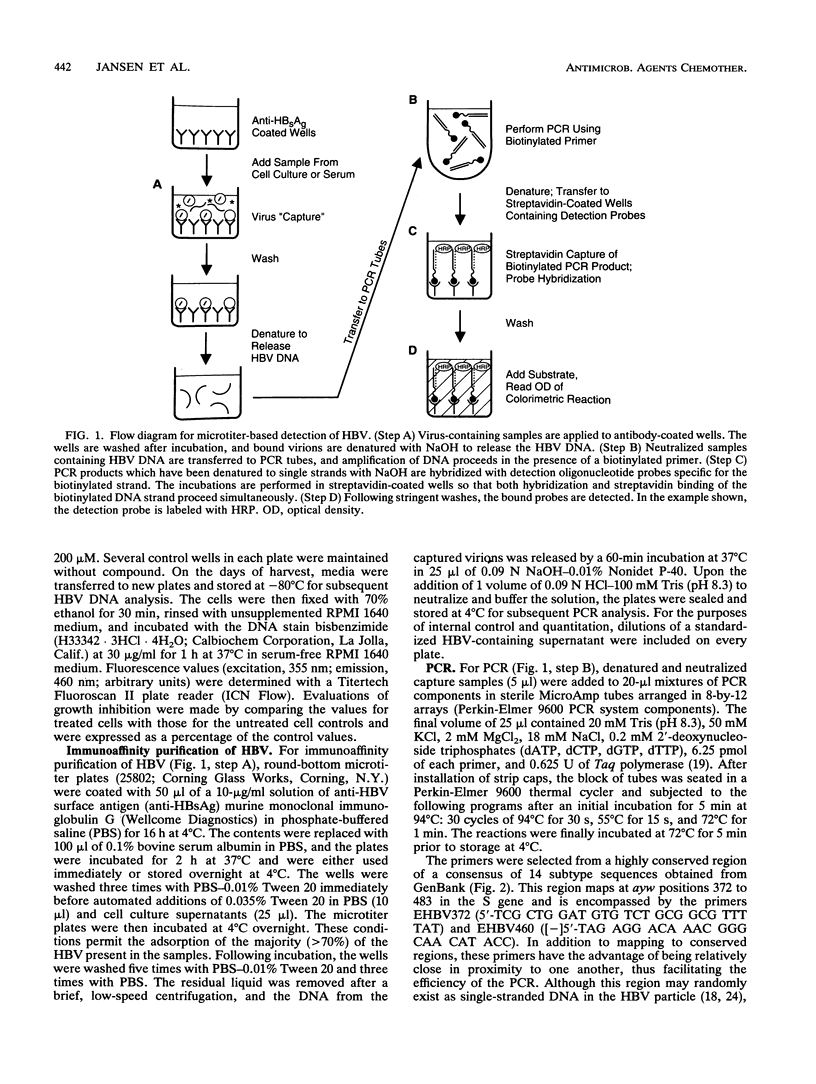
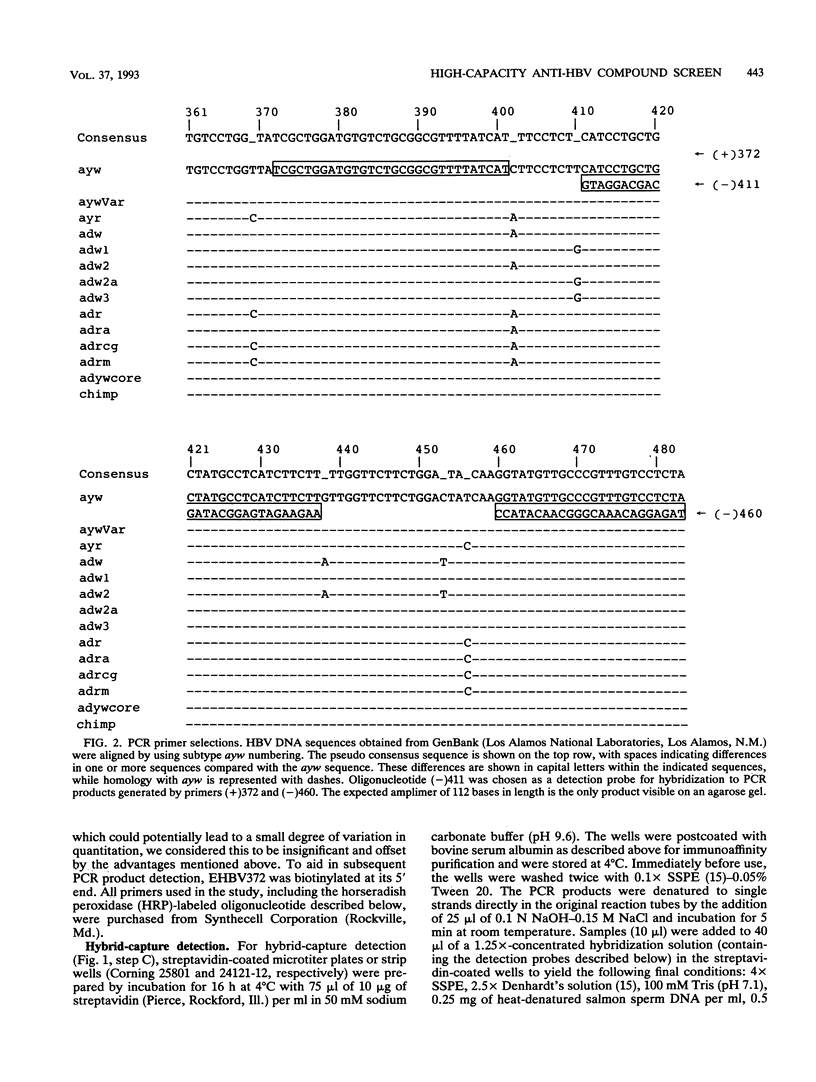
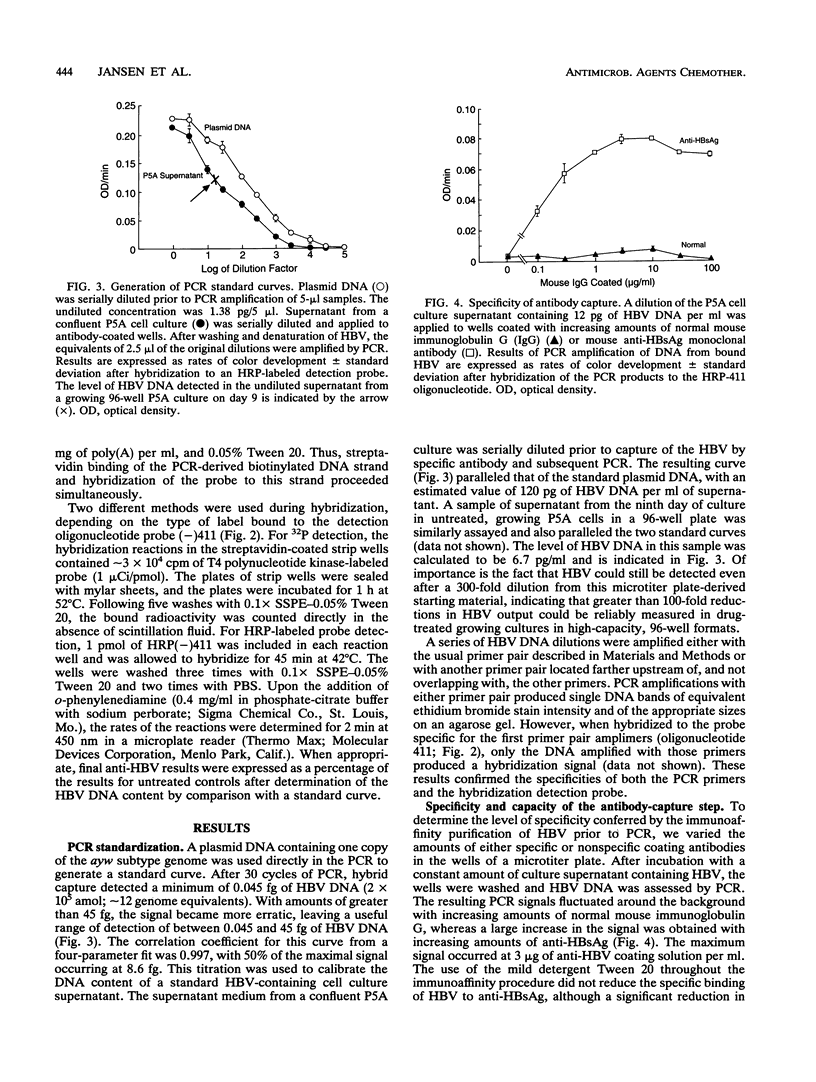
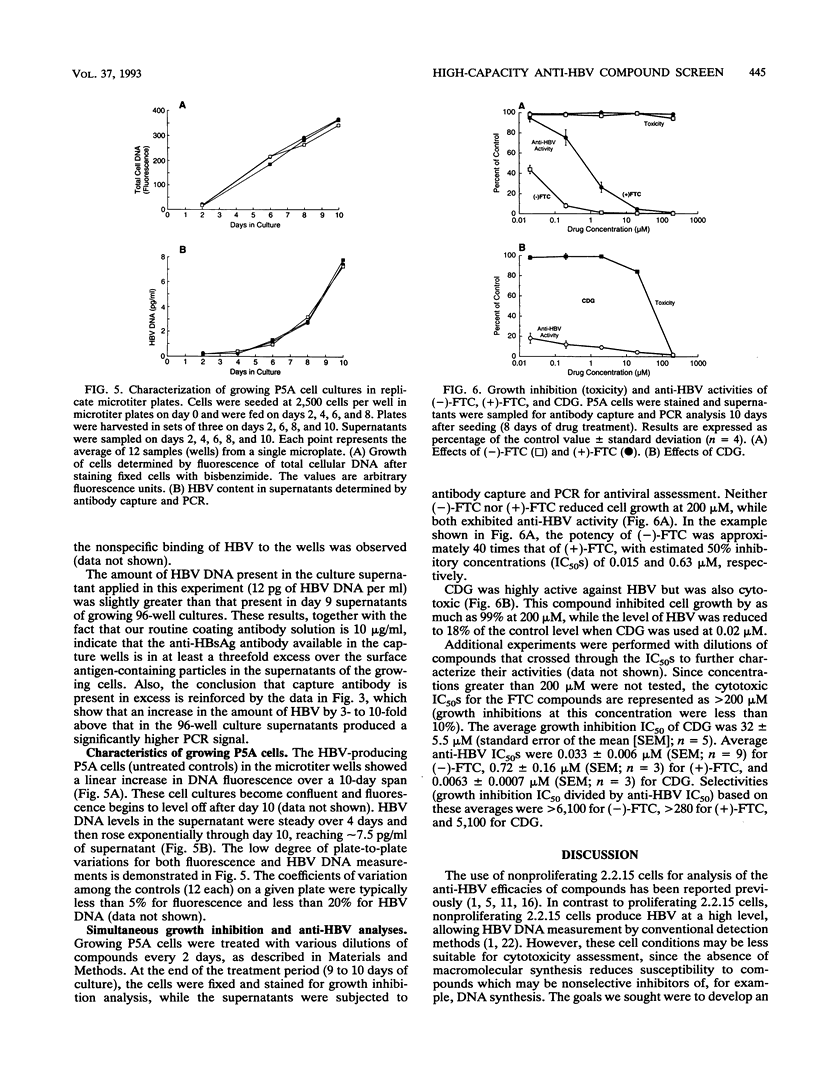
An integrated assessment system specific for hepatitis B virus (HBV) Dane particle DNA was developed to examine the activity of potential anti-HBV compounds in chronic HBV-producing HepG2-derived 2.2.15 cells. Cell culture, immunoaffinity purification, polymerase chain reaction, and hybrid-capture detection were performed in the microtiter format to facilitate increased throughput by automation. The high sensitivity afforded by the assay provided quantitative detection of less than 0.5 fg of extracellular HBV DNA from 25 microliters of cell culture supernatants, and drug-induced reductions in HBV titers greater than 100-fold were easily measured. Fluorometric determination of total cellular DNA from the same 96-well proliferating cell cultures allowed simultaneous evaluation of inhibition of cell growth, thus providing the ability to assess the overall selectivities of candidate compounds in a single experiment. The potent activities of three anti-HBV compounds, the (+) and (-) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxythiolane-5-yl]cytosine (FTC) and D-carbocyclic-2'-deoxyguanosine (CDG), were confirmed by this method. (-)-FTC was more active than its (+) enantiomer (50% inhibitory concentrations, 0.033 +/- 0.006 and 0.723 +/- 0.160 microM [standard error of the mean; SEM], respectively), while both enantiomers demonstrated a lack of cytotoxicity at 200 microM. CDG was more potent (50% inhibitory concentration, 0.0063 +/- 0.0007 microM [SEM]) but was also significantly more toxic, inhibiting cell growth by 50% at 32 +/- 6 microM (SEM). These results demonstrate the usefulness of this immunoaffinity-based, quantitative polymerase chain reaction system as a high-capacity in vitro tool for assessment of anti-HBV compound selectivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki-Sei S., O'Brien M. C., Ford H., Fujii H., Gilbert D. A., Cooney D. A., Johns D. G., Broder S., Mitsuya H. In vitro inhibition of hepatitis B virus replication by 2',3'-dideoxyguanosine, 2',3'-dideoxyinosine, and 3'-azido-2',3'-dideoxythymidine in 2.2.15 (PR) cells. J Infect Dis. 1991 Nov;164(5):843–851. doi: 10.1093/infdis/164.5.843. [DOI] [PubMed] [Google Scholar]
- Davis G. L., Hoofnagle J. H. Interferon in viral hepatitis: role in pathogenesis and treatment. Hepatology. 1986 Sep-Oct;6(5):1038–1041. doi: 10.1002/hep.1840060537. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Davis M., Liotta D. C., Paff M., Frick L. W., Nelson D. J., Dornsife R. E., Wurster J. A., Wilson L. J., Fyfe J. A. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Dec;36(12):2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodniy M., Katzenstein D. A., Sengupta S., Wang A. M., Casipit C., Schwartz D. H., Konrad M., Groves E., Merigan T. C. Detection and quantification of human immunodeficiency virus RNA in patient serum by use of the polymerase chain reaction. J Infect Dis. 1991 Apr;163(4):862–866. doi: 10.1093/infdis/163.4.862. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Winters M. A., Merigan T. C. Detection and quantification of gene amplification products by a nonisotopic automated system. Biotechniques. 1992 Jan;12(1):36, 38-9. [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Combined immunoaffinity cDNA-RNA hybridization assay for detection of hepatitis A virus in clinical specimens. J Clin Microbiol. 1985 Dec;22(6):984–989. doi: 10.1128/jcm.22.6.984-989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Milman G. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antiviral Res. 1991 Mar-Apr;15(3):217–228. doi: 10.1016/0166-3542(91)90068-3. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lampertico P., Malter J. S., Gerber M. A. Development and application of an in vitro model for screening anti-hepatitis B virus therapeutics. Hepatology. 1991 Mar;13(3):422–426. [PubMed] [Google Scholar]
- Landgraf A., Reckmann B., Pingoud A. Direct analysis of polymerase chain reaction products using enzyme-linked immunosorbent assay techniques. Anal Biochem. 1991 Oct;198(1):86–91. doi: 10.1016/0003-2697(91)90510-z. [DOI] [PubMed] [Google Scholar]
- Price P. M., Banerjee R., Acs G. Inhibition of the replication of hepatitis B virus by the carbocyclic analogue of 2'-deoxyguanosine. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8541–8544. doi: 10.1073/pnas.86.21.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Khanna B., Nainan O. V., Margolis H. S. Epidemiologic patterns of wild-type hepatitis A virus determined by genetic variation. J Infect Dis. 1991 Feb;163(2):286–292. doi: 10.1093/infdis/163.2.286. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Greenman R. L. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974 Jun;13(6):1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sells M. A., Chen M. L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Zelent A. Z., Shvartsman M., Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988 Aug;62(8):2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy Y. F., O'Dell C. A., Shannon W. M., Arnett G. Synthesis and antiviral activity of carbocyclic analogues of 2'-deoxyribofuranosides of 2-amino-6-substituted-purines and of 2-amino-6-substituted-8-azapurines. J Med Chem. 1984 Nov;27(11):1416–1421. doi: 10.1021/jm00377a007. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Bengtström M., Tenhunen J., Söderlund H. Quantification of polymerase chain reaction products by affinity-based hybrid collection. Nucleic Acids Res. 1988 Dec 9;16(23):11327–11338. doi: 10.1093/nar/16.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Buendia M. A. Hepatitis B virus. Sci Am. 1991 Apr;264(4):116–123. doi: 10.1038/scientificamerican0491-116. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]



