Abstract
Oral administration of interleukin-6 (IL-6) has been shown to reduce hemorrhage-induced bacterial translocation from the gut in mice and rats. To examine the intestinal microvasculature, mice were given the electron-dense tracer horseradish peroxidase (HRP) after hemorrhage and IL-6 or vehicle administration. In normal mice and in those hemorrhaged and given IL-6, the electron-dense marker, administered intravenously, could be found in intestinal capillaries and between mucosal epithelial cells, suggesting that the microvasculature was patent. In mice given saline after shock, however, no marker was present in the gut, suggesting that the intestinal microvasculature was unable to deliver the marker to the epithelia. When mice were given HRP intralumenally (il) the tracer was able to penetrate between intestinal epithelial cells only in mice given vehicle after hemorrhage. This finding suggests that hemorrhaged mice were susceptible to sepsis and endotoxic shock from the leaky gut. In normal and IL-6-treated mice, the tracer was unable to pass from the lumen between mucosal epithelial cells, because the presence of an intact zonula occludens prevented passage. Functional studies supported the electron microscopy findings. Bacteria were cultured from the livers of mice fed vehicle after hemorrhage, but not from those fed IL-6. These data support the conclusions that parts of the intestinal microvasculature remain diminished after hemorrhage and resuscitation and that oral IL-6 restores this circulation.
Maintenance of intestinal barrier function is essential for health and survival. Sequestration of bacteria and their products in the intestine is maintained by both mucin and a layer of epithelial cells. These gut cells are in constant division, metabolizing rapidly and forming an impermeable barrier to harmful intestinal contents. Because they are metabolically extremely active, they are also susceptible to oxygen deprivation. Following severe hemorrhage, as occurs under a number of clinical conditions, the intestinal microcirculation remains decreased by 35 to 50%, even with adequate fluid resuscitation, 1-3 resulting in severe necrotic 4 and apoptotic 5 damage. Subsequent damage, perhaps due to neutrophil activation, oxy-gen free radical formation, or induction of nitric oxide synthase, leads to increases in intestinal permeability, endotoxin leakage, and bacterial translocation to extraintestinal sites. 6
Our laboratory has been exploring orally administered cytokines, particularly interleukin-6 (IL-6), as agents to affect gut function. We have shown that IL-6, when given at the time of infection, can dramatically reduce the numbers of infectious Campylobacter organisms in the mouse. 7 This reduction takes place well in advance of the onset of antigen-specific IgA in the intestinal secretions of infected mice. These data suggest that, in addition to its traditional role in augmenting B cell responses, IL-6 has additional, not yet described, effects on the intestinal mucosa.
Further examination of the role of IL-6 in intestinal function has shown that it is instrumental in the reduction or elimination of bacterial translocation after hemorrhagic shock in mice 8 and rats. 9 Because IL-6 has been shown to be a vasorelaxer, 8,10 we postulated that its role in the prevention of gut injury following hemorrhage is dependent on its ability to increase intestinal circulation, thereby decreasing the total ischemia time and allowing for nearly full recovery of the intestine after hemorrhage.
Materials and Methods
Mice
BALB/CByJ female mice were obtained from The Jackson Laboratory (Bar Harbor, ME). They were certified pathogen-free, and screening by Charles River Testing Services (Portage, MI) showed no serological evidence of viral or parasitic infection. Mice were housed in AAALAC-approved laminar flow cages in animal facilities at the Naval Medical Research Institute (Bethesda, MD). Standard laboratory animal chow and water were provided ad libitum.
Hemorrhagic Shock Model
The hemorrhagic shock model used has been described previously. 8 Briefly, nonfasted, anesthetized mice were cannulated in both femoral arteries to measure blood pressure in one and to bleed from the other. Mice were bled to and maintained at a mean arterial pressure of 35 mm Hg for 1 hour and then resuscitated (over 15 minutes) with shed blood and a twofold volume of lactated Ringer’s solution. After regaining consciousness, 30 minutes after resuscitation, they were fed 0.5 ml phosphate-buffered saline vehicle alone or vehicle containing 300 units rIL-6 (Genzyme, Boston, MA). After overnight recovery (16 hours), the mice were prepared for horseradish peroxidase (HRP) and bacterial translocation studies as described below.
HRP label, mol wt 40 kd, was prepared as a solution of 4 mg in 0.25 ml 0.9% NaCl. 11 In the HRP iv groups, the label was injected into the tail vein, and 10 minutes later the mice were sacrificed by cervical dislocation under methoxyfluorane anesthesia. In the HRP il groups, it was injected into a ligated loop of distal ileum and allowed to penetrate the mucosa for 15 minutes in methoxyfluorane-anesthetized mice before sacrifice. 1,3 For each experiment, three mice were used: one for hemorrhage plus IL-6, one for hemorrhage plus vehicle, and one for sham. The bacterial translocation experiments were repeated at least 50 times. The HRP experiments were performed 6 times.
Bacterial Translocation
Enumeration of bacteria in solid organs has been published previously. 8 Briefly, tissues were removed from mice, weighed, and homogenized in 4 ml phosphate-buffered saline. Serial dilutions of the homogenate were plated on sheep blood agar plates and incubated at 37°C for 24 to 48 hours. Bacterial colonies were counted and calculated as colonies per gram of tissue.
Histology
The ileum of each mouse was removed immediately, flushed, cut into 0.5-cm rings, and fixed in 2% glutaraldehyde, 1% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.25, for 1 hour at 4°C. All of the intestines were rinsed overnight at 4°C in 0.1 mol/L sodium cacodylate buffer, pH 7.5. Sections of intestinal rings 100 mm thick were cut for localization of label. Demonstration of peroxidase location was achieved by incubating the sections in the dark for 20 minutes in 5 ml DAB (3, 3′-diaminobenzidine) substrate medium (Sigma Immuno Chemicals, St. Louis, MO). Sections were then rinsed in Tris(hydroxymethyl)aminomethane buffer and postfixed in 1% sodium cacodylate buffered osmium tetroxide for 60 minutes. After dehydration with ethanol, the sections were treated with propylene oxide and embedded in Epon polybed 812 (Poly/Bed; Polysciences, Warrington, PA). Sections 1 mm thick were examined by light microscopy. Ultrathin sections prepared with a diamond knife were lightly stained with lead citrate and examined in a JEOL 100 CXII electron microscope (JEOL, Peabody, MA).
Results
General electron microscopy findings were similar to those reported previously. 9 In normal mice, microvilli were even and intact, and mitochondria appeared active. In shocked mice fed vehicle alone, focal areas of uneven microvilli were seen and cells appeared more highly vacuolated with mitochondrial breakdown. In shocked mice fed vehicle plus IL-6, the microvilli were again even and mitochondria appeared more like those of normal mice.
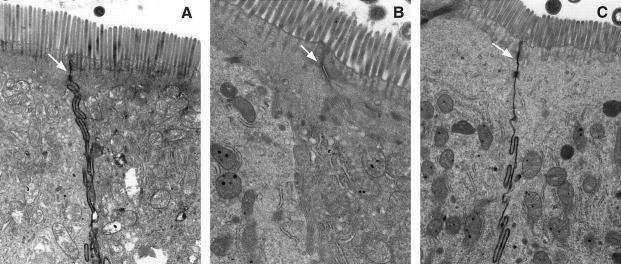
The effect of hemorrhage on intestinal circulation can be seen very clearly in mice receiving an iv injection of HRP. In normal, unhemorrhaged mice, the HRP is seen to pass between intestinal epithelial cells from the circulation to the lumen (arrows, Figure 1A ▶ ) suggesting that the intestinal circulation is patent, allowing the label to reach the intestinal lumen by intravascular pressure. Vesicles containing HRP are also present in the cytoplasm as has been previously noted. 12
Figure 1.
A: Intestinal epithelium from normal mouse. HRP administered intravenously (iv) before sacrifice. Label can be seen between the individual cells. B: Intestinal epithelium from mouse hemorrhaged and fed control fluid. HRP administered iv. Absence of label between epithelial cells. C: Intestinal epithelium from mouse hemorrhaged and fed IL-6. HRP administered iv. Label is again apparent between epithelial cells.
In mice hemorrhaged and fed vehicle, there is no evidence that HRP has reached the intestine (Figure 1B) ▶ . Intercellular channels are apparent (arrows) but not filled with electron-dense material. No HRP containing vesicles are present in cells of this group. Mice hemorrhaged and fed IL-6 have restored circulation to their intestines (Figure 1C) ▶ , since HRP is able to again penetrate between the cells (arrows). It appears that circulation is not restored completely, however, since the label is less dense with focal areas of patchiness when compared with normal mice (Figure 1A) ▶ .
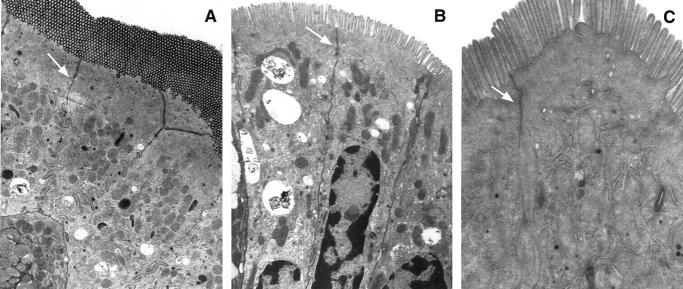
When mice were given HRP il, the distribution pattern is opposite that seen in mice injected iv. In normal mice, HRP penetrated from the lumen only as far as the zonula occludens suggesting that the intestinal barrier function was well maintained (Figure 2A) ▶ . Bacterial products, to the extent that they are the size of HRP, cannot penetrate past the zonula occludens. Large amounts of HRP are seen between intestinal epithelial cells only in mice hemorrhaged and fed vehicle (Figure 2B) ▶ . The prominent cytoplasmic vacuoles and swelling of mitochondria seen in epithelial cells of shocked mice fed vehicle are indicative of cell degeneration. Intestines of mice hemorrhaged and fed IL-6, although not completely normal, show a reduced pattern of HRP distribution, suggesting the reestablishment of barrier function (Figure 2C) ▶ .
Figure 2.
A: Intestinal epithelium from normal mouse. HRP administered intralumenally (il). Label can be seen to penetrate a short distance (zonula occludens) between epithelial cells. B: Intestine from a mouse hemorrhaged and fed vehicle. HRP administered il. Large amounts of label penetrate between the epithelial cells. C: Intestine from a mouse hemorrhaged and fed IL-6. HRP administered il. Label is scant between cells.
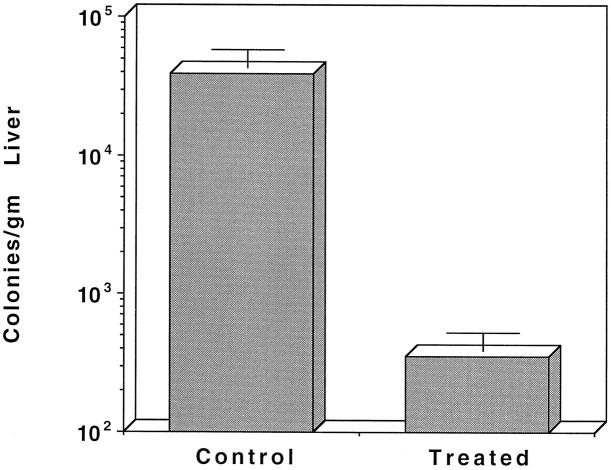
The data of Figure 3 ▶ support functionally the histological findings seen using the HRP tracer. It can be seen that mice hemorrhaged and fed saline have significant numbers of bacteria in their livers at 16 hours post-hemorrhage, a condition which persists for many days (not shown). Mice hemorrhaged and fed IL-6, however, showed much reduced bacterial translocation and sepsis after hemorrhage, as was shown previously. 8,9
Figure 3.
Bacterial translocation following hemorrhage in the mouse. Mice were hemorrhaged and fed either saline or IL-6. At 18 hours post-hemorrhage, mice were sacrificed and analyzed for the presence of bacteria in their livers.
Discussion
After an ischemic event, blood is shunted from the central organs to the brain and heart. Even though blood pressure is reestablished in the periphery, splanchnic ischemia may be maintained for long periods. 2 This ischemia can result in breakdown of the intestinal barrier, leading to the leakage of intestinal contents into the periphery, inducing the onset of endotoxic shock and systemic sepsis. 1 The exact route of penetration and elimination of this material, however, has not been demonstrated. Since we have shown that orally administered IL-6 can reduce or eliminate bacterial translocation in hemorrhagic shock models, 5,8,9 a detailed investigation of cellular events in the intestine after hemorrhage was carried out using the electron-dense marker HRP. HRP has been widely used to document blood flow and permeability in a number of models. 13,14 The data presented in this report show that orally administered IL-6 restores intestinal circulation and reduces leakage of marker from the lumen to the circulation after hemorrhage.
Although IL-6 might be thought to be limited in availability by stomach and intestinal digestive processes, it has been shown to be extraordinarily resistant to acid denaturation and proteolysis by its unique structure and glycosylation. It has been shown that mutant IL-6 adopts a molten globule structure in an acid environment and, while in that configuration, is resistant to proteolysis. 15 Similarly, the cytokine granulocyte-colony stimulating factor (G-CSF) is capable of assuming this same kind of molten globule structure in an acid environment. 16 It has been postulated that cytokines of the 4-α chain amino acid class, which includes IL-6 and G-CSF, are all similarly resistant to acid proteolysis (deFellipis, personal communication). Inasmuch as intestinal epithelial cells express IL-6R on their lumenal surfaces 17 (F. M. Rollwagen, unpublished data), it can be supposed that IL-6 survives passage through the stomach and proximal small intestine to maintain its functions in the ileum.
This report demonstrates that the intestinal barrier is compromised after hemorrhage, suggesting a mode of transport by which lipopolysaccharide and perhaps live bacteria can move freely from the lumen between intestinal epithelial cells into the sterile interior. HRP administered to normal mice seems to travel from the blood vessels to the lumen of the intestine. The route of transport appears to flow between intestinal epithelial cells, despite the presence of tight junctions, rather than being transported through the cells. This particular route of travel not only establishes a positive pressure gradient which may prevent backflow of intestinal contents between cells, it also is a marker of vascular patency, in that the label can travel through the vasculature to reach the epithelium. Evidence for this positive flow is seen in Figure 1A ▶ , and has been shown in rats by Andersen et al. 18 In shocked mice fed saline (Figure 1B) ▶ , the label is prevented from reaching the lumen, perhaps because vascular spasm reduced blood flow to the area. The subsequent loss of blood flow to the intestinal microvasculature reverses this positive pressure gradient leading to retrograde flow from the lumen between intestinal epithelial cells and into the systemic circulation (Figure 2B) ▶ . These results may explain the existence of sepsis syndrome in trauma patients who lack a focus of infection. 4 After IL-6 administration, HRP administered iv is again present between the epithelial cells (Figure 1C) ▶ . Restoration of intestinal blood flow, shown by the data of this group of mice, suggests that orally administered IL-6 allows blood flow to return to the intestine after hemorrhage. Similarly, passage of HRP from the lumen was blocked at the zonula occludens in normal mice (Figure 2A) ▶ and in those hemorrhaged and fed IL-6 (Figure 2C) ▶ . These data support the work of Langer et al, 14 who showed that intralumenally administered HRP can permeate between the intestinal epithelia of shocked animals, and extend it to show our IL-6 effects. Prevention of the passage of intralumenally administered HRP at the zonula occludens also supports the work of Rhodes and Karnovsky, 19 who first demonstrated this secondary barrier function.
It has been suggested that IL-6 produced by intraepithelial lymphocytes is responsible for the loss of intestinal barrier function following hemorrhage, and the extent of loss can be correlated with plasma levels of this cytokine. 20 Since it has been shown that cytokines such as tumor necrosis factor, 21 IL-1, 22 interferon-γ, 23 and IL-6 22 can be secreted by epithelial cells in culture, and may affect epithelial cell function, 24 attributing the intestinal cytokine source to intraepithelial lymphocytes alone may be limiting. IL-6 alone may not be solely responsible for the deleterious effects of hypoxia/reoxygenation on the gut, since levels of other inflammatory cytokines also play an important role in loss of barrier function. Correlation of intestinal damage with measurement of serum cytokine levels by enzyme-linked immunosorbent assay 25,26 or bioassay 27,28 may also be ambiguous, because the presence of soluble cytokine receptor (especially IL-6R) and other cytokine carrier proteins (known as chaperones 25 ) may compromise the results. 27,28 Finally, the correlation between levels of a single cytokine in serum and mucosal permeability is limiting, because it is known that cytokines are seldom secreted singly and are usually found as part of a spectrum of inflammation.
Although it has been difficult to show bacterial translocation in clinical cases, patients suffering from hemorrhagic shock or post-surgical syndrome are quite prone to endotoxemia and multiple organ failure. 29 These patients almost assuredly become endotoxemic from intestinal leakage, even though a peripheral focus of bacteremia is not found. Endotoxin administered iv can induce intestinal damage, as evidenced by the presence of increased intestinal hemoglobin and other serum proteins. 30 It is possible, therefore, that once a small amount of intestinal damage is sustained by focal lesions after hemorrhage, the response can be self-stimulating, in that the small amounts of endotoxin released can induce further epithelial damage. 31 Such damage can result in a reduction in net ion transport across the epithelium, 32 leading to further metabolic impairment.
The data of this communication suggest that oral IL-6 administration partially restores the positive pressure gradient seen in normal mice and establishes a barrier to the migration of bacteria and their products into the circulation. The establishment of such a gradient could be brought about by any number of physiological changes induced by IL-6, such as increase in local blood pressure via relaxation of upstream blood vessels, constriction of downstream blood vessels, which would slow blood outflow, or an effect on lymphatic drainage. Our work 8 and that of Ohkawa et al 10 suggest that IL-6 acts as a vasorelaxer in the intestine, allowing more blood to flow into previously constricted vessels, 2 leading to improved intestinal mucosal pH. 32
The data presented in this report, as well as our previously published work, clearly show that orally administered IL-6 can be of benefit in the restoration of intestinal health after hemorrhage. We have shown that IL-6 reduces hypoxia-induced apoptosis in vivo and in vitro, most likely by increasing bcl-2 gene expression. 5 These apoptosis findings support the work of Ikeda, 33 who showed that apoptosis is induced in rat intestinal epithelium after hemorrhage. The hypoxia-induced apoptosis may be eliminated by a reduction in total ischemic time in vivo. If oral IL-6 restores intestinal perfusion, a theory supported by the work of others, 32 then the reduced ischemia time of tissue may also reduce the apoptosis seen in hemorrhaged mice. It seems that orally administered IL-6 is of benefit in the restoration of intestinal health through its vasoactivity and its effects on hypoxia-induced apoptosis.
Acknowledgments
We thank Mr. Robert Williams for invaluable assistance with the electron microscopy and Dr. Elliott Kagan for constructive suggestions on the manuscript. F. M. R. is particularly grateful to the Organ Transplant Service, Walter Reed Army Medical Center, Washington, D.C., for extraordinary patient care after liver transplant.
The experiments reported herein were conducted according to the principles set forth in the current edition of the Guide for Care and Use of Laboratory Animals. Institute for Laboratory Animal Resources, National Research Council, DHHS Publication No. (NIH) 86–23.
The opinions and assertions contained herein are not to be construed as official or as reflecting the views of the Department of the Navy or the naval service at large.
Footnotes
Address reprint requests to Florence M. Rollwagen, Department of Pathology, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814-4799. E-mail: frollag@usuhs.mil.
Funded by Naval Medical Research and Development Command DC DD 1498 No. 61153N.MR04120.001.1470.
Y.-H. K. is deceased.
References
- 1.Deitch EA: Bacterial translocation of the gut flora. J Trauma 1990, 30:S184-S189 [DOI] [PubMed] [Google Scholar]
- 2.Scannell G, Clark L, Waxman K: Regional flow during experimental hemorrhage and crystalloid resuscitation: persistence of low flow to the splanchnic organs. Resuscitation 1992, 23:217-225 [DOI] [PubMed] [Google Scholar]
- 3.Xu D, Qi L, Guillory D, Cruz N, Berg R, Deitch EA: Mechanisms of endotoxin-induced intestinal injury in an hyperdynamic model of sepsis. J Trauma 1993, 34:676-683 [DOI] [PubMed] [Google Scholar]
- 4.Deitch EA: Multiple organ failure. Ann Surg 1992, 216:117-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollwagen FM, Li Y-Y, Yu EZ-Y, Pacheco N: IL-6 rescues enterocytes from hemorrhage induced apoptosis in vivo and in vitro by a bcl-2 mediated mechanism. Clin Immunol Immunopathol 1998, 89:205-213 [DOI] [PubMed] [Google Scholar]
- 6.Chavez AM, Menconi MJ, Hodin RA, Fink MP: Cytokine induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med 1999, 27:2246-2251 [DOI] [PubMed] [Google Scholar]
- 7.Baqar S, Pacheco ND, Rollwagen FM: Modulation of mucosal immunity against Campylobacter jejuni by orally administered cytokines. Antimicrob Agents Chemother 1993, 37:2688-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollwagen FM, Li Y-Y, Pacheco ND, Nielsen TB: Systemic bacteraemia following haemorrhagic shock in mice: alleviation with oral interleukin-6. Cytokine 1996, 8:121-129 [DOI] [PubMed] [Google Scholar]
- 9.Rollwagen FM, Li Y-Y, Pacheco ND, Baqar S: Systemic sepsis following hemorrhagic shock: alleviation with oral interleukin-6. Mil Med 1997, 162:366-370 [PubMed] [Google Scholar]
- 10.Ohkawa F, Ikeda U, Kawasaki K, Kusano E, Igarashi M, Shimada K: Inhibitory effect of interleukin-6 on vascular smooth muscle contraction. Am J Physiol 1994, 266:H898-H902 [DOI] [PubMed] [Google Scholar]
- 11.Graham RC, Jr, Karnovsky MJ: The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney, ultrastructural cytochemistry by a new technique. J Histochem Cytochem 1966, 11:291-302 [DOI] [PubMed] [Google Scholar]
- 12.Westergaard E, Go G, Klatzo I, Spatz M: Increased permeability of cerebral vessels to horseradish peroxidase induced by ischemia in Mongolian gerbils. Acta Neuropathol Berl 1976, 35:307-325 [PubMed] [Google Scholar]
- 13.Kang Y-H, Williams R: Endotoxin-induced endothelial injury and subendothelial accumulation of fibronectin in rat aorta. Anat Rec 1991, 229:86-102 [DOI] [PubMed] [Google Scholar]
- 14.Langer JC, Sohal SS, Blennerhasset P: Mucosal permeability after subclinical intestinal ischemia-reperfusion injury: an exploration of possible mechanisms. J Pediatr Surg 1995, 30:568-572 [DOI] [PubMed] [Google Scholar]
- 15.de Filippis V, Polverino P, de Laurento, Toniutti N, Fontana A: Acid-induced molten globule state of a fully active mutant of human interleukin-6. Biochemistry 1996, 35:11503–11511 [DOI] [PubMed]
- 16.Kolvenbach CG, Narhi LO, Philo JS, Li T, Zhang M, Arakawa T: Granulocyte-colony stimulating factor maintains a thermally stable, compact, partially folded structure at pH 2. J Pept Res 1997, 50:310-318 [DOI] [PubMed] [Google Scholar]
- 17.Molmenti EP, Ziambaras T, Perlmutter DH: Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem 1993, 268:14116-14124 [PubMed] [Google Scholar]
- 18.Andersen V, Hansen GH, Olsen J, Poulsen MD, Horen O, Sjostrom H: On the transfer of serum proteins to the rat intestinal juice. Scand J Gastroenterol 1994, 29:430-436 [DOI] [PubMed] [Google Scholar]
- 19.Rhodes RS, Karnovsky MJ: Loss of macromolecular barrier function associated with surgical trauma to the intestine. Lab Invest. 1971, 25:220-229 [PubMed] [Google Scholar]
- 20.Wang W, Smail N, Wang P, Chaudry IH: Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res 1998, 79:39-46 [DOI] [PubMed] [Google Scholar]
- 21.Taylor CT, Dzus AL, Colgan SF: Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology 1998, 114:657-668 [DOI] [PubMed] [Google Scholar]
- 22.Mascarenhas JO, Goodrich ME, Eichelberger H, McGee DW: Polarized secretion of IL-6 by IEC-6 intestinal epithelial cells: differential effects of IL-1 beta and TNF-alpha. Immunol Invest 1996, 25:333-340 [DOI] [PubMed] [Google Scholar]
- 23.Youakim A, Ahdieg M: Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999, 276:G1279-G1288 [DOI] [PubMed] [Google Scholar]
- 24.Fish SM, Proujansky R, Reenstra WW: Synergistic effects of interferon gamma and tumor necrosis factor alpha on T84 cell function. Gut 1999, 45:191-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndubuisi MI, Patel K, Rayande RJ, Mittelman A, May LT, Seghal PB: Distinct classes of chaperoned IL-6 in human blood: differential immunological and biological availability. J Immunol 1998, 160:494-501 [PubMed] [Google Scholar]
- 26.May LT, Viguet H, Kenney JS, Ida N, Allison AC, Seghal PB: High levels of “complexed” interleukin-6 in human blood. J Biol Chem 1992, 267:19698-19704 [PubMed] [Google Scholar]
- 27.Diamant M, Hansen MB, Rieneck K, Svenson M, Yasukawa K, Bendtzen K: Stimulation of the B9 hybridoma cell line by soluble interleukin-6 receptors. J Immunol Meth 1994, 173:229-235 [DOI] [PubMed] [Google Scholar]
- 28.Halimi H, Eisenstein M, Oh J-W, Revel M, Chebath J: Epitope peptides from interleukin-6 receptor which inhibit the growth of human myeloma cells. Eur Cytokine Netw 1995, 6:135-143 [PubMed] [Google Scholar]
- 29.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV: Outcome after hemorrhagic shock in trauma patients. J Trauma 1998, 45:454-459 [DOI] [PubMed] [Google Scholar]
- 30.Shindo N, Najima MM, Ohno T, Sugimoto K, Ohwada T: Induction mechanism of small intestinal lesions caused by intravenous injection of endotoxin in rats. Surg Today 1996, 26:610-617 [DOI] [PubMed] [Google Scholar]
- 31.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Reicken EO, Schultzke JD: Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116:301-319 [DOI] [PubMed] [Google Scholar]
- 32.Oldner A, Wanecek M, Goiny M, Weitzberg E, Rudehill A, Alving K, Sollevi A: The endothelin receptor antagonist bosentan restores gut oxygen delivery and reverses intestinal mucosal acidosis in porcine endotoxin shock. Gut 1998, 42:696-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G: Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 1998, 42:530-537 [DOI] [PMC free article] [PubMed] [Google Scholar]