Abstract
The dystrophin-glycoprotein complex, which comprises α- and β-dystroglycan, sarcoglycans, and utrophin/dystrophin, links the cytoskeleton to agrin and laminin in the basal lamina in muscle and epithelial cells. Recently, agrin was identified as a major heparan sulfate proteoglycan in the glomerular basement membrane. In the present study, we found mRNA expression for agrin, dystroglycan, and utrophin in kidney cortex, isolated glomeruli, and cultured podocytes and mesangial cells. In immunofluorescence, agrin was found in the glomerular basement membrane. The antibodies against α- and β-dystroglycan and utrophin revealed a granular podocyte-like staining pattern along the glomerular capillary wall. With immunoelectron microscopy, agrin was found in the glomerular basement membrane, dystroglycan was diffusely found over the entire cell surface of the podocytes, and utrophin was localized in the cytoplasm of the podocyte foot processes. In adriamycin nephropathy, a decrease in the glomerular capillary wall staining for dystroglycan was observed probably secondary to the extensive fusion of foot processes. Immunoelectron microscopy showed a different distribution pattern as compared to the normal kidney, with segmentally enhanced expression of dystroglycan at the basal side of the extensively fused podocyte foot processes. In passive Heymann nephritis we observed no changes in the staining intensity and distribution of the dystrophin-glycoprotein complex by immunofluorescence and immunoelectron microscopy. From these data, we conclude that agrin, dystroglycan, and utrophin are present in the glomerular capillary wall and their ultrastructural localization supports the concept that these molecules are involved in linking the podocyte cytoskeleton to the glomerular basement membrane.
Dystroglycan (DG) is an important member of the dystrophin-glycoprotein complex (DGC) which links the subsarcolemmal cytoskeleton to the basal lamina in skeletal muscle. 1 The importance of this link becomes clear from the severe muscular dystrophies resulting from mutations in genes that encode different members of the DGC. 2-5 DG is synthesized as a large precursor protein and is posttranslationally cleaved into α-DG, a heavily glycosylated peripheral membrane protein and the transmembrane protein β-DG. In skeletal muscle, α-DG is a major binding protein for agrin as well as for laminins. 6-9 α-DG remains noncovalently linked to β-DG, which through its cytoplasmic tail, binds directly to the C-terminal portion of dystrophin 10,11 whereas the N-terminal domain of dystrophin binds to the subsarcolemmal actin cytoskeleton. 12,13 Utrophin is an autosomal homologue of dystrophin and can also bind β-DG. 14 Recent in vitro studies support the role of the DGC in adhesion. Adhesion of a rat schwannoma cell line to laminin could be inhibited by antibodies against α-DG in vitro. 15 Furthermore, myotubules of patients with Duchenne muscular dystrophy were unable to adhere to laminin α2 in vitro. 16
Recently, we showed that agrin is a major heparan sulfate proteoglycan in the glomerular basement membrane (GBM). 17 Various other components of the DGC were also identified in the kidney. 18-20 Therefore, we hypothesized that glomerular visceral epithelial cells or podocytes are linked to the GBM in a similar way as the myocyte to the basal lamina. The podocytes and GBM, together with the fenestrated endothelium form the glomerular capillary wall (GCW), the barrier preventing passage of plasma proteins into the urinary space during glomerular ultrafiltration. During heavy proteinuria in various human and experimental glomerulopathies, the podocytes show dramatic morphological changes like fusion of foot processes and/or detachment from the GBM. 21-26 Several observations suggest that podocyte dysfunction and subsequent detachment contributes to the development of proteinuria. In adriamycin nephropathy (ADN), puromycin aminonucleoside nephrosis, and serum sickness nephritis, proteinuria correlates with podocyte detachment, 27,28 but not with changes in GBM charge density. 29 Passage of albumin through the GCW was localized to regions of saponin-induced detachment of podocytes in the single nephron model. 30 In another study it was found that injection of a monoclonal antibody (mAb) directed against a component of the slit diaphragm resulted in an acute massive proteinuria. 31,32 Therefore, it is generally assumed that the extent of podocyte detachment is related to the severity of proteinuria. However, no conclusive data are available on the mechanism of podocyte detachment during proteinuria.
The present study focuses on the distribution of agrin, α- and β-DG, and utrophin in the kidney. Expression and localization was evaluated by reverse transcriptase-polymerase chain reaction (RT-PCR) on RNA isolated from renal cortex, isolated glomeruli, and cultured podocytes and mesangial cells, and by immunofluorescence (IF) and immunoelectron microscopy (IEM) with monoclonal antibodies against the N- and C-terminus of agrin, α-DG, β-DG, and utrophin. These studies show that apart from agrin, α- and β-DG and utrophin are also present in the GCW and suggest a link between the podocyte and the GBM. Furthermore, the expression of the DGC was studied in two experimental models of proteinuric glomerulopathy: ADN in rats, which serves as a model for the nephrotic syndrome, characterized by extensive fusion of podocyte foot processes; and passive Heymann nephritis (PHN), a model for human membranous nephropathy, characterized by IgG depositions subepithelially in the GCW. Only in ADN were changes seen in the distribution of DG.
Materials and Methods
Animal Studies
The experimental protocol for the animal studies was approved by the local ethical committee. For induction of ADN and PHN and for the isolation of mesangial cells, we used male Wistar-Unilever rats that were bred at our animal laboratory and weighed ∼150 g at the start of the experiments. The animals were given standard food and tap water ad libitum. For primary podocyte culture, female Sprague-Dawley rats (Charles-River, Sulzfeld, Germany) ∼120 g were used.
Adriamycin Nephropathy
ADN was induced in four rats as described. 33 As controls, four rats were injected with the same volume of saline.
Passive Heymann Nephritis
Antibodies against renal tubular epithelium raised in a sheep were used for the induction of PHN. Serum of four normal sheep was pooled and used as control. The immunoglobulin (Ig) from the antiserum and the normal sheep serum was purified by ammonium sulfate precipitation followed by ion-exchange chromatography using DEAE-Sepharose. The Ig was dialyzed extensively against phosphate-buffered saline (PBS), concentrated to one-fifth of the original volume, passed through a 0.2-μm filter and used for injection. PHN was induced in four rats by three intravenous injections on subsequent days of 40 mg IgG of this purified sheep anti-renal tubular epithelium antiserum. As a control, four rats were injected with the same amount of normal sheep IgG.
Determination of Urine Albumin Concentration
Urinary albumin excretion was measured as described previously. 34
Isolation and Culture of Glomerular Cells
Primary Culture of Rat Podocytes
The primary culture of rat podocytes was performed as described by Mundel and co-workers. 35 Briefly, glomeruli were isolated from female Sprague-Dawley rats by the differential sieving procedure and cultured for 4 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mmol/L glutamine, 1 mmol/L pyruvate (all from Gibco, Paisley, Scotland). The outgrowing epithelial cells were trypsinized and passed through a sieve with a pore size of 32 μm to remove the remaining glomerular cores. The epithelial cells were cultured in plastic tissue culture flasks (Greiner, Frickenhausen, Germany) or plastic slide flasks (Nunc, Roskilde, Denmark) coated with collagen I (Seromed, Berlin, Germany) for 3 to 4 weeks without splitting. Medium was changed twice a week.
Isolation and Culture of Rat Mesangial Cells
Isolation and culture of rat mesangial cells was performed as described by Wolthuis and co-workers. 36 For IF and RT-PCR mesangial cells between passage 12 and 15 were used.
Reverse Transcriptase-Polymerase Chain Reaction and Analysis of Polymerase Chain Reaction Products
Isolation of RNA
Total RNA was extracted from kidney cortex and glomeruli by RNAzol (Campro Scientific, Veenendaal, The Netherlands) according to the instructions of the manufacturer. RNA extractions from podocytes and mesangial cells were performed using the guanidinium isothiocyanate acid phenol chloroform procedure 37 with small modifications. In all samples, RNA was dissolved in DEPC-treated water for 10 minutes at 65°C and stored at −80°C. RNA concentration was determined by measuring the absorbance of a diluted aliquot at 260 nm. Quality of the RNA samples was checked by electrophoresis on a 0.8% agarose gel and staining with ethidium bromide. No degradation was observed.
Reverse Transcriptase-Polymerase Chain Reaction
Primers and probes listed in Table 1 ▶ were designed using the Primer Express 1.0 program (Perkin-Elmer Cetus, Norwalk, CT) based on the rat agrin and dystroglycan cDNA sequence 38-40 and the homological stretches of mouse and human utrophin cDNA sequence. 41,42 The primer sets for the agrin N-terminus and C-terminus are exon-spanning (based on the mouse gene), with introns of 165 and 270 bp, respectively. Primers and probes were synthesized and purified by the Eurogentec Corporation (Seraing, Belgium). Synthesis of single-stranded cDNA was performed in a solution containing 1 μg of total RNA, 50 mmol/L Tris-HCl (pH 8.3), 7.5 mmol/L KCl, 6 mmol/L MgCl2, 10 mmol/L DTT, 0.2 mmol/L of dNTPs (Boehringer Mannheim, Mannheim, Germany), 2.5 μmol/L of random hexamers (Perkin-Elmer Cetus), 1000 U/ml RNase inhibitor (RNasin; Promega, Madison, WI), 20,000 U/ml Moloney murine leukemia virus reverse transcriptase (Life Technologies, Inc., Gaithersburg, MD) in a total volume of 20 μl. Reactions were overlaid with mineral oil and were subsequently incubated in a DNA thermal cycler (Eppendorf Master Cycler 5330, Eppendorf, Hamburg, Germany) at 20°C for 10 minutes, 42°C for 50 minutes, and 95°C for 5 minutes. Amplification of cDNA was performed by addition of 80 μl of a mixture containing 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.9), various concentrations of MgCl2 ranging between 0.1 and 2.5 mmol/L (see Table 1 ▶ ), 0.2 mmol/L of dNTPs, 150 nmol/L of upstream primer, 150 nmol/L of downstream primer, and 2.5 U/ml Thermoperfect DNA polymerase (Integro, Zaandam, The Netherlands). Amplification started with an initial denaturation step at 94°C for 2 minutes, followed by 34 cycles of denaturation at 94°C for 1.5 minutes, annealing at various temperatures ranging between 54 and 62°C (see Table 1 ▶ ) for 1.5 minutes, and extension at 72°C for 1.5 minutes. After the last cycle, the extension phase was prolonged for 10 minutes at 72°C and the samples were cooled to 15°C. To optimize the PCR reaction, for each product the MgCl2 concentration and the annealing temperature were varied and the conditions yielding maximal signal together with minimal background were chosen for the experiments.
Table 1.
Primers and Probes Used in RT-PCR and Southern Blot
| Reference no. | GenBank no. | Oligo sequence 5′→3′ | Base pair number | Fragment length | MgCl2 concentration | Annealing temp. (°C) | ||
|---|---|---|---|---|---|---|---|---|
| Agrin N-terminus | 38, 39 | M64780 | Forward primer | GCC GTA TAG GTG CAA CCC G | 998-1016 | 101 | 2.5 | 62 |
| Reverse primer | TAC GGA GTT AAA CTG GCA GGT CT | 1098-1076 | ||||||
| Probe | AAA GTA CGC TCT GGT CAA TGC CAA | 1033-1056 | ||||||
| Agrin C-terminus | 38, 39 | M64780 | Forward primer | TTC GAA TCA GGG CTC ACA GG | 5744-5763 | 101 | 0.5 | 62 |
| Reverse primer | GTC CAG TTG CGT GGC ACC | 5844-5827 | ||||||
| Probe | AGC CCC TGT GAC TGG ATC TTC C | 5799-5820 | ||||||
| Dystroglycan | 40 | H35660 | Forward primer | TTT GGA AGA AAC CAT TTT GAG CAT | 95-118 | 101 | 1.0 | 54 |
| Reverse primer | CAG GGA AGG GAT ACA TTA TTG CA | 195-173 | ||||||
| Probe | GTA CCT TTT AGG GAG GAA TGC CTT TT | 148-173 | ||||||
| Utrophin | 41, 42 | Y12229/ | Forward | GCC CAC AAT GAC ATA TTT AAA AGC AT | 7492-7517 (Y12229) | 154 | 1.0 | 54 |
| X69086 | primer | 7501-7526 (X69086) | ||||||
| Reverse | CCC TGA TGC TAG CAG ATT TTG C | 7645-7624 (Y12229) | ||||||
| primer | 7654-7633 (X69086) | |||||||
| Probe | CGG CAG AAG ATG GTG AAA GCT CT | 7528-7550 (Y12229) |
Analysis of Polymerase Chain Reaction Products
After amplification, 25 μl of PCR products were analyzed on a 1.5% agarose gel and stained with ethidium bromide. To check the specificity of the PCR products, they were blotted to a Hybond N+ nylon membrane (Amersham, Buckinghamshire, UK), using the alkali blotting procedure according to the manufacturer. Probes were 5′ end-labeled with γ-32P-dATP using T4 kinase (Boehringer Mannheim). Unincorporated radioactivity was removed using a Sephadex G-25 spin column. The Southern blots were prehybridized with 1 mg of herring sperm DNA in 0.5 mol/L NaH2PO4/Na2HPO4, 7% SDS, 1 mmol/L EDTA buffer (pH 7.2; hybridization solution) for 1 hour at 58°C, and then 50 pmol/L radiolabeled oligomer probe was added and incubation was continued overnight at 58°C. To remove unbound probes, blots were washed with 0.5 mol/L NaH2PO4/Na2HPO4, 1% SDS, 1 mmol/L EDTA (pH 7.2), for 10 minutes at 58°C and with 0.25 mol/L NaH2PO4/Na2HPO4, 1% SDS, 1 mmol/L EDTA (pH 7.2), for 10 minutes at 58°C and exposed to X-ray film (Eastman-Kodak, Rochester, NY) for 1 hour at −80°C.
Immunohistology
Immunofluorescence
Indirect IF was performed as described previously 34 on 2-μm cryostat sections of rat or human kidney and rat soleus muscle or human quadriceps muscle. Sections were fixed in acetone at 4°C during 10 minutes, except for incubations with the anti-dystrophin and anti-sarcoglycan antibodies, which was performed on nonfixed sections. Details on the used antibodies are given in Table 2 ▶ . 17,43-48 Primary antibodies were diluted in PBS containing 1% bovine serum albumin (BSA) and 0.05% sodium azide (IF-buffer), fluorescein isothiocyanate-labeled secondary antibodies were diluted in IF-buffer containing 10% normal rat serum (for IF on rat tissue), all antibodies were incubated during 45 minutes at room temperature. To evaluate the deposition of sheep and rat IgG and complement factor C3c in the GCW of rats with PHN, sections were directly incubated with fluorescein isothiocyanate-labeled rabbit anti-sheep IgG (Southern, Birmingham, AL) diluted 1:100 in IF-buffer, goat anti-rat IgG and goat anti-rat C3c (both from Nordic, Tilburg, The Netherlands) both diluted 1:50 in IF-buffer. After incubation with antibodies, the sections were washed with PBS, fixed with 1% paraformaldehyde in PBS for 15 minutes, washed, and embedded in Vectashield mounting medium H-1000 (Vector Laboratories Inc., Burlingame, CA) and examined with a Zeiss Axioskop microscope equipped with an epi-illuminator. The staining of the antibodies in the GCW of the rats (four ADR rats and four controls; four PHN rats and four controls) were evaluated in 25 glomeruli on a scale between 0 and 4+ by two independent observers on coded sections and the mean of the two scores was used for further analysis.
Table 2.
Antibodies Used to Localize the Various Members of the DGC by IF and IEM
| Antibody | Antigen | Reference no. | Dilution IF | Dilution IEM | Conjugate* | Dilution |
|---|---|---|---|---|---|---|
| MI-90 | Agrin N-terminus | 17 | 400 | 100 | Goat anti-hamster‡ | 50 |
| Agr-131 | Agrin C-terminus | 43 | 1000 | 250 | Goat anti-mouse IgG2a§ | 80 |
| 45 | Agrin C-terminus | 44 | 50 | 50 | Swine anti-rabbit IgG/p | 100 |
| IIH6 | α-dystroglycan | 45 | 1 | 1 | Goat anti-mouse IgM§ | 80 |
| 43-DAG† | β-dystroglycan | 46 | 1 | 1 | Goat anti-mouse IgG1§ | 80 |
| DRP-2† | Utrophin | 47 | 1 | 1 | Goat anti-mouse IgG1§ | 80 |
| 50-DAG† | α-sarcoglycan | 10 | Goat anti-mouse IgG1§ | 80 | ||
| DYS1† | Dystrophin rod | 48 | 1 | Goat anti-mouse IgG2a§ | 80 | |
| DYS2† | Dystrophin C-term | 48 | 2 | Goat anti-mouse IgG1§ | 80 | |
| DYS3† | Dystrophin N-term | 48 | 4 | Goat anti-mouse IgG2a§ | 80 |
*Conjugated to FITC for IF, to peroxidase for IEM.
†Novo Castra Laboratories, Newcastle upon Tyne, UK.
‡Jackson, West Grove, PA.
§Southern, Birmingham, AL.
/pDAKO, Glostrup, Denmark.
To verify the phenotype of the cultured podocytes and mesangial cells by IF, cells were grown on plastic slide flasks. Cells were fixed in 2% paraformaldehyde and 4% sucrose in PBS for 10 minutes at room temperature, washed in PBS, permeabilized with 0.3% Triton X-100 in PBS for 10 minutes at room temperature, and washed in PBS. Aspecific binding was blocked by pre-incubation with 2% fetal calf serum, 2% BSA, and 0.2% fish gelatin blocking reagent (Amersham, Braunschweig, Germany) in PBS (blocking solution) for 30 minutes at room temperature. Primary and fluorescein isothiocyanate- or cy3-labeled secondary antibodies which are detailed in Table 3 ▶ 45,49,50 were diluted in blocking solution and incubated at room temperature for 60 and 45 minutes, respectively. Thereafter, the sections were washed in PBS, fixed with 1% paraformaldehyde in PBS for 15 minutes, washed, and mounted in Vectashield mounting medium. To stain vital cells, the cells were incubated first with primary antibodies on ice, washed in PBS, fixed in 2% paraformaldehyde and 4% sucrose in PBS, washed, blocked, incubated with secondary antibodies, washed, fixed with 1% paraformaldehyde in PBS, washed, and mounted in Vectashield mounting medium as described above. The whole procedure was performed on ice.
Table 3.
Antibodies Used to Phenotype Cultured Podocytes and Mesangial Cells
| Antibody | Antigen | Reference no. | Dilution IF | Conjugate | Dilution |
|---|---|---|---|---|---|
| C19* | WT-1 | 50 | Swine anti-rabbit IgG-FITC† | 40 | |
| G1 | Synaptopodin | 49 | 1 | Goat anti-mouse IgG-cy3∥ | 200 |
| 5A | Podocalyxin | 50 | 10 | Goat anti-mouse IgG-cy3∥ | 200 |
| 27A | O-acetylated ganglioside | 50 | 10 | Goat anti-mouse IgG-cy3∥ | 200 |
| V9† | Vimentin | 800 | Goat anti-mouse IgG-cy3∥ | 200 | |
| D33† | Desmin | 400 | Goat anti-mouse IgG-cy3∥ | 200 | |
| OX7‡ | Thy1.1 | 400 | Goat anti-mouse IgG-cy3∥ | 200 | |
| 1A4§ | Smooth muscle actin | 5000 | Goat anti-mouse IgG-cy3∥ | 200 | |
| Anti-Factor VIII/p | Factor VIII | 300 | Swine anti-rabbit IgG-FITC† | 40 | |
| IIH6 | α-dystroglycan | 45 | 1 | Goat anti-mouse IgG-cy3∥ | 200 |
*Santa Cruz Biotechnology, Heidelberg, Germany.
†DAKO, Glostrup, Denmark.
‡PharMingen, San Diego, CA.
§Sigma, St. Louis, MO.
¶Central Laboratory Blood Transfusion Service, Amsterdam, The Netherlands.
∥Biotrend, Cologne, Germany.
Immunoelectron Microscopy
Normal rats or rats with ADN or PHN were anesthetized with 60 mg/kg pentobarbital. One kidney was removed and snap-frozen in liquid nitrogen for IF, the other kidney was perfused for 10 minutes via the retrograde aortic route with PBS, followed by perfusion with a mixture of 10 mmol/L sodium periodate, 75 mmol/L lysine HCl, and 2% paraformaldehyde (PLP) for 10 minutes. Small pieces of renal cortex were immersed in the same fixative for another 3 hours, washed in PBS for 1 hour, cryoprotected in 2.3 mol/L sucrose in PBS for 45 minutes, and snap-frozen in liquid nitrogen. Twenty-five-μm cryostat sections of PLP-fixed renal tissue were incubated with mAbs MI-90, Agr-131, IIH6, 43-DAG, and DRP-2 (Table 2) ▶ , diluted in PBS containing 1% BSA for 18 hours at 4°C and washed with PBS. The sections were then incubated with peroxidase-labeled secondary antibodies diluted in PBS containing 1% BSA and 10% normal rat serum for 90 minutes. After washing with PBS, the sections were preincubated with 0.05% diaminobenzidine medium containing 0.6% Tris for 10 minutes, followed by the same medium with H2O2 in a final concentration of 0.0001%. After three washes in distilled water, the sections were postfixed in 1% OsO4, pH 7.4, for 30 minutes at room temperature, dehydrated, and embedded in Epon 812. Ultrathin sections were prepared on a LKB Ultratome and examined in a Jeol 1200 EX2 electron microscope (Jeol Ltd, Tokyo, Japan).
Results
Presence of mRNA for Agrin, Dystroglycan, and Utrophin in Kidney Cortex, Glomeruli, Podocytes, and Mesangial Cells
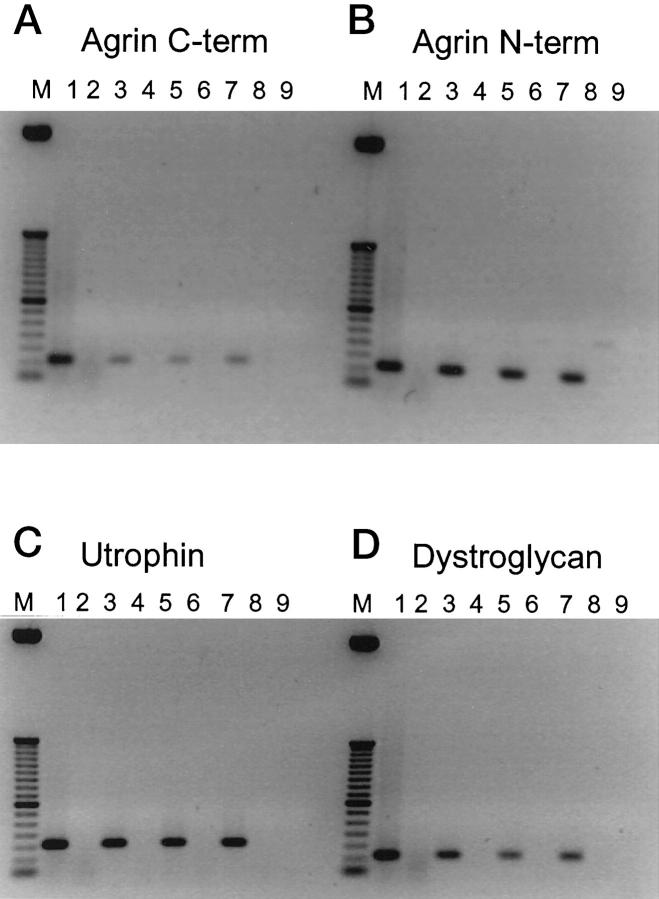
First, we tested whether mRNA for agrin, DG, and utrophin was present in kidney cortex. Amplification of cDNA with primers for C-terminal agrin yielded a PCR product of 101 bp (Table 1 ▶ and Figure 1 ▶ A, lane 1). Similarly, amplification with primers for N-terminal agrin and DG yielded products of 101 bp (Figure 1 ▶ , B and D, lanes 1) and with primers for utrophin yielded a product of 154 bp (Figure 1C ▶ , lane 1). This shows that mRNA for agrin, DG, and utrophin is present in the kidney cortex. No PCR product was obtained in samples that were not reverse-transcribed (Figure 1 ▶ , A–D, lanes 2), confirming that the RNA samples were not contaminated with genomic DNA. Omitting RNA from the samples also yielded no PCR product (Figure 1 ▶ , A–D, lanes 9). To confirm the identity of the PCR products, the products were transposed to nylon blots that were hybridized with radiolabeled probes specific for the PCR products. All probes specifically hybridized with their PCR product (not shown).
Figure 1.
Reverse transcriptase-polymerase chain reaction for C-terminal agrin (A), N-terminal agrin (B), utrophin (C), and dystroglycan (D) on cDNA from cortex, glomeruli, and cultured podocytes and mesangial cells. M50 bp marker. Lanes 1 and 2, kidney cortex; lanes 3 and 4, glomeruli; lanes 5 and 6, podocytes; lanes 7 and 8, mesangial cells; lane 9, buffers only. Lanes 1, 3, 5, and 7: cDNA (reverse-transcribed RNA); lanes 2, 4, 6, and 8: nonreverse-transcribed RNA.
Then, RNA was extracted from isolated glomeruli and also in this preparation amplification with all four primer sets yielded PCR products of the same size as for kidney cortex RNA, indicating that RNA for agrin, DG, and utrophin is present in glomeruli as well (Figure 1 ▶ , A–D, lanes 3). No PCR product was obtained in samples that were not reverse-transcribed (Figure 1 ▶ , A–D, lanes 4).
To find out which cells in the glomerulus are responsible for the production of agrin, DG, and utrophin, we cultured rat podocytes and rat mesangial cells. The phenotype of the cells was confirmed by IF with various markers as shown in Table 4 ▶ . The podocytes showed staining for WT-1, synaptopodin, vimentin, and smooth muscle actin, and a weak staining for α-DG early after isolation, but this staining disappeared with prolonged culture, probably as a result of dedifferentiation. The podocytes were negative for podocalyxin, O-acetylated ganglioside, desmin, Thy 1.1, and coagulation factor VIII. The mesangial cells showed staining for desmin, vimentin, Thy 1.1, smooth muscle actin, and α-DG, but were negative for WT-1, synaptopodin, podocalyxin, O-acetylated ganglioside, and factor VIII. Thus, both cultured cell types had some but not all features expressed by podocytes and mesangial cells in vivo, which is consistent with a lower differentiation state of these cells in vitro. 51 Amplification of RNA extracted from both podocytes and mesangial cells with all four primer sets yielded PCR products comparable to kidney cortex and glomeruli (Figure 1 ▶ , A–D, lanes 5, podocytes and lanes 7, mesangial cells). RNA samples from podocytes and mesangial cells that were not reverse-transcribed yielded weak bands of 266 bp for N-terminal agrin, indicating that these samples were slightly contaminated with genomic DNA (Figure 1B ▶ , lanes 6 and 8). For C-terminal agrin, DG, and utrophin, however, no PCR product was obtained in the samples that were not reverse-transcribed (Figure 1 ▶ , A, C, and D, lanes 6 and 8). These data show that both podocytes and mesangial cells contain message for agrin, DG, and utrophin.
Table 4.
Expression of Various Antigens in Glomeruli of Normal Rats and on Cultured Podocytes and Mesangial Cells
| Antibody | Antigen | In situ staining | In vitro staining | ||||
|---|---|---|---|---|---|---|---|
| GVEC | GPEC | MC | Endo | GVEC | MC | ||
| C19 | WT1 | + | − | − | − | + | ± |
| G1 | Synaptopodin | + | − | − | + | + | − |
| 5A | Podocalyxin | + | − | − | + | − | − |
| 27A | O-acetylated ganglioside | + | − | − | − | − | − |
| V9 | Vimentin | + | + | − | + | + | + |
| D33 | Desmin | − | − | + | − | − | + |
| OX7 | Thy 1.1 | − | − | + | − | − | + |
| 1A4 | Smooth muscle actin | − | − | + | − | + | + |
| Anti-Factor VIII | Factor VIII | − | − | − | + | − | − |
| IIH6 | α-dystroglycan | + | − | − | − | ± | + |
GVEC, glomerular visceral epithelial cell or podocyte; GPEC, glomerular parietal epithelial cell; MC, mesangial cell; Endo, endothelial cell.
+, positive staining; −, negative staining
Expression of Agrin, Dystroglycan, and Utrophin in Normal Kidney Tissue
In indirect IF, anti-α-sarcoglycan (adhalin) mAb 50-DAG showed clear staining of myocyte cell membranes of rat soleus muscle, whereas no staining was seen on rat kidney sections. Anti-α-dystrophin mAbs DYS1 and DYS2 also clearly stained myocyte cell membranes of rat muscle and the vascular myocyte membranes in kidney sections, however, no staining was observed in the glomeruli and in the tubuli. DYS1, DYS2, and DYS3 (human-specific) also stained human quadriceps muscle and human kidney tissue in the same way as described for rat tissue. These observations are in agreement with previous studies, which have shown that α-sarcoglycan is specifically expressed in muscle 52 and dystrophin is only expressed in muscle and neural tissue. These antibodies were not used in further studies.
On rat kidney cryostat sections, anti-N-terminal agrin mAb MI-90 and anti-C-terminal agrin mAb Agr-131 stain the GBM in a bright linear way as described previously. 17 mAb MI-90 stains most tubular basement membranes (TBMs) in a linear fashion, but somewhat less intense than the GBM, whereas the TBMs are negative or weakly stained by mAb Agr-131. Bowman’s capsule, basement membranes (BMs) of endothelial cells in peritubular capillaries, and smooth muscle cells of arteries and arterioles are negative for both mAbs (Figure 2 ▶ , A and B). Anti-α-DG mAb IIH6 and anti-β-DG mAb 43-DAG both show a granular staining along the GCW in a podocyte-like staining pattern (Figure 2C) ▶ . Some tubular epithelial cells show a coarse granular staining of IIH6 at the basal side, whereas most tubular epithelial cells show a granular staining of 43-DAG (anti-β-DG) at the basal side facing the tubular BMs. However, the staining of both mAbs in the tubuli is less intense than in the GCW. The basal sides of parietal epithelial cells near Bowman’s capsule are negative or weakly stained by both mAbs. The basal cell surface of smooth muscle cells in arterioles is weakly stained by 43-DAG (not shown). Anti-utrophin mAb DRP-2 shows a granular staining along the GCW and sometimes of the podocyte cell body. There is a variable and coarse granular staining of the basal side of the tubular epithelial cells. The basal sides of parietal epithelial cells near Bowman’s capsule are negative or weakly stained and the basal cell surface of smooth muscle cells in arteries and arterioles is stained brightly (Figure 2D) ▶ . These experiments show that agrin, DG, and utrophin are present in the GCW. On sections of normal human kidney, anti-N-terminal agrin mAb MI-90, anti-β-dystroglycan mAb 43-DAG, and anti-utrophin mAb DRP-2 showed the same staining pattern as shown for normal rat kidney (Figure 3) ▶ , indicating that these components are also important for human glomerular physiology.
Figure 2.
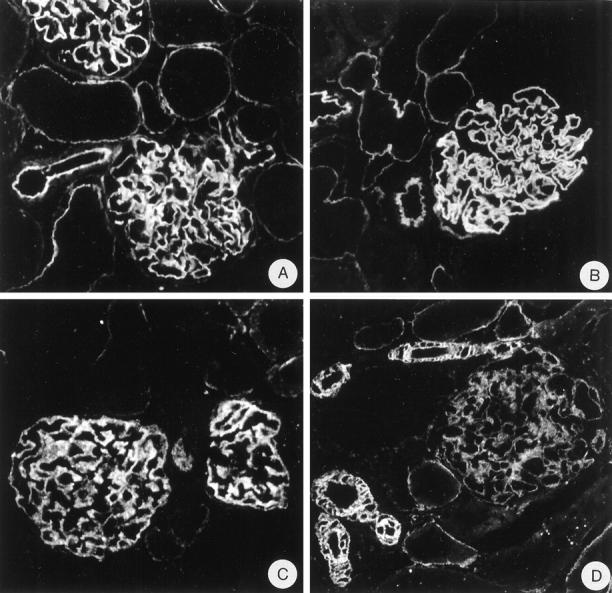
Indirect IF on normal rat kidney tissue with anti-N-terminal agrin mAb MI-90 (A), anti-C-terminal agrin mAb Agr-131 (B), anti-α-dystroglycan mAb IIH6 (C), and anti-utrophin mAb DRP-2 (D). Magnification, ×375.
Figure 3.
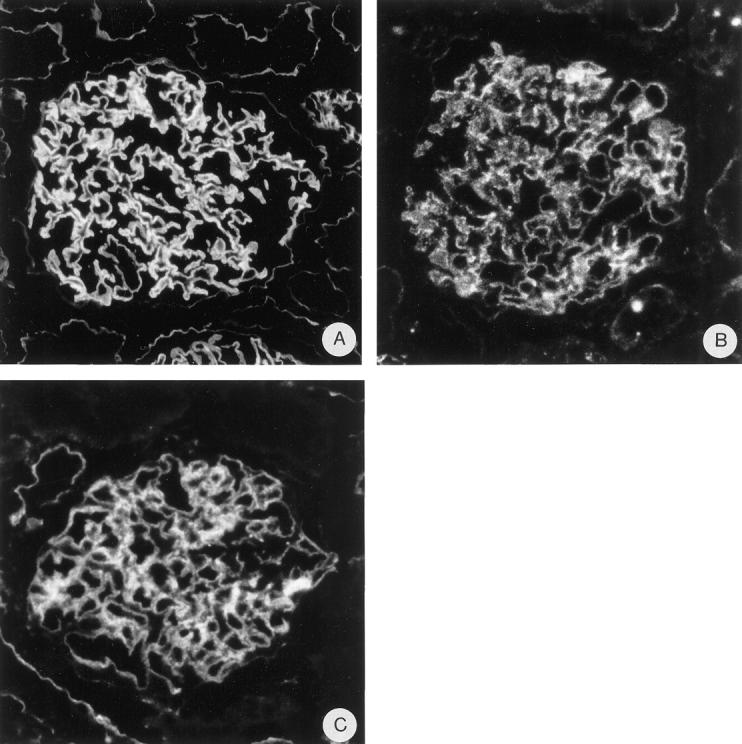
Indirect IF on normal human kidney tissue with anti-N-terminal agrin mAb MI-90 (A), anti-β-dystroglycan mAb 43-DAG (B), and anti-utrophin mAb DRP-2 (C). Magnification, ×475.
To obtain more information about their ultrastructural localization in the GCW, we performed IEM with these mAbs. In IEM, both the anti-N-terminal agrin mAb MI-90 and the anti-C-terminal agrin mAb Agr-131 stained the GBM, with the highest intensity at the endothelial and epithelial sides of the GBM (Figure 4 ▶ ,A, B, D, and E). Furthermore, mAb MI-90 stained most TBMs, whereas mAb Agr-131 only stained TBMs of distal tubuli. Both mAbs slightly stain the basal in-foldings of the tubular epithelial cells (Figure 4 ▶ , C and F). The regions of the GBM covering the mesangial areas were not stained by mAb Agr-131 (C-terminal agrin), whereas this part of the GBM was positive for mAb MI-90 (N-terminal agrin). This suggested that the perimesangial part of the GBM lacks the C-terminus of agrin. Alternatively, this lack of staining with mAb Agr-131 could be due to hampered penetration of the mAb. Therefore, we performed additional IEM using a rabbit polyclonal antiserum against the recombinant C-terminal 95 amino acids of chick agrin, 44 which closely corresponds with the C-terminus of rat agrin and cross-reacts with rat tissues. Also this antiserum exclusively stained the peripheral GBM loops and not the perimesangial GBM parts (Figure 5) ▶ . This similar staining pattern revealed by a mouse IgG2b mAb and a polyclonal rabbit antiserum toward the C-terminus of agrin suggests that the perimesangial GBM expresses a truncated variant of agrin, lacking the C-terminus. Both MI-90 and Agr-131 showed minor staining of mesangial cells, predominantly in the regions where a mesangial cell adheres to another mesangial or endothelial cell (Figure 4 ▶ , A and D). Anti-α-DG mAb IIH6 stained the cell membrane of the podocyte cell bodies as well as the foot processes with high intensity, however, the regions where the foot processes face the GBM were stained less intense than the rest of the podocyte cell membrane (Figure 4 ▶ , G and H). mAb IIH6 also stains the basolateral cell membranes of the epithelial cells of tubuli, including the basal membrane in-foldings (Figure 4I) ▶ . Anti-β-DG mAb 43-DAG showed a similar but weaker staining pattern than mAb IIH6 (not shown), which also corresponds to the staining observed in IF. Anti-utrophin mAb DRP-2 stained the cytoplasm of some podocyte foot processes, where the strongest intensity was observed in the regions near the GBM (Figure 4 ▶ , J and K). At the basal side of epithelial cells of distal tubuli some staining for utrophin was also observed (Figure 4L) ▶ . The staining patterns of these mAbs obtained by IEM are in agreement with the staining observed in IF.
Figure 4.
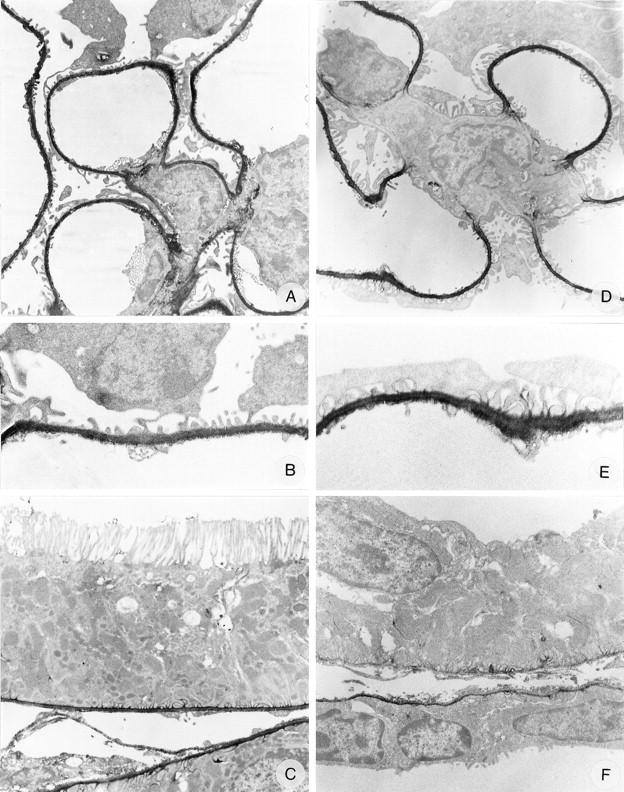
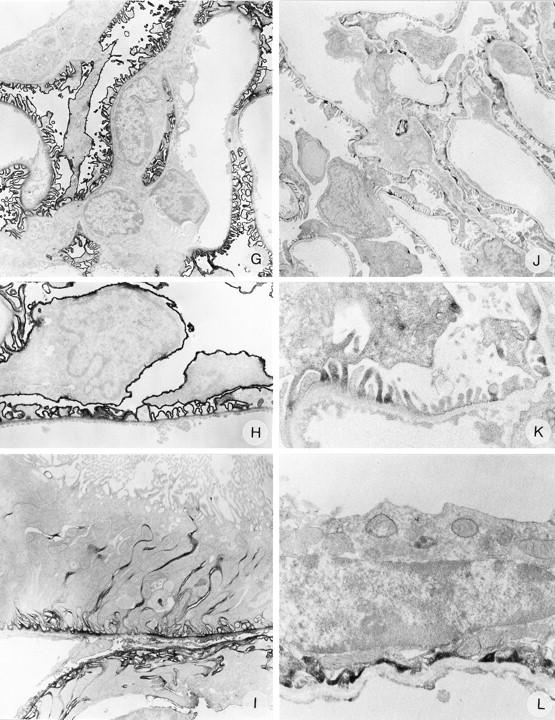
Immunoelectron microscopy on normal rat kidney tissue with anti-N-terminal agrin mAb MI-90 (A, B, and C), anti-C-terminal agrin mAb Agr-131 (D, E, and F), anti-α-dystroglycan mAb IIH6 (G, H, and I), and anti-utrophin mAb DRP-2 (J, K, and L). Magnifications: A, ×4500; B, ×12000; C, ×5500; D, ×6000; E, ×12,000; F, ×7000; G, ×3500; H, ×7000; I, ×6000; J, ×3500; K, ×12,000; L, ×12,000.
Figure 5.
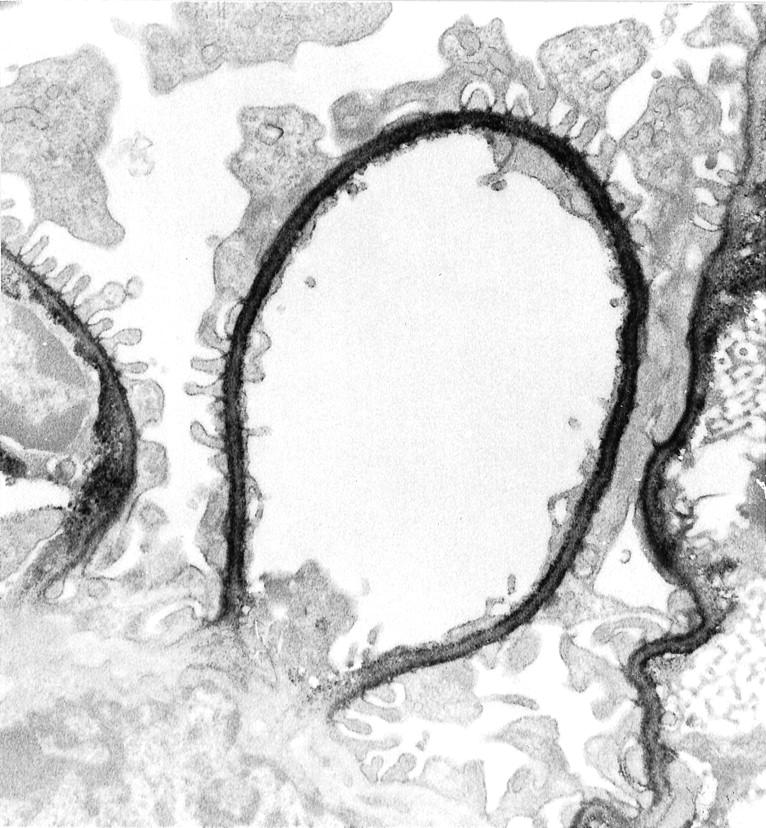
Immunoelectron microscopy on normal rat kidney tissue with anti-C-terminal agrin antibody 45. Note that the perimesangial GBM is negative. Magnification, ×8000.
These experiments show that agrin, DG and utrophin are present in the GCW. Furthermore, IEM shows that N- and C-terminal agrin are GBM components, α- and β-DG are podocyte cell membrane components, and that utrophin is localized in the cytoplasm of the podocyte foot processes.
Agrin, Dystroglycan, and Utrophin Staining in Adriamycin Nephropathy and Passive Heymann Nephritis
To evaluate the correlation between the development of albuminuria and the expression of agrin, DG, and utrophin, we analyzed glomerular staining of these components in rats with ADN and PHN.
Adriamycin Nephropathy
The injection of 5 mg of adriamycin per kg of bodyweight induced a heavy albuminuria of 344 ± 106 mg per 24 hours similarly as previously documented. 34 The rats injected with saline had an albumin excretion of less than 1 mg per 24 hours throughout the experiment. In ADN, ∼20% of the glomeruli showed a slight segmental decrease in staining of mAb MI-90 against the N-terminus of agrin and of mAb Agr-131 against the C-terminus of agrin. More notable, however, was the decreased staining of mAb IIH6 against α-DG in ∼70% of the glomeruli. This decreased staining was due to a segmentally or generally reduced staining of the capillary loops from 3+ to 2+ (Figure 6A) ▶ . In ∼50% of the glomeruli the staining with mAb 43-DAG against β-DG was decreased in a pattern similar to mAb IIH6 (not shown). The staining of mAb DRP-2 against utrophin was slightly decreased in ∼20% of the glomeruli.
Figure 6.
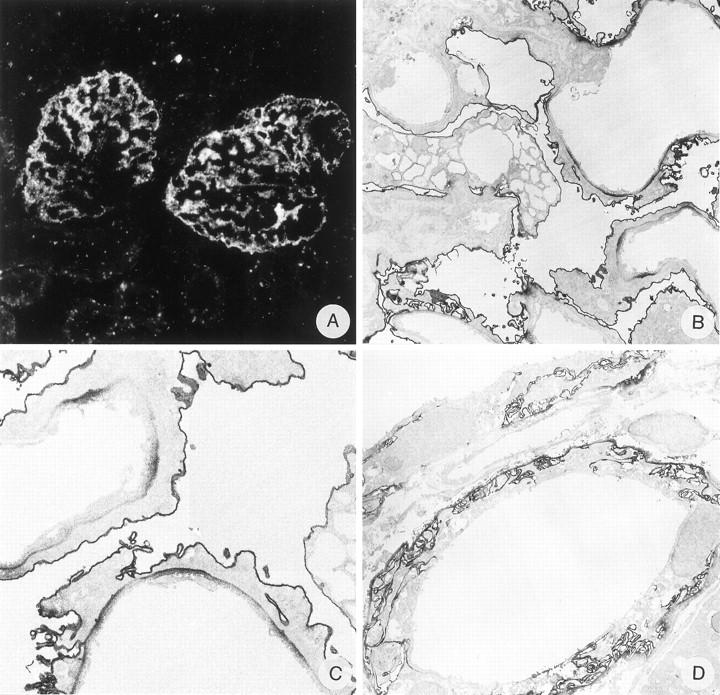
Staining of anti-α-dystroglycan mAb IIH6 in ADN. Indirect IF (A) and IEM (B, C, and D). Magnifications: A, ×375; B, ×3500; C, ×7500; D, ×3500.
Because the decrease in staining in ADN was most clear for α-DG, we also performed IEM with this mAb. There were striking morphological changes in ADN because podocytes showed vacuolization and extensive fusion of foot processes, however, no detachment of podocyte foot processes from the GBM was observed (Figure 6B) ▶ . Furthermore, the expression of α-DG on the basal side of the podocytes was locally more pronounced than in the normal rat kidney, whereas the staining of the podocyte cell membrane was unaltered (Figure 6 ▶ , B and C). There was also a more pronounced expression of α-DG at the basal side of tubular epithelial cells and the membrane in-foldings (Figure 6D) ▶ .
Passive Heymann Nephritis
Injection of rats with 40 mg of anti-renal tubular epithelium IgG on 3 subsequent days, induced in a mild albuminuria starting at week 2, that increased steadily, and resulted after 18 weeks in an albumin excretion of 115 ± 76 mg per 24 hours. The rats injected with the same volume of normal sheep IgG had an albumin excretion of less than 1 mg per 24 hours throughout the experiment. In rats with PHN, the GCW was stained in a bright, coarse granular pattern for sheep IgG (Figure 7A) ▶ and was weakly positive for rat IgG and rat C3c (not shown). In PHN we did not observe alterations in the staining of mAb MI-90 against the N-terminus of agrin, mAb Agr-131 against the C-terminus of agrin, mAb IIH6 against α-DG (Figure 7B) ▶ , mAb 43-DAG against β-DG, and mAb DRP-2 against utrophin, despite the presence of subepithelial immune deposits as shown by a bright course granular staining for sheep IgG (Figure 7A) ▶ . IEM was performed with IIH6 (α-DG). No obvious changes were observed. Podocytes displayed a rather normal phenotype and barely any effacement of the foot processes was seen. Occasionally, large immune deposits were visible subepithelially. IIH6 stained the cell membranes of the podocytes very prominently, the basal side of the foot processes were rather weak, identical to the staining in the control kidney (Figure 7 ▶ , C and D). In contrast to ADN, no up-regulation of DG was observed between the GBM and the podocyte foot processes. Staining of α-DG in the tubular areas of PHN kidneys did not differ from control renal tissue (data not shown). We conclude that in PHN the distribution of α-DG is not altered.
Figure 7.
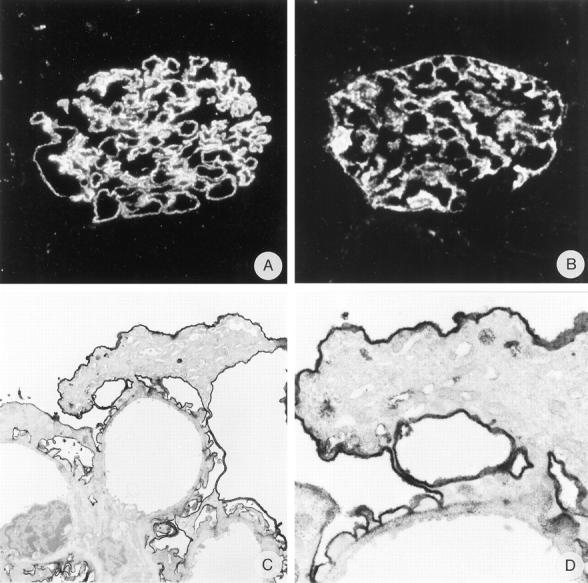
Staining of anti-α-dystroglycan mAb IIH6 in PHN. Glomerular localization of immune complexes is demonstrated by IF staining of sheep IgG (A). Distribution of α-dystroglycan is shown by IF (B) and IEM (C and D). Magnification: A and B, ×530; C, ×4200; D ×13,500.
Discussion
The present study demonstrates the glomerular expression of agrin, DG, and utrophin. The main findings are summarized as follows: 1) by RT-PCR, mRNA expression for agrin, DG, and utrophin was found in kidney cortex, isolated glomeruli, and cultured podocytes and mesangial cells; 2) IF showed that besides agrin, DG and utrophin are also present in the GCW in both rat and human kidney; 3) IEM revealed the presence of agrin in the GBM, of DG on the basal and luminal sides of the cell membrane of the podocytes, and of utrophin in the cytoplasm of the podocyte foot processes. To our knowledge, this is the first ultrastructural localization of DG and utrophin in kidney tissue.
Agrin has been identified in the BM in many tissues, 53 including the developing kidney. 54 Furthermore, agrin is a major heparan sulfate proteoglycan in the adult GBM. 17,55 The anionic heparan sulfate side chains of agrin play an important role in the maintenance of the charge-dependent permeability of the GBM. 56-58 In the present study, we observed a difference in ultrastructural localization between the N- and C-terminus of agrin. The N-terminus of agrin was found in the entire GBM whereas with two different antibodies the C-terminus was not found in the regions of the GBM covering mesangial cells. In a previous study, we also observed a difference in tissue distribution with these antibodies. Most TBMs lacked staining for the C-terminus of agrin, whereas the N-terminus of agrin was present in most TBMs. 17 The difference in GBM staining pattern for N- and C-terminal agrin in the present study could be due to a reduced accessibility of the perimesangial GBM regions for the antibodies. This is, however, not very likely because the mAb against N-terminal agrin clearly stained both peripheral and perimesangial GBM parts. Alternatively, we suggest the presence of a truncated agrin isoform, missing the C-terminus, in the perimesangial GBM. Because the C-terminus of agrin contains the binding site for α-DG, 6-9 the agrin-DGC link between the GBM and the podocyte cytoskeleton may only be important in peripheral GCW regions that are involved in ultrafiltration.
DG has been shown to be present adjacent to BMs in many tissues including the kidney. 18,19 The staining patterns that we observed for α- and β-DG correspond to the findings of Durbeej and co-workers, 19 who have shown that an antiserum recognizing DG stains the GCW and the basal side of tubular epithelial and glomerular parietal epithelial cells near Bowman’s capsule in human and mouse kidney. The importance of DG in development is underlined by the observation that DG-deficient mice, which die early in development, fail to form a functional Reichert’s membrane, suggesting that DG is involved in organizing BMs. 59 Furthermore, antibodies directed against α-DG, that block the binding of α-DG to agrin and laminin, 7 perturb kidney development in vitro. 18 Several investigators reported co-localization of agrin and β-DG in the developing kidney, lung, and brain 60 and of β-DG and dystrophin in human, rat, and mouse myofibers, near or at the plasma membrane. 61-63
Several studies have already shown that utrophin is present near BMs in many tissues, 64-67 including the kidney. 20 In the present study we found staining for utrophin in some but not all of the podocyte foot processes. That not all foot processes were positive could be due to low affinity of the mAb (DRP-2), because staining in IF was weak as well (see Figure 2D ▶ ). The observation in IEM that staining for utrophin was strongest in the regions near the GBM confirms the hypothesis that via utrophin the DGC links the cytoskeleton to the GBM.
By RT-PCR, we could show that cultured podocytes express mRNA for agrin, DG, and utrophin. mRNA for these components was, however, also present in the mesangial cells. In IEM however, no staining was observed in the mesangium for DG and utrophin and only a weak staining for agrin. By in situ hybridization on adult mouse kidney, Durbeej et al 19 suggested that DG mRNA expression was predominantly localized in epithelial cells. With this technique however, it cannot be excluded that mesangial cells also express mRNA for DG. As known, PCR does not provide information about protein expression. With IEM we found in the mesangium some staining for the N-terminus of agrin and also a weaker staining for the C-terminus of agrin. This staining was seen in regions where mesangial cells adhere to other mesangial or endothelial cells. Because the mAbs against β-DG and utrophin showed a rather weak staining both in IF and IEM, the possible expression of these proteins by mesangial cells could have been missed. The staining intensity on the podocyte cell membrane for α-DG was much higher, but no staining was observed with this mAb in mesangial areas. Furthermore, cultured mesangial cells have a lower differentiation state than in vivo (Table 4) ▶ and this dedifferentiation may result in an altered gene expression. Therefore, it is possible that mesangial cells are to some extent able to synthesize agrin, DG, and utrophin. On the other hand, we found cultured podocytes to be weakly positive or negative for DG. Based on the data obtained by IEM however, it can be concluded that within the glomerulus the podocyte is the major source for DG and utrophin. With respect to DG, we conclude that glomerular cell culture systems are not reliable models for the in vivo situation.
Several investigators have suggested a role for the DGC in linking the cytoskeleton to the ECM, not only for muscle cells but also for epithelial cells. 1,19 The results from the present study underline the possibility that GBM agrin is linked via α- and β-DG to utrophin (Figure 8) ▶ . This hypothesis, however, is weakened by the observation that although both α- and β-DG are present at the basal side of the podocytes, the strongest expression is seen outside the contact sites with the GBM at the lateral and apical regions of the podocyte membrane. If α-DG on podocytes serves as a receptor for agrin (and laminin), it is unclear why it is more abundantly present on the luminal than on the basal side of the cells. Furthermore, it is unlikely that α- and β-DG are complexed to utrophin at the luminal sides, because we only observed utrophin expression in the foot processes of the podocytes. The weaker staining for DG at the basal side of the foot processes could also be the result of a differential accessibility of the basal and lateral sides of the podocyte cell membrane to the antibodies. At present, it is unclear what the function could be of α-DG on the luminal membrane of the podocyte cell body. A similar membrane distribution on the podocyte is found for podocalyxin, glomerular epithelial protein 1 (GLEPP1), and podoplanin which all are more heavily expressed on the luminal podocyte membrane than on the basal side. 68-73 Also the functions of these proteins are not clear yet. The anionic sialoglycoprotein podocalyxin may contribute to the negative charge of the GCW. 74 GLEPP1 is a transmembrane receptor-like protein-tyrosine phosphatase, which may have a function in podocyte structure by regulating tyrosine phosphorylation of podocyte proteins. 71,72 Because α-DG is very heavily glycosylated, 75 it may have a function similar to that of podocalyxin and contribute to the negative charge of the GCW. However, it may also be a receptor for a presently unknown substance in Bowman’s space. Agrin or laminin fragments that are released in situations of GBM damage could appear in the urinary space and bind to α-DG on the podocyte cell surface. This interaction could lead via intracellular signaling to fusion and/or detachment of foot processes.
Figure 8.
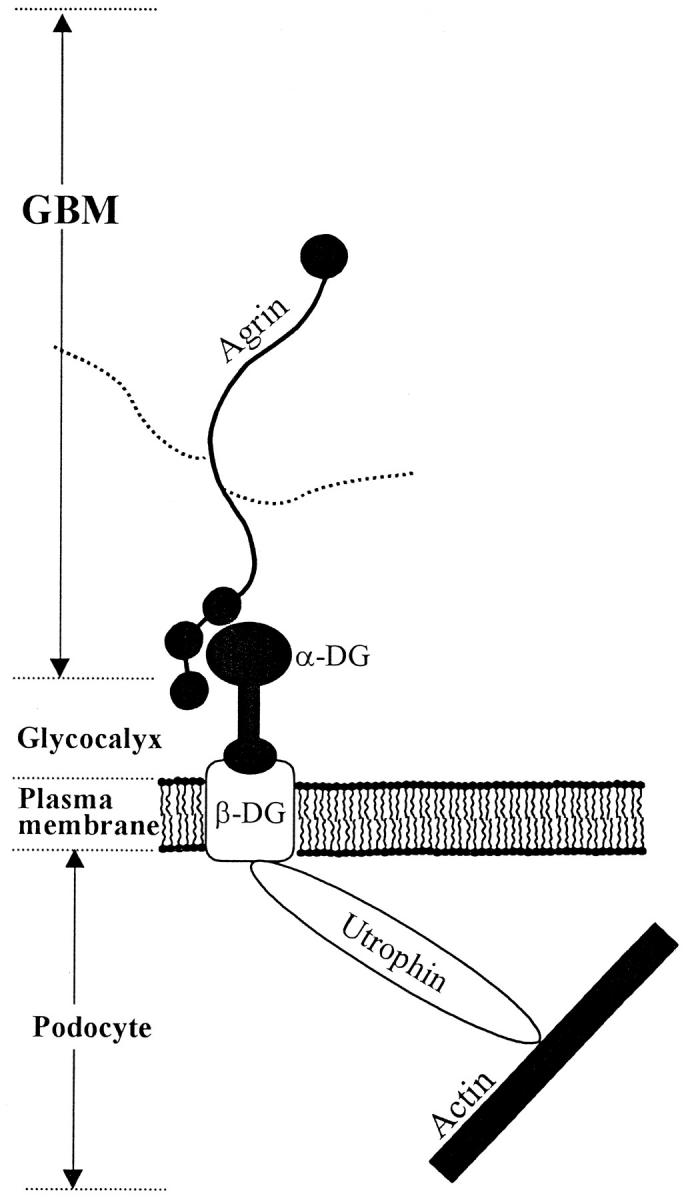
Schematic representation of the possible interaction between agrin, DG, and utrophin in the glomerular capillary wall. Reproduced with permission from Groffen et al. 79
To investigate the role of agrin, DG, and utrophin in glomerular pathology, we studied the expression of these proteins in ADN and PHN. Rats with ADN had a heavy proteinuria, which was previously shown to be inversely correlated with the GBM staining for the heparan sulfate side chain. 34 These animals showed a segmental decrease in GCW staining for both α- and β-DG. In IEM, an alteration was observed in the distribution pattern of α-DG, with locally a more pronounced expression at the basal side of the fused podocyte foot processes. The decrease in staining intensity in IF can be explained by the fusion of the foot processes in ADN, which results in a decreased cell membrane surface near the GCW and therefore in a decreased density of membrane-associated proteins. The significance of the agrin-DG-utrophin complex for the development of proteinuria is not clear. Although it has been reported that podocytes detach from the GBM in ADN, 24,29 we did not observe this in the present study. It is possible that in later stages of ADN podocytes detach, and it may be interesting to study the relation between the sites of detachment and α-DG expression. In PHN the staining intensity for agrin, DG, and utrophin did not change. In contrast to ADN, IEM of PHN barely showed foot process effacement. Besides that, there is no up-regulation of DG on the basal sides of the podocytes. These data suggest that in this model of immune-complex-mediated proteinuria, DGC expression and distribution is not affected. This suggests that alterations in the DGC complex are not uniformly associated with proteinuria. Variations in DG expression might be related to differences in the induction of podocyte injury (toxic in ADN versus immune-complex mediated in PHN). However, alterations in the expression of agrin, DG, and utrophin may also be related to the degree of proteinuria, because rats with ADN had a heavier proteinuria than rats with PHN.
Apart from the DGC which is presently recognized as a possible link between muscle or epithelial cells and the ECM, 1,19 another well-characterized adhesion receptor system for epithelial cells are the integrin matrix receptors. In the GBM α3β1 integrin is predominantly found. 76 Because it has been shown that integrins interact with agrin and sarcoglycans, the DGC may also act synergistically to integrins to link podocytes to the GBM. 77,78
Taken together, the present study shows that agrin, DG, and utrophin are present in the GCW. Further studies are needed to elucidate their function in normal podocyte physiology and their role in increased glomerular permeability for proteins. They may have a role in maintaining the intricate relation between podocytes and the GBM. It remains to be determined whether alterations in the DGC complex occur in human glomerular diseases and whether specific alterations are related to certain glomerulopathies.
Note Added in Proof
After acceptance of this manuscript, Regele et al (J Am Soc Nephrol 2000, 11:403–412) reported that in minimal change nephrosis, alpha and beta dystroglycan expression was reduced in contrast to focal segmental glomerulosclerosis. After remission induction with steroids, dystroglycan expression returned to normal.
Acknowledgments
The authors are grateful to Prof. K. P. Campbell and Dr. M. Durbeej (Howard Hughes Medical Institute, University of Iowa College of Medicine, Iowa City, IA) for the gift of mAb IIH6, to Dr. W. Hoch (Department of Biochemistry, Max Planck Institute for Developmental Biology, Tübingen, Germany) for the gift of mAb Agr-131, to Dr. S. Kröger (Max Planck Institute for Brain Research, Frankfurt, Germany) for the gift of antiserum 45, and to Dr. E. de Heer (Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands) for the gift of renal tubular epithelium. Mr. W. P. M. Tamboer is gratefully acknowledged for the purification of sheep anti-renal tubular epithelium antiserum. Dr. P. Mundel and Dr. J. Reiser (Department of Anatomy and Cell Biology, University of Heidelberg, Heidelberg, Germany) are gratefully acknowledged for their help to culture podocytes.
Footnotes
Address reprint requests to J. H. M. Berden, M.D., Ph.D., Division of Nephrology (545), University Hospital St. Radboud, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail: j.berden@nefro.azn.nl.
Supported by the Dutch Kidney Foundation (Grants 93.1318, 95.1530, and 95.1513) and the Dutch Diabetes Foundation (Grant DFN 940–10-009).
Jacob van den Born’s present address is Department of Cell Biology and Immunology, Free University, Amsterdam, The Netherlands.
References
- 1.Henry MD, Campbell KP: Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol 1996, 8:625-631 [DOI] [PubMed] [Google Scholar]
- 2.Campbell KP: Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 1995, 80:675-679 [DOI] [PubMed] [Google Scholar]
- 3.Worton R: Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science 1995, 270:755-756 [DOI] [PubMed] [Google Scholar]
- 4.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP: Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990, 345:315-319 [DOI] [PubMed] [Google Scholar]
- 5.Cote PD, Moukhles H, Lindenbaum M, Carbonetto S: Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet 1999, 23:338-342 [DOI] [PubMed] [Google Scholar]
- 6.Bowe MA, Deyst KA, Leszyk JD, Fallon JR: Identification and purification of an agrin receptor from torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron 1994, 12:1173-1180 [DOI] [PubMed] [Google Scholar]
- 7.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S: Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell 1994, 77:675-686 [DOI] [PubMed] [Google Scholar]
- 8.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP: Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992, 355:696-702 [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LVB, Fujita S, Fukuta-Ohi H, Shimizu T, Ruegg MA, Matsumura K: Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J Biol Chem 1996, 271:23418-23423 [DOI] [PubMed] [Google Scholar]
- 10.Rosa G, Ceccarini M, Cavaldesi M, Zini M, Petrucci TC: Localization of the dystrophin binding site at the carboxyl terminus of beta-dystroglycan. Biochem Biophys Res Commun 1996, 223:272-277 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Yoshida M, Hayashi K, Mizuno Y, Hagiwara Y, Ozawa E: Molecular organization at the glycoprotein-complex-binding site of dystrophin. Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur J Biochem 1994, 220:283-292 [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Ozawa E: Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem 1990, 108:748-752 [DOI] [PubMed] [Google Scholar]
- 13.Way M, Pope B, Cross RA, Kendrick Jones J, Weeds AG: Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett 1992, 301:243-245 [DOI] [PubMed] [Google Scholar]
- 14.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP: Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 1992, 360:588-591 [DOI] [PubMed] [Google Scholar]
- 15.Matsumura K, Chiba A, Yamada H, Fukuta-Ohi H, Fujita S, Endo T, Kobata A, Anderson LVB, Kanazawa I, Campbell KP, Shimizu T: A role for dystroglycan in schwannoma cell adhesion to laminin. J Biol Chem 1997, 272:13904-13910 [DOI] [PubMed] [Google Scholar]
- 16.Angoli D, Corona P, Baresi R, Mora M, Wanke E: Laminin-alpha-2 but not -alpha-1-mediated adhesion of human (Duchenne) and murine (mdx) dystrophic myotubes is seriously defective. FEBS Lett 1997, 408:341-344 [DOI] [PubMed] [Google Scholar]
- 17.Raats CJI, Bakker MAH, Hoch W, Tamboer WPM, Groffen AJA, Van den Heuvel LPWJ, Berden JHM, Van den Born J: Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem 1998, 273:17832-17838 [DOI] [PubMed] [Google Scholar]
- 18.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P: Non-muscle alpha-dystroglycan is involved in epithelial development. J Cell Biol 1995, 130:79-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P: Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem 1998, 46:449-457 [DOI] [PubMed] [Google Scholar]
- 20.Love DR, Morris GE, Ellis JM, Fairbrother U, Marsden RF, Bloomfield JF, Edwards YH, Slater CP, Parry DJ, Davies KE: Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc Natl Acad Sci USA 1991, 88:3243-3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grishman E, Churg J: Focal glomerular sclerosis in nephrotic patients: an electron microscopic study of glomerular podocytes. Kidney Int 1975, 7:111-122 [DOI] [PubMed] [Google Scholar]
- 22.Powell HR: Relationship between proteinuria and epithelial cell changes in minimal lesion glomerulopathy. Nephron 1976, 16:310-317 [DOI] [PubMed] [Google Scholar]
- 23.Murphy WM, Moretta FL, Jukkola AF: Epithelial foot-process effacement in patients with proteinuria. Am J Clin Pathol 1979, 72:529-532 [DOI] [PubMed] [Google Scholar]
- 24.Bertani T, Poggi A, Pozzoni R, Delaini F, Sacchi G, Thoua Y, Mecca G, Remuzzi G, Donati MB: Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest 1982, 46:16-23 [PubMed] [Google Scholar]
- 25.Bohman SO, Jaremko G, Bohlin AB, Berg U: Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int 1984, 25:696-700 [DOI] [PubMed] [Google Scholar]
- 26.Gabbai FB, Gushwa LC, Wilson CB, Blantz RC: An evaluation of the development of experimental membranous nephropathy. Kidney Int 1987, 31:1267-1278 [DOI] [PubMed] [Google Scholar]
- 27.Messina A, Davies DJ, Dillane PC, Ryan GB: Glomerular epithelial abnormalities associated with the onset of proteinuria in aminonucleoside nephrosis. Am J Pathol 1987, 126:220-229 [PMC free article] [PubMed] [Google Scholar]
- 28.Inokuchi S, Shirato I, Kobayashi N, Koide H, Tomino Y, Sakai T: Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney Int 1996, 50:1278-1287 [DOI] [PubMed] [Google Scholar]
- 29.Whiteside C, Prutis K, Cameron R, Thompson J: Glomerular epithelial detachment, not reduced charge density, correlates with proteinuria in adriamycin and puromycin nephrosis. Lab Invest 1989, 61:650-659 [PubMed] [Google Scholar]
- 30.Laurens W, Battaglia C, Foglieni C, De Vos R, Malanchini B, Van Damme BJC, Vanrenterghem YF, Remuzzi G, Remuzzi A: Direct podocyte damage in the single nephron leads to albuminuria in vivo. Kidney Int 1995, 47:1078-1086 [DOI] [PubMed] [Google Scholar]
- 31.Orikasa M, Matsui K, Oite T, Shimizu F: Massive proteinuria in rats by a single intravenous injection of a monoclonal antibody. J Immunol 1988, 141:807-814 [PubMed] [Google Scholar]
- 32.Kawachi H, Matsui K, Orikasa M, Morioka T, Oite T, Shimizu F: Quantitative studies of monoclonal antibody 5–1-6-induced protein-uric state in rats. Clin Exp Immunol 1992, 87:215-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desassis JF, Raats CJI, Bakker MAH, Van den Born J, Berden JHM: Anti-proteinuric effect of cyclosporin A in adriamycin nephropathy in rats. Nephron 1997, 75:336-341 [DOI] [PubMed] [Google Scholar]
- 34.Raats CJI, Bakker MAH, Van den Born J, Berden JHM: Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem 1997, 272:26734-26741 [DOI] [PubMed] [Google Scholar]
- 35.Mundel P, Reiser J, Kriz W: Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 1997, 8:697-705 [DOI] [PubMed] [Google Scholar]
- 36.Wolthuis A, Boes A, Rodemann HP, Grond J: Vasoactive agents affect growth and protein synthesis of cultured rat mesangial cells. Kidney Int 1992, 41:124-131 [DOI] [PubMed] [Google Scholar]
- 37.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 38.Rupp F, Payan DG, Magill-Solc C, Cowan DM, Scheller RH: Structure and expression of a rat agrin. Neuron 1991, 6:811-823 [DOI] [PubMed] [Google Scholar]
- 39.Rupp F, Ozcelik T, Linial M, Peterson K, Francke U, Scheller RH: Structure and chromosomal localization of the mammalian agrin gene. J Neurosci 1992, 12:3535-3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee NH, Weinstock KG, Kirkness EF, Earle-Hughes JA, Fuldner RA, Marmaras S, Glodek A, Gocayne JD, Adams MD, Kerlavage AR, Fraser CM, Venter JC: Comparative expressed sequence tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment. Proc Natl Acad Sci U S A 1995, 92:8303-8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo WX, Nichol M, Merlie JP: Cloning and expression of full length mouse utrophin: the differential association of utrophin and dystrophin with AChR clusters. FEBS Lett 1996, 398:259-264 [DOI] [PubMed] [Google Scholar]
- 42.Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR, Edwards YH, Davies KE: Primary structure of dystrophin-related protein. Nature 1992, 360:591-593 [DOI] [PubMed] [Google Scholar]
- 43.Hoch W, Campanelli JT, Harrison S, Scheller RH: Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J 1994, 13:2814-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsen G, Halfter W, Kroger S, Cole GJ: Agrin is a heparan sulfate proteoglycan. J Biol Chem 1995, 270:3392-3399 [DOI] [PubMed] [Google Scholar]
- 45.Ervasti JM, Campbell KP: A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 1993, 122:809-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bewick GS, Nicholson LVB, Young C, Slater CR: Relationship of a dystrophin-associated glycoprotein to junctional acetylcholine receptor clusters in rat skeletal muscle. Neuromuscul Disord 1993, 3:503-506 [DOI] [PubMed] [Google Scholar]
- 47.Bewick GS, Nicholson LVB, Young C, O’Donnell E, Slater CR: Different distributions of dystrophin and related proteins at nerve-muscle junctions. Neuroreport 1992, 3:857-860 [DOI] [PubMed] [Google Scholar]
- 48.Nicholson LV, Johnson MA, Davison K, O’Donnell E, Falkous G, Barron M, Harris JB: Dystrophin or a “related protein” in Duchenne muscular dystrophy? Acta Neurol Scand 1992, 86:8-14 [DOI] [PubMed] [Google Scholar]
- 49.Mundel P, Gilbert P, Kriz W: Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem 1991, 39:1047-1056 [DOI] [PubMed] [Google Scholar]
- 50.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 1990, 137:929-944 [PMC free article] [PubMed] [Google Scholar]
- 51.Holthofer H, Sainio K, Miettinen A: Rat glomerular cells do not express podocytic markers when cultured in vitro. Lab Invest 1991, 65:548-557 [PubMed] [Google Scholar]
- 52.Roberds SL, Anderson RD, Ibraghimov Beskrovnaya O, Campbell KP: Primary structure and muscle-specific expression of the 50-kDa dystrophin-associated glycoprotein (adhalin). J Biol Chem 1993, 268:23739-23742 [PubMed] [Google Scholar]
- 53.Godfrey EW, Dietz ME, Morstad AL, Wallskog PA, Yorde DE: Acetylcholine receptor-aggregating proteins are associated with the extracellular matrix of many tissues in Torpedo. J Cell Biol 1988, 106:1263-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoch W, Ferns MJ, Campanelli JT, Hall ZW, Scheller RH: Developmental regulation of highly active alternatively spliced forms of agrin. Neuron 1993, 11:479-490 [DOI] [PubMed] [Google Scholar]
- 55.Groffen AJA, Ruegg MA, Dijkman HBPM, Van der Velden TJ, Buskens CA, Van den Born J, Assmann KJM, Monnens LAH, Veerkamp JH, Van den Heuvel LPWJ: Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem 1998, 46:19-27 [DOI] [PubMed] [Google Scholar]
- 56.Farquhar MG, Lemkin MC, Stow JL: Role of proteoglycans in glomerular function and pathology. Robinson RR eds. Nephrology. 1985, :pp 580-590 Springer-Verlag, New York [Google Scholar]
- 57.Kanwar YS, Liu ZZ, Kashihara N, Wallner EI: Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol 1991, 11:390-413 [PubMed] [Google Scholar]
- 58.Van den Born J, Van den Heuvel LPWJ, Bakker MAH, Veerkamp JH, Assmann KJM, Berden JHM: A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int 1992, 41:115-123 [DOI] [PubMed] [Google Scholar]
- 59.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP: Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet 1997, 6:831-841 [DOI] [PubMed] [Google Scholar]
- 60.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA: Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem 1998, 273:600-605 [DOI] [PubMed] [Google Scholar]
- 61.Wakayama Y, Inoue M, Murahashi M, Shibuya S, Jimi T, Kojima H, Oniki H: Ultrastructural localization of adhalin in normal murine skeletal myofiber. Ann Neurol 1996, 39:217-223 [DOI] [PubMed] [Google Scholar]
- 62.Inoue M, Wakayama Y, Murahashi M, Shibuya S, Jimi T, Kojima H, Oniki H: Electron microscopic observations of triple immunogold labelling for dystrophin, beta-dystroglycan and adhalin in human skeletal myofibers. Acta Neuropathol (Berl) 1996, 92:569-575 [DOI] [PubMed] [Google Scholar]
- 63.Cullen MJ, Walsh J, Stevenson SA, Rothery S, Severs NJ: Co-localization of dystrophin and beta-dystroglycan demonstrated in en face view by double immunogold labeling of freeze-fractured skeletal muscle. J Histochem Cytochem 1998, 46:945-953 [DOI] [PubMed] [Google Scholar]
- 64.Mizuno Y, Yoshida M, Yamamoto H, Hirai S, Ozawa E: Distribution of dystrophin isoforms and dystrophin-associated proteins 43DAG (A3a) and 50DAG (A2) in various monkey tissues. J Biochem 1993, 114:936-941 [DOI] [PubMed] [Google Scholar]
- 65.Khurana TS, Hoffman EP, Kunkel LM: Identification of a chromosome 6-encoded dystrophin-related protein. J Biol Chem 1990, 265:16717-16720 [PubMed] [Google Scholar]
- 66.Thi Man N, Ellis JM, Love DR, Davies KE, Gatter KC, Dickson G, Morris GE: Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol 1991, 115:1695-1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen TM, Le TT, Blake DJ, Davies KE, Morris GE: Utrophin, the autosomal homologue of dystrophin, is widely-expressed and membrane-associated in cultured cell lines. FEBS Lett 1992, 313:19-22 [DOI] [PubMed] [Google Scholar]
- 68.Sawada H, Stukenbrok H, Kerjaschki D, Farquhar MG: Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am J Pathol 1986, 125:309-318 [PMC free article] [PubMed] [Google Scholar]
- 69.Kerjaschki D, Poczewski H, Dekan G, Horvat R, Balzar E, Kraft N, Atkins RC: Identification of a major sialoprotein in the glycocalyx of human visceral glomerular epithelial cells. J Clin Invest 1986, 78:1142-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dekan G, Miettinen A, Schnabel E, Farquhar MG: Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection. Localization of antigens and bound IgGs by immunoelectron microscopy. Am J Pathol 1990, 137:913-927 [PMC free article] [PubMed] [Google Scholar]
- 71.Wiggins RC, Wiggins JE, Goyal M, Wharram BL, Thomas PE: Molecular cloning of cDNAs encoding human GLEPP1, a membrane protein tyrosine phosphatase: characterization of the GLEPP1 protein distribution in human kidney and assignment of the GLEPP1 gene to human chromosome 12p12–p13. Genomics 1995, 27:174-181 [DOI] [PubMed] [Google Scholar]
- 72.Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC: GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem 1994, 269:19953-19962 [PubMed] [Google Scholar]
- 73.Breiteneder Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D: Podoplanin novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 1997, 151:1141-1152 [PMC free article] [PubMed] [Google Scholar]
- 74.Gelberg H, Healy L, Whiteley H, Miller LA, Vimr E: In vivo enzymatic removal of alpha 2–>6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest 1996, 74:907-920 [PubMed] [Google Scholar]
- 75.Ervasti JM, Campbell KP: Membrane organization of the dystrophin-glycoprotein complex. Cell 1991, 66:1121-1131 [DOI] [PubMed] [Google Scholar]
- 76.Kerjaschki D, Ojha PP, Susani M, Horvat R, Binder S, Hovorka A, Hillemanns P, Pytela R: A beta-1 integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol 1989, 134:481-489 [PMC free article] [PubMed] [Google Scholar]
- 77.Martin PT, Sanes JR: Integrins mediate adhesion to agrin and modulate agrin signaling. Development 1997, 124:3909-3917 [DOI] [PubMed] [Google Scholar]
- 78.Yoshida T, Pan Y, Hanada H, Iwata Y, Shigekawa M: Bidirectional signaling between sarcoglycans and the integrin adhesion system in cultured L6 myocytes. J Biol Chem 1998, 273:1583-1590 [DOI] [PubMed] [Google Scholar]
- 79.Groffen AJA, Veerkamp JH, Monnens LA, Van den Heuvel LPWJ: Recent insights in the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant 1999, 14:2119-2129 [DOI] [PubMed] [Google Scholar]