Abstract
Previous investigations have shown that cytotoxic T lymphocytes (CTLs) contribute to muscle pathology in the dystrophin-null mutant mouse (mdx) model of Duchenne muscular dystrophy through perforin-dependent and perforin-independent mechanisms. We have assessed whether the CTL-mediated pathology includes the promotion of eosinophilia in dystrophic muscle, and thereby provides a secondary mechanism through which CTLs contribute to muscular dystrophy. Quantitative immunohistochemistry confirmed that eosinophilia is a component of the mdx dystrophy. In addition, electron microscopic observations show that eosinophils traverse the basement membrane of mdx muscle fibers and display sites of close apposition of eosinophil and muscle membranes. The close membrane apposition is characterized by impingement of eosinophilic rods of major basic protein into the muscle cell membrane. Transfer of mdx splenocytes and mdx muscle extracts to irradiated C57 mice by intraperitoneal injection resulted in muscle eosinophilia in the recipient mice. Double-mutant mice lacking dystrophin and perforin showed less eosinophilia than was displayed by mdx mice that expressed perforin. Finally, administration of prednisolone, which has been shown previously to reduce the concentration of CTLs in dystrophic muscle, produced a significant reduction in eosinophilia. These findings indicate that eosinophilia is a component of the mdx pathology that is promoted by perforin-dependent cytotoxicity of effector T cells. However, some eosinophilia of mdx muscle is independent of perforin-mediated processes.
Eosinophils are generally associated with immune responses to infection by parasites or with allergic reactions. 1 However, eosinophils can play a significant role in the cellular immune response in which their involvement appears to be tightly coupled both temporally and functionally to T lymphocyte activity. For example, eosinophils and lymphocytes are recruited to sites of inflammation simultaneously, 2,3 lymphocytes are the most potent source of chemoattractants for eosinophils, 3,4 and both cytotoxic T cells (CTLs) and helper T cells (Th cells) produce interleukin-5 which promotes eosinophil differentiation and possibly activation. 5-7
T-cell-promoted eosinophilia has been demonstrated in experimental delayed-type hypersensitivity in which CD8+ T cells are injected into the footpads of mice. 8 The eosinophilia produced in this experimental delayed-type hypersensitivity is attributable to specific cytotoxic actions of T cells because T cells from perforin-null mutants caused less hypersensitivity than wild-type T cells. Although the mechanism through which CD8+ T cells induce eosinophilia is unknown, the finding that CD8+ cells which express the Th2 repertoire of cytokines cause more eosinophilia than CD8+ cells that express the Th1 repertoire, suggests that cytokines involved in humoral responses may underlie the T cell promotion of eosinophilia.
Recent provocative findings have also shown a potentially significant functional link between eosinophilia and T cell involvement in autoimmune diseases. Experimental autoimmune encephalomyopathy (EAE), the mouse model of the human autoimmune disease multiple sclerosis, results from a T-cell-mediated autoimmune response to myelin basic protein. 9,10 Eosinophils have been noted previously in the immune cell population present in multiple sclerosis tissue, 11 although their presence has been generally neglected as a substantial contributor to the pathology. However, adoptive transfer experiments in which activated splenocytes from EAE mice were transplanted into control mice showed that not only did EAE pathology appear in the optic nerves of the recipient animals, but eosinophilia of the optic nerve also resulted. 12 This finding suggests that eosinophilia in autoimmune disease may result from T-cell-mediated activities.
Muscle pathology in the mdx mouse model of Duchenne muscular dystrophy (DMD) has been shown to involve a substantial and important contribution of CTLs to the mdx pathology. Evidence that supports this role for CTLs in muscular dystrophy includes the finding that antibody depletions of CTLs from dystrophic muscle produce large reductions in histologically-discernible pathology. 13 Furthermore, a significant portion of the cytotoxicity induced by T cells has been shown to occur through perforin-mediated mechanisms that involve both apoptotic and necrotic cell death. 13 However, it is unknown whether perforin-mediated pathology may also promote eosinophilia in dystrophic muscle.
In the present investigation, we test the hypothesis that eosinophilia is a component of the pathology of muscular dystrophy and that it is induced by perforin-mediated actions of T lymphocytes. We test this hypothesis by quantifying the presence of eosinophils in mdx hindlimb muscles, which undergo a single prominent bout of necrosis at 4 weeks of age, and in mdx diaphragms, which undergo progressive necrosis through the animals’ lives, in comparison to age-matched controls. We also assay for specific cell-cell contacts between eosinophils and mdx muscle fibers that indicate eosinophil-mediated cytotoxicity. We then assess whether muscle eosinophilia can be induced in wild-type animals by splenocyte transplantation from mdx animals at the peak of pathology. The possibility that mdx muscle eosinophilia is promoted by perforin-dependent mechanisms is tested by assaying whether double-mutant mice, that lack both dystrophin and perforin, exhibit lower eosinophil concentrations than mdx mice. Finally, we examine whether treating dystrophic mice with a prednisone derivative, which is one of the few known, useful therapies for dystrophin-deficient muscular dystrophy, may influence eosinophilia, and thereby support the proposal that reduction of eosinophil populations may ameliorate the pathology of muscular dystrophy.
Materials and Methods
Animals
Dystrophin-null mutant mice (mdx) and wild-type controls (C57) were obtained from Jackson Laboratories (Bar Harbor, ME). Perforin-null mutant mice were provided by Dr. William R. Clark (UCLA). All mice were maintained in the UCLA vivarium until killing for tissue collection. The Animal Research Committee at UCLA approved all animal treatments. Perforin-deficient, dystrophin-deficient mice were generated by crossing mdx mice with perforin-null mutants, according to previously described breeding and screening strategies. 13 Animals were used at 4 weeks or at 30 to 32 weeks of age. The hindlimb musculature is at the peak of necrosis in 4-week-old animals and the muscles have regenerated at 30 to 32 weeks of age. 14 Diaphragm pathology is also apparent at 4 weeks of age, but it continues to progress throughout the life of the animal.
Assays to Confirm Identity of Perforin-Deficient mdx Mice
Presence of the perforin mutation was confirmed by polymerase chain reaction (PCR) analysis of genomic DNA isolated from tail tissue. Primer pairs and PCR conditions used to identify the homozygous mutants have been described previously. 13 Mice in the F2 and F3 generations in the breeding strategy used were dystrophin-deficient 13 although this was confirmed in select mice by immunoblot analysis. 15
Adoptive Transfer Procedures
Splenocytes were isolated from 4-week-old, female mdx mice by sterile dissection of spleens after which the spleens were forced through a stainless steel mesh in PBS (50 mmol/L sodium phosphate buffer at pH 7.4 containing 150 mmol/L sodium chloride). The filtrate was then pressed through a 70-μm nylon filter and red cells were lysed with Tris-ammonium chloride. The splenocytes in the filtrate were then counted with a hemocytometer.
Four-week-old female C57 mice that had been γ-irradiated with 800 rads received an injection of 1.4 × 10 7 mdx splenocytes into the tail vein 4 days after irradiation. In addition, the mice received intraperitoneal (ip) injections of sterile extracts of mdx muscle to prime transplanted T cells. Control mice were irradiated and received ip injections of muscle extract but received tail vein injections of PBS only. The host animals’ hindlimb muscles and diaphragms were then analyzed 14 days later for eosinophilia.
Electron Microscopy
Hindlimb muscles from 4-week-old C57 and mdx mice were fixed in situ at physiological length in 1.4% glutaraldehyde in 0.2 mol/L sodium cacodylate, pH 7.4, for 30 minutes and dissected free from the hindlimb and fixed for an additional 30 minutes. The muscles were then sliced into small strips and placed in 1.0% osmium tetroxide for 40 minutes, after which they were rinsed in 0.2 mol/L sodium cacodylate, pH 7.4, dehydrated in a series of ethanols, followed by propylene oxide, and then embedded in epoxy resin. Thin sections were cut at 70 nm, stained with uranyl acetate and lead citrate and viewed in a transmission electron microscope.
Prednisolone Treatments
mdx mice received ip injections of 0.75 mg of prednisolone (a derivative of prednisone) per kilogram mouse body mass in sterile PBS daily, beginning at 2 weeks of age and continuing until sacrifice for tissue collection at 4 weeks of age. Control mdx mice received injections of equal volumes of PBS ip.
Assay for Eosinophils
Hindlimb muscles and diaphragms were collected from mice and prepared and sectioned as described previously. All sections used for analysis were cross-sections taken at the mid-belly of the hindlimb muscle or through the coronal plane of the diaphragm muscles. Cationic vesicles that are characteristic of eosinophils were identified by fluorescein binding, as described by Nonaka et al. 16 The concentration of eosinophils in each sample was determined by quantitative microscopic techniques in which the number of eosinophils per total cross-section of muscle was measured and then the number of eosinophils per unit volume was calculated for 10-μm-thick sections. 13
Statistical Analysis
All quantitative data were analyzed using Student’s t-test with confidence level at 0.05.
Results
Mdx Dystrophy Involves Eosinophilia
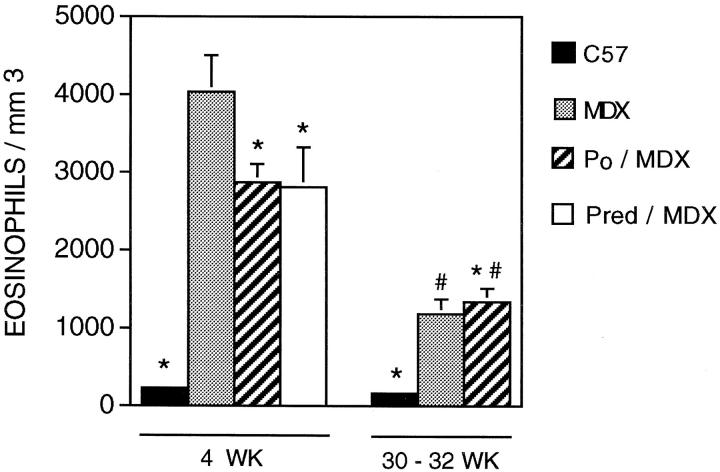
Fluorescein binding assays show that eosinophilia is a prominent feature of the necrotic phase of muscular dystrophy in mdx mice (Figure 1) ▶ . At the peak of hindlimb pathology in 4-week-old mice, mdx quadriceps contains ∼4033 eosinophils/mm 3 (SE = 467; n = 22) which is a highly significant (P < 0.001), nearly 20-fold increase over the concentration present in age-matched C57 mice (228 eosinophils/mm3; SE = 190; n = 11) (Figures 1 and 2) ▶ ▶ . This eosinophil concentration in mdx muscle decreases significantly during the regenerative period of the pathology to ∼1184 eosinophils/mm 3 (SE = 190; n = 11), which is still greater than the concentration present in age-matched C57 mice (167 eosinophils/mm3; SE = 44; n = 5).
Figure 1.
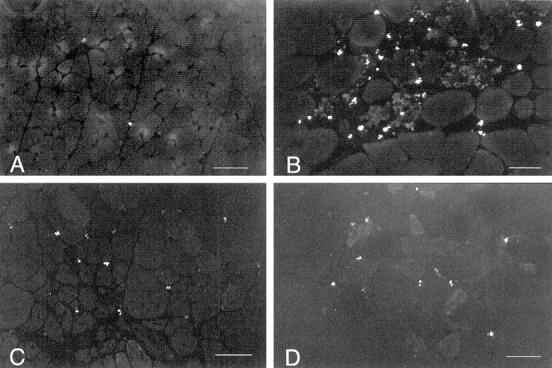
Fluorescent microscopy of eosinophils in cross-sections of mouse quadriceps muscle. Bright cells are eosinophils. Micrographs are printed so that muscle fibers can be discerned by faint background fluorescence. A: Representative section of 4-week-old C57 quadriceps. One eosinophil is present in the field. Scale bar, 60 μm. B: Representative section of necrotic region of 4-week-old mdx quadriceps. Scale bar, 50 μm. C: Representative section of a regenerated region of 30-week-old mdx quadriceps. Scale bar, 50 μm. D: Representative section of quadriceps from a 4-week-old perforin-deficient, mdx mouse. Scale bar, 60 μm.
Figure 2.
Concentration of eosinophils in quadriceps muscle. Data from C57 (black bars), mdx (stippled bars), perforin-deficient mdx (striped bars), and prednisolone-treated mdx (white bars) are shown for 4-week-old (left) and 30- to 32-week-old (right) groups are shown. Asterisks indicate those groups that differ significantly from the age-matched mdx at P = 0.05. #, Indicates those groups that differ significantly (P = 0.05) from 4-week animals receiving the same treatment.
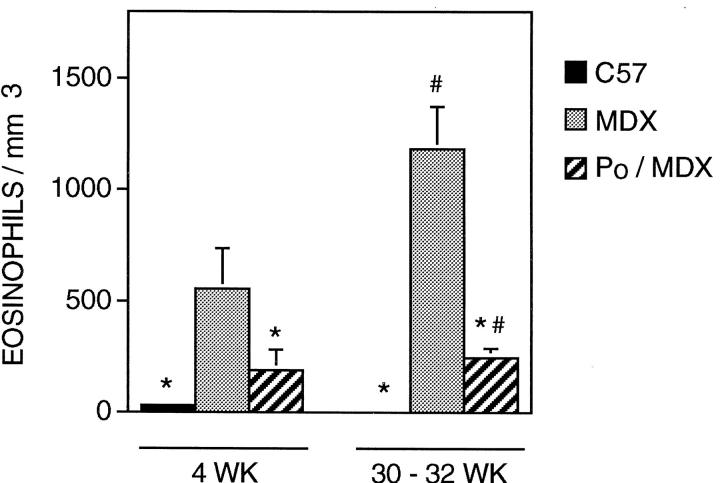
The progression of muscle pathology in mdx mouse diaphragms is more similar than mdx hindlimb muscle to the pathology in DMD muscle in that it is progressive, unlike mdx hindlimb musculature that undergoes one prominent bout of necrosis and then regenerates. Consistent to the general course of diaphragm pathology, we found that eosinophil concentration was elevated at 4 weeks of age in the diaphragm (556 eosinophils/mm3; SE = 181; n = 16), but the concentration did not decline during regeneration as we observed to occur in mdx hindlimbs (Figure 3) ▶ . The concentration of eosinophils in the mdx diaphragm was significantly lower than the concentration in mdx hindlimbs at both 4 weeks and 30 to 32 weeks of age. However, we also found that control C57 diaphragms contain significantly fewer eosinophils than present in C57 hindlimbs at both 4 weeks and 30 to 32 weeks of age.
Figure 3.
Concentration of eosinophils in diaphragm muscle. Symbols are identical to those used in Figure 2 ▶ .
Electron microscopic observations also showed elevated concentrations of eosinophils in mdx tissue. Mononucleated cells containing vesicles with dense, rod-like inclusions that are diagnostic of major basic protein (MBP) in eosinophil vesicles, 17,18 were frequently observed in mdx hindlimb muscle (Figures 4–6) ▶ ▶ ▶ . Hindlimb muscles from each of five mdx mice at 4 weeks of age showed eosinophils at the surface of necrotic fibers. No eosinophils were observed by electron microscopy in C57 hindlimb muscles. Electron microscopic observations showed three different relationships between eosinophils and mdx muscle fibers: 1) some eosinophils were separated from the surface of apparently healthy muscle fibers by basement membrane and other connective tissue components (Figure 4) ▶ ; 2) other eosinophils were closely apposed to the intact surface membrane of intact mdx fibers (Figure 5) ▶ ; or 3) other eosinophils were located at the surface of mdx fibers that had already experienced cell membrane dissolution (Figure 6) ▶ . In the first instance, no obvious signs of pathology were evident in the fibers near the eosinophils, and the MBP rods tended to be oriented along the long axis of the eosinophils (Figure 4) ▶ . In the second instance, the eosinophil and muscle cell membranes were closely apposed at sites where the eosinophil had crossed the muscle cell’s basement membrane. At these sites of close apposition, MBP rods in the eosinophils were oriented approximately normal to the membranes, and the eosinophil membrane protruded toward the muscle fiber membrane, and impinged on the muscle cell surface (Figure 5) ▶ . In the latter case when the muscle fibers were frankly necrotic, the eosinophils remained at the surface of the necrotic fiber, but the fiber to which they were adjacent showed membrane lysis and disruption of organelles (Figure 6) ▶ .
Figure 4.

Electron micrograph of 4-week-old mdx muscle showing an eosinophil lying within the endomysial connective tissue that separates two muscle fibers that show no signs of pathology. The eosinophil is separated from the muscle fiber surface by ∼0.5 μm (arrows) and the MBP rods within the eosinophil are oriented along the length of the eosinophil. Scale bar, 1.0 μm.
Figure 5.
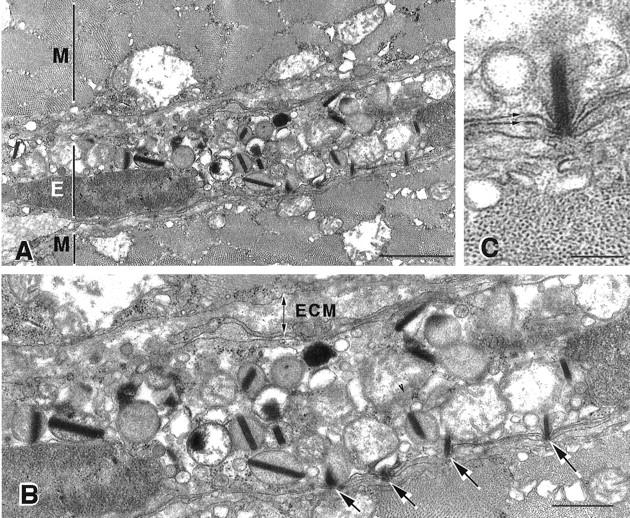
Electron micrograph of 4-week-old mdx muscle showing invading eosinophil. A: Portions of two muscle fibers (M) between which an eosinophil (E) has invaded are shown. Scale bar, 1.0 μm. B: Higher magnification of invading eosinophil illustrated in A. Note that the extracellular matrix (ECM), which includes the basement membrane that envelops the muscle fiber, lies between the eosinophil and the upper muscle fiber. The eosinophil lies in close apposition to the lower muscle fiber, and no basement membrane or other connective tissue separates the two cells. Note that the rod-like inclusions of MBP impinge on the surface of the lower muscle fiber (arrows). Vesicles containing MBP are not similarly distributed at the opposite surface of the eosinophil, where direct contacts between the eosinophil and muscle membranes have not been formed. Scale bar, 400 nm. C: Higher magnification view of single MBP rod impinging on muscle fiber surface where the close association between the eosinophil and muscle cell membranes (arrows) can be resolved. Note that no connective tissue lies between the two cells. Scale bar, 150 nm.
Figure 6.
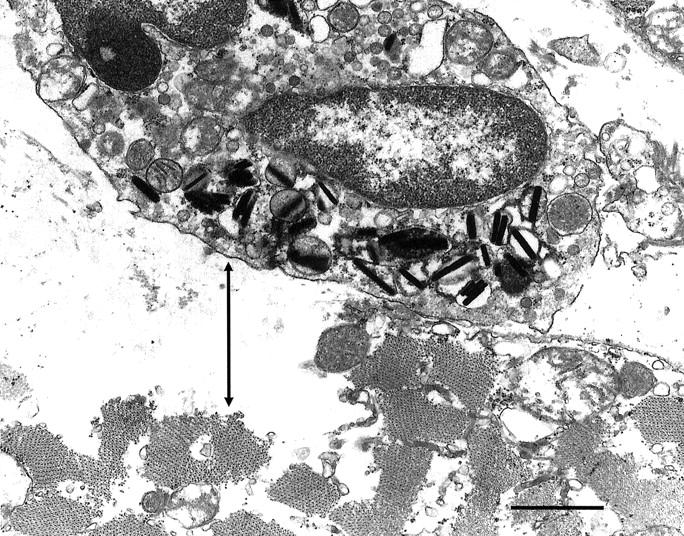
Electron micrograph of 4-week-old mdx muscle showing an eosinophil at the surface of a necrotic fiber. Note that the muscle cell membrane is absent or disrupted adjacent to the site of eosinophil apposition and that the region of the muscle fiber subjacent to the site of membrane loss (arrow) shows dissolution of cytosolic contents. Also note that the eosinophilic granules are aggregated at the region of the eosinophil that is closely applied to the necrotic fiber surface. Scale bar, 1.0 μm.
Adoptive Transfer of mdx Splenocytes to C57 Recipients Increases Muscle Eosinophil Concentrations
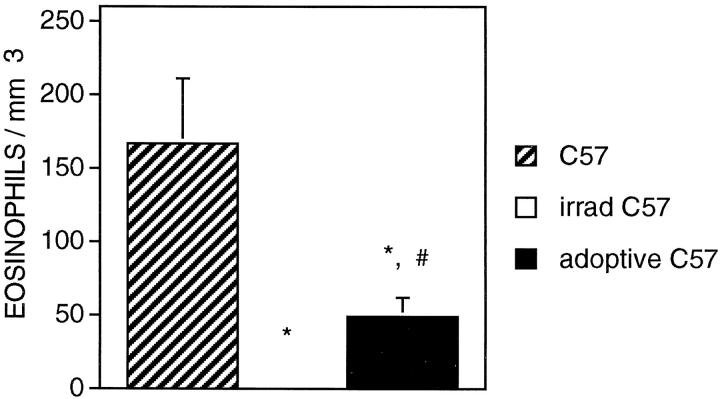
If eosinophilia of mdx muscle were a specific response to the actions of autoreactive T cells to dystrophic muscle, we postulated that the transplantation into wild-type mice of splenocytes from mdx mice collected at the peak of pathology would induce eosinophilia in the muscles of the recipient animals. Injection of mdx splenocytes into the tail veins of irradiated C57 mice that had also received a sterile injection of mdx muscle extract produced a significant increase in eosinophils in the recipient mouse hindlimb muscles at 2 weeks after injection (49 eosinophils/mm3; SE = 13; n = 14) (Figure 7) ▶ . C57 mice that received muscle extract injections ip and but received buffer only in tail vein injections, showed no eosinophils in their muscles at 2 weeks after injection (0 eosinophils/mm3; SE = 50; n = 6). Both the control mice and the mice undergoing splenocyte transplantation contained eosinophil concentrations that were significantly much lower than nonirradiated, age-matched C57 mouse muscles, but this is attributable to the irradiation of the experimental animals and their buffer injected controls.
Figure 7.
Concentration of eosinophils in quadriceps muscles of control C57 mice (striped bars), irradiated C57 mice receiving injection of mdx muscle extract (no eosinophils were present) and irradiated C57 mice receiving injection of mdx muscle extract and mdx splenocytes. Asterisks indicate groups that differ significantly from C57 control at P = 0.05. #, significant difference (P = 0.05) from irradiated animals receiving injection of muscle extract but no splenocyte injection.
Perforin-Deficient Mdx Mice Show Reduced Eosinophil Concentrations in Muscle
All animals used in this analysis were shown to be homozygous for the perforin mutation in that the PCR analysis yielded PCR products at 350 and 1600 bp, whereas the wild-type PCR product is at 500 bp. 13 Quantitative histochemical analysis of 4-week-old double-mutant mice showed that they contain significantly fewer eosinophils than mdx muscles, although the eosinophil concentration in double-mutant mice remained much higher than that observed in age-matched C57 (Figures 1–3) ▶ ▶ ▶ . The reduction in eosinophil concentration in 4-week hindlimb muscle of double-mutants was ∼30%, whereas their reduction in diaphragm was ∼65% compared to age-matched mdx muscles. Eosinophil concentrations in 30- to 32-week-old diaphragms of double-mutant mice were significantly (∼65%) less than the concentration in age-matched mdx diaphragms, and did not differ significantly from age-matched C57 diaphragms. However, the concentration of eosinophils in the hindlimb musculature of 30- to 32-week-old double-mutants did not differ from that found in mdx.
Prednisolone Treatments Reduce Eosinophilia in mdx Mice
Administration of prednisolone to mdx mice beginning in prenecrotic, 2-week-old animals until the peak of necrosis at 4 weeks of age caused a significant reduction in eosinophilia (Figure 2) ▶ . Quadriceps muscle of prednisolone-treated animals showed a significant, 30% decrease in the concentration of eosinophils compared to age-matched, nontreated controls. The concentration of eosinophils in prednisolone-treated animals did not differ from the concentration present in age-matched, perforin-deficient mdx hindlimb muscles.
Discussion
The findings of the present investigation show that eosinophilia is a component of muscular dystrophy that is promoted by the perforin-dependent activities of CTLs. These findings contribute to expanding the general view of eosinophil function to include their participation in the cellular immune response in addition to their better-characterized role in the immune response to parasitic infection or allergens. Other investigations that have provided support for this larger view of eosinophilia include: 1) the finding that transplantation of autoreactive T cells from EAE mice to naïve mice can induce EAE pathology and eosinophilia in the recipient animals; 12 and 2) the report that delayed tissue hypersensitivity involving eosinophilia results from T cell transplantation, but the severity of the response is diminished if the T cells are null mutants for perforin. 8 Thus, T cells can promote eosinophilia by at least two mechanisms: through perforin-dependent mechanisms and through the T cell production of interleukin-5, which promotes the differentiation and activation of eosinophils.
The electron microscopic observations reported here that show close associations between eosinophil and muscle cell membranes also provide strong evidence that eosinophils specifically recognize the dystrophic muscle cell surface. Muscle fibers are ensheathed in basement membranes that form a barrier between their cell membrane and those of all other cell types except satellite cells in healthy tissue, 19 and immune cells during disease states in which specific receptor-ligand interactions between the muscle and immune cells are expected to occur. 20 The ability of immune cells to cross this connective tissue barrier indicates specific chemoattractant activity that may result from muscle injury. 21 Furthermore, the close apposition between the membranes of the two cells reflects specific cell-cell interactions, and is not observed to occur between cells that do not associate via specific molecular interactions. The 15-nm gap observed between the eosinophil and muscle membrane at sites of close cellular apposition is similar to that observed at adherens junctions (about 15 to 20 nm 22 ) or desmosomes (about 20 to 30 nm 23 ), and is substantially narrower than the gap that occurs between the membranes of adjacent cells that do not associate via specific molecular associations (50 nm or more). This spacing between neighboring cells that do not share direct adhesive associations is largely attributable to the negative charge carried by cell membranes that precludes close apposition of membranes in the absence of specific interactions between surface molecules. 24 For example, the distance between apposing membranes of CTLs and their targets does not reach values less than 10 nm which is attributable to glycoconjugates present on the cells’ surfaces. 24 Finally, the restricted distribution of dense material in the space between the two apposing cell membranes is a consistent feature of sites of specific cell-cell adhesion. 22,23
Previous electron microscopic analyses have shown that the rods present in eosinophils consist of MBP, 17,18 which is a cytolytic protein that directly disrupts plasma membrane structure 25,26 and accounts for much of the eosinophilia of the vesicles in which it is stored. Eosinophil cationic protein is colocalized in the same vesicles that contain the MBP and is also capable of contributing to the cytolytic actions of eosinophils in that eosinophil cationic protein can form pores in target cell membranes. 27 Together, eosinophil cationic protein and MBP-induced damage to target cell membranes result in swelling of the target cell, influx of Ca2+, and loss of cytosolic proteins into the extracellular space. 25 All of these pathological changes are consistent with previous reports of the pathological changes that occur in dystrophin-deficient muscle during its necrosis. 14,15 The finding in the present investigation that each of the sites of close apposition between eosinophil and muscle cell membrane is associated with a MBP rod that impinges on the muscle cell surface, indicates that cytolysis of the muscle cell may occur at these sites of cell adhesion.
These findings contribute to the growing evidence that the immune system plays an important role in the pathophysiology of dystrophin-deficient muscular dystrophy. Death of dystrophin-deficient muscle has been primarily attributed to mechanical damage incurred by the cell membrane which lacks the support of the cytoskeletal protein, dystrophin. 28 However, this mechanical defect hypothesis for dystrophic muscle death has been inadequate to explain many aspects of the pathophysiology of the disease. For example, the onset of severe muscle cell necrosis does not occur when ambulation first begins in humans or other mammals that are null mutants for dystrophin. 29 Instead, overt muscle weakness and necrosis does not occur until ∼3 years of age in humans and ∼3.5 weeks of age in mice. The mechanical defect hypothesis is also inadequate to explain the effectiveness of nonanabolic steroids in slowing the progress of muscle weakness or necrosis, and current evidence indicates that the beneficial effects of prednisone on DMD muscle result from suppression of the activities of immune cells. 30
The observation that prednisone treatments significantly reduce the concentration of eosinophils in dystrophic muscle suggests that this response may underlie some of the beneficial effects of prednisone in the treatment of DMD. Currently, prednisone treatments are the most effective therapeutic that is approved in the United States for use in slowing the progress of muscle weakness in DMD, although there is no clear, pharmacological understanding for the beneficial effects of this drug in DMD. 31 Previous investigators have speculated that prednisone could increase muscle regenerative capacity, stabilize lysosomal proteases and thereby reduce wasting, or stabilize the muscle cell membrane and reduce muscle damage as a result. However, there is little support for those speculations and several investigations show that these proposals are not well-founded. 30,32 Prednisone or its derivatives have been shown to reduce necrosis of dystrophin-deficient muscle fibers and to reduce the concentrations of CD8+ T cells 30 and eosinophils (present study) in dystrophin-deficient muscle, although it has no significant effect on the concentration of other immune cells in DMD muscle, for example B cells, macrophages, or CD4+ T cells. 30 Thus, the apparently selective effect of prednisone in diminishing CTL and eosinophil concentrations in dystrophic muscle, while improving strength of dystrophic muscle, further supports the proposal that both of these cell types contribute to the pathology of dystrophin deficiency and that CTLs promote eosinophilia in muscular dystrophy.
As examination of the potential involvement of the immune system in the pathology of dystrophin-deficient muscle proceeds, evidence continues to accumulate which indicates that the immune system may play an important role in promoting dystrophic muscle death. For example, T cell receptors of CTLs isolated from DMD patients show a conserved peptide in the hypervariable domain of the receptor that interacts with antigen, and this peptide does not occur in the receptors of CTLs obtained from patients with other neuromuscular diseases. 33 This suggests that a specific antigen may activate T cells in DMD. More recently, antibody depletions of CTLs from mdx mice were found to cause significant reductions in muscle pathology, 13 which shows that CTLs promote the pathology of muscular dystrophy. As the present results now show, some of this CTL-mediated pathology may occur through muscle cytolysis by eosinophils.
Footnotes
Address reprint requests to Dr. James G. Tidball, Department of Physiological Science, 621 Young Drive South, University of California, Los Angeles, CA 90095-1527. E-mail: jtidball@physci.ucla.edu.
Dr. Cai and Dr. Spencer contributed equally to this investigation.
References
- 1.Silberstein D: Eosinophil function in health and disease. Crit Rev Oncol Hematol 1994, 19:47-77 [DOI] [PubMed] [Google Scholar]
- 2.Laitinen L, Laitenen A, Neino M, Haahtela T: Eosinophilic airway inflammation during exacerbation of asthma and its treatment with inhaled corticosteroids. Am Rev Respir Dis 1991, 143:423-427 [DOI] [PubMed] [Google Scholar]
- 3.Kornfield H, Berman J, Beer D, Center D: Induction of human T-lymphocyte motility by interleukin-2. J Immunol 1985, 134:3887-3890 [PubMed] [Google Scholar]
- 4.Rand T, Silberstein D, Kornfield H, Weller P: Human eosinophil express functional interleukin-2 receptors. J Clin Invest 1991, 88:825-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu A: Highly purified murine interleukin-5 stimulates eosinophil function and prolongs survival. IL-5 as an eosinophil chemotactic factor. J Exp Med 1988, 167:1737-1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez A, Sanderson C, Gamble J, Campbell H, Young I, Vadas M: Recombinant human interleukin-5 is a selective activator of human eosinophil function. J Exp Med 1988, 167:219-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson C, Gleich C: Regulator effect of cytokines on eosinophil degranulation. J Immunol 1990, 144:642-646 [PubMed] [Google Scholar]
- 8.Li L, Sad S, Kagi D, Mosman T: CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol 1997, 158:4152-4161 [PubMed] [Google Scholar]
- 9.Fujinami R, Oldstone M: Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 1985, 230:1043-1045 [DOI] [PubMed] [Google Scholar]
- 10.Siram S, Carroll L, Fortin S, Cooper S, Ranges G: In vivo immunomodulation by monoclonal anti-CD-4 antibody. II. Effect on T cell response to myelin basic protein and experimental allergic encephalomyelitis. J Immunol 1988, 141:464-468 [PubMed] [Google Scholar]
- 11.Tanpahichitr K: Multiple sclerosis associated with eosinophilic vasculitis, pericarditis, and hypocomplementemia. Arch Neurol 1980, 37:314-315 [DOI] [PubMed] [Google Scholar]
- 12.Milici A, Carroll L, Stukenbrok H, Shay A, Gladue R, Showell H: Early eosinophil infiltration into the optic nerve of mice with experimental allergic encephalomyelitis. Lab Invest 1998, 78:1239-1244 [PubMed] [Google Scholar]
- 13.Spencer M, Walsh C, Dorshkind K, Rodriguez E, Tidball J: Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest 1997, 99:2745-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson J, Bressler B, Ovalle W: Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. Cell Motil 1988, 9:499-515 [DOI] [PubMed] [Google Scholar]
- 15.Spencer M, Croall D, Tidball J: Calpains are activated in necrotic fibers from mdx mice. J Biol Chem 1995, 270:10909-10914 [DOI] [PubMed] [Google Scholar]
- 16.Nonaka M, Nonaka R, Woolley K, Aldelroth E, Miura K, Okhawara Y, Glibetic M, Nakano K, O’Byrne P, Dolovich J, Jordana M: Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. J Immunol 1995, 155:3234-3244 [PubMed] [Google Scholar]
- 17.Peters M, Rodriguez M, Gleich G: Localization of eosinophil granule major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin by immunoelectron microscopy. Lab Invest 1986, 54:656-662 [PubMed] [Google Scholar]
- 18.Egesten A, Alumets J, von Mecklenburg C, Palmegren M, Olsson I: Localization eosinophil cationic protein, major basic protein and eosinophil peroxidase in human eosinophils by immunoelectron microscope technique. J Histochem Cytochem 1986, 34:1399-1403 [DOI] [PubMed] [Google Scholar]
- 19.Schultz E: Fine structure of satellite cells in growing skeletal muscle. Am J Anat 1976, 147:49-70 [DOI] [PubMed] [Google Scholar]
- 20.Hohlfeld R, Engel A, Ii K, Harper M: Polymyositis mediated by T lymphocytes that express the γ/δ receptor. N Engl J Med 1991, 324:877-881 [DOI] [PubMed] [Google Scholar]
- 21.Robertson T, Maley M, Grounds M, Papadimitriou J: The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 1993, 207:321-331 [DOI] [PubMed] [Google Scholar]
- 22.Staehelin L: Structure and function of intercellular junctions. Int Rev Cytol 1974, 39:191-283 [DOI] [PubMed] [Google Scholar]
- 23.Farquhar M, Palade G: Junctional complexes in epithelia. J Cell Biol 1963, 17:375-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foa C, Mege J, Capo C, Benoliel A, Galindo J, Bongrand P: Electron microscopic study of the surface topography of isolated and adherent cells. Bongrand P eds. Physical Basis of Cell Adhesion. 1988, :pp 191-225 CRC Press, Boca Raton, FL, [Google Scholar]
- 25.Kroegel C, Costabel U, Matthys H: Mechanism of membrane damage mediated by eosinophil major basic protein. Lancet 1987, i:1380-1381 [DOI] [PubMed] [Google Scholar]
- 26.Abu-Ghazaleh R, Gleich C, Pendergast F: Interaction of eosinophil granule major basic protein with synthetic lipid bilayers. J Membr Biol 1992, 128:153-164 [DOI] [PubMed] [Google Scholar]
- 27.Young J, Peterson C, Venge P, Cohn Z: Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature 1986, 321:613-616 [DOI] [PubMed] [Google Scholar]
- 28.Weller B, Karpati G, Carpenter S: Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci 1990, 100:9-13 [DOI] [PubMed] [Google Scholar]
- 29.Iannaccone S: Current status of Duchenne muscular dystrophy. Pediatr Neurol 1992, 39:879-894 [DOI] [PubMed] [Google Scholar]
- 30.Kissel J, Burrow K, Rammohan K, Mendell J: Mononuclear cell analysis of muscle biopsies in prednisone-treated and untreated Duchenne muscular dystrophy. Neurology 1991, 41:667-672 [DOI] [PubMed] [Google Scholar]
- 31.Fenichel G, Florence J, Pestronk A, Mendell J, Moxley R, Griggs R, Brooke M, Miller J, Robison J, King W, Signore L, Pandya S, Schierbecker J, Wilson B: Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology 1991, 41:1874-1877 [DOI] [PubMed] [Google Scholar]
- 32.Haycock J, Falkous G, Maltin C, Delday M, Mantle D: Effect of prednisone on protease activities and structural protein levels in rat muscles in vivo. Clin Chim Acta 1996, 249:47-58 [DOI] [PubMed] [Google Scholar]
- 33.Gussoni E, Pavlath G, Miller R, Panzara M, Posell M, Blau H, Steinman L: Specific T cell receptor gene rearrangements at the site of muscle degeneration in Duchenne muscular dystrophy. J Immunol 1994, 153:4798-4805 [PubMed] [Google Scholar]