Abstract
Vascular endothelial growth factor receptor-3 (VEGFR-3) is essential for embryonic cardiovascular development, but thereafter becomes confined to the lymphatic endothelium in adult tissues. We have here studied VEGFR-3 expression in experimental wounds of pigs and chronic inflammatory wounds of humans. In healing incisional and punch biopsy wounds made in the dorsal skin of pigs, angiogenic blood vessels, identified by use of the blood vascular endothelial markers vWF and PAL-E and the basal lamina protein laminin, developed into the granulation tissue stroma from day 4 onward, being most abundant on days 5 and 6 and regressing thereafter. VEGFR-3-positive vessels were observed in the granulation tissue from day 5 onward. These vessels were distinct from the PAL-E/laminin/vWF-positive vessels and fewer in number, and they appeared to sprout from pre-existing VEGFR-3-positive lymphatic vessels at the wound edge. Unlike the blood vessels, very few VEGFR-3-positive lymphatic vessels persisted on day 9 and none on day 14. In chronic wounds such as ulcers and decubitus wounds of the lower extremity of humans, VEGFR-3 was also weakly expressed in the vascular endothelium. Our results suggest that transient lymphangiogenesis occurs in parallel with angiogenesis in healing wounds and that VEGFR-3 becomes up-regulated in blood vessel endothelium in chronic inflammatory wounds.
Angiogenesis, the formation of new blood vessels from pre-existing ones, is essential in many physiological processes such as embryonic development, tissue and organ regeneration, the female reproductive cycle, and wound healing and inflammation. 1 It has been also implicated in a wide variety of pathological processes such as tumor growth and metastasis, psoriasis, and rheumatoid arthritis. In normal physiology, there appears to be a carefully maintained balance between blood vessel growth stimulatory and inhibitory signals, and this balance is disturbed in pathological angiogenesis. 2 Vascular endothelial growth factor (VEGF) is considered to be a major angiogenic molecule, which also strongly increases vascular permeability. 3 VEGF binds to its receptors, VEGFR-1 (Flt1) and VEGFR-2 (KDR/Flk1), which are important in embryonic vasculogenesis but considerably down-regulated in many adult tissues. 4 Recently, additional members of the VEGF gene family have been characterized: placenta growth factor, VEGF-B, VEGF-C, VEGF-D, and VEGF-E. 5 VEGF-C was discovered as the ligand for the receptor fms-like tyrosine kinase, Flt4, or VEGFR-3. 6,7
VEGFR-3 is essential in embryonic angiogenesis, as homozygous knockout mice lacking a functional VEGFR-3 gene die in utero from cardiovascular failure. 8 During embryogenesis, VEGFR-3 is expressed in blood vascular and lymphatic endothelium, whereas in adults, it is largely restricted to the lymphatic endothelium 9,10 and a few fenestrated endothelia of endocrine organs (Partanen TA, Saaristo A, Jussila L, Ora A, Miettinen M, Alitalo K, submitted). VEGFR-3 is also up-regulated in the endothelium of blood vessels in breast adenocarcinomas 11 and various other tumors. 12 This indicates that VEGFR-3 is present in vessels undergoing angiogenesis in tumors and is thus a potential target for anti-angiogenic therapy.
Although tumor angiogenesis is erratic with abnormally high vessel permeability, inflammation, vessel growth, and regression, 13 angiogenic processes occur in an organized fashion in wound healing. 14 In wounds, acute inflammation with increased permeability is followed by the deposition of a provisional fibrin and connective tissue matrix, proliferation and migration of endothelial cells, vessel formation, and remodeling. Most blood vessels regress as the wound is remodeled into scar tissue. Many factors have angiogenic activity and possibly participate in wound repair. These include VEGF, fibroblast growth factors, transforming growth factors, tumor necrosis factor-α, and interleukin-8. 15 VEGF has been found in tissue fluid in wounds, 16 and it is up-regulated in the epidermis overlying healing wounds, 17 whereas VEGFR-1 and VEGFR-2 are up-regulated in angiogenic vessels. 18,19
We studied the presence of VEGFR-3 in the vasculature of healing wounds of the domestic pig, which has previously been shown to be a valuable model of angiogenesis. 20 We also examined VEGFR-3 expression in the neovasculature of ulcers and decubitus lesions of the lower extremity of humans.
Materials and Methods
Experimental Wounds
Five young adult domestic pigs were used for the wound experiments. After their skin was shaved and cleaned, the anesthetized pigs were wounded on one side of the back with 20 incisions or punch biopsies. The incisions were 3 cm long and cut through the whole epidermis and dermis. Punch biopsy wounds (diameter 6 mm) were made with a circular cut or a punch biopsy tool (Fray Products Corp., Buffalo, NY). Incisional wounds were sutured and punch biopsies were allowed to heal by secondary intention. Hemostasis was assured by applying gentle pressure when necessary. The pigs were anesthetized with i.v. ketamine hydrochloride 20 mg/kg, atropine sulfate 0.05 mg/kg, and diazepam 0.1 mg/kg. Adequate pre- and postoperative pain medication (diclofenac 75 mg i.m.) was given during the wounding and the harvesting on different days. Triplicate samples were processed for 10% neutral formalin fixation or 4% paraformaldehyde fixation or were snap-frozen in liquid nitrogen. Wounds displaying swelling or infection were excluded from the experiments. After the experiments, the animals were allowed free access to food and drink. After all harvesting procedures the pigs were given a lethal dose of pentobarbital i.v., and then disposed of according to rules set by the Helsinki University Central Hospital (HUCH) ethics committee. The experiments were approved by the HUCH ethics committee and by the Provincial State Office of Southern Finland.
Human Samples
Samples of granulation tissue from a human decubitus lesion, a diabetic ulcer, and a leg ulcer were obtained with the patients’ consent from four patients undergoing plastic surgery in the HUCH Department of Plastic Surgery. The skin samples were snap-frozen in liquid nitrogen and processed like the other frozen samples.
Immunohistochemistry
The cryosections and paraffin-embedded sections were processed as previously described. 21 Primary antibodies used included monoclonal anti-VEGFR-3 (clone 9D9F9, affinity-purified, 1.1 μg/ml), monoclonal PAL-E (a molecularly undefined endothelial antigen specific for blood vascular endothelium, excepting arterioles and arteries, 22 0.15 μg/ml, Monosan, Uden, The Netherlands), monoclonal anti-laminin (1:3000, clone LAM-89, Sigma, St. Louis, MO), monoclonal anti-smooth muscle α-actin (1:7000, clone 1A4, Sigma), or polyclonal anti-factor VIII-related antigen/von Willebrand factor (vWF; 1:300, DAKO Immunoglobulins, Glostrup, Denmark). Negative controls were done by omitting the primary antibody, by using irrelevant primary antibodies of the same isotype (negative control IgG1, DAKO), or by using a 20-fold molar excess of the extracellular portion of VEGFR-3. In some samples the Tyramide Signal Amplification kit (NEN Life Sciences, Boston, MA) was used. Results were viewed with an Olympus light microscope and photographed. For quantification, vessels were counted from 4 to 7 square grids (area = 0.16 mm2, 40× magnification) and the mean value score and SD were calculated.
Results
The specificity of the monoclonal anti-VEGFR-3 antibodies was verified by immunoprecipitation. Anti-VEGFR-3 precipitated protein bands of 195 kd and 125 kd from pig lymph nodes, corresponding to the VEGFR-3 full-length protein and to the proteolytically processed form, 23 respectively (data not shown).
In a panel of all major pig tissues VEGFR-3 localized to lymphatic vessels, as has been previously shown in human skin samples. 24 VEGFR-3 was also found in few fenestrated endothelia in endocrine glands, in the ductus thoracicus, and in liver sinusoids. In the majority of pig tissues studied, though, VEGFR-3 localized predominantly to a subset of vessels that were negative for PAL-E, laminin, smooth muscle actin, and vWF, and thus presumably lymphatic vessels (Figure 1) ▶ . For instance, in the ileum, VEGFR-3 was detected in the lacteals (arrowheads in Figure 1 ▶ , A–D) and in lymphatic ducts but not in the blood vessels (arrows in Figure 1 ▶ , A–D). In pharyngeal mucosa and in lymphatic tissue near the pharyngeal tonsils, VEGFR-3 was also stained in the lymphatic vessels (Figure 1 ▶ , E and F) and in the myocardium, in perivascular lymphatic vessels (Figure 1 ▶ , G and H). In the pig dermis, the VEGFR-3-stained lymphatic vessels (Figure 1I) ▶ were PAL-E-negative (Figure 1J) ▶ and smooth muscle actin-negative (Figure 1L) ▶ . In the skin, the lymphatic vessels stained only very weakly for laminin (Figure 1K) ▶ , as previously described for human tissues. 25
Figure 1.
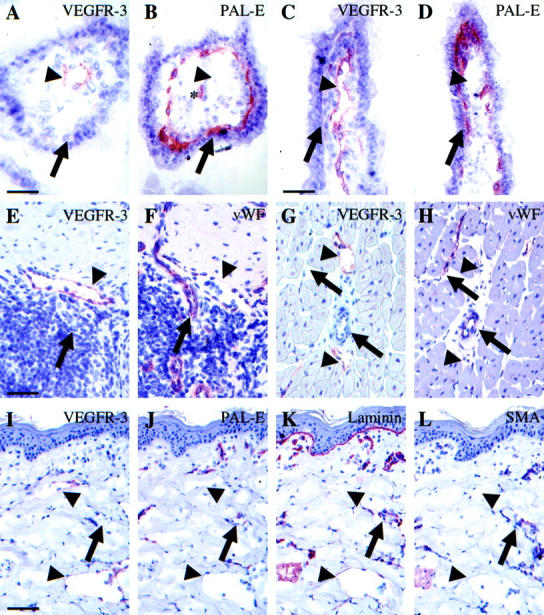
Immunoperoxidase staining of serial sections of normal pig tissues. In pig ileum (A−D), VEGFR-3 stains vessels (arrowheads) that do not stain for the vascular endothelial marker PAL-E (arrows). The star (B) denotes the central artery of the villus. In pig lymphatic tissue of the tonsilla (E and F) and in the myocardium (G and H) the VEGFR-3-stained vessels (arrowheads) do not stain for vWF (arrows). In normal skin (I−L), VEGFR-3-positive vessels are negative for PAL-E and for smooth muscle actin and stain only very slightly for laminin (arrowheads, I−L), whereas PAL-E-, laminin-, and smooth muscle actin-positive blood vessels do not stain for VEGFR-3 (arrows, I−K). Scale bars, 100 μm (A, B, E−H), 200 μm (C and D), 300 μm (I−L).
In the skin, the incisional wounds and the punch biopsy wounds healed by ingrowth of granulation tissue and re-epithelialization. During the inflammatory phase on days 1 to 3, the vascular ingrowth had not yet penetrated the granulation tissue (data not shown), but from day 4 onwards, angiogenic blood vessels negative for VEGFR-3 (arrow in Figure 2A ▶ ), but positive for PAL-E (Figure 2B) ▶ and laminin (Figure 2C) ▶ invaded into the granulation tissue. On day 5, many blood vessels and fewer VEGFR-3-positive but PAL-E-negative vessels (arrowheads, Figure 2 ▶ , E and I) negative also for laminin and smooth muscle actin (data not shown) were observed especially in the peripheral parts of the granulation tissue. The blood vessels remained VEGFR-3-negative during their further maturation on days 7 to 14 (arrows in Figure 2 ▶ , F–H). In distinction, the VEGFR-3-positive vessels were often single, dilated vessels traversing the wound close to the blood vessels (arrowheads in Figure 2 ▶ , E and F). Simultaneously, sprouting was also observed from the pre-existing VEGFR-3-positive vessels alongside blood vessels in the granulation tissue (arrowheads in Figure 2 ▶ , M and N) and from pre-existing lymphatic vessels at the wound edge (arrowheads in Figure 2 ▶ , O and P). Quantitation of the number of VEGFR-3-expressing vessels in comparison with the number of PAL-E-positive blood vessels after day 5 of healing in incisional wounds (PPI) and punch biopsy wounds (PSI) is shown in Figure 3 ▶ . This analysis shows that the VEGFR-3-positive vessels were fewer in number than the blood vessels and they regressed earlier than did the blood vessels. On day 9, very few VEGFR-3-positive vessels remained and none could be detected in the granulation tissue on day 14 (Figure 2H) ▶ , although the remaining blood vessels were stabilized at this point (Figure 2L) ▶ . Very weak staining for vWF was observed in lymphatic vessels (Figure 2N) ▶ .
Figure 2.
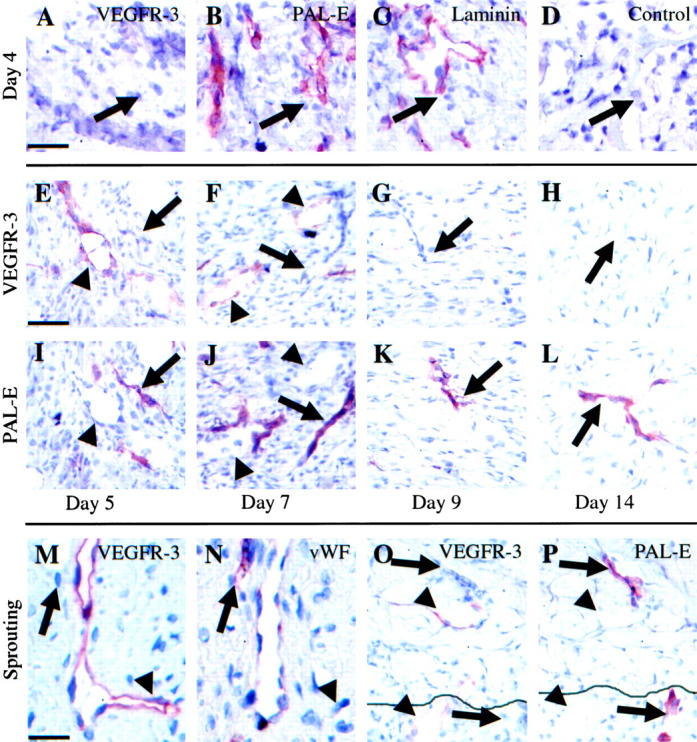
Analysis of VEGFR-3-positive vessels and blood vessels in wound healing. The angiogenic vessels at the leading edge on day 4 stained with VEGFR-3 (A), PAL-E (B), laminin (C), or control antibodies (D). Granulation tissue on days 5, 7, 9, and 14 stained for VEGFR-3 (E−H) and PAL-E (I−L). Note that the VEGFR-3-positive vessels (arrowheads) do not stain for PAL-E (arrows) and vice versa. Sprouting of the VEGFR-3-positive vessel in granulation tissue of a day 7 punch biopsy wound stained for VEGFR-3 (M) and vWF (N). Note absence of VEGFR-3 on blood vessel endothelium (arrows) and the very slight staining of vWF in lymphatic endothelium. New lymphatic vessel (arrowheads) and blood vessel (arrows) sprouting from pre-existing vessels at the edge of a day 5 punch biopsy wound stained for VEGFR-3 (O) and PAL-E (P). The continuous line in O and P marks the wound edge. Scale bars, 50 μm (A−D, M, N) and 70 μm (E−L, O, P).
Figure 3.
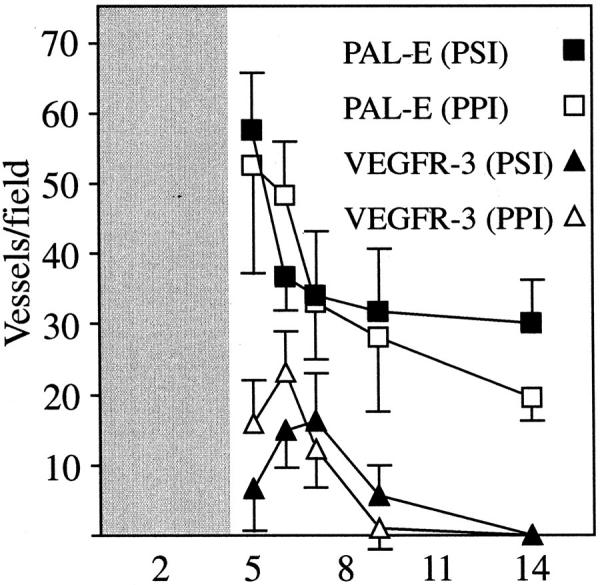
Vessels were counted on days 5, 6, 7, 9, and 14 in incisional wounds (PPI) and in punch biopsy wounds (PSI) from 4 to 7 square grids (area = 0.16 mm2) in a magnification field (40×), and the mean value score and SD were calculated. Only vessels with a lumen or consisting of more than a single endothelial cell were calculated. The granulation tissue did not develop into the wound area until day 4 to 5.
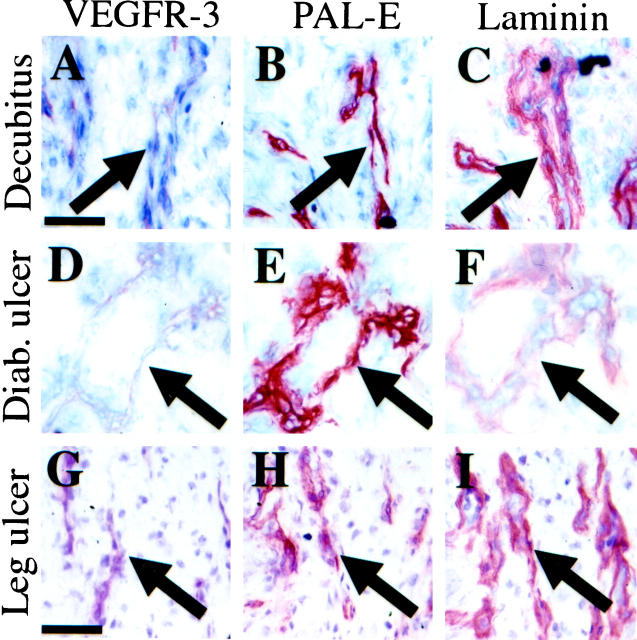
In human skin samples (Figure 4) ▶ , faint expression of VEGFR-3 was detected in the endothelium of the PAL-E- and laminin-positive blood vessels in a decubitus wound (Figure 4 ▶ , A–C), in a diabetic leg ulcer (Figure 4 ▶ , D–F) and in a nondiabetic leg ulcer (Figure 4 ▶ , G–I), suggesting that VEGFR-3 is up-regulated in vessels involved in chronic inflammation. The endothelium of capillaries in healthy skin of a skin transplant patient did not express VEGFR-3 (data not shown). Few PAL-E-, laminin-, and smooth muscle actin-negative, but VEGFR-3-positive, vessels were observed in these chronic inflammatory states. These lymphatic vessels were located within the deeper layers of the chronic wounds (data not shown).
Figure 4.
Immunoperoxidase staining of serial sections of a decubitus lesion (A−C), a diabetic leg ulcer (D−F), and a nondiabetic leg ulcer (G−I). Blood vessels (arrows) stain very weakly for VEGFR-3 (A, D, and G) and strongly for PAL-E (B, E, and H) and laminin (C, F, and I). Scale bars, 100 μm (A−F) and 200 μm (G−I).
Discussion
We show here that angiogenic blood vessels are negative for VEGFR-3 during normal blood vessel regeneration in wounds. In contrast, we and others have shown that VEGFR-3 is up-regulated in blood vessel endothelia in the tumor stroma. 11,12 Taken together, these findings suggest that in tumors VEGFR-3 is induced by factors that are not present in wound healing angiogenesis and that VEGFR-3 expression in wound healing angiogenesis differs from that of VEGFR-1 and VEGFR-2.
Earlier studies have shown at least some lymphatic vessel sprouting in experimental rabbit ear wounds. 26,27 The growth of new lymphatics has also been demonstrated in autotransplants of the rat small bowel 28 and of the rat hindleg. 29 In the present work we analyzed wound healing in pig skin and found that VEGFR-3-positive lymphatic vessels appear in the wound concurrently with blood vessels but regress earlier. To our knowledge this is the first time lymphangiogenesis has been demonstrated by immunohistochemistry in healing wounds. The transient presence of the VEGFR-3-positive vessels may be an integral part of normal wound healing and the relative absence of lymphatic vessels in the chronic wounds studied may be one of the reasons for their impaired healing. On the other hand, the low levels of VEGFR-3 detected in the blood vascular endothelium of chronic wounds may depend on, eg, growth factor or cytokine signals similar to those that have been found to up-regulate VEGFR-3 in tumor vessels. 11,12
Lymphangiogenesis may be an important yet poorly studied phenomenon in both wound healing and in tumor angiogenesis. The rapid growth and regression of lymphatic vessels shown here suggests that tumors may be able to induce their growth. However, lymphatic vessels cannot in general penetrate the tumor stroma because of increased interstitial pressure. 30 Tumor lymphangiogenesis may be a process operating via similar mechanisms to the wound lymphangiogenesis demonstrated here.
Acknowledgments
We thank Dr. Berndt Enholm for help with the experiments and Pirkko Uhlbäck, Eija Koivunen, Pipsa Ylikantola, and Tapio Tainola for excellent technical assistance. We also thank Dr. Ulpu Saarialho-Kere and Dr. Jouni Lauronen for providing samples for the study.
Footnotes
Address reprint requests to Kari Alitalo, Molecular/Cancer Biology Laboratory, Haartman Institute, P.O. Box 21, Haartmaninkatu 3, 00014 University of Helsinki, Helsinki, Finland. E-mail: kari.alitalo@helsinki.fi.
Supported by the Finnish Academy, the Sigrid Juselius Foundation, and the Finnish State Technology Development Centre. K. P. was supported by the Finnish Medical Foundation and the Finnish Cancer Institute.
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J: Seminars in medicine of the Beth Israel Hospital, Boston: clinical applications of research on angiogenesis. N Engl J Med 1995, 333:1757-1763 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999, 77:527-543 [DOI] [PubMed] [Google Scholar]
- 4.Shibuya M, Ito N, Claesson-Welsh L: Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr Top Microbiol Immunol 1999, 237:59-83 [DOI] [PubMed] [Google Scholar]
- 5.Korpelainen EI, Alitalo K: Signaling angiogenesis and lymphangiogenesis. Curr Opin Cell Biol 1998, 10:159-164 [DOI] [PubMed] [Google Scholar]
- 6.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Gray A, Yuan J, Louth S-M, Avraham H, Wood W: Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci USA 1996, 93:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont D, Jussila L, Taipale J, Mustonen T, Pajusola K, Breitman M, Alitalo K: Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282:946-949 [DOI] [PubMed] [Google Scholar]
- 9.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VM, Fang G-H, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to endothelium of lymphatic vessels during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K: VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1996, 122:3829-3837 [DOI] [PubMed] [Google Scholar]
- 11.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999, 154:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partanen TA, Alitalo K, Miettinen M: Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor-3 in 185 vascular tumors. Cancer 1999, 86:2406-2412 [PubMed] [Google Scholar]
- 13.Jain RK: Barriers to drug delivery in solid tumors. Sci Am 1994, 271:58-65 [DOI] [PubMed] [Google Scholar]
- 14.Clark RAF: The Molecular and Cellular Biology of Wound Repair, ed 2 1996, Plenum Press, New York, London,
- 15.Singer AJ, Clark RA: Cutaneous wound healing. N Engl J Med 1999, 341:738-746 [DOI] [PubMed] [Google Scholar]
- 16.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA: Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998, 152:1445-1452 [PMC free article] [PubMed] [Google Scholar]
- 17.Brown LF, Yeo K-T, Berse B, Yeo T-K, Senger DR, Dvorak HF, van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plate KH, Breier G, Millauer B, Ullrich A, Risau W: Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res 1993, 53:5822-5827 [PubMed] [Google Scholar]
- 19.Hatva E, Kaipainen A, Mentula P, Jääskeläinen J, Paetau A, Haltia M, Alitalo K: Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumors. Am J Pathol 1995, 146:368-378 [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, Tonnesen MG, Gailit J, Cheresh DA: Transient functional expression of alphaVbeta3 on vascular cells during wound repair. Am J Pathol 1996, 148:1407-1421 [PMC free article] [PubMed] [Google Scholar]
- 21.Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K: Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res 1998, 58:1599-1604 [PubMed] [Google Scholar]
- 22.Schlingemann RO, Dingjan GM, Emeis JJ, Blok J, Warnaar SO, Ruiter DJ: Monoclonal antibody PAL-E specific for endothelium. Lab Invest 1985, 52:71-76 [PubMed] [Google Scholar]
- 23.Pajusola K, Aprelikova O, Armstrong E, Morris S, Alitalo K: Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene 1993, 8:2931-2937 [PubMed] [Google Scholar]
- 24.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher CD, de Waal RM, Kaipainen A, Alitalo K: Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998, 153:395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autio-Harmainen H, Karttunen T, Apaja-Sarkkinen M, Dammert K, Risteli L: Laminin and type IV collagen in different histological stages of Kaposi’s sarcoma and other vascular lesions of blood vessel or lymphatic vessel origin. Am J Surg Pathol 1988, 12:469-476 [DOI] [PubMed] [Google Scholar]
- 26.Clark E, Clark E: Observations on the new growth of lymphatic vessels as seen in transparent chambers introduced into the rabbit’s ear. Am J Anat 1932, 51:43-87 [Google Scholar]
- 27.Oden B: A micro-lymphangiographic study of experimental wounds by second intention. Acta Chir Scand 1960, 120:100-114 [PubMed] [Google Scholar]
- 28.Schier F, Uner A, Waldschmidt J: Microlymphography of spontaneous lymph vessel anastomosis in small bowel transplantation in the rat. J Pediatr Surg 1991, 26:1239-1242 [DOI] [PubMed] [Google Scholar]
- 29.Anthony JP, Foster RD, Price DC, Mahdavian M, Inoue Y: Lymphatic regeneration following microvascular limb replantation: a qualitative and quantitative animal study. J Reconstr Microsurg 1997, 13:327-330 [DOI] [PubMed] [Google Scholar]
- 30.Boucher Y, Leunig M, Jain RK: Tumor angiogenesis and interstitial hypertension. Cancer Res 1996, 56:4264-4266 [PubMed] [Google Scholar]