Abstract
The complement system plays an important role in mediating tissue injury after oxidative stress. The role of mannose-binding lectin (MBL) and the lectin complement pathway (LCP) in mediating complement activation after endothelial oxidative stress was investigated. iC3b deposition on hypoxic (24 hours; 1% O2)/reoxygenated (3 hours; 21% O2) human endothelial cells was attenuated by N-acetyl-D-glucosamine or D-mannose, but not L-mannose, in a dose-dependent manner. Endothelial iC3b deposition after oxidative stress was also attenuated in MBL-deficient serum. Novel, functionally inhibitory, anti-human MBL monoclonal antibodies attenuated MBL-dependent C3 deposition on mannan-coated plates in a dose-dependent manner. Treatment of human serum with anti-MBL monoclonal antibodies inhibited MBL and C3 deposition after endothelial oxidative stress. Consistent with our in vitro findings, C3 and MBL immunostaining throughout the ischemic area at risk increased during rat myocardial reperfusion in vivo. These data suggest that the LCP mediates complement activation after tissue oxidative stress. Inhibition of MBL may represent a novel therapeutic strategy for ischemia/reperfusion injury and other complement-mediated disease states.
Oxidative stress results in the activation and deposition of complement on the vascular endothelium. 1-4 Tissue injury after ischemia/reperfusion is significantly reduced by complement inhibition 1,5-8 and depletion 9,10 or in complement-deficient animals. 11,12 Specifically, complement inhibition has been shown to preserve endothelium-dependent relaxation 13,14 and to decrease endothelial leukocyte adhesion molecule expression 4,6 and pro-inflammatory cytokine secretion. 15 Thus, inhibition of complement activation after oxidative stress may significantly decrease inflammation and vascular injury.
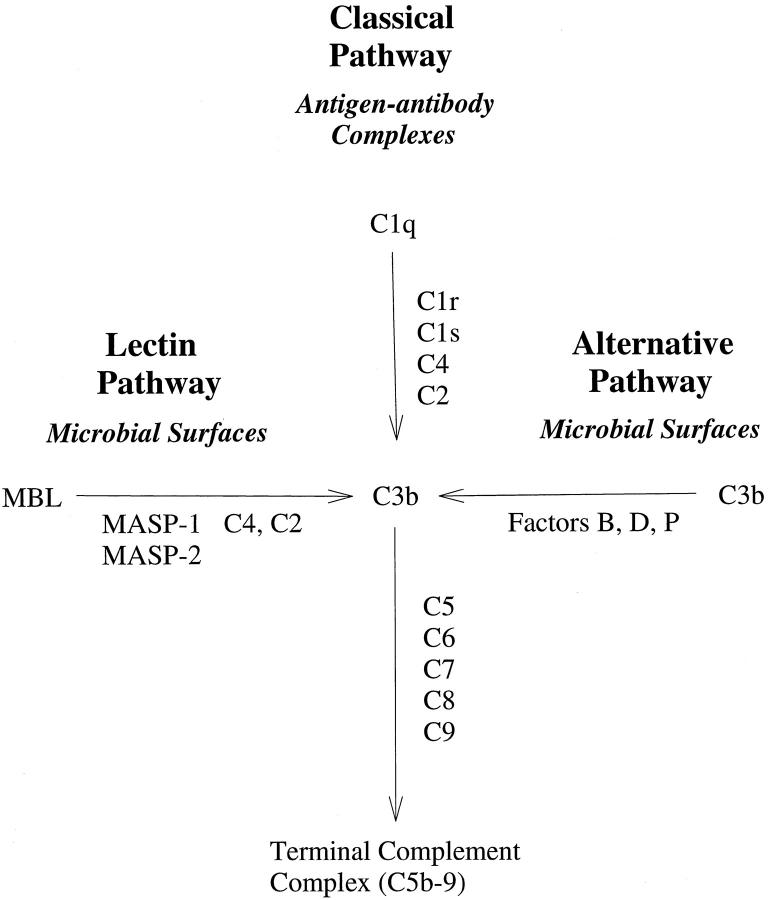
The complement system is comprised of three different cascades: the classical, the alternative, and the recently described lectin complement pathway (LCP). 16,17 The LCP is an antibody-independent cascade initiated by binding of mannose-binding lectin (MBL) to cell surface carbohydrates. 18 MBL, also known as mannose-binding protein (MBP), is structurally similar to C1q and may predate C1q evolutionarily. 19 Associated with MBL are two novel C1r2C1s2-like serine proteases, MBL-associated serine protease (MASP)-1 and MASP-2, 16 that cleave C2 and C4 to form the classical pathway C3 convertase. 20 Thus, unlike the classical complement pathway, activation of the LCP does not require antibody or C1 (Figure 1) ▶ . Although there is experimental evidence linking the classical and alternative complement pathways to human disease, the role of the LCP is just beginning to be evaluated. 21 Further, potent, selective inhibitors of the LCP have not yet been described.
Figure 1.
Three pathways of complement activation. A simplified schematic figure depicting the antigen/antibody-dependent classical complement pathway and the antibody-independent alternative and lectin complement pathways. Note that activation of the classical or LCP is C2-dependent.
We have previously demonstrated that endothelial oxidative stress activates complement via a C2-dependent mechanism and not via the alternative complement pathway. 2 However, preliminary studies in our laboratory demonstrated that IgG, IgM, and C1q deposition on normoxic endothelial cells did not differ from those undergoing oxidative stress, despite increased C2-dependent iC3b deposition. Further, reoxygenation of anoxic endothelial cells has also been shown to increase complement activation and deposition independent of IgG and IgM. 22 Because C2 is necessary for lectin or classical complement pathway activation (Figure 1) ▶ , we investigated whether the LCP could mediate complement activation following endothelial oxidative stress. Further, we designed specific, functionally inhibitory monoclonal antibodies (mAbs) against human MBL to inhibit MBL-dependent complement activation.
Materials and Methods
Human Umbilical Vein Endothelial Cells (HUVECs)
HUVECs were isolated and cultured as previously described. 2,3 Cells were used during passages 1 through 3.
Isolation and Purification of MBL
MBL and MASPs were purified from fresh-frozen plasma as previously described. 23 Western blot analysis confirmed the absence of IgG and/or IgM contamination.
MBL-Deficient Human Serum
MBL-deficient human serum (HS) was produced by treatment with 2 mmol/L phenylmethlysulfonyl fluoride and affinity chromatography using mannan cross-linked to agarose (Sigma Chemical Co., St. Louis, MO). The resultant MBL-deficient HS was dialyzed against Hanks’ balanced salt solution (HBSS) containing Mg2+ and Ca2+.
Immunization and mAb Production
Immunization, fusion, and production of anti-MBL mAbs were performed as previously described with a modification. 24 Female Balb/C mice were immunized initially with human or rat MBL (50 μg, i.p.) in TiterMax (Sigma) and then with MBL (25 μg, i.p.) in PBS once a week for 4 weeks. Four days after the last immunization, the splenocytes were aseptically removed and fused with P301. 24 The hybridoma supernatant was first screened against human or rat MBL in a solid phase antibody capture enzyme-linked immunosorbent assay (ELISA) consisting of human or rat MBL plated onto 96-well microtiter plates. Positive clones were subcloned to monoclonal status by limiting dilution.
Hybridomas were grown in Dulbecco’s modified Eagle’s medium (DMEM, Irvine Scientific, Santa Ana, CA) containing 10% Hyclone fetal bovine serum, 4 mmol/L L-glutamine, 1% penicillin/streptomycin, and 1% nonessential amino acids. Monoclonal antibodies were isotyped with a commercially available kit (Gibco BRL, Grand Island, NY). Antibodies were purified by protein G affinity chromatography, eluted with 100 mmol/L glycine, pH 3.0, and immediately neutralized in 1 mol/L Tris (1:10; v:v). All antibodies were dialyzed against PBS, concentrated, and sterile filtered.
Western Blot Analysis of MBL
To demonstrate that the anti-MBL mAbs were specific for human or rat MBL, and not MASP-1 or MASP-2, Western blot analysis was performed. Reduced human or rat MBL was resolved by 9% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gel was transferred to nitrocellulose and blocked with 10% nonfat dry milk overnight. Each anti-MBL mAb (10 μg/ml) was then incubated with the nitrocellulose in 3% nonfat dry milk for 1 hour at 4°C. The nitrocellulose was then washed and incubated with horseradish peroxidase-conjugated goat anti-mouse polyclonal antibody (1:3000, ICN, Aurora, OH) for 1 hour at 4°C. The nitrocellulose was then washed and developed with the ECL system (Amersham) and X-ray films (Kodak).
Complement Hemolytic Assays
HS was incubated with 0 or 50 μg/ml of the anti-human MBL mAbs for 30 minutes at 37°C. The HS was then diluted serially 1:2 (v:v) in gelatin veronal buffer. Classical complement pathway hemolytic assays (CH50) using sensitized chicken red blood cells were performed as described. 7 Hemolytic assay of MBL-deficient HS was also performed to determine the effect of MBL removal on classical complement pathway activity.
MBL-Dependent C3 Deposition on Mannan-Coated Plates
A MBL-dependent C3 deposition ELISA was used as previously described. 25 Briefly, mannan (Sigma, 500 μg/ml in 15 mmol/L sodium carbonate, pH 9.6) was added to 96 well microtiter plates for 12 to 16 hours at 4°C and then washed. Human or rat serum (2% final concentration) was incubated with (i) 0.1 to 100 mmol/L GlcNAc; (ii) various doses of mAb; or (iii) vehicle (veronal buffered saline containing Ca2+/Mg2+) for 30 minutes at room temperature. The plates were inoculated with 100 μl of treated or untreated human or rat serum and then incubated for 30 minutes at 37°C. The plates were then washed and 50 μl of horseradish peroxidase-conjugated goat anti-human or rat C3 polyclonal antibody (1:2000; ICN) was used to determine the relative amounts of C3 deposited on the wells. MBL-dependent C3 deposition was determined to be the amount of C3 deposited that could be inhibited by 100 mmol/L N-acetyl-D-glucosamine (GlcNAc). C3 deposition in each well was calculated as a percentage of C3 deposition compared to untreated wells (100%) and wells not receiving HS (0%). IC50 concentrations were calculated for each individual experiment. C3 deposition was evaluated using six wells per experimental group, and each experiment was repeated 4 to 5 times.
C3 Deposition ELISA
C3 deposition was measured on hypoxic/reoxygenated HUVECs as previously described. 2,26 We have previously shown that the predominant C3 species present on HUVECs in this model is iC3b. 2 Therefore, the data are presented as changes in iC3b deposition. HUVECs were grown to confluence and then subjected to 0 (normoxia) or 24 hours of hypoxia (1% O2). The cell media were aspirated and 100 μl of one of the following was added to each well: 1) 30% HS, 2) HBSS, 3) 30% HS + 3, 30, or 300 mmol/L GlcNAc, 4) 30% HS + 3, 30, or 300 mmol/L D-mannose, 5) 30% HS + 3, 30, 300 mmol/L L-mannose, 6) 30% MBL-deficient HS, or 7) 30% MBL-deficient HS + 0.6 μg/ml MBL. The cells were then reoxygenated for 3 hours, washed, and then fixed with 1% paraformaldehyde (Sigma) for 30 minutes. The cells were then washed and incubated with peroxidase-conjugated polyclonal goat anti-human C3 antibody (Cappel, West Chester, PA) for 1 hour at 4°C. The plates were washed and developed with ABTS, and the absorbances (405 nm) were measured using a microtiter plate reader (Molecular Devices, Sunnyvale, CA). Background optical density for the C3 deposition ELISA was obtained from cells to which only the anti-human C3 antibody was added or cells incubated with 30% heat-inactivated HS, and was subtracted from all groups. All ELISA experiments were performed 3 times using 6 wells per experimental group (n = 3).
Confocal Microscopy
HUVECs were grown on LabTech tissue culture microscope slides (NUNC) and exposed to hypoxia for 24 hours as described above. The slides were removed from the hypoxia chamber and reoxygenated for 3 hours in 30% HS treated with PBS (ie, vehicle), 3F8 (5 μg/ml), 2A9 (5 μg/ml), or 1C10 (50 μg/ml). The slides were then washed in PBS containing calcium and magnesium and fixed in 4% paraformaldehyde for 15 minutes, washed again, and blocked with 10% goat serum to prevent nonspecific secondary antibody staining. Fixation is necessary to avoid the loss of MBL from the cell surface from the multiple washing steps because, unlike covalently attached iC3b, MBL attachment is calcium-dependent and not covalent. Human MBL deposition was identified using biotinylated 1C10 and streptavidin-conjugated CY5 (blue; Jackson Immunoresearch, West Grove, PA). Human C3 deposition (green) was evaluated with a FITC-conjugated goat anti-human C3 F(ab′)2 antibody (ICN). Following incubation with the appropriate antibodies, the slides were washed 3× 10 minutes, coated with anti-fade mounting media (Molecular Probes, Eugene, OR), covered and analyzed with a Zeiss confocal microscope as described. 26 Controls with streptavidin-conjugated CY5 only were processed as above, omitting the primary antibody to determine nonspecific binding. All analyses were conducted at 40× magnification at the same pinhole, voltage, and laser settings. This experiment was performed three times (n = 3).
Animal Preparation and Protocols
The study protocol was approved by the ethical committee of the Meilahti Theoretical Institutes (Helsinki, Finland) and by the local health authority. Adult male Lewis rats (220–260 g) were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and xylazine (6 mg/kg, i.m.). Rats were tracheostomized and ventilated with a SAR-380 small animal respirator (CWE Inc., PA). Expired CO2 was monitored continuously with a microcapnometer (CWE Inc.) and maintained at 4 to 5% by adjusting the respiratory rate and/or tidal volume. Myocardial ischemia and reperfusion was produced as described previously. 27 Briefly, a left thoracotomy was performed and the left anterior descending coronary artery (LAD) was ligated 3 to 4 mm from its point of origin with 6–0 silk. Ischemia was produced by tightening the previously placed reversible ligature to occlude completely the vessel. Sham-operated animals underwent the same surgical procedures, but without ligation of the LAD suture. Animals were randomly divided into the following three groups: (i) 30 minutes ischemia followed by 30 minutes reperfusion (n = 4); (ii) 60 minutes ischemia, but no reperfusion (n = 4); and (iii) sham controls (n = 4). The rats were sacrificed by carbon dioxide inhalation and decapitation. Ischemia was confirmed in all rats by the presence of ventricular ectopy, discoloration of the ischemic area, and left ventricular (LV) dyskinesia. Ventricular ectopy and the return of color to the ischemic area confirmed reperfusion. The great vessels, atria, and right ventricle were removed at the end of the experimental protocol. The LV cavity was embedded in Tissue-Tek embedding medium (Ames Corp., Elkhart, IN), frozen with dry ice, and stored at −80°C.
Rat Myocardial Immunohistochemistry
Frozen sections (5 μm) were fixed for 5 minutes in cold (−20°C) acetone and then stained with a primary fluorescein isothiocyanate (FITC)-conjugated rabbit anti-rat C3 polyclonal antibody (pAb; Cappel) or a primary mouse anti-rat MBL mAb followed by a secondary FITC-conjugated goat anti-mouse pAb (Jackson Immunoresearch). Control incubations were done either by omitting the primary antibody or by using FITC-conjugated rabbit anti-horse IgG. The slides were mounted with Mowiol 28 and examined with an Olympus Standard microscope equipped with a filter specific for FITC fluorescence.
Statistical Analysis
All data presented represent the mean and SE for n determinations. Data analyses were performed using Sigma Stat (Jandel Scientific, San Rafael, CA) and a P value of <0.05 was considered significant. Endothelial C3 deposition on normoxic versus hypoxic HUVECs was analyzed by two-way analysis of variance. All pairwise multiple comparisons were made using the Student-Newman-Keuls test. C3 deposition on treated cells (ELISA; Figures 2 and 3 ▶ ▶ ) after hypoxia/reoxygenation was normalized to hypoxic HUVECs reoxygenated in untreated 30% HS. Means ± SE of the raw data used for normalization are presented in Results and/or figure legends.
Figure 2.
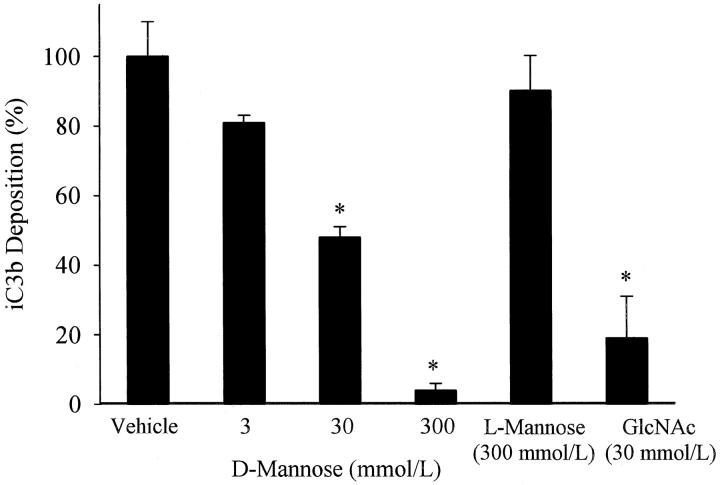
Mannose inhibition of endothelial iC3b deposition. iC3b deposition on hypoxic (24 hours) HUVECs reoxygenated (3 hours) in the presence of 30% HS treated with 0, 3, 30, or 300 mmol/L D-mannose or L-mannose (300 mmol/L) was measured by ELISA. D-mannose, but not L-mannose, reduced endothelial iC3b deposition in a dose-dependent manner. Similarly, GlcNAC (30 mmol/L) inhibited iC3b deposition. Data are normalized to hypoxic HUVECs reoxygenated in 30% HS (vehicle; OD405 = 0.14 ± 0.01). n = 3; error bars = SE; *P < 0.05 compared to vehicle.
Figure 3.
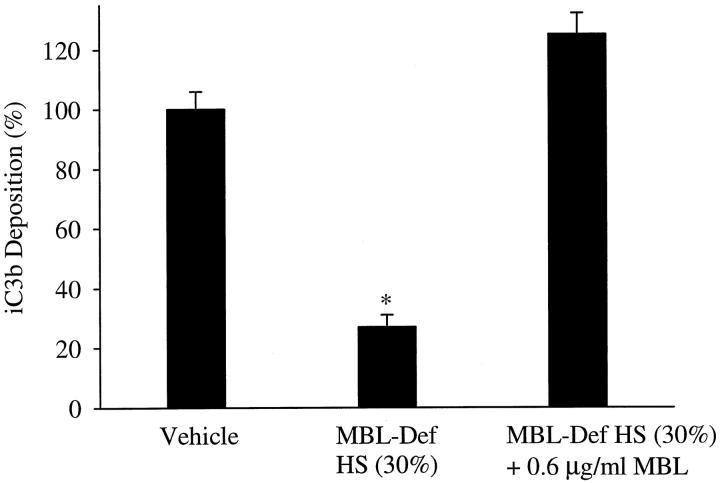
Endothelial iC3b deposition is attenuated in MBL-deficient serum. iC3b deposition on hypoxic HUVECs reoxygenated in 30% MBL-deficient HS was significantly less (P < 0.05) than hypoxic HUVECs reoxygenated in 30% HS (vehicle). In contrast, iC3b deposition on hypoxic HUVECs reoxygenated in 30% MBL-deficient HS reconstituted with MBL did not significantly differ from 30% HS (vehicle). Data are normalized to hypoxic HUVECs reoxygenated in 30% HS (vehicle; OD405 = 0.16 ± 0.01). n = 3; error bars = SE; *P < 0.05 compared to vehicle.
Results
Complement Activation and Deposition after HUVEC Oxidative Stress
Consistent with previous studies, 2,3 a significant increase in iC3b deposition was observed after oxidative stress compared to normoxic HUVECs (OD405 = 0.14 ± 0.01 vs. 0.05 ± 0.01, respectively; P < 0.05). The MBL inhibitory sugar, D-mannose, attenuated iC3b deposition in a dose-dependent manner (Figure 2) ▶ . Similarly, GlcNAc (30 mmol/L) inhibited iC3b deposition by 81 ± 2%. L-mannose (300 mmol/L) did not significantly inhibit iC3b deposition. These data demonstrate that GlcNAc or D-mannose significantly attenuates complement activation and iC3b deposition in a dose-dependent, stereospecific manner, suggesting a possible role for MBL.
Deposition of iC3b after MBL Depletion and Reconstitution
MBL-deficient serum was produced to further evaluate the role of MBL in this model. iC3b deposition (Figure 3) ▶ on hypoxic HUVECs reoxygenated in MBL-deficient HS was approximately 25% that in vehicle control. Reconstitution of MBL-deficient HS with purified MBL restored iC3b deposition. A complement hemolytic assay (CH50) demonstrated that the classical complement pathway activity of the MBL-deficient HS did not significantly differ from that of complete HS (data not shown). Thus, these data suggest that the C2-dependent LCP is primarily responsible for complement activation and deposition after endothelial oxidative stress.
Anti-MBL mAb Production and Characterization
To demonstrate that MBL was responsible for complement activation in this study, we developed novel, functionally inhibitory mAbs against human MBL. Eleven parent hybridoma clones that recognized human MBL were identified in a solid phase antibody-capture ELISA. After limiting dilution and isotyping, eight mAbs that recognized human MBL in the antibody-capture ELISA were identified. Clones 3F8, 2A9, and hMBL1.2 were isotyped as mouse IgG1κ, whereas clone 1C10 was a mouse IgG2b. The other hybridomas produced IgM antibodies and were not included in this study.
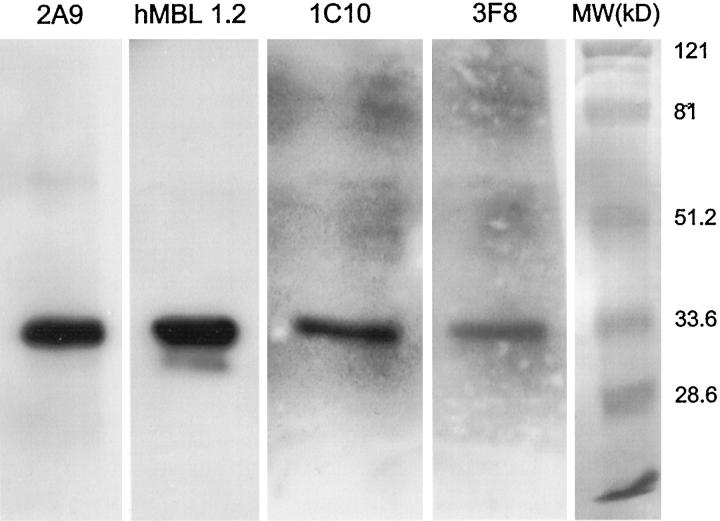
To demonstrate that the anti-human MBL mAbs recognized human MBL, Western blot analysis of reduced and nonreduced MBL was performed. Monoclonal antibodies 2A9, hMBL1.2, 1C10, and 3F8 recognized nonreduced human MBL (MW ∼600 kd; data not shown). As shown in Figure 4 ▶ , mAbs 2A9 (Lane 1), hMBL1.2 (Lane 2), 1C10 (Lane 3), and 3F8 (Lane 4) recognized reduced human MBL (MW ∼32 kd). In particular, mAbs 2A9 and hMBL1.2 easily recognized reduced and denatured human MBL. Although mAbs 1C10 and 3F8 also recognized reduced and denatured human MBL, longer exposure times were necessary, resulting in higher backgrounds. Addition of purified human MBL to each of the mAbs before Western blot analysis inhibited detection of immobilized MBL on the membranes. Further, immunoprecipitation of human MBL with each of these mAbs yielded a 32-kd protein under reducing conditions that was recognized by anti-human MBL pAbs (data not shown). Thus, these antibodies are specific for human MBL and do not recognize MASP-1 or MASP-2. In particular, clones 2A9 and hMBL1.2 are good mAbs for Western blot analysis of reduced human MBL. Clones hMBL1.2, 2A9, and 3F8 have been deposited at the International Depository Authority with American Type Culture Collection designations of HB-12619, HB-12620, and HB-12621, respectively.
Figure 4.
Western blot analysis of human MBL. Monoclonal anti-human MBL antibodies 3F8, hMBL1.2, 2A9, or 1C10 were used for Western blot analysis of reduced human MBL. Lanes 1–4 represent staining of reduced human MBL with 10 μg/ml of mAb 2A9, hMBL1.2, 1C10, or 3F8, respectively. A single band with an approximate molecular weight of 32 kd (ie, consistent with reduced MBL) was observed with each mAb. This figure is representative of 3 separate experiments.
Inhibition of MBL-Dependent Complement Activation with Anti-Human MBL mAbs
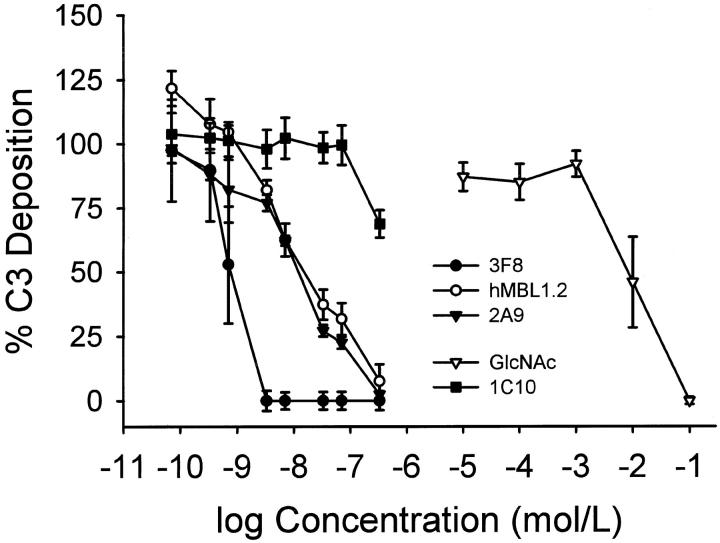
Selective activation of the LCP using mannan-coated plates and human serum was performed as described 25 to characterize whether the anti-human MBL mAbs functionally inhibited MBL-dependent complement activation. Monoclonal antibodies 3F8, 2A9, or hMBL1.2 inhibited C3 deposition on the mannose-coated plates in a dose-dependent manner (Figure 5) ▶ . Clone 1C10 failed to inhibit MBL-dependent C3 deposition in this assay. The IC50 for 3F8, 2A9, hMBL1.2 and GlcNAc were 0.9 ± 0.3 nmol/L, 13.3 ± 3.3 nmol/L, 18.9 ± 7.8 nmol/L, and 15 ± 6 mmol/L, respectively. Thus, the functionally inhibitory anti-human MBL mAbs (eg, 3F8, 2A9, and hMBL1.2) were about 6 orders of magnitude more effective than GlcNAc in this assay.
Figure 5.
C3 deposition on mannan-coated plates. Monoclonal anti-human MBL antibodies 3F8, hMBL1.2 and 2A9, as well as N-acetylglucosamine (GlcNAc) inhibited C3 deposition on mannan coated plates in a dose-dependent manner. The control anti-human MBL mAb, 1C10, did not inhibit C3 deposition. Each symbol represents the mean of 4 to 5 individual experiments. Concentrations of antibodies (shown in mol/L) represent 0.01, 0.05, 0.1, 0.5, 1, 5, 10, and 50 μg/ml, respectively. Brackets represent SE.
Seven IgG anti-rat MBL mAbs were also identified by solid phase antibody-capture ELISA in the present study. Although six of the seven anti-rat MBL mAbs recognized reduced and nonreduced rat MBL by Western blot analysis, none of the antibodies functionally inhibited rat C3 deposition on mannan-coated plates (data not shown). Clone 14C3.74 was the best mAb for Western blot analysis of reduced rat MBL.
Complement Hemolysis Assays
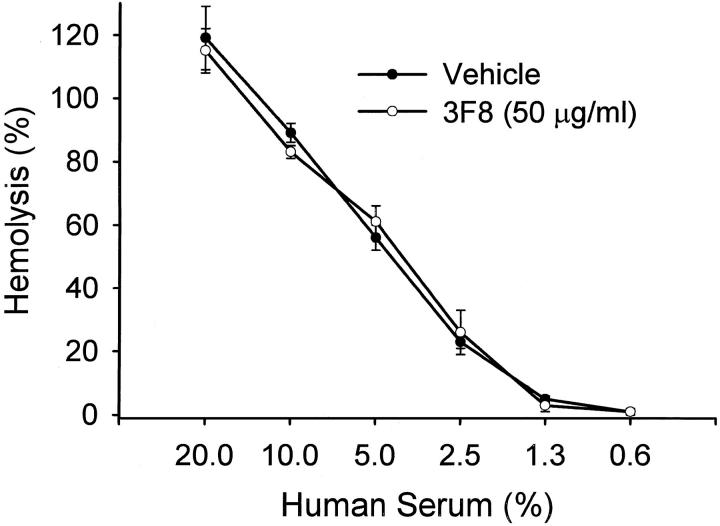
Hemolysis assays were performed to demonstrate that the anti-human MBL mAbs did not inhibit or activate (ie, immune complex formation) the classical complement pathway. As demonstrated in Figure 6 ▶ , mAb 3F8 (eg, the best MBL inhibitory antibody in the mannan assay in concentrations up to 50 μg/ml) did not attenuate classical complement pathway activation (ie, hemolytic activity of human serum). Similar findings were observed for mAbs hMBL1.2, 1C10, and 2A9 (data not shown). Thus, the mechanism of LCP inhibition is neither through activation (ie, immune complex formation) or inhibition of the classical complement pathway.
Figure 6.
Anti-human MBL mAbs do not attenuate HS hemolytic activity. HS treated with vehicle (PBS) or mAb 3F8 (50 μg/ml) demonstrated similar hemolytic activity to sensitized chicken red blood cells. Similar data were obtained with clones 2A9, hMBL1.2 and 1C10 (data not presented). Thus, these mAbs do not inhibit classical complement pathway activation. Each symbol represents the mean of 3 individual experiments. Brackets represent SE.
Binding of MBL to Hypoxic HUVECs
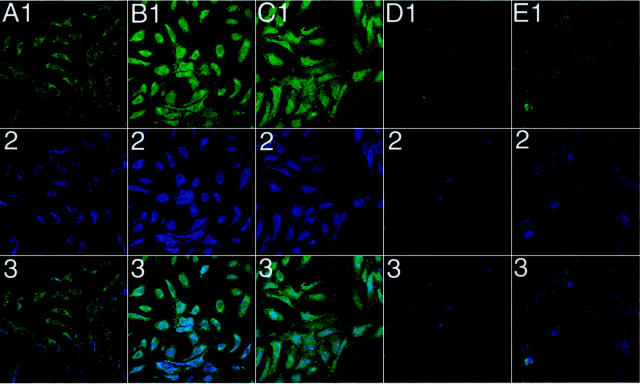
Dual labeling for MBL (blue) and C3 (green) deposition on HUVECs was performed to demonstrate MBL and C3 colocalization after oxidative stress (Figure 7) ▶ . Normoxic and hypoxic HUVECs were reoxygenated in 30% HS treated with and without the anti-human MBL mAbs 3F8 (5 μg/ml), 2A9 (5 μg/ml), or 1C10 (50 μg/ml). Small amounts of C3 (Figure 7A ▶ 1) and MBL (Figure 7A ▶ 2) staining were observed under normoxic conditions, confirming our previous finding of low level C3 deposition under normoxic conditions. 2,3 C3 (Figure 7B ▶ 1) and MBL (Figure 7B ▶ 2) staining on hypoxic/reoxygenated HUVECs was increased compared to normoxic cells. Clone 1C10 failed to inhibit C3 (Figure 7C ▶ 1) or MBL (Figure 7C ▶ 2) deposition after oxidative stress, confirming its inability to inhibit MBL. C3 (Figure 7 ▶ , D1 and E1) and MBL (Figure 7 ▶ , D2 and E2) staining was attenuated on hypoxic/reoxygenated HUVECs treated with mAbs 3F8 or 2A9, respectively (similar results were observed with mAb hMBL1.2). Row 3 demonstrates that MBL and C3 colocalize on human endothelial cells under the conditions outlined above. These data demonstrate that inhibition of MBL deposition with a functionally inhibitory mAb decreases C3 deposition after endothelial oxidative stress.
Figure 7.
Colocalization of C3 and MBL on HUVECs after oxidative stress. Immunofluorescent confocal microscopic demonstration of MBL (blue) and C3 (green) deposition on normoxic and hypoxic (24 hours) HUVECs reoxygenated (3 hours) in 30% HS with and without the functionally inhibitory anti-human MBL mAbs 3F8 or 2A9 (5 μg/ml), or the non-functionally inhibitory anti-human MBL mAb, 1C10 (50 μg/ml). Columns A-E represent normoxic untreated, hypoxic/reoxygenated untreated, 1C10-treated, 3F8-treated, and 2A9-treated cells, respectively. Rows 1–3 represent staining for C3, MBL, and C3 + MBL, respectively. C3 (B1) and MBL (B2) staining on hypoxic/reoxygenated HUVECs was significantly increased compared to normoxic HUVECs (A1 and A2, respectively). Treatment of HS with clone 1C10 (50 μg/ml) did not attenuate C3 or MBL deposition (C1 and C2, respectively) after oxidative stress. Treatment of HS with clone 3F8 or 2A9 (5 μg/ml) significantly decreased C3 and MBL deposition (D1 or E1, and D2 or E2, respectively). Row 3 represents colocalization of MBL and C3 under normoxic untreated, hypoxic/reoxygenated untreated, 1C10-treated, 3F8-treated, and 2A9-treated conditions, respectively. These panels are representative of 3 different experiments. Original magnification, ×40.
Immunohistochemistry of Ischemic/Reperfused Rat Hearts
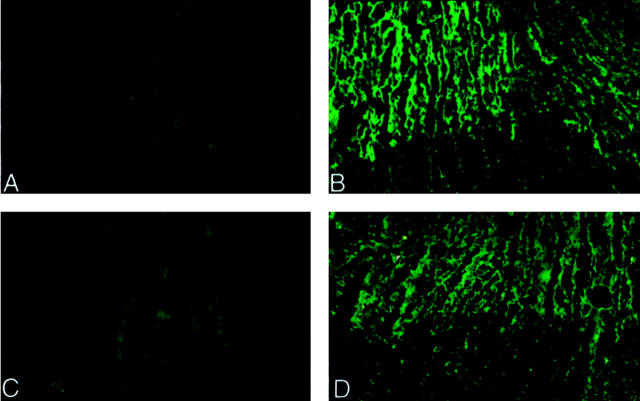
A previous study has demonstrated that complement activation and deposition during the early phase of myocardial ischemia/reperfusion is limited to the coronary vascular endothelium. 1 We investigated MBL (Figure 8 ▶ , C and D) and C3 (Figure 8 ▶ , A and B) deposition in the ischemic/reperfused rat myocardium by indirect immunofluorescence microscopy. Strong C3 and MBL (clone 14C3.74) staining were observed throughout the ischemic area at risk (Figure 8 ▶ , B and D, upper part) in rat hearts (n = 4) subjected to 30 minutes ischemia followed by 30 minutes reperfusion (Figure 8 ▶ , B and D, respectively). In contrast, minimal C3 or MBL deposition was observed in rat hearts (n = 4) subjected to 60 minutes of ischemia without reperfusion (Figure 8 ▶ , A and C, respectively). No staining for C3 or MBL was observed in the sham-operated rat hearts (data not shown). These data are the first to demonstrate MBL deposition during the early phase of myocardial ischemia/reperfusion. Further, reperfusion appears to augment MBL deposition and complement activation. Thus, these in vivo data are consistent with our in vitro findings using isolated human endothelial cells.
Figure 8.
Immunofluorescence analysis of rat C3 and MBL. The left ventricular free wall of rats undergoing 60 minutes of ischemia and no reperfusion (A and C) or 30 minutes of ischemia and 30 minutes of reperfusion (B and D) were processed for immunohistochemical staining for rat C3 (A and B) or MBL (clone 14C3.74; C and D). Positive staining for C3 (B) and MBL (D) is observed in the ischemic/reperfused hearts compared to the ischemia-only hearts (A and C, respectively). Furthermore, the straining is localized to the ischemic area at risk. No staining for C3 or MBL was observed in the sham-operated rat hearts (data not shown). Original magnification, ×100.
Discussion
Growing evidence suggests that complement plays an important role in the pathogenesis of cardiovascular injury after oxidative stress. 1,5,7,8,11 However, the role of the LCP in human cardiovascular disease is unknown. We demonstrate that oxidative stress of human endothelial cells in vitro leads to MBL deposition and LCP activation. Further, rat myocardial MBL and C3 deposition is increased by reperfusion in vivo. Human MBL and complement deposition after endothelial oxidative stress in vitro is attenuated by novel, functionally inhibitory anti-MBL mAbs. Thus, these data suggest a novel pathophysiological role for the LCP after oxidative stress.
Anoxic human endothelial cells were recently shown to activate complement in the absence of immunoglobulin-binding, suggesting antibody-independent complement activation. 22 Additionally, we have shown that complement activation after endothelial oxidative stress is C2-dependent. 2 Because the lectin or classical complement pathway is C2-dependent (Figure 1) ▶ , we investigated whether the antibody-independent LCP mediates complement activation after oxidative stress. In the present study, inhibition of MBL deposition with D-mannose, GlcNAc, or MBL-deficient serum significantly attenuated iC3b deposition after endothelial oxidative stress. Further, immunofluorescent confocal microscopy demonstrated increased deposition and colocalization of MBL and C3 on hypoxic/reoxygenated human endothelial cells. Inhibition of MBL deposition with the anti-human MBL mAbs significantly attenuated C3 deposition. Together, these in vitro data suggest that MBL initiates complement activation following endothelial oxidative stress. Further, these data suggest that oxidative stress leads to the formation of a complement-activating MBL ligand on human endothelial cells. We are currently identifying and characterizing this complement-activating MBL ligand.
Experimental in vivo data strongly support the role of the reperfused endothelium in activating complement after ischemia/reperfusion. 1,11 It has been hypothesized that novel endothelial epitopes are up-regulated and that natural antibodies then activate the classical complement pathway. 11 Indeed, complement activation after reperfusion of ischemic myocardium is initially limited to the coronary endothelium. 1 In the present study, strong C3 and MBL staining were observed throughout the ischemic area at risk during the early phase of rat myocardial reperfusion. Furthermore, myocardial reperfusion augmented MBL deposition and complement activation in vivo compared to sham-operated or ischemia-only animals. This study is the first to demonstrate MBL deposition during the early phase of myocardial ischemia/reperfusion. This is an important observation, insofar as several previous studies using C4-deficient or C1 esterase inhibitor-treated animals have suggested that the classical complement pathway mediates complement activation after ischemia/reperfusion. 6,11,29 However, the possible involvement of the LCP in these studies cannot be ruled out, as C1 esterase inhibitor also attenuates MASP-1 and MASP-2 activity, and LCP activation is inhibited in C4-deficient animals. 30 Thus, the specific roles of the lectin and/or classical complement pathways in ischemia/reperfusion injury have not been fully elucidated. Generation of species-specific MBL inhibitors/antibodies or MBL/MASP knockout mice will aid in the elucidation of the role of the lectin versus classical complement pathways in animal models. To date, we have not been able to generate a functionally inhibitory antibody to rat MBL. This difficulty may be due to the presence of two rat MBL isoforms. 31
Inhibition of MBL and the LCP can be accomplished by using specific carbohydrates including mannose, N-acetylglucosamine (GlcNAc), or oligomers of these sugars. Although these sugars are somewhat specific in attenuating MBL deposition/binding, 32,33 their efficacy and half-life are relatively poor. 34 Further, intravenous treatment with carbohydrate oligomers (ie, mannan) may directly activate complement in vivo, creating a complement-deprived animal. We thus designed specific, functionally inhibitory mAbs against human MBL to increase the efficacy and specificity of MBL inhibition. Using an MBL-dependent C3 deposition ELISA, 25 these mAbs (eg, 3F8, 2A9, and hMBL1.2) were about 6 orders of magnitude more effective than GlcNAc in this assay. Furthermore, these antibodies were very potent inhibitors of MBL deposition after endothelial oxidative stress. Thus, anti-human MBL mAb treatment may represent a novel, specific therapeutic strategy for LCP-mediated tissue injury.
In summary, MBL and iC3b deposition are increased following human endothelial oxidative stress in vitro, rat MBL and C3 deposition is increased following myocardial reperfusion in vivo, and inhibition of human MBL deposition with GlcNAc, D-mannose, MBL-deficient serum, or anti-MBL mAbs attenuates complement activation after endothelial oxidative stress in vitro. These data suggest that MBL deposition on the vascular endothelium after oxidative stress may lead to LCP activation. Future studies with specific inhibitors of MBL in animal models will help define the role of MBL and the LCP in cardiovascular disease.
Footnotes
Address reprint requests to Gregory L. Stahl, Ph.D., Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesiology, Pain and Perioperative Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115. E-mail: gstahl@zeus.bwh.harvard.edu.
Supported by HL-03854 (to C. D. C.), the Foundation for Anesthesia Education and Research (to C. D. C.), the Academy of Finland, the Sigrid Juselius Foundation, the State Subsidy to the Helsinki University Central Hospital, the Finnish Foundation for Cardiovascular Research (to A.V and S.P.), HL-52886 (to G. L. S.), and an American Heart Association Established Investigator Award (to G. L. S.).
References
- 1.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT: Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 1990, 249:146-151 [DOI] [PubMed] [Google Scholar]
- 2.Collard CD, Vakeva A, Bukusoglu C, Zünd G, Sperati CJ, Colgan SP, Stahl GL: Reoxygenation of hypoxic human umbilical vein endothelial cells activates the classic complement pathway. Circulation 1997, 96:326-333 [DOI] [PubMed] [Google Scholar]
- 3.Collard CD, Agah A, Stahl GL: Complement activation following reoxygenation of hypoxic human endothelial cells: role of intracellular reactive oxygen species, NF-κB and new protein synthesis. Immunopharmacology 1998, 39:39-50 [DOI] [PubMed] [Google Scholar]
- 4.Collard CD, Agah A, Reenstra WR, Buras JA, Stahl GL: Endothelial nuclear factor-κB translocation and vascular cell adhesion molecule-1 induction by complement: Inhibition with anti-C5 therapy or cGMP analogues. Arterioscler Thromb Vasc Biol 1999, 19:2623-2629 [DOI] [PubMed] [Google Scholar]
- 5.Buerke M, Murohara T, Lefer AM: Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation 1995, 91:393-402 [DOI] [PubMed] [Google Scholar]
- 6.Buerke M, Prüfer D, Dahm M, Oelert H, Meyer J, Darius H: Blocking of classical complement pathway inhibits endothelial adhesion molecule expression and preserves ischemic myocardium from reperfusion injury. J Pharmacol Exp Ther 1998, 286:429-438 [PubMed] [Google Scholar]
- 7.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL: Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation 1998, 97:2259-2267 [DOI] [PubMed] [Google Scholar]
- 8.Amsterdam EA, Stahl GL, Pan H-L, Rendig SV, Fletcher MP, Longhurst JC: Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol Heart Circ Physiol 1995, 268:H448-H457 [DOI] [PubMed] [Google Scholar]
- 9.Hill JH, Ward PA: The phlogistic role of C3 leukotactic fragments in myocardial infarcts in rats. J Exp Med 1971, 133:885-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinckard RN, O’Roarke RA, Crawford MH: Complement localization and mediation of ischemic injury in baboon myocardium. J Clin Invest 1980, 66:1050-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma MH, Hechtman HB, Carroll MC: Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 1996, 183:2343-2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito W, Schäfer HJ, Bhakdi S, Klask R, Hansen S, Schaarschmidt S, Schofer J, Hugo F, Hamdoch T, Mathey D: Influence of the terminal complement-complex on reperfusion injury, no-reflow and arrhythmias: a comparison between C6-competent and C6-deficient rabbits. Cardiovasc Res 1996, 32:294-305 [DOI] [PubMed] [Google Scholar]
- 13.Stahl GL, Reenstra WR, Frendl G: Complement mediated loss of endothelium-dependent relaxation of porcine coronary arteries: role of the terminal membrane attack complex. Circ Res 1995, 76:575-583 [DOI] [PubMed] [Google Scholar]
- 14.Lennon PF, Collard CD, Morrissey MA, Stahl GL: Complement-induced endothelial dysfunction in rabbits: mechanisms, recovery, and gender differences. Am J Physiol Heart Circ Physiol 1996, 270:H1924-H1932 [DOI] [PubMed] [Google Scholar]
- 15.Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS: The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol 1996, 149:953-961 [PMC free article] [PubMed] [Google Scholar]
- 16.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen S, Holmskov U, Reid KB, Jensenius JC: A second serine protease associated with mannan-binding lectin that activates complement. Nature 1997, 386:506-510 [DOI] [PubMed] [Google Scholar]
- 17.Turner MW: The lectin pathway of complement activation. Res Immunol 1996, 147:110-115 [DOI] [PubMed] [Google Scholar]
- 18.Turner MW: Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today 1996, 17:532-540 [DOI] [PubMed] [Google Scholar]
- 19.Matsushita M, Endo Y, Nonaka M, Fujita T: Complement-related serine proteases in tunicates and vertebrates. Curr Opin Immunol 1998, 10:29-35 [DOI] [PubMed] [Google Scholar]
- 20.Swanson AF, Kuo CC: Identification of lectin-binding proteins in Chlamydia species. Infect Immun 1990, 58:502-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner MW: Mannose-binding lectin (MBL) in health and disease. Immunobiology 1998, 199:327-339 [DOI] [PubMed] [Google Scholar]
- 22.Väkevä A, Meri S: Complement activation and regulator expression after anoxic injury of human endothelial cells. APMIS 1998, 106:1149-1156 [DOI] [PubMed] [Google Scholar]
- 23.Tan SM, Chung MCM, Kon OL, Thiel S, Lee SH, Lu J: Improvements on the purification of mannan-binding lectin and demonstration of its calcium-independent association with a C1s-like serine protease. Biochem J 1996, 319:329-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tofukuji M, Stahl GL, Agah A, Metais C, Simons M, Sellke FW: Anti-C5a monoclonal antibody reduces cardiopulmonary bypass and cardioplegia-induced coronary endothelial dysfunction. J Thorac Cardiovasc Surg 1998, 116:1060-1068 [DOI] [PubMed] [Google Scholar]
- 25.Super M, Levinsky RJ, Turner MW: The level of mannan-binding protein regulates the binding of complement-derived opsonins to mannan and zymosan at low serum concentrations. Clin Exp Immunol 1990, 79:144-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collard CD, Bukusoglu C, Agah A, Colgan SP, Reenstra WR, Morgan BP, Stahl GL: Hypoxia-induced expression of complement receptor type 1 (CR1, CD35) in human vascular endothelial cells. Am J Physiol Cell Physiol 1999, 276:C450-C458 [DOI] [PubMed] [Google Scholar]
- 27.Stahl GL, Terashita Z-I, Lefer AM: Role of platelet activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther 1988, 244:898-904 [PubMed] [Google Scholar]
- 28.Heimer GV, Taylor CED: Improved mountant for immunofluorescence preparations. J Clin Pathol 1974, 27:254-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochilas L, Campbell B, Scalia R, Lefer AM: Beneficial effects of C1 esterase inhibitor in murine traumatic shock. Shock 1997, 8:165-169 [DOI] [PubMed] [Google Scholar]
- 30.Lambris JD, Reid KBM, Volanakis JE: The evolution, structure, biology and pathophysiology of complement. Immunol Today 1999, 20:207-211 [DOI] [PubMed] [Google Scholar]
- 31.Oka S, Ikeda K, Kawasaki T, Yamashina I: Isolation and characterization of two distinct mannan-binding proteins from rat serum. Arch Biochem Biophys 1988, 260:257-266 [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I: Serum lectin with known structure activates complement through classical pathway. J Biol Chem 1987, 262:7451-7454 [PubMed] [Google Scholar]
- 33.Kawasaki N, Kawasaki T, Yamashina I: A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J Biochem (Tokyo) 1989, 106:483-489 [DOI] [PubMed] [Google Scholar]
- 34.Setnikar I, Palumbo R, Canali S, Zanolo G: Pharmacokinetics of glucosamine in man. Arzneimittelforschung 1993, 43:1109-1113 [PubMed] [Google Scholar]