Abstract
Physiological scrotal hypothermia is necessary for normal spermatogenesis and fertility in mammals. Human RNA binding motif protein 3 (RBM3) is structurally highly similar to the cold-inducible RNA-binding protein (Cirp), and both mRNAs are induced in human cells at the scrotal temperature (32°C). We report here the cloning of mouse Rbm3 cDNA, which encoded an 18-kd protein with 94% identity in amino acid sequence to that of human RBM3. In the testis of adult mice, Rbm3 mRNA and protein were detected in Sertoli cells, but not germ cells, of seminiferous tubules at all stages. The expression was not observed in Sertoli cells of fetuses, but was observed in newborn and older mice. In the TAMA26 mouse Sertoli cell line, the Rbm3 expression level was increased or decreased within 12 hours after temperature shift from 37°C to 32°C or 39°C, respectively. In contrast to Cirp, the cold-induced growth suppression of TAMA26 cells was not affected by suppression of the Rbm3 expression. When mouse testis was exposed to heat stress by experimental cryptorchidism, the level of Rbm3 was decreased in Sertoli cells. Rbm3 may play important roles distinct from those played by Cirp in spermatogenesis and cryptorchidism by regulating the gene expression in Sertoli cells.
In mammals, male germ cells cannot develop independently but are heavily dependent on both chemical and physical interactions with somatic cells of the testis. 1-4 Sertoli cells play especially prominent roles in the cell-to-cell interactions necessary for germ cell development by their physical association with and the transfer of molecules to the developing germ cells. Sertoli cells also establish an important physiological separation between premeiotic and postmeiotic germ cells by the formation of the Sertoli cell barrier, which allows bidirectional movement of Sertoli cell products to different populations of the developing germ cells.
Most mammals have testes within a scrotum, the temperature of which is maintained 2 to 8°C lower than the body cavity temperature. 5,6 The low temperature is believed to be important for normal testicular function. 7,8 In experimental animals, surgical induction of cryptorchidism or exposure to heat stress causes disruption of spermatogenesis, leading to infertility. 7,9 Various exogenous thermal factors, including those observed in welders and paraplegic patients in wheelchairs, are proposed to be risk factors for human male infertility. 10-12 Cryptorchidism and varicocele of the spermatic veins are associated with male infertility, and their pathogenesis is attributed to thermal factors. 13,14 Although all cell types in the testis including germ cells, Sertoli cells, and Leydig cells may be affected by the elevated temperature, effects on the germ cells have been most extensively investigated. The earliest cellular changes noticed in experimentally cryptorchid testes are in early pachytene spermatocytes and early spermatids. 15-17 Especially in pachytene spermatocytes, apoptotic cell death is induced within 2 to 4 days. 17 The initial phase of germ-cell apoptosis has been reported to be p53-dependent and the subsequent apoptosis to be p53-independent. 18 The molecular mechanisms of the thermal effect on spermatogenesis are, however, just beginning to be clarified.
Recently we have isolated the cold-inducible RNA-binding protein (Cirp) from a mouse testis cDNA library. 19 In the testis of mice and humans, Cirp is constitutively expressed in the germ cells, which is decreased at an elevated temperature. 20 In somatic cells, Cirp is induced by mild cold stress (32°C), and mediates the cold-induced suppression of cell growth. 19 Human RNA-binding motif protein 3 (RBM3), originally isolated from the Xp11.2 region of chromosome during positional cloning of disease-causing genes, encodes a protein structurally related to Cirp, 21 and both Cirp and RBM3 transcripts are induced by cold stress in human cells. 22 In the present study, we report an isolation of mouse Rbm3 cDNA. We identified the cells expressing Rbm3 in mouse testis and assessed the effect of temperature stress on its expression.
Materials and Methods
Mice and Cells
Sterile WBB6F1-W/Wv mutant mice were obtained through the mating between congenic C57BL/6-Wv/+ and WB-W/+ mice. Two-month-old and 4-month-old C57BL/6 wild-type (+/+) mice and 4-month-old WBB6F1-W/Wv mutant mice were purchased from Japan SLC Company (Hamamatsu, Japan).
BALB/3T3 fibroblasts, BMA1 bone marrow stromal cells, and TAMA26 Sertoli cells were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) supplemented with 10% calf serum at 37°C in a humidified atmosphere of 5% CO2 in air as described. 23 In experiments where effects of growth temperature were examined, cells were grown at 37°C for 96 hours and then shifted to 32°C, 37°C, or 39°C. After 12 hours, cells were scraped, frozen in liquid nitrogen, and stored at −80°C until analysis.
Antisense and sense oligodeoxynucleotides (ODNs) containing phosphorothioates were purchased from Cruachem (Kyoto, Japan). The sequence of the sense ODN was (5′-GCCTTCTGCCATGTCGTCTG-3′) spanning the translation initiation site of Rbm3 mRNA, and that of antisense ODN was complementary to this sequence. Cell numbers were determined after 2 days of culture at 32°C or 37°C in the presence of 0.5, 1.0, or 1.5 μmol/L of ODN as described previously. 19
Experimental Cryptorchidism
To induce unilateral cryptorchidism, 4-month-old +/+ male mice were anesthetized by intraperitoneal injection of 25 mg/kg pentobarbital sodium, and a small incision was made in the abdomen. On one side, the testis was fixed in the abdominal cavity by suturing its capsule to peritoneum. On the other side of the same animal, the testis was not fixed and used as a control. The testes of sham-operated mice were used as another control. The animals were sacrificed at different intervals after surgery. The testes were removed, frozen in liquid nitrogen, and stored at −80°C until analysis.
Cloning of Mouse Rbm3 cDNA
Mouse EST clone (AA218202) was obtained from Research Genetics, Inc. (Huntsville, AL). The insert was excised from the clone and ligated into the NotI and EcoRI sites of pBluescript SK(-) vectors. The nucleotide sequence was determined by the dideoxy method as described. 19
RNA Extraction and Northern Blot Hybridization
Mice tissues and cells were dissolved in TRIzol reagent (Life Technologies, Tokyo, Japan) and RNA was extracted by following the manufacturer’s instructions. Twenty μg of total RNA of each sample was separated in 1.0% agarose/formaldehyde gels by electrophoresis and was blotted onto nylon filters (Hybond-N+; Amersham, Buckinghamshire, UK). The filters were hybridized with [α-32P] dCTP-labeled random-primed cDNA fragments, then washed under stringent conditions (65°C for 30 minutes in a washing buffer composed of 0.1× standard saline citrate and 0.1% sodium dodecyl sulfate), and detected by autoradiography. A fragment of Rbm3 cDNA (nucleotide positions 86 to 547) was used as a probe. The filters were stripped and rehybridized with a cDNA probe for the S26 ribosomal protein as an internal control.
Western Blot Analysis
The anti-Rbm3 polyclonal antibody was produced by immunizing rabbits with a carboxyl-terminal oligopeptide (SGGNYRDNYDN) common to mice and humans. For Western blot analysis, 20 μg of proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto polyvinyl difluoride membranes (Millipore, Tokyo, Japan), and treated with the anti-Rbm3 antiserum (1: 20,000 diluted). Protein concentrations were determined using the Bradford reagent (BioRad Labs, Hercules, CA). Bound antibody was detected using a goat anti-rabbit immunoglobulin G (IgG) conjugated with horseradish peroxidase (BioRad) and the enhanced chemiluminescence system (ECL, Amersham Life Science).
Immunohistochemistry
Immunohistochemistry was performed using sections from the mouse testes fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and embedded in wax. Five-μm sections were mounted on siran-coated glass slides. Rbm3 was immunolocalized using the indirect immunoperoxidase or immunofluorescence method. Briefly, tissue sections were incubated with 5% bovine serum albumin in PBS, pH 7.5, for 1 hour. They were subsequently reacted with the anti-Rbm3 antibody at a dilution of 1: 20,000 overnight at 4°C. After washing in PBS containing 0.2% Tween-20 and blocking with 5% bovine serum albumin, the sections were incubated with peroxidase-conjugated anti-rabbit IgG for 1 hour at room temperature. Diaminobenzidine was used as substrate for the peroxidase reaction. Sections were counterstained with hematoxylin. As a control, the preimmune antiserum was used at the same dilution. In some experiments, nuclei of testicular germ cell were specifically stained by the rat monoclonal antibody TRA 98, kindly provided by Dr. Y. Nishimune, Osaka University. 24 Detection was with fluorescein isothiocyanate-conjugated goat anti- rat IgG (DAKO, Kyoto, Japan) diluted 1:1,000 and a fluorescence microscope (Olympus, Tokyo, Japan) equipped with the fluorescein-isothiocyanate filter set.
Results
Cloning of Mouse Rbm3
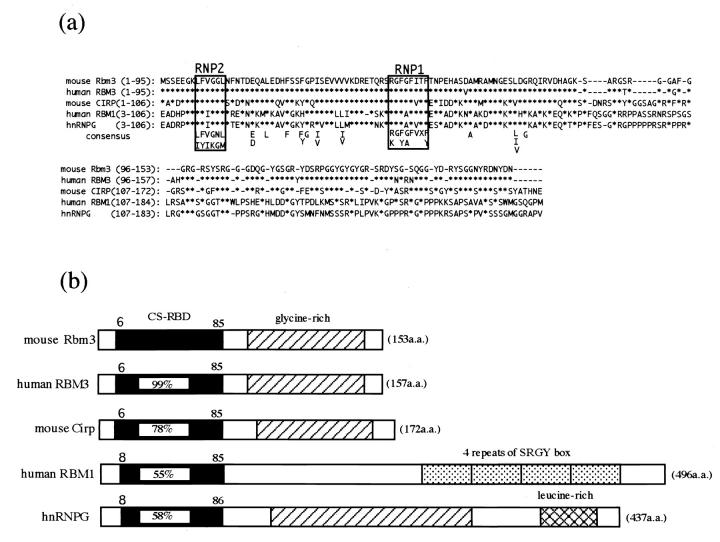
To isolate the mouse homolog of human RBM3 cDNA, we searched the GenBank database for similar cDNA sequences and identified a mouse EST clone (AA218202). Sequencing of the EST clone revealed that it contained a 1158-bp insert potentially encoding 153 amino acids (Figure 1) ▶ . The sequence around the first ATG codon provided a favorable context for translation initiation. 25 The predicted amino acid sequence displayed two main features: the presence of an amino-terminal consensus sequence RNA-binding domain (CS-RBD) and a carboxyl-terminal part rich in glycine. The predicted amino acid sequence of this clone was compared to known sequences in GenBank using the Wisconsin Package (Genetics Computer Group, Madison, WI). The overall amino acid sequence was 94% identical to that of human RBM3. 21 The CS-RBD of Rbm3 contained consensus sequences of RNP1 and RNP2, and a number of highly conserved segments that could be perfectly aligned with those of other CS-RBDs (Figure 2) ▶ . Together with Cirp and some others, Rbm3 constitutes a glycine-rich RNA-binding protein subgroup in the family of proteins with CS-RBD. 26
Figure 1.
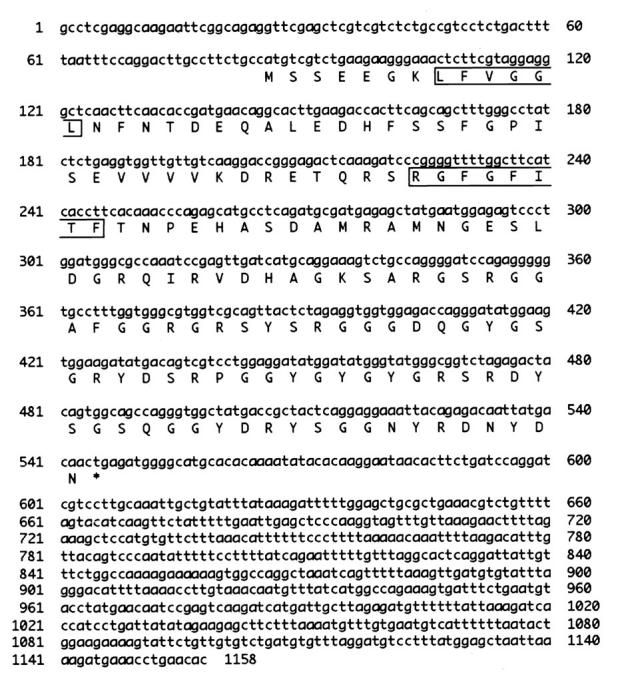
Nucleotide and deduced amino acid sequences of mouse Rbm3. Amino acid sequence is shown in single-letter code below the nucleotide sequence. Putative RNP motifs are boxed. *, terminal codon. The mouse Rbm3 cDNA sequence data are available from GenBank under accession number AB016424.
Figure 2.
Comparison of mouse Rbm3 with its related proteins. a: Comparison of amino acid sequences of mouse Rbm3, human RBM3, 21 mouse Cirp, 19 human YRRM/RBM1, 28 and human hnRNPG. 33 The consensus sequence for the RNA-binding domain as determined by Burd and Dreyfuss 34 is also shown. *, amino acid identical to the mouse Rbm3 sequence. Bars mark gaps introduced to optimize the alignment. RNP1 and RNP2 sequences are boxed. b: Structural comparison. The length of each bar does not reflect the actual length of the sequences. The numbers in the black boxes indicate percentage identity of amino acid sequence to mouse Rbm3 in the CS-RBD.
Expression of Rbm3 in Various Tissues of Adult Mouse
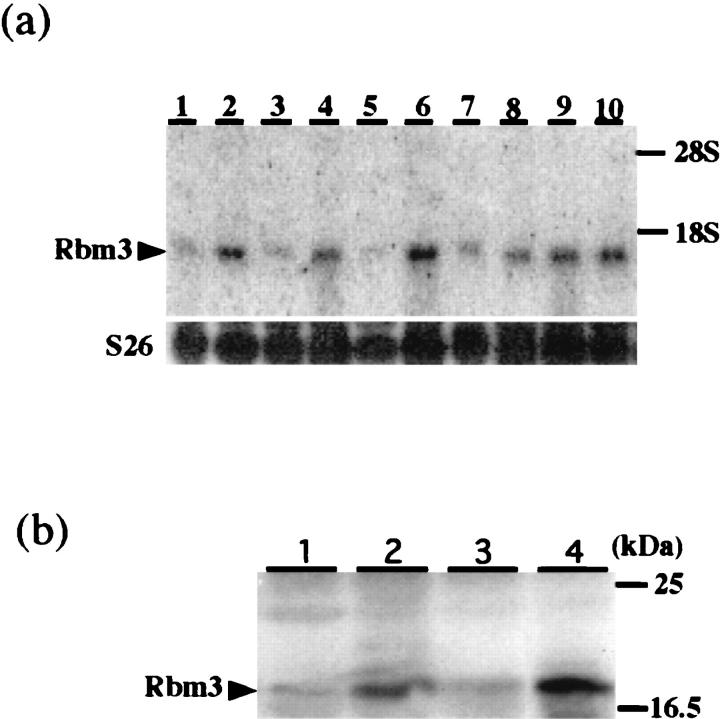
When we analyzed the expression of Rbm3 mRNA in various mouse tissues by Northern blot analysis, a single band of 1.1 kb was detected in all tissues examined including the testis and spleen (Figure 3a) ▶ .
Figure 3.
Expression of Rbm3 mRNA and protein in various mouse tissues. a: Northern blot analysis using mouse Rbm3 cDNA (upper) or S26 ribosomal protein cDNA (lower) as a probe. Total RNAs were from the cerebrum (lane 1), cerebellum (lane 2), heart (lane 3), lung (lane 4), liver (lane 5), spleen (lane 6), kidney (lane 7), testis (lane 8), intestine (lane 9), and uterus (lane 10). b: Western blot analysis of lysates from various tissues using the anti-Rbm3 polyclonal antibody. Lysates were made from the kidney (lane 1), testis (lane 2), liver (lane 3), and spleen (lane 4).
As shown in Figure 3b ▶ , the antibody raised against the carboxyl-terminal peptide sequence of mouse Rbm3 recognized an 18-kd protein in all lysates from the examined mouse tissues. The size of the band was as expected from the predicted amino acid sequence of Rbm3, and was not detectable when preimmune serum was used (data not shown). Consistent with the results of Northern blot analysis, the levels of Rbm3 were high in the testis and spleen.
Expression of Rbm3 in Sertoli Cells
To identify the cells expressing Rbm3 in the testis, adult mouse testis was analyzed by immunohistochemistry using the anti-Rbm3 antibody. As shown in Figure 4 ▶ , a and c, strong signals were detected in the nuclei of Sertoli cells. Stage-dependency of the signal intensity was not detected under the present conditions. Expression of Rbm3 in Sertoli cells was further confirmed by analysis of the testis of the adult W/Wv mutant mice which are deficient in germ cells. 20 As shown in Figure 4d ▶ , Sertoli cells were positively stained with the anti-Rbm3 antibody in all of the tubules examined. To determine whether the signals were present in germ cells as well as Sertoli cells, serial sections of testis were stained with the germ cell-specific TRA 98 antibody 24 or the anti-Rbm3 antibody. The cells stained with the anti-Rbm3 antibody (Figure 4e) ▶ were not stained with the TRA 98 antibody (Figure 4f) ▶ , indicating that the germ cells did not express Rbm3.
Figure 4.
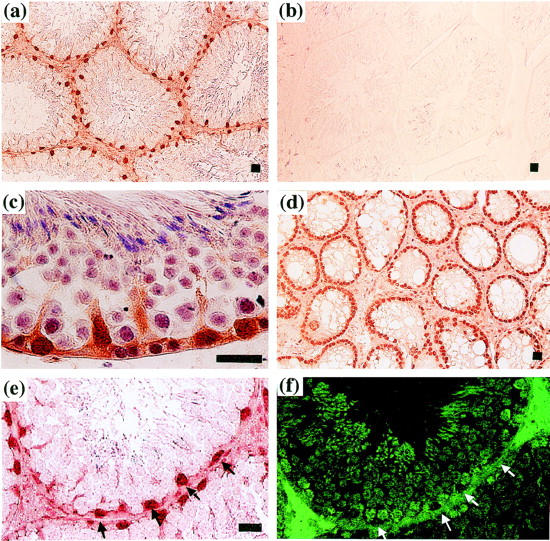
Localization of Rbm3 protein expression in mouse testis. Immunohistochemical staining of the testes of 4-month-old C57BL/6 mice (a, b, c, e, and f) and W/Wv mutant mice deficient in germ cells (d). Sections were incubated with the rabbit anti-Rbm3 antibody (a, c, d, and e) or preimmune antiserum (b), and bound antibody was detected using the anti-rabbit IgG conjugated with horseradish peroxidase. f: A serial section of (e) was incubated with the TRA 98 germ cell-specific antibody, 24 and bound antibody was detected using the fluorescein isothiocyanate-conjugated secondary antibody and fluorescence microscopy. Arrowheads indicate the Rbm3-positive Sertoli cells. Scale bar, 20 μm.
Expression of Rbm3 during Testicular Development
Testes at several developmental stages were immunohistochemically analyzed for expression of Rbm3. Rbm3 was not detected in the testis of 17-day-old fetus (Figure 5a) ▶ . A low-level expression was detected in the Sertoli cells of newborn mice (Figure 5b) ▶ . At this age, signals were also observed in the interstitial cells of the testis. In the testis of 8-day-old mice, strong signals were present only in the Sertoli cells (Figure 5c) ▶ . Sertoli cells were located in the periphery of the seminiferous tubules in the testis of 17-day-old mice, and they were strongly positive for the Rbm3 expression (Figure 5e) ▶ . The strong signals were also observed in the Sertoli cells of 21-day-old mice (Figure 5f) ▶ . Thus, during testicular development, distinct Rbm3 expression was first observed at early neonatal period and continued thereafter in Sertoli cells.
Figure 5.
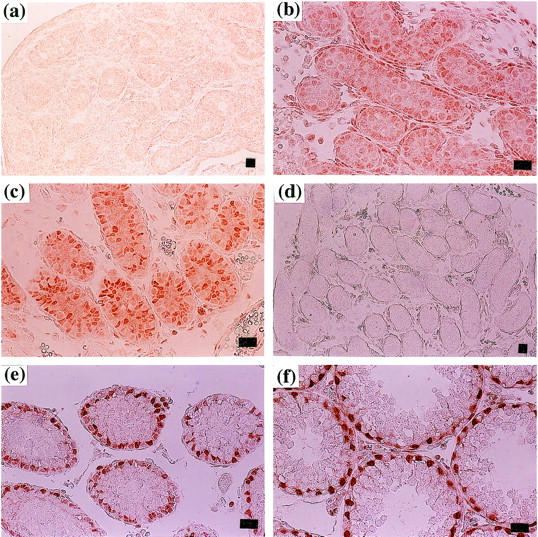
Expression of Rbm3 during testicular development. Testes were isolated from 17 days postcoitus fetus (a), newborn (b), 8-day (c and d), 17-day (e), and 21-day (f) -old mice. Immunohistochemical staining was performed using the anti-Rbm3 antibody (a, b, c, e, and f) or preimmune antiserum (d). Scale bar, 20 μm.
Effects of Temperature Stress on Rbm3 Expression in Mouse Cell Lines
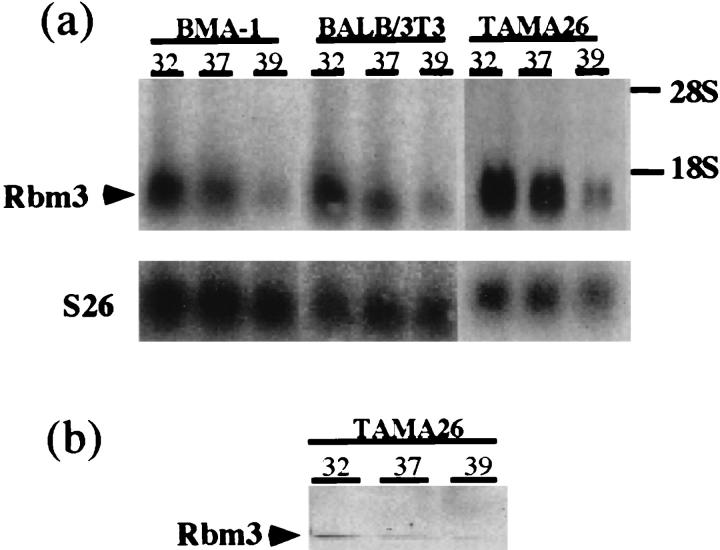
We exposed the mouse cell lines BMA-1 bone marrow stromal cells, BALB/3T3 fibroblasts, and TAMA26 Sertoli cells to cold (32°C) or heat (39°C) stress. As shown in Figure 6a ▶ , Northern blot analysis demonstrated that the levels of Rbm3 mRNA were increased in response to the cold stress in all of the cell lines examined. By contrast, the heat stress decreased the levels of Rbm3 expression. The expression was also affected at the protein level (Figure 6b) ▶ . The anti-Rbm3 antibody specifically recognized the 18-kd protein in the lysates of TAMA26 cells. In agreement with the results of Northern blot analysis, the protein level was increased after the temperature shift from 37°C to 32°C.
Figure 6.
Temperature sensitivity of Rbm3 expression in mouse cell lines. a: Northern blot analysis using Rbm3 cDNA (upper) or S26 ribosomal protein cDNA (lower) as a probe. Total RNAs were from BMA-1 bone marrow stromal cells (lanes 1–3), BALB/3T3 fibroblasts (lanes 4–6), and TAMA26 Sertoli cells (lanes 7–9). Cells were cultured at 37°C, transferred to the indicated temperatures, and harvested after 12 hours of incubation at the respective temperature. The positions of 18S and 28S ribosomal RNAs are indicated on the right. b: Western blot analysis using the anti-Rbm3 antibody. Lysates were from TAMA26 cells incubated at 32°C (lane 1), 37°C (lane 2), or 39°C (lane 3) for 12 hours.
Downshift of temperature from 37°C to 32°C is known to suppress the proliferation of cultured mammalian cells and induce expression of Cirp and RBM3. 19,22 By suppressing the induction of Cirp with the antisense ODN, this growth impairment can be alleviated. 19 When culture temperature was shifted from 37°C to 32°C, the growth of TAMA26 Sertoli cells was impaired, which was partly prevented by the presence of ODN antisense to Cirp mRNA as expected (data not shown). The presence of the ODN antisense to Rbm3 mRNA in the culture medium, however, had no effect on the growth of TAMA26 cells, although the induction of Rbm3 was partly inhibited (data not shown). Thus, in contrast to Cirp, Rbm3 does not seem to play a significant role in cold-induced modification of cell proliferation.
Effect of Cryptorchidism on Rbm3 Expression
To examine the effect of heat stress on Rbm3 expression in the testis in vivo, we made use of experimental cryptorchidism. As shown in Figure 7a ▶ , Northern blot analysis revealed that Rbm3 transcripts were decreased in the cryptorchid testes within 12 hours after the surgery as compared with the contralateral testis of the same animal as well as the sham-operated testis. By immunohistochemistry using the anti-Rbm3 antibody, the decrease in Rbm3 protein expression was detected in Sertoli cells of the cryptorchid testis (Figure 7c) ▶ .
Figure 7.
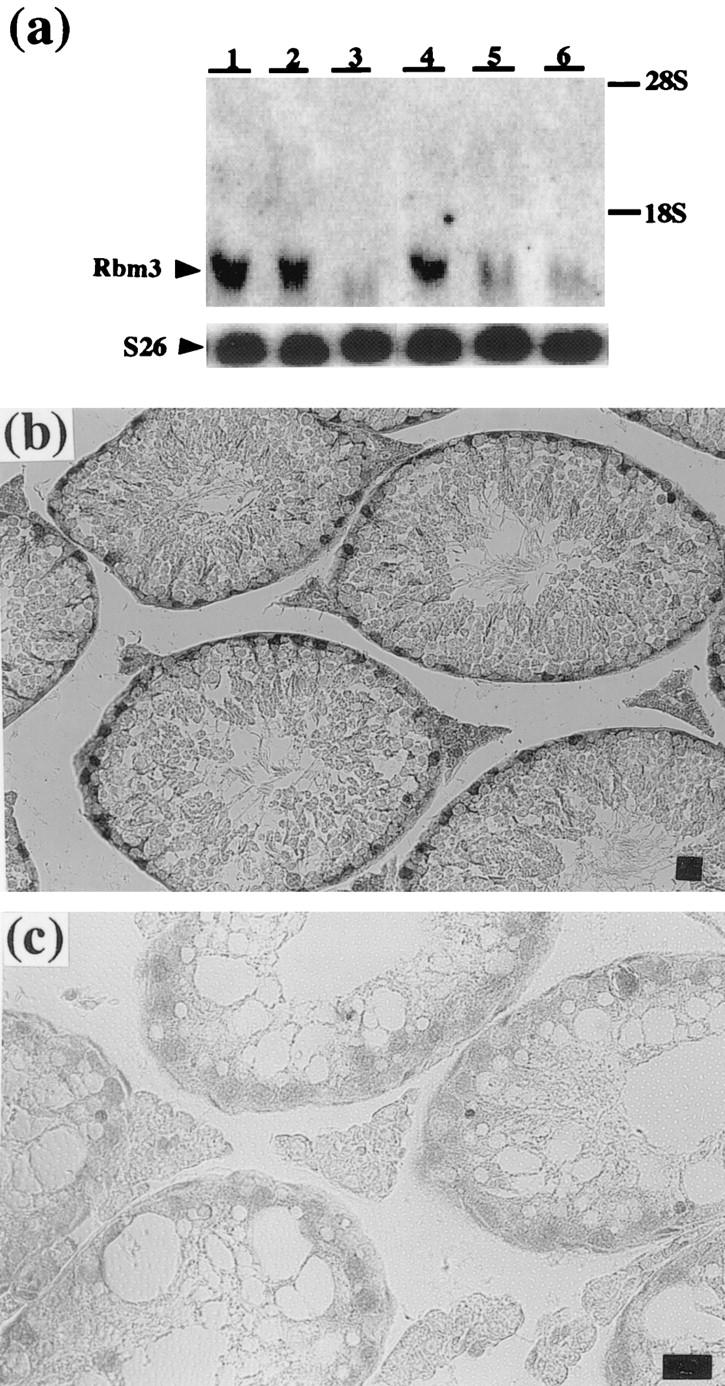
Effect of experimental cryptorchidism on Rbm3 expression in mouse testis. a: Northern blot analysis of total RNAs from cryptorchid testis (lanes 3 and 6), contralateral testis of the same animal (lanes 2 and 5) or sham-operated testis (lanes 1 and 4) 12 hours (lanes 1 to 3) or 1 week (lanes 4 to 5) after the operation. Rbm3 cDNA (upper) or S26 ribosomal protein cDNA (lower) was used as a probe. b and c: Immunohistochemical analysis of Rbm3 expression in the cryptorchid testis (c) and sham-operated testis (b) 2 weeks after the operation. Sections were stained using the anti-Rbm3 antibody. Scale bar, 20 μm.
Discussion
In the present study, we isolated the mouse homologue of human RBM3 cDNA. The expression of mouse Rbm3 was induced by mild cold stress in cultured somatic cells. In the mouse testis it was expressed in Sertoli cells, and the level of expression was decreased by experimental cryptorchidism.
It is known that some proteins with CS-RBD play important roles in spermatogenesis. For example, chromosomal deletion of the Rb97D gene results in azoospermia in Drosophila. 27 The Y-chromosome genes YRRM/RBM1 and DAZ (deleted in azoospermia) are candidate genes for azoospermia factor that controls human spermatogenesis. 28,29 The X chromosome gene RBM3 has been found to encode a protein with high amino acid identity to YRRM/RBM1 (51%), and suggested to have a related function. 21 Subsequent isolation of Cirp, however, has demonstrated that RBM3 is most similar to Cirp in amino acid sequence, and together they belong to a glycine-rich RNA-binding protein family. 26 Cirp is an RNA-binding protein with a growth suppressing activity and its expression is inducible at 32°C in mouse somatic cells. 19 It is constitutively expressed in various tissues and most strongly in the testis. In the mouse testes, Cirp is expressed in the germ cells, and the level varies depending on the stage of differentiation. 20 Similar to Cirp, the expression of Rbm3 was induced at 32°C in mouse somatic cells. However, Rbm3 did not seem to mediate the cold-induced growth suppression. The kinetics of induction by cold stress is slightly different between Cirp and RBM3. 22 The tissue distribution patterns of constitutive expression were different. Although expressions of both Cirp and Rbm3 were decreased in the cryptorchid testis, Cirp is expressed in the germ cells 20 whereas Rbm3 was in Sertoli cells. These findings suggest that Cirp and Rbm3 play distinct roles under physiological and stress conditions.
Sertoli cells are known to proliferate most actively in the fetal testis shortly before birth. 30 The rate of the Sertoli cell growth declines linearly thereafter and this proliferation ceases entirely by approximately 3 weeks after birth. In contrast, development of mature Sertoli cell function begins in the testis at approximately 2 weeks of age. 31 The testis of 2-month-old mice contains fully differentiated, mature Sertoli cells. We observed a high level of Rbm3 expression in Sertoli cells of 8-day-old mice and thereafter. Thus, the timing of the emergence of Rbm3 expression during mouse testis development shows correlation with the Sertoli cell acquisition of developmental function.
Sertoli cells provide essential physical and trophic support for developing spermatogonia, spermatocytes, and spermatids. 1-4 This trophic support in turn depends on a network of reciprocal signaling interactions between Leydig cells, Sertoli cells, peritubular cells and germ cells within the testis, and on upstream hormonal regulation from the pituitary. Recently the importance of Sertoli cells in spermatogenesis has definitely been demonstrated by analysis of null mutations in the mouse genes encoding three structurally related tyrosine kinase receptors: Tyro 3, Axl, and Mer. 32 They are normally expressed by Sertoli cells during postnatal development, and male animals lacking all three receptors show progressive death of differentiating germ cells. The temperature of the testis is maintained at a specific temperature between 30 to 33°C depending on the species. 5,6 The difference of a few degrees between body and scrotum is critical for spermatogenesis. The present study demonstrated that the level of Rbm3 expression was regulated by temperature in Sertoli cells. The Rbm3 mRNA and protein expressions were decreased in the cryptorchid testis. The histological analysis confirmed that the decrease was not due to a decrease in the number of cells. At the body cavity temperature, male germ cells are easily damaged and our findings suggest a possibility that effects of heat on Sertoli cell functions related to those of Rbm3 contribute to the observed damages. Because proteins with CS-RBD are involved in posttranscriptional regulation of gene expression, identification of targets for Rbm3 will help elucidate the molecular mechanisms of spermatogenesis and its sensitivity to heat .
Acknowledgments
We thank Dr. Y. Nishimune, Osaka University and Dr. H. Nishiyama, Kyoto University for helpful suggestions, and Ms. S. Takatori for skillful technical assistance.
Footnotes
Address reprint requests to Dr. Jun Fujita, Department of Clinical Molecular Biology, Faculty of Medicine, Kyoto University, 54 Shogoin Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan. E-mail: jfujita@virus.kyoto-u.ac.jp.
Supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture and the Ministry of Agriculture, Forestry, and Fishery of Japan, and Kansai Medical University Research Grants.
References
- 1.Hecht NB: The making of a spermatozoon: a molecular perspective. Dev Genet 1995, 16:95-103 [DOI] [PubMed] [Google Scholar]
- 2.Skinner MK: Cell-cell interactions in the testis. Endocrine Rev 1991, 12:45-77 [DOI] [PubMed] [Google Scholar]
- 3.Russell LD: Morphological and functional evidence for Sertoli-germ cell relationships. Russell LD Griswold MD eds. The Sertoli Cell. 1993, :365-390 Cache River Press, Clearwater [Google Scholar]
- 4.Griswold MD: Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 1995, 52:211-216 [DOI] [PubMed] [Google Scholar]
- 5.Harrison R, Weiner J: Abdomino-testicular temperature gradients. J Physiol 1948, 107:48P [Google Scholar]
- 6.Setchell BP: Spermatogenesis and spermatozoa. Reproduction in mammals. Germ cells and fertilization, book 1: Edited by CR Austin, RV Short. Cambridge, Cambridge University Press, 1982, 63–101
- 7.Chowdhury AK, Steinberger E: Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil 1970, 22:205-212 [DOI] [PubMed] [Google Scholar]
- 8.Mieusset R, Bujan L, Mondinat C, Mansat A, Pontonnier F, Grandjean H: Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil Steril 1987, 48:1006-1011 [DOI] [PubMed] [Google Scholar]
- 9.Rommerts FF, Jong F, Grootegoed JA, Molen VH: Metabolic changes in testicular cells from rats after long-term exposure to 37 degrees C in vivo or in vitro. J Endocrinol 1980, 85:471-479 [DOI] [PubMed] [Google Scholar]
- 10.Brindley GS: Deep scrotal temperature and the effect on it of clothing, air temperature, activity, posture and paraplegia. Br J Urol 1982, 54:49-55 [DOI] [PubMed] [Google Scholar]
- 11.Rachootin P, Olsen J: The risk of infertility and delayed conception associated with exposures in the Danish workplace. J Occup Med 1983, 25:394-402 [PubMed] [Google Scholar]
- 12.Thonneau P, Ducot B, Bujan L, Mieusset R, Spira A: Heat exposure as a hazard to male infertility. Lancet 1996, 347:204-205 [PubMed] [Google Scholar]
- 13.Zorgniotti AW, Macleod J: Studies in temperature, human semen quality, and varicocele. Fertil Steril 1973, 24:854-863 [PubMed] [Google Scholar]
- 14.Hezmall HP, Lipshultz LI: Cryptorchidism and infertility. Urol Clin North Am 1982, 9:361-369 [PubMed] [Google Scholar]
- 15.Young WC: The influence of high temperature on the guinea-pig testis. J Exp Zool 1927, 49:459-499 [Google Scholar]
- 16.Parvinen M: Observations on freshly isolated and accurately identified spermatogenic cells of the rat. Early effects of heat and short-time experimental cryptorchidism. Virchows Arch B Cell Pathol 1973, 13:38-47 [DOI] [PubMed] [Google Scholar]
- 17.Shikone T, Billig H, Hsueh AJ: Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol Reprod 1994, 51:865-872 [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, DeWolf WC, Morgentaler A: Experimental cryptorchidism induces testicular germ cell apoptosis by p53-dependent and -independent pathways in mice. Biol Reprod 1998, 58:492-496 [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J: A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 1997, 137:899-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, Okuno H, Millan JL, Matsuda T, Yoshida O, Fujita J: Decreased expression of cold- inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol 1998, 152:289-296 [PMC free article] [PubMed] [Google Scholar]
- 21.Derry JM, Kerns JA, Francke U: RBM3, a novel human gene in Xp11.23 with a putative RNA-binding domain. Hum Mol Genet 1995, 4:2307-2311 [DOI] [PubMed] [Google Scholar]
- 22.Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, Fujita J: Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 1997, 236:804-807 [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Nishiyama H, Nonoguchi K, Higashitsuji H, Kishishita M, Fujita J: A novel hsp110-related gene, apg-1, that is abundantly expressed in the testis responds to a low temperature heat shock rather than the traditional elevated temperatures. J Biol Chem 1997, 272:2640-2645 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H, Pereira LA, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y: A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int J Androl 1997, 20:361-366 [DOI] [PubMed] [Google Scholar]
- 25.Kozak M: The scanning model for translation: an update. J Cell Biol 1989, 108:229-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato N: A family of cold-regulated RNA-binding protein genes in the cyanobacterium Anabaena variabilis M3. Nucleic Acids Res 1994, 23:2161-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karsch MI, Haynes SR: The Rb97D gene encodes a potential RNA-binding protein required for spermatogenesis in Drosophila. Nucleic Acids Res 1993, 21:2229-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma K, Inglis JD, Sharkey A, Bickmore WA, Hill RE, Prosser EJ, Speed RM, Thomson EJ, Jobling M, Taylor K, Wolfe J, Cooke HJ, Hargreave TB, Chandley AC: A Y chromosome gene family with RNA-binding protein homology: candidates for the azoospermia factor AZF controlling human spermatogenesis. Cell 1993, 75:1287-1295 [DOI] [PubMed] [Google Scholar]
- 29.Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, Chapelle ADL, Silber S, Page DC: Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 1995, 10:383-393 [DOI] [PubMed] [Google Scholar]
- 30.Pelliniemi LJ, Frojdman K, Paranko J: Embryological and postnatal development and function of Sertoli cells. Russell LD Griswold MD eds. The Sertoli Cell. 1993, :pp 387-114 Cache River Press, Clearwater [Google Scholar]
- 31.Gondos B, Berndtso WE: Postnatal and pubertal development. Russell LD Griswold MD eds. The Sertoli Cell. 1993, :pp 115-154 Cache River Press, Clearwater [Google Scholar]
- 32.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp S, Goff SP, Lemke G: Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999, 398:723-728 [DOI] [PubMed] [Google Scholar]
- 33.Soulard M, Della Valle V, Siomi MC, Pinol-Roma S, Codogno P, Bauvy C, Bellini M, Lacroix JC, Monod G, Dreyfuss G, Larsen CJ: hnRNP G: sequence and characterization of a glycosylated RNA-binding protein. Nucleic Acids Res 1993, 21:4210-4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burd CG, Dreyfuss G: Conserved structures and diversity of functions of RNA-binding proteins. Science 1994, 265:615-621 [DOI] [PubMed] [Google Scholar]