Abstract
Recent studies have shown that airway inflammation dominated by neutrophils, ie, polymorphonuclear cells (PMN) was observed in infants and children with cystic fibrosis (CF) even in the absence of detectable infection. To assess whether there is a CF-related anomaly of PMN migration across airway epithelial cells, we developed an in vitro model of chemotactic migration across tight and polarized CF15 cells, a CF human nasal epithelial cell line, seeded on porous filters. To compare PMN migration across a pair of CF and control monolayers in the physiological direction, inverted CF15 cells were infected with increasing concentrations of recombinant adenoviruses containing either the normal cystic fibrosis transmembrane conductance regulator (CFTR) cDNA, the ΔF508 CFTR cDNA, or the β-galactosidase gene. The number of PMN migrating in response to N-formyl-Met-Leu-Phe across inverted CF15 monolayers expressing β-galactosidase was similar to that seen across CF15 monolayers rescued with CFTR, whatever the proportion of cells expressing the transgene. Moreover, PMN migration across monolayers expressing various amounts of mutated CFTR was not different from that observed across matched counterparts expressing normal CFTR. Finally, PMN migration in response to adherent or Pseudomonas aeruginosa was equivalent across CF and corrected monolayers. The possibility that mutated CFTR may exert indirect effects on PMN recruitment, via an abnormal production of the chemotactic cytokine interleukin-8, was also explored. Apical and basolateral production of interleukin-8 by polarized CF cells expressing mutated CFTR was not different from that observed with rescued cells, either in baseline or stimulated conditions. CF15 cells displayed a CF phenotype that could be corrected by CFTR-containing adenoviruses, because two known CF defects, Cl− secretion and increased P. aeruginosa adherence, were normalized after infection with those viruses. Thus, we conclude that the presence of a mutated CFTR does not per se lead to an exaggerated inflammatory response of CF surface epithelial cells in the absence or presence of a bacterial infection.
Cystic fibrosis (CF), a hereditary disease caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, is associated with chronic airway inflammation dominated by neutrophils (PMN) that release large amounts of factors contributing to tissue destruction. 1,2 The mechanisms involved in PMN immigration into the airway lumen are still poorly understood. Although the inflammatory response is thought to be maintained by the presence of characteristic bacterial pathogens and the release of factors chemotactic for PMN, 3 recent observations suggest that the initiation of PMN immigration into CF airways may result, at least in part, from a different mechanism. Indeed, airway inflammation was observed in infants and children with CF even in the absence of detectable bacterial, viral, or fungal colonization or infection. 4-6 Because PMN and the chemotactic cytokine interleukin-8 (IL-8) can be found in CF bronchial secretions in the absence of a detectable infection, the question arises as to whether there is a CF-related anomaly of PMN migration across the airway epithelium. Some manifestations of the CF phenotype may be due to the absence of functional CFTR at the plasma membrane, whereas others may be related to the presence of certain amounts of mutated CFTR in the endoplasmic reticulum. 7 Thus, we addressed two related questions: 1) Does correction of CF cells with CFTR lead to a difference in PMN migration across CF airway epithelia; and 2) Does the presence of different amounts of mutated CFTR lead to an increase in PMN migration and/or an aberrant IL-8 production?
To explore these questions, we developed an in vitro model to measure PMN migration in the physiological direction, across a tight-polarized human CF airway epithelial cell line, the CF15 cells, 8 corrected or not with CFTR. Matched CF and non-CF monolayers were created by infecting the CF epithelial sheets with adenoviral vectors containing either the wild-type or ΔF508 CFTR cDNA, or a β-galactosidase gene reporter. Our data show that a similar number of PMN migrated across the different types of monolayers, and that no difference in production of IL-8 was observed whatever the proportion of cells expressing the transgenes.
Materials and Methods
Cell Culture and Seeding on Permeable Filters
The CF15 human nasal airway epithelial cell line, derived from a CF patient homozygous for the ΔF508 mutation, was transformed and characterized by Jefferson et al. 8 CF15 cells were passaged once a week, plated in flasks coated with human placental collagen IV (50 μg/ml) and cultured in Dulbecco’s minimal essential medium/Ham F-12 (3:1) supplemented with 10% fetal calf serum (FCS) and seven growth factors. 8 For culture on permeable filters, the cells were seeded at a density of 0.6 × 10 6 on 1-cm 2 polycarbonate 3-μm-pore filters (Transwell inserts, Costar, Badhoevedorp, The Netherlands). When airway cells were seeded on inverted inserts, 200 μl of medium containing the cells were disposed on the lower surface of the filter and the cells were allowed to attach overnight at 37°C, before turning the inserts again. PMN migration and transepithelial electrical measurements were performed on day 8 after seeding. The cells were regularly tested for the presence of mycoplasma, and only pathogen-free cells were used for this study. All tissue culture supplies were obtained from Life Technologies, Inc. (Basel, Switzerland), FCS was from SeraTech (Griesbach, Germany), epidermal growth factor was from Collaborative Biomedical Products (Bedford, MA), and all other reagents were purchased from Sigma Chemical Co. (Buchs, Switzerland).
Recombinant Adenoviruses
Transgenes Driven by Rous Sarcoma Virus (RSV) or Cytome Galovirus (CMV) Promoters
The replication-defective adenoviruses were derived from the human adenovirus serotype 5, and contained either the CFTR cDNA controlled by the RSV promoter (AdTG 6429) or the CMV promoter (AdTG 6418), the CMV promoter-driven eGFP (enhanced green fluorescent protein) gene (AdTG 6297), or the RSV promoter-driven lacZ gene. 9 All vectors were constructed as infectious plasmids by homologous recombination in E. coli as described. 10,11 The vectors contain a deletion in E1 (Δ nucleotides 459-3327) and in E3 (Δ nucleotides 28592–30470). All vectors have the transgene incorporated in place of the viral E1 gene. For the generation of viruses, the viral genomes were released from their respective plasmids by PacI digestion and transfected into E1-complementing 293 cells as described. 10 Viral stocks were prepared from the transfected cells, purified, and stored in viral storage buffer (1 mol/L sucrose; 10 mmol/L Tris-HCl, pH 8.5; 1 mmol/L MgCl2; 150 mmol/L NaCl; and 0.005% Tween 80).
Transgenes Driven by the β-Actin Promoter
These serotype-5-derived recombinant adenoviruses contained either the wild-type CFTR (Ad CB CFTR) or the mutated CFTR (Ad CB ΔF508) cDNA controlled by a CMV enhancer/β-actin promoter. They were engineered by Yang et al 12 and provided by the Vector Core of the Institute for Human Gene Therapy of the University of Pennsylvania Health System (Philadelphia, PA).
Viral Infections and 5-Bromo-4-Chloro-3-Indolyl-β-d-Galactopyranoside (X-Gal) Staining
Confluent CF15 monolayers on inserts were infected with adenoviruses on the day 6 of culture for 16 hours (unless otherwise indicated) with 200 μl of OptiMeM (Life Technologies, Inc.) supplemented with 2.5% FCS and containing or not the viruses at a multiplicity of infection (MOI) of 1 to 1000 (for RSV- and CMV-based viruses, a MOI of 1 = 1 infectious unit/cell (∼1/2 plaque-forming unit/cell), whereas for β-actin-based viruses, a MOI of 1 = 1 plaque-forming unit/cell). For infection of inverted monolayers, the inserts were placed directly on a 200-μl drop of medium containing the viruses. The cells were then rinsed and cultured for an additional 24 hours in normal medium before the experiment. Expression of β-galactosidase was detected by light microscopy as nuclear-localized blue staining using the X-Gal substrate. Infected cells were rinsed with phosphate-buffered saline (PBS), fixed with 0.5% glutaraldehyde for 10 minutes, and incubated for 6 hours at 37o with 1 mg/ml X-Gal, 5 mmol/L K+ ferricyanide, 5 mmol/L K+ ferrocyanide, and 1 mmol/L MgCl2 in PBS.
Transepithelial Electrical Measurements
Right-side-up or inverted CF15 monolayers on inserts were placed in a modified and thermostatized Ussing chamber (manufactured by J. Pahud, CHUV, Lausanne, Switzerland) containing Hanks’ balanced salt solution (HBSS) supplemented with 1.3 mmol/L Ca2+, 1 mmol/L Mg2+, and 10 mmol/L (N-[Hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]) (HEPES), pH 7.4 (HBSS+), heated to 37°C. Transepithelial potential difference (ΔV) and short circuit current (Isc) were measured with apical and basolateral agar bridges connected to Ag/AgCl electrodes of a current/voltage clamp apparatus (VCC 600, Physiological Instruments, San Diego, CA). Transepithelial electrical resistance (TER) was calculated from Ohm’s law, after measuring the difference in current induced by voltage pulses of 1 mV. The sequential electrical responses to various drugs were determined: 100 μmol/L amiloride, added to the mucosal side of the epithelium, mucosal Cl− replacement with HBSS+ containing 3.6 mmol/L Cl− (chloride replaced by gluconate), 50 μmol/L forskolin, and 200 to 500 μmol/L 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid. The TER of CF15 monolayers varied from week to week (from 200 to 800 Ω·cm2), but all monolayers displayed a similar TER at a given time in culture.
Whole-Cell Patch-Clamp Recordings of CFTR-Dependent Cl− Currents
CF15 cells seeded on Petri dishes were infected for 16 hours with either Ad RSV CFTR or Ad RSV β-galactosidase at a MOI of 500. Whole-cell patch-clamp experiments were performed as described. 13 Briefly, the cells were superfused with a solution containing 136 mmol/L NaCl; 4 mmol/L KCl; 1 mmol/L CaCl2; 1 mmol/L MgCl2; 2.5 mmol/L glucose; and 10 mmol/L HEPES, pH 7.4, supplemented with 100 μmol/L amiloride. Patch electrodes were filled with a solution containing 1 mmol/L NaCl; 138 mmol/L KCl; 2.9 mmol/L CaCl2; 5.5 mmol/L EGTA; 3 mmol/L MgATP; 0.1 mmol/L GTP; and 10 mmol/L HEPES, pH 7.2. Cells were held at 0 mV and membrane capacitance was determined using the automatic compensation procedure of an EPC-9 patch-clamp amplifier (Heka Elektronic, Lambrecht, Germany). Every 5 seconds, cells were depolarized from the holding potential to −70 mV for 600 milliseconds. CFTR-dependent currents were then stimulated with 50 μmol/L forskolin (or the solvent ethanol) added to the superfusing solution.
Isolation of Neutrophils and Transmigration Experiments
PMN from buffy coats of citrated blood collected from healthy donors were isolated by dextran sedimentation followed by density gradient centrifugation in Ficoll-Hypaque (Amersham, Uppsala, Sweden). 14 Contaminating red blood cells were lysed by hypotonic shock with cold water for 40 seconds, PMN were washed twice and resuspended at a concentration of 107/ml in HBSS+ (without phenol red). The inserts with monolayers were lifted from wells, drained of media by inverting, and gently rinsed by dipping in HBSS+ heated to 37°C. They were then placed in new 12-well plates with 1 ml HBSS+ containing the chemotactic factor N-formyl-Met-Leu-Phe (fMLP) or solvent in the lower compartment, before adding 5 × 10 6 PMN to the upper compartment. PMN were then allowed to transmigrate for various periods of time at 37°C. For migration experiments in response to adherent bacteria, 150 μl containing 5 × 10 7 colony-forming units (cfu) P. aeruginosa (strain PAO1, see culture details below) were disposed on the apical surface of inverted monolayers and allowed to adhere for 2 hours. The monolayers were then turned again and placed in 12-well plates, rinsed four times with HBSS+, and PMN were added to their basolateral side.
The number of PMN having migrated into the lower compartment was quantified by coloration of the PMN-specific azurophil granule marker, myeloperoxidase, with a modification of the technique of Madara et al. 15 After stopping PMN migration by placing the 12-well plates on ice, the lower surface of the inserts was rinsed 10 times with the liquid present in the lower compartment to remove attached PMN. Myeloperoxidase was then solubilized after PMN lysis by 100 μl of 10% Triton X-100, and the remaining myeloperoxidase trapped within PMN-derived DNA was dissolved by adding 20 μl of 10 mg/ml DNase (200 μg/ml final). After 15 minutes of shaking on ice, 100 μl of 1 mol/L citrate, pH 4.2, was added before transferring 100 μl of each sample in a 96-well microtiter plate and adding 100 μl of substrate (2 mmol/L 2,2′-azino-bis[3-ethylbenzthiazoline 6-sulfonic acid] diammonium; [Sigma] and 0.06% H2O2 in 100 mmol/L of citrate buffer, pH 4.2). The colorimetric reaction was stopped by adding 25 μl of 5.5% sodium dodecyl sulfate (0.5% final) and read at 405 nm after centrifugation of the plates at 500 rpm for 5 minutes. Standards were made with serial dilutions of the same PMN, in 1 ml of HBSS+, and processed in the same way as described above. The assay was linear in the range of 4 to 600 × 10 3 PMN/ml.
IL-8 Production by Polarized Monolayers
To determine IL-8 production at the apical or basolateral side of the CF15 monolayers, the cells were seeded on 1-cm 2 inserts and infected for 16 hours on day 6. Ten hours after the end of infection, the monolayers were incubated overnight with culture medium without FCS and with 0.1% BSA, before being challenged on their mucosal side with or without tumor necrosis factor-α (TNF-α) for 1 or 4 hours. Apical and basolateral supernatants (500 μl each) were collected after 4 hours or 16 hours. For IL-8 production in response to adherent P. aeruginosa, the monolayers were exposed on their apical side to 5 × 10 7 cfu PAO1 for 2 hours. After rinsing four times with HBSS+, the monolayers were incubated for 4 hours with culture medium without FCS or antibiotics, supplemented with 100 μg/ml cycloserine and 0.1% BSA, before supernatant collection. IL-8 was measured using an ELISA kit (CLB, Amsterdam, The Netherlands).
Pseudomonas aeruginosa Adherence
Confluent CF15 cells in 24-well plates were infected with adenoviruses for 16 hours on day 6, and bacteria were allowed to adhere 1 day after the end of infection. P. aeruginosa strain PAO1 was grown to a density of 5 × 10 8 cfu/ml and labeled with 35S-methionine (Amersham, Zürich, Switzerland) to a specific activity of ∼5000 cfu/cpm for 15 minutes. After being washed in PBS, the bacteria were suspended in CF15 culture medium without FCS or antibiotics, supplemented with 100 μg/ml cycloserine. PAO1 was then added to confluent CF15 cells (5 × 10 7 cfu/well) for 2 hours at 37°C. Unbound bacteria were removed by rinsing the monolayers three times with PBS. CF15 cells and bacteria were solubilized in 0.5 ml sodium dodecyl sulfate 2% by shaking at 110 rpm and scintillations counted. The experiments were done in quadruplicates.
All data are means ± SEM and compared using a two-tailed unpaired Student’s t-test.
Results
Neutrophil Migration across CF15 Monolayers in the Physiologically Relevant Direction
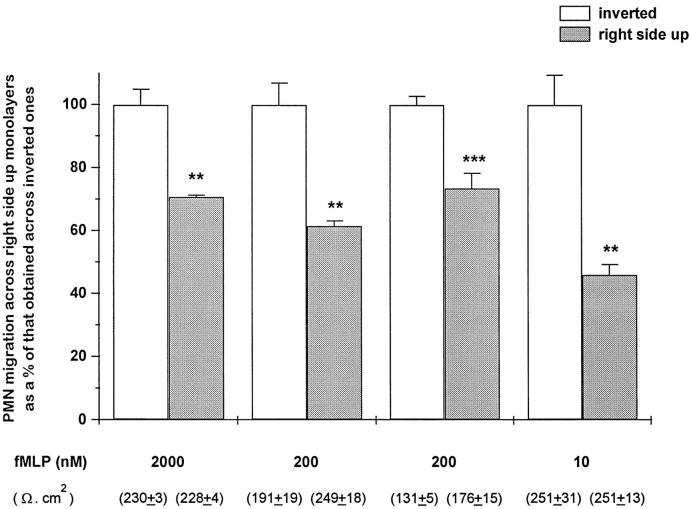
To determine whether PMN migration across electrically-tight CF airway monolayers is affected by the orientation of the epithelium, CF15 cells were seeded on either side of the permeable inserts, before being exposed to PMN that were allowed to cross the monolayers in response to fMLP. Thus, PMN migrating across right-side-up monolayers first encounter their apical membrane, whereas those migrating across inverted monolayers move in the physiologically relevant direction, ie, from the basolateral to the apical side of the monolayer. Figure 1 ▶ shows that the number of PMN having migrated across right-side-up monolayers was 27% to 54% lower than that observed across inverted monolayers, in conditions of similar initial transepithelial resistance (TER). Many experiments of comparison were performed, but only those in which the TER of the monolayers was not significantly different were interpreted and reported.
Figure 1.
Comparison of PMN migration across right-side-up or inverted CF15 monolayers. PMN were either added to the basolateral or the apical side of confluent monolayers and driven to transmigrate for 1 hour 30 minutes in response to various concentrations of fMLP. PMN migration across right-side-up monolayers is expressed as a percentage of that obtained across inverted monolayers. Values are means ± SEM of three to four monolayers of four different experiments. Absolute values (in millions) of PMN recovered across the inverted epithelia were, from left to right: 1.17 ± 0.06, 3.7 ± 0.25, 2.79 ± 0.07, 1.18 ± 0.11. The TER, measured at the beginning of the experiment (ie, without fMLP or PMN) and shown in parentheses (means ± SEM, n = 3 to 4), are not significantly different between both groups. **P < 0.05, ***P < 0.001 versus corresponding controls.
Expression of Adenoviral-Derived Transgenes by Inverted Polarized CF15 Monolayers and by Single Cells
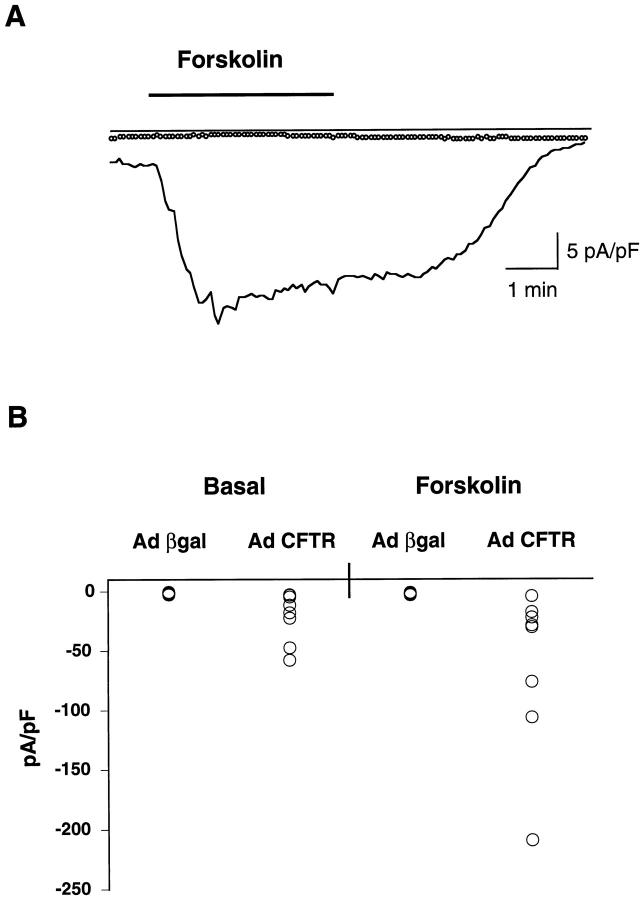
To compare PMN migration in the physiologically relevant direction, a pair of matched CF and non-CF airway monolayers was created. For this purpose, inverted CF15 monolayers were infected with adenoviruses containing the CFTR (Ad RSV CFTR) or β-galactosidase (Ad RSV βgal) gene. We determined whether cyclic adenosine monophosphate (cAMP)-mediated Cl− secretion was restored in the group of cells infected with Ad RSV CFTR using various drugs (Figure 2A) ▶ . This typical recording shows the correction of defective transepithelial Cl− secretion in a monolayer exposed to Ad CFTR, with an important increase in Isc in the presence of a low Cl−-containing solution. The cAMP-stimulating drug forskolin promoted a further increase of the response. In contrast, the cells infected with Ad βgal displayed only a small increase of Isc in response to low Cl−, without any change when challenged with forskolin (Figure 2B) ▶ . The fact that inverted monolayers maintained their polarity when seeded on the lower side of the filter is shown by comparing Figure 2A to 2C ▶ . The latter shows the transepithelial Cl− current obtained with a CF15 right-side-up monolayer infected with Ad RSV CFTR. The trace mirrored that obtained with inverted cells, because Isc was of opposite sign. This demonstrates that the direction of transport of ions across the epithelium is maintained whatever the orientation of the monolayer. The Cl− channel blocker 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid significantly reduced the forskolin-mediated effect, indicating that the observed increase of Isc was due to the activation of Cl− channels.
Figure 2.
Transepithelial Cl− current of inverted or right-side-up CF15 monolayers infected with Ad RSV βgal or Ad RSV CFTR. Inverted CF15 monolayers were infected for 16 hours at a MOI of 500 of Ad CFTR (A) or Ad βgal (B), and short-circuit current (Isc) was measured 1 day after. Baseline Isc values were 7.0 and 1.7 μA/cm2, respectively. The following drugs were added: 100 μmol/L amiloride (A), followed by a low Cl− solution containing amiloride (low Cl−), and 50 μmol/L forskolin (F). The monolayers had a TER higher than 400 Ω.cm 2 and, in the Ad βgal group, ∼60% of blue cells were revealed after X-Gal staining. Right-side-up monolayers were infected for 8 hours at a MOI of 500 of Ad RSV CFTR (C) and were processed as described above, except that forskolin was 100 μmol/L, followed by the addition of 200 μmol/L 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS). Matched monolayers infected with Ad βgal displayed ∼35% of blue cells.
Patch-clamp measurements of Cl− currents were done to assess the effect of CFTR expression on plasma membrane conductance in CF15 cells. Figure 3A ▶ shows a typical recording of Cl− currents in a CF15 cell infected with Ad RSV CFTR or Ad RSV βgal. The combined data obtained with all cells tested (Figure 3B) ▶ reveal that, in 5 out of 8 cells infected with Ad CFTR, Cl− current was already increased at the basal level (−20.89 ± 7.4 pA/pF, mean ± SEM, n = 8), whereas no increase was observed in the Ad βgal-infected cells (−1.36 ± 0.19, n = 8). Likewise, when stimulated with forskolin, 6 out of 8 cells displayed an increase in Cl− conductance in the former group (Δ = 40.73 ± 24.5, n = 8), whereas none responded to this agent in the latter group (Δ = 0.5 ± 0.23, n = 8).
Figure 3.
A: Examples of membrane currents recorded from a cell infected with Ad RSV CFTR (solid line) or Ad RSV βgal (dotted line) in the presence of 100 μM amiloride. Whereas exposure of the CFTR-expressing cells to 50 μM forskolin induced reversible inward currents, the drug was without effect on membrane currents of the cells infected with Ad βgal. Bar indicates the duration of forskolin superfusion. B: Distribution of basal and forskolin-stimulated membrane currents (pA/pF) as recorded in cells infected with Ad CFTR (n = 8) or Ad βgal (n = 8). Basal and stimulated currents were markedly enhanced in cells expressing normal CFTR. More than 90% of the cells infected with Ad βgal were blue.
Taken together, these results demonstrate that the Cl− secretion defect was corrected by CFTR-containing adenoviruses.
Comparison of PMN Migration across Inverted CF15 Monolayers Corrected or Not with CFTR
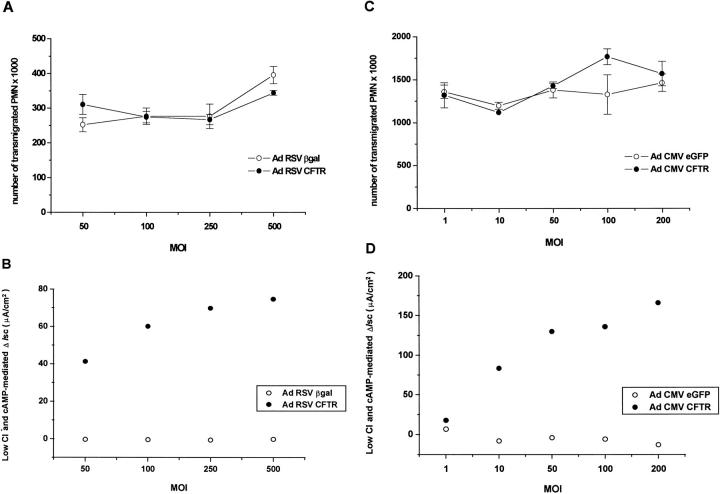
We determined whether the presence of a functional CFTR affects cellular processes involved in PMN migration. Inverted CF15 monolayers were infected with various MOI of two different pairs of viruses containing either the RSV (Figure 4,A and B) ▶ or the CMV (Figure 4, C and D) ▶ promoter, and assayed for chemotactic migration. Figure 4A ▶ shows that a similar number of PMN migrated across monolayers of cells infected with Ad RSV CFTR or Ad RSV βgal, at all of the viral MOI tested. Moreover, no significant difference of migration could be observed between the monolayers infected with increasing MOI of a given virus. Ad RSV CFTR-infected cells exhibited a response to low Cl− and forskolin that increased with the virus load (Figure 4B) ▶ , whereas this response remained almost undetectable in monolayers infected with Ad βgal. The level of transgene expression in the latter group also varied with the MOI, because the number of blue cells increased from ∼20% to 90%. PMN migration experiments were also performed with monolayers expressing higher levels of transgenes (Figure 4, C and D) ▶ . Likewise, the number of PMN having migrated across monolayers infected with Ad CMV CFTR was not different from that having crossed their counterpart infected with Ad CMV eGFP. The group of cells expressing CFTR displayed a MOI-dependent increase of response to low Cl− and forskolin ∼2.5- to 3-fold higher than that seen with the same MOI of Ad RSV CFTR (Figure 4, D and B ▶ , respectively). Monolayers expressing eGFP did not respond to those challenges, but their fluorescence increased with the viral load (data not shown).
Figure 4.
A and C: PMN migration across inverted CF and corrected monolayers. The monolayers were infected with various MOI of either Ad RSV CFTR or Ad RSV βgal (A), or Ad CMV CFTR or Ad CMV eGFP (C) before assessing PMN migration. PMN were added to the basolateral side of the monolayers and driven to transmigrate for 1 hour (A) or 1 hour 30 minutes (C) in response to 50 nmol/L fMLP present in the opposite compartment. Values are means ± SEM of three monolayers. B and D: effect of increasing viral MOI on transepithelial Cl− currents. Part of the monolayers were used in parallel to perform electrical measures as described for Figure 2, A and B ▶ . The combined effect of low Cl− and forskolin is presented for both groups of monolayers infected with viruses containing either the RSV (B) or the CMV (D) promoter. In the Ad βgal-infected group, the number of blue cells after X-Gal staining increased from ∼20% (MOI 50) to ∼90% (MOI 500).
Other experiments, performed with adenoviruses containing the RSV promoter (with MOI ranging from 0 to 1000), confirmed the lack of difference of PMN migration between inverted CF and corrected monolayers, all groups displaying similar results. Moreover, the data were identical to those obtained across parental noninfected monolayers, indicating that the adenoviral infection per se does not influence this process.
Comparison of PMN Migration across CF15 Monolayers Expressing Various Amounts of Normal or Mutated CFTR
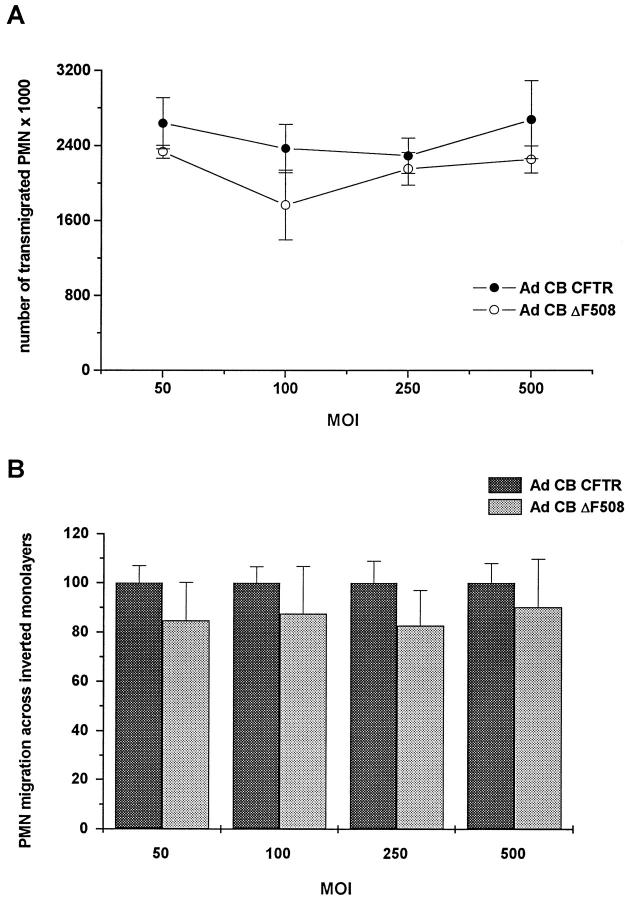
To determine whether the amount of mutated CFTR plays a role in cellular events involved in the migratory process, inverted CF15 epithelia were infected with increasing MOI of matched adenoviruses containing either the normal or ΔF508 CFTR cDNA (Ad CB CFTR and Ad CB ΔF508, respectively). As shown in Figure 5 ▶ , no significant difference in the number of transmigrated PMN was observed across monolayers expressing various quantities of either normal or mutated CFTR. The data were also similar to those obtained across uninfected monolayers (n = 2 experiments, data not shown). These adenoviruses also displayed a very efficient level of infection, because the response to low Cl− and forskolin showed a dose-dependent increase when the Ad CB CFTR viral load was increased; indeed, over a MOI range of 25 to 500, Δ Isc increased from 22 to 243 μA/cm2.
Figure 5.
PMN migration across inverted CF15 monolayers expressing normal or mutated CFTR. The monolayers were infected or not with various MOI of either Ad CB CFTR or Ad CB ΔF508 before measuring PMN migration. PMN were added to the basolateral side of the monolayers and allowed to transmigrate for 1 hour 30 minutes in response to 50 nmol/L fMLP. A: Values of a typical experiment done in triplicate. B: The pooled data of three experiments. Each group of monolayers infected with a given MOI of Ad CB ΔF508 is expressed as a percentage of its corresponding group (ie, infected with the same MOI of Ad CB CFTR). For each condition, data are means ± SEM of the nine samples pooled. Electrical measures done in parallel showed that monolayers infected with a MOI of 250 of Ad CB CFTR or Ad CB ΔF508 displayed a ΔIsc of 49 ± 6 μA/cm 2 (n = 8) and 1 ± 0.3 μA/cm 2 (n = 5), respectively, in response to low Cl− and forskolin.
Comparison of PMN Migration in Response to P. aeruginosa across Inverted CF15 Monolayers Corrected or Not with CFTR
To determine whether PMN migration in the physiological direction in response to adherent P. aeruginosa is influenced by the presence of a functional CFTR, experiments were done with monolayers rescued or not with CFTR and challenged with PAO1 (Figure 6) ▶ . Our data show that the presence of adherent bacteria on the apical surface of monolayers expressing CFTR does not induce a difference in PMN migration, as compared to that seen across monolayers either uninfected or expressing βgal.
Figure 6.
PMN migration across inverted monolayers rescued or not with CFTR, in response to adherent PAO1. Confluent CF15 cells were infected or not with Ad RSV CFTR or Ad RSV βgal (MOI 500), before being incubated with PAO1 on their apical surface. The absolute values of three experiments done in quadruplicate were pooled. Data are means ± SEM of the 12 samples pooled. In parallel, monolayers that were neither infected with viruses nor challenged with PAO1 were assessed for migration in response to 50 nmol/L fMLP as a means of comparison. In the Ad βgal-infected groups, X-Gal staining revealed between ∼40% and ∼80% of blue cells and, in the Ad CFTR-infected groups, the response to low Cl− and forskolin varied between 28 and 57 μA/cm2.
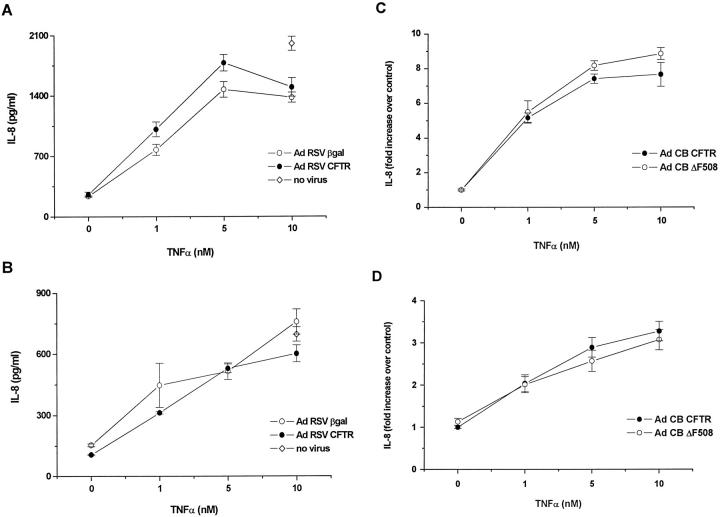
IL-8 Secretion by Polarized CF15 Cells Expressing Various Amounts of Normal or Mutated CFTR
Polarized CF15 monolayers corrected or not with CFTR were tested for IL-8 secretion from their apical or basolateral side (Figure 7) ▶ . IL-8 present in the supernatant collected from monolayers infected or not with Ad RSV CFTR or Ad RSV βgal showed a dose-dependent increase in response to TNF-α, in both the apical (Figure 7A) ▶ and basolateral (Figure 7B) ▶ compartment. However, no significant difference in IL-8 secretion was detected between cells expressing CFTR or β-galactosidase, in the presence or absence of TNF-α, in either compartment. The presence of the adenoviral vector did not significantly affect IL-8 production in cells challenged with 10 nM TNF-α, as compared to parental uninfected cells. The adenoviruses did not either affect baseline IL-8 production (n = 2 experiments done in quadruplicate, data not shown). Other experiments of IL-8 secretion done with monolayers infected with adenoviral MOI ranging from 0 to 1000 demonstrated that the production of this cytokine in Ad CFTR-infected cells was similar to that observed in Ad βgal-infected cells, with no increase paralleling the increasing adenoviral load (data not shown). Experiments of the same type were done with monolayers expressing similar amounts of either normal or mutated CFTR (Figure 7, C and D) ▶ . The profile of IL-8 secretion by CF15 cells infected with either Ad CB CFTR or Ad CB ΔF508 was comparable, demonstrating that the production of this cytokine is not affected by the presence or amount of either type of CFTR. To measure IL-8 production in response to a bacterial stimulus, polarized monolayers infected with Ad RSV CFTR or β-galactosidase (MOI 500) were challenged with PAO1 on their apical surface. The data obtained with two experiments show that, after 4 hours, no difference in IL-8 production in either the apical or basolateral supernatants was observed between both groups of monolayers (data not shown). These preliminary data do not confirm those obtained by DiMango et al. 7 This may be due to the fact that IL-8 production has already reached a plateau in CFTR-corrected monolayers, and is therefore not further stimulated in uncorrected ones. Alternatively, there may be conditions in which a difference in IL-8 production between both groups occurs, but this would necessitate a thorough investigation that is beyond the scope of this project.
Figure 7.
IL-8 production by polarized CF15 cells expressing normal or mutated CFTR. CF15 monolayers on inserts were infected or not with Ad RSV CFTR or Ad RSV βgal (MOI 500) (A, B) or Ad CB CFTR or Ad CB ΔF508 (MOI 250) (C, D) before incubation with various concentrations of TNF-α. The apical (A, C) and basolateral (B, D) supernatants were collected after 4 hours and assayed for IL-8 production. A and B: Data are means ± SEM (n = 3) of a typical experiment. C and D: The data of three experiments were pooled and presented as a fold increase over controls (monolayers corrected with CFTR and not submitted to TNF-α) and are means ± SEM of the eight to nine samples.
P. aeruginosa Adherence to CF15 Cells Corrected or Not with CFTR
To confirm that CF15 cells indeed displayed a CF phenotype, experiments were done to test the adherence of P. aeruginosa to the plasma membrane, a function reported to be increased in CF. 16-18 Figure 8 ▶ shows that there was no difference of adherence between uninfected cells or cells infected with Ad RSV βgal. However, a significant decrease of adherence was observed with the cells rescued with CFTR, in each of the three experiments performed. This demonstrates that the CF15 cells display a CF phenotype that can be corrected by CFTR.
Figure 8.
PAO1 adherence to CF15 cells rescued or not with CFTR. Confluent CF15 cells were infected or not with Ad RSV CFTR or Ad RSV βgal (MOI 500), before being incubated with PAO1. The data of three experiments were pooled and presented as a percentage of bacteria adhering to uninfected CF15 cells (means ± SEM of the 12 samples pooled). The number of bacteria adhering to uninfected cells, determined in the third experiment, was of 13 ± 1 PAO1/CF15 cell (mean ± SEM, n = 4 wells). ***P < 0.001 versus uninfected CF15 cells, §§P < 0.05 versus cells infected with Ad βgal.
Discussion
Research on PMN migration across CF airway epithelium and its comparison to that occurring across non-CF epithelia has been hampered by the lack of adequate models of human tight airway epithelial monolayers displaying a TER characteristic of epithelial barriers. Although tight primary cultures of human tracheal epithelia can be successfully obtained, 19 their use is limited by the difficulty in obtaining simultaneously CF and non-CF monolayers of similar tightness to compare PMN migration. Several CF transformed human airway epithelial cells lines have been isolated and characterized 20 but, until now, no CF cell line has been described to retain differentiated features after several passages in culture. Here we describe a model of PMN migration in the physiologically relevant direction, across tight human CF airway epithelial monolayers, that has been developed with possible use in long-term culture. The CF15 cell line is, to our knowledge, the only CF airway epithelial cell line that could be induced to maintain tight junctions and vectorial ion transport, without undergoing a well-known process of dedifferentiation. 20 CF15 cells did not loose their CF characteristics either, because the presence of the homozygous ΔF508 mutation, the production of endogenous mutated CFTR mRNA, as well as the lack of response to forskolin measured on Isc, have been reconfirmed (data not shown).
In our model, PMN migration was shown to be greater in the physiological direction than in the apical-to-basolateral direction. This is in agreement with other studies suggesting that the polarity of the epithelium plays a role in PMN migration across intestinal monolayers such as T84 epithelial cells 21-23 and airway epithelial barriers. 24-26 However, no comparisons of the initial TER of inverted and right-side-up monolayers were done in the former studies, whereas only qualitative TER measures were provided in the latter ones. To address this issue, we compared PMN migration across right-side-up and inverted CF15 monolayers with similar initial TER and showed that the increase of migration observed in the physiological direction still persisted. This increase may be related to a difference in the polarity of epithelial receptors. Alternatively, CF15 cells may also have features similar to those of T84 intestinal epithelial cells, which were shown to display a luminal retention signal influencing PMN migration asymmetrically by cytoskeletal reorganization. 23
The fact that airway inflammation was observed in infants and children with CF even in the absence of detectable pathogens 4-6 has led to the suggestion that the CFTR defect may play a direct role in the initiation of PMN immigration into CF airways. To compare PMN migration across CF and control monolayers, the use of ex vivo primary CF airway epithelial cultures makes it difficult to distinguish between the responses due to the primary defect or secondary to the microenvironment to which the cells were formerly exposed. To address this issue, we set up an in vitro model of a matched pair of CF and control monolayers differing only by the presence or absence of a normal CFTR. With this model, it was also possible to create matched pairs expressing increasing amounts of transgene by varying the adenoviral load. Moreover, within a given experiment, the monolayers displayed a comparable tightness, because they originated from the same source. Our data demonstrated that a similar number of PMN migrated across inverted monolayers of CF15 cells expressing wild-type CFTR or β-galactosidase, whatever the level of transgene expression. In the group of monolayers infected with Ad RSV βgal, a MOI of 500 generally yielded ∼50% of blue cells when detected by X-Gal staining. This value is certainly underestimated, because the sensitivity of detection with this method was reported to be relatively low. 27 In the group of monolayers infected with Ad RSV CFTR, the defective Cl− current was corrected to levels observed in non-CF airway monolayers at a MOI of ∼100 to 500, depending on the experiment. PMN migration experiments were therefore always performed with various MOI. This allowed us to confirm the lack of difference in PMN migration across monolayers displaying from 0 to >90% of transgenic cells. We therefore conclude that correction with CFTR does not lead to a difference in PMN migration across CF airway epithelial cells.
Although part of the cellular manifestations of the CF phenotype has been attributed to the absence of a functional CFTR in airway epithelial cells, other ones have been suggested to be the consequence of the presence of a mutated CFTR. For example, an abnormal activation of nuclear factor-κB in CF bronchial epithelial cells has been reported by DiMango et al 7 and suggested to be a consequence of cell stress caused by the accumulation of mutant CFTR in the endoplasmic reticulum. To determine whether the amount of ΔF508 CFTR affects intracellular pathways involved in the interactions of epithelial cells with PMN, the migratory process was done across CF15 monolayers expressing various amounts of either normal or ΔF508 CFTR. Because no significant difference was observed in the number of PMN having crossed the different types of monolayers, we suggest that there is no direct link between the CF genetic defect and the process of PMN migration across airway epithelial barriers, in the absence of pathogens.
The fact that adherent P. aeruginosa promoted no difference in PMN migration across monolayers rescued or not with CFTR further strengthens the results of migration we obtained in response to fMLP. Indeed, those bacteria were chosen as a specific stimulus encountered in CF patients. The data acquired with our model suggest that the combined presence of a mutated CFTR and of P. aeruginosa is not enough to explain the excessive amount of PMN found in CF airways colonized by these bacteria. This also further underlines the complexity of the CF inflammatory response in vivo.
We then explored the possibility that CFTR may exert indirect effects on PMN recruitment, via an abnormal production of the potent chemotactic cytokine IL-8. Whereas some studies have reported high concentrations of this cytokine in bronchoalveolar lavage fluid from uninfected infants and children with CF, as compared to controls, 4-6 another study found no difference of IL-8 in bronchoalveolar lavage from uninfected infants with or without CF, both levels being very low. 28 Contradictory data were also published with in vitro experiments. Indeed, subcultures of primary human CF bronchial gland cells were recently shown to spontaneously release much higher levels of IL-8 than non-CF ones, 29 although no difference of baseline or stimulated IL-8 production was observed in primary or immortalized airway epithelial cells, in other studies. 30-32 Massengale et al 33 recently even reported a defective IL-8 secretion by CF airway cells. We addressed this issue with polarized CF15 cells expressing various amounts of either normal or mutated CFTR cDNA, or the β-galactosidase transgene. The cells were grown as tight monolayers on filters because differentiation of epithelial cells was shown to regulate CFTR expression, 34 and because it is possible that part of the effects of CFTR dysfunction on intracellular processes are not revealed unless cell polarization occurs. Our data demonstrate that correction of CF15 monolayers with CFTR did not induce a change in the pattern of IL-8 secretion in either the basolateral or apical compartments. The fact that monolayers overexpressing ΔF508 CFTR displayed a similar profile of IL-8 production as matched counterparts rescued with normal CFTR provides hitherto unreported evidence that the accumulation of mutated CFTR in the ER does not lead to an aberrant synthesis of this cytokine in polarized airway surface epithelial cells.
To confirm the validity of our model, in particular the fact that CF15 cells display a biological dysfunction (other than the Cl− secretion defect) that can be corrected by CFTR, parallel experiments were done as a positive control. We tested the adherence of P. aeruginosa, which has been shown to be increased at the surface of epithelial CF cells, 16-18 and observed that the number of bacteria bound to CF15 cells was indeed corrected by CFTR-containing adenoviruses.
In summary, our data suggest that there is no intrinsic defect of the airway surface epithelial cells predisposing to PMN infiltration into the lung. Although these results are consistent with the studies of Armstrong et al, 28 they do not exclude other possibilities. For example, the inflammation observed in CF airways 4-6 may have been initiated by poorly cleared airborne particles and/or members of the respiratory flora. Alternatively, CF cells other than surface epithelial cells may deliver a signal mediating PMN transmigration. 29 We conclude that the presence of a mutated CFTR does not per se lead to an exaggerated inflammatory response of CF surface epithelial cells.
Acknowledgments
We wish to thank Dr. Susanna Schlegel-Haueter for participating in the conception of the project; Dr. Douglas Jefferson for providing the CF15 cells; Dr. Joëlle Coclet-Ninin for help with electrophysiology; and Drs. Viktor Steimle, Irène Garcia, and Isabelle Moix for their participation in molecular biology experiments. We are also indebted to Drs. Serge Poitry, Marie-Christine Broillet, and Francis Mingard for their help with the electrical setting, and Dr. Arbans Sandhu for amplification and isolation of viruses. We also thank Drs. Christian Vandelden, Thilo Kohler, and Lasta Kocjancic for assistance with PAO1 experiments.
Footnotes
Address reprint requests to Lara Pizurki, Laboratory of Clinical Investigation III, Case postale 14, Hôpital Cantonal Universitaire, 1211 Geneva 14, Switzerland. lara_pizurki@hotmail.com.
Supported by Swiss National Foundation for Scientific Research grants 32–36338.92 and 32–45845.95 .
References
- 1.Suter S, Schaad UB, Tegner H, Ohlsson K, Desgrandchamps D, Waldvogel F: Free granulocyte elastase levels in bronchial secretions from patients with cystic fibrosis: effect of antimicrobial treatment against Pseudomonas aeruginosa. J Infect Dis 1986, 153:902-907 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein W, Döring G: Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis 1986, 134:49-56 [DOI] [PubMed] [Google Scholar]
- 3.Pilewski JM, Frizzell RA: Role of CFTR in airway disease. Physiol Rev 1999, 79:S215-S255 [DOI] [PubMed] [Google Scholar]
- 4.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R: The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 1995, 20:63-70 [DOI] [PubMed] [Google Scholar]
- 5.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DWH: Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995, 151:1075-1082 [DOI] [PubMed] [Google Scholar]
- 6.Noah TL, Black HR, Cheng P, Wood RE, Leigh MW: Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 1997, 175:638-647 [DOI] [PubMed] [Google Scholar]
- 7.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A: Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest 1998, 101:2598-2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson DM, Valentich JD, Marini FC, Grubman SA, Iannuzzi MC, Dorkin HL, Li M, Klinger KW, Welsh MJ: Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol 1990, 259:L496-L505 [DOI] [PubMed] [Google Scholar]
- 9.Stratford-Perricaudet LD, Makeh I, Perricaudet M, Briand P: Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest 1992, 90:626-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chartier C, Degryse E, Gantzer M, Dieterlé A, Pavirani A, Mehtali M: Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol 1996, 70:4805-4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lusky M, Christ M, Rittner K, Dieterlé A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M: In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol 1998, 72:2022-2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Janich S, Cohn JA, Wilson JM: The common variant of CFTR is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA 1993, 90:9480-9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanson M, Scerri I, Suter S: Defective regulation of gap junctional coupling in cystic fibrosis pancreatic duct cells. J Clin Invest 1999, 103:1677-1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyum A: Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 1976, (suppl.5):S9-S15 [PubMed] [Google Scholar]
- 15.Madara JL, Colgan S, Nusrat A, Delp-Archer C, Parkos C: A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil-epithelia interactions. J Tissue Culture Methods 1992, 14:209-216 [Google Scholar]
- 16.Saiman L, Prince A: Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelia cells. J Clin Invest 1993, 92:1875-1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zar H, Saiman L, Quittell L, Prince A: Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr 1995, 126:230-233 [DOI] [PubMed] [Google Scholar]
- 18.Davies JC, Stern M, Dewar A, Caplen NJ, Munkonge FM, Pitt T, Sorgi F, Huang L, Bush A, Geddes DM, Alton EWFW: CFTR gene transfer reduces the binding of Pseudomonas aeruginosa to cystic fibrosis respiratory epithelium. Am J Respir Cell Mol Biol 1997, 16:657-663 [DOI] [PubMed] [Google Scholar]
- 19.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH: Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol 1992, 262:L713-L724 [DOI] [PubMed] [Google Scholar]
- 20.Gruenert DC, Finkbeiner WE, Widdicombe JH: Culture and transformation of human airway epithelial cells. Am J Physiol 1995, 268:L347-L360 [DOI] [PubMed] [Google Scholar]
- 21.Parkos CA, Delp-Archer C, Arnaout MA, Madara JL: Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 1991, 88:1605-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colgan SP, Parkos CA, Delp-Archer C, Arnaout MA, Madara JL: Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-γ in a highly polarized fashion. J Cell Biol 1993, 120:785-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman P, D’Andrea L, Carnes D, Colgan SP, Madara JL: Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am J Physiol 1996, 271:C312-C320 [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Mul FPJ, Lutter R, Roos D, Knol EF: Transmigration of human neutrophils across airway epithelial cell monolayers is preferentially in the physiologic basolateral-to-apical direction. Am J Respir Cell Mol Biol 1996, 15:771-780 [DOI] [PubMed] [Google Scholar]
- 25.Carolan EJ, Casale TB: Neutrophil transepithelial migration is dependent upon epithelial characteristics. Am J Respir Cell Mol Biol 1996, 15:224-231 [DOI] [PubMed] [Google Scholar]
- 26.Carolan EJ, Mower DA, Casale TB: Cytokin-induced neutrophil transepithelial migration is dependent upon epithelial orientation. Am J Respir Cell Mol Biol 1997, 17:727-732 [DOI] [PubMed] [Google Scholar]
- 27.Zabner J, Couture LA, Smith AE, Welsh MJ: Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther 1994, 5:585-593 [DOI] [PubMed] [Google Scholar]
- 28.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutièrrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD: Lower airway inflammation in infants and young children with cystic fibrosis. Am J Resp Crit Care Med 1997, 156:1197-1204 [DOI] [PubMed] [Google Scholar]
- 29.Tabary O, Zahm J, Hinrasky J, Couetil J, Cornillet P, Guenounou M, Gaillard D, Puchelle E, Jacquot J: Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol 1998, 153:921-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bédard M, McClure CD, Schiller NL, Francoeur C, Cantin A, Denis M: Release of Interleukin-8, Interleukin-6, and Colony-stimulating factors by upper airway epithelial cells: implication for cystic fibrosis. Am J Respir Cell Mol Biol 1993, 9:455-462 [DOI] [PubMed] [Google Scholar]
- 31.Black HR, Yankaskas JR, Johnson LG, Noah TR: Interleukin-8 production by cystic fibrosis nasal epithelial cells after Tumor Necrosis Factor-α and Respiratory Syncytial Virus stimulation. Am J Respir Cell Mol Biol 1998, 19:210-215 [DOI] [PubMed] [Google Scholar]
- 32.Schwiebert LM, Estell K, Propst SM: Chemokine expression in CF epithelia : implications for the role of CFTR in RANTES expression. Am J Physiol 1999, 276:C700-C710 [DOI] [PubMed] [Google Scholar]
- 33.Massengale ARD, Quinn F, Yankaskas J, Weissman D, McClellan WT, Cuff C, Aronoff SC: Reduced Interleukin-8 production by cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 1999, 20:1073-1080 [DOI] [PubMed] [Google Scholar]
- 34.Montrose-Rafizadeh C, Guggino WB, Montrose MH: Cellular differentiation regulates expression of Cl- transport and CFTR mRNA in human intestinal cells. J Biol Chem 1991, 266:4495-4499 [PubMed] [Google Scholar]