Abstract
Extracellular matrix (ECM) is known to provide signals controlling cell shape, migration, proliferation, differentiation, morphogenesis, and survival. Recent data shows that some of these signals are derived from biologically active cryptic sites within matrix molecules (matricryptic sites) that are revealed after structural or conformational alteration of these molecules. We propose the name, matricryptins, for enzymatic fragments of ECM containing exposed matricryptic sites. Mechanisms regulating the exposure of matricryptic sites within ECM molecules include the major mechanism of enzymatic breakdown as well as others including ECM protein multimerization, adsorption to other molecules, cell-mediated mechanical forces, and ECM denaturation. Such matrix alterations occur during or as a result of tissue injury, and thus, the appearance of matricryptic sites within an injury site may provide important new signals to regulate the repair process. Here, we review the data supporting this concept and provide insight into why the increased exposure of matricryptic sites may be an important regulatory step in tissue responses to injury.
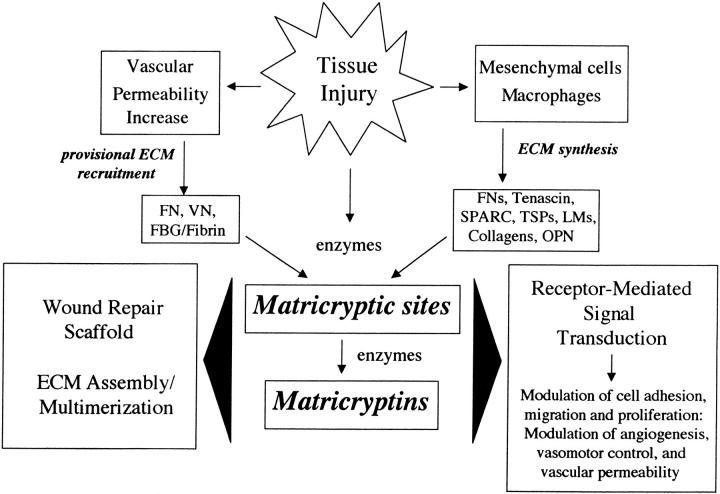
The extracellular matrix (ECM) contains signals that control cell shape, migration, proliferation, differentiation, morphogenesis, and survival. 1-3 It appears that ECM signals act in concert with other signaling pathways, such as those initiated by growth factors, to regulate cell behavior. Cells use a series of receptors for ECM including integrins, cell surface proteoglycans, and a newly described class of cell-surface-expressed tyrosine kinase receptors with direct affinity for ECM. 3-6 The components of ECM include insoluble ECM proteins (ie, collagens, laminins, fibronectin, proteoglycans), matricellular ECM proteins that modulate cell-matrix interactions and other cellular responses such as cell proliferation (ie, SPARC, thrombospondins, osteopontin, tenascin) 7,8 and ECM-associated proteins such as growth factors. 9 Recent reviews discuss the unique properties of these individual insoluble or matricellular ECM proteins in detail. 3,8,10-15 During tissue injury, the composition of ECM and its cellular recognition sites are altered in a number of significant ways (Figure 1) ▶ . Increased vascular permeability results in the recruitment of plasma-derived proteins (eg, fibronectin, vitronectin, fibrinogen) into ECM, whereas cells in the injury site are induced to release or synthesize new ECM components (eg, osteopontin, SPARC, thrombospondins, tenascins, alternatively spliced fibronectins) which regulate tissue repair. 16-19 Furthermore, tissue injury may result in alterations in existing ECM proteins within tissues or in recruited ECM that reveal cryptic biologically active (matricryptic) sites that provide important signals within the injury site. Recent work has implicated the potential importance of these matricryptic sites. 20-25 In this review, we discuss the data supporting this concept and provide insight into why increased exposure of matricryptic sites may be a critical step during tissue injury responses.
Figure 1.
Schematic diagram illustrating how tissue injury leads to the generation of matricryptic sites and matricryptins which participate in the regulation of key aspects of tissue injury responses. FN, fibronectin; VN, vitronectin; FBG, fibrinogen; OPN, osteopontin; LM, laminin; TSP, thrombospondin.
Matricryptic Sites and Matricryptins Regulate Cell and Tissue Responses
Matricryptic sites are defined here to be biologically active sites that are not exposed in the mature, secreted form of ECM molecules (including both proteins and carbohydrates such as glycosaminoglycans), but which become exposed after structural or conformational alterations. These sites can be derived from insoluble ECM molecules that are deposited in tissues, matricellular ECM proteins, and plasma-derived ECM molecules. The term is limited to those sites derived directly from ECM molecules and does not refer to sites derived from other ECM-associated molecules such as proteases, protease inhibitors, or growth factors. We propose the term matricryptins to refer to biologically active fragments from ECM molecules (as defined above) which expose functional matricryptic sites (Figure 1) ▶ . This term refers specifically and is limited to biologically active ECM fragments that contain a cryptic domain that is not normally exposed in the intact molecule.
An accumulating number of studies have suggested that matricryptic sites exist within ECM molecules and that these sites regulate biological phenomena such as ECM matrix assembly, formation of a wound repair scaffold, and receptor-mediated signal transduction which can induce a variety of important biological effects (Table 1 ▶ and Figure 1 ▶ ). Matricryptic sites and matricryptins have been reported within protein components of ECM as well as in glycosaminoglycans such as hyaluronic acid. Listed in Table 1 ▶ are ECM molecules with known matricryptic sites or which contain biologically active matricryptins. In addition, information is provided concerning the known activities and structure of individual matricryptic sites as well as mechanisms involved in their generation.
Table 1.
Matricryptic Sites and Matricryptins in Extracellular Matrix Molecules
| ECM molecule | Matricryptic site(s) | Matricryptic site function | Mechanism(s) of matricryptic site exposure | References |
|---|---|---|---|---|
| Fibronectin | Type III (1st and 10th) repeats | FN matrix assembly | Cell-mediated mechanical forces, FN multimerization | 10, 26-33 |
| Type III repeat (10th)-RGD site | Cell adhesion | Cell-mediated mechanical forces, adsorption | 34-36 | |
| 120-kd cell-binding domain, 40-kd gelatin-binding domain | Stimulates cell migration | Enzymatic degradation | 37, 38 | |
| N- and C-terminal heparin-binding fragments | Inhibits cell proliferation | Enzymatic degradation | 39 | |
| Vitronectin | RGD site | Cell adhesion (αvβ3, αvβ5) | VN multimerization, adsorption | 40 |
| Collagens | RGD site(s) | Cell adhesion (αvβ3), arteriolar vasoactivity | Enzymatic degradation (MMP-1, elastase), thermal denaturation | 20-22, 41 |
| (Pro-Pro/Hyp-Gly)5 synthetic peptides | Stimulates cell migration | Enzymatic degradation (Cathepsin G, bacterial collagenase) | 42, 43 | |
| Unknown | FN, MMP-2, MMP-9 binding | Enzymatic degradation, thermal denaturation | 44, 45 | |
| Unknown | Decreased VSMC adhesion and focal contacts | Enzymatic degradation (bacterial collagenase) | 46 | |
| Laminins | LM-5, γ2 chain fragment | Stimulates tumor cell motility | Enzymatic degradation (MMP-2) | 23, 47 |
| RGD site | Cell adhesion | Enzymatic degradation | 48 | |
| Collagen XVIII | C-terminal globular domain (∼20 kd) | Angiogenesis inhibitor (endostatin) | Enzymatic degradation (MMPs) | 24 |
| Fibrinogen/fibrin | γ chain, D domain | Fibrin polymerization | Enzymatic degradation (thrombin), fibrinogen multimerization | 49 |
| Fragment D and fibrinogen B chain (β peptides) | Increased vascular permeability | Enzymatic degradation (plasmin) | 50, 51 | |
| Hyaluronic acid | Fragments of 3–16 disaccharide units in length | Stimulation of angiogenesis, increased metalloelastase production | Enzymatic degradation (hyaluronidase) | 52-54 |
| SPARC | KGHK site | Stimulation of angiogenesis, copper binding | Enzymatic degradation | 55 |
| Helix loop A | Increased affinity for collagens | Enzymatic degradation (MMPs) | 56, 57 | |
| Thrombospondin | Unknown | ECM interactions with FN and heparin, increased protease resistance | Heterotypic binding to FN or heparin | 58 |
| Osteopontin | N-terminal fragment | Cell adhesion (α9β1) | Enzymatic degradation (thrombin) | 59 |
| Tenascin | RGD site | Cell adhesion (α8β1) | Enzymatic degradation | 60 |
| Elastin | VGVAPG sites | Stimulates cell migration | Enzymatic degradation | 61 |
Abbreviations: VSMC, vascular smooth muscle cell; MMP, matrix metalloprotease; FN, fibronectin; VN, vitronectin; LM, laminin.
Some examples of matricryptic sites and matricryptins include plasmin-derived fibrin fragments which increase vascular permeability, 50,51 and collagen, fibronectin, and elastin fragments that 1) stimulate directed cell migration (eg, leukocytes), 37,42,43,61 2) affect cell proliferation, 39 3) induce focal contact disassembly, 46 and 4) induce arteriolar vasodilation. 22 In addition, the N-terminal thrombin fragment of osteopontin as well as proteolytic fragments of laminin or tenascin all contain cryptic cell adhesion sites that are not exposed in the intact molecules. 48,59,60 Also, ECM fragments or peptides derived from SPARC, hyaluronate, and collagen type XVIII (ie, endostatin) have been shown to affect important biological processes such as angiogenesis. 24,25,52,53,55 Furthermore, recent studies have shown that proteolysis of laminin can stimulate directed cell migration of either epithelial tumor cells or epithelial cells undergoing morphogenesis, and also can stimulate hemidesmosome formation. 23,47,62 Overall, these studies show that alterations of ECM molecules can generate new signals for cells that influence important cellular events.
Matricryptic Sites Are Revealed in Extracellular Matrix after Enzymatic Degradation, Heterotypic Binding to Other Molecules, Multimerization, Cell-Mediated Mechanical Forces, or Denaturation
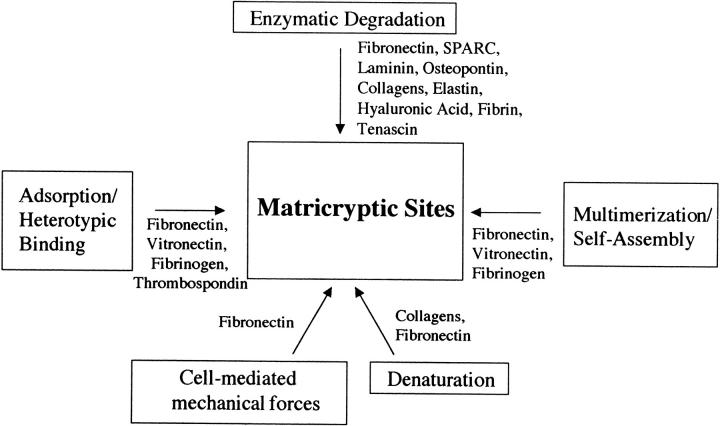
The mechanisms regulating the exposure of matricryptic sites may constitute an important step in the control of many biological processes (Figure 2 ▶ and Table 1 ▶ ). At least five mechanisms may play a role in generating these new sites and all share a common step that involves a change in ECM molecule structure or conformation. These changes can be induced in ECM molecules after 1) enzymatic degradation, 2) heterotypic binding to other molecules (adsorption), 3) multimerization (ie, self-assembly), 4) cell-mediated mechanical force, and 5) denaturation.
Figure 2.
Mechanisms for the generation of matricryptic sites during tissue injury responses. Individual ECM proteins and known mechanisms for the generation of matricryptic sites are indicated.
Heterotypic binding of ECM molecules to other molecules as well as their adsorption to surfaces is known to alter protein conformation. 34,36,49,58,63,64 These interactions can lead to the exposure of matricryptic sites which likely play a role during deposition of ECM proteins after synthesis and secretion as well as in the generation of the provisional ECM that occurs after increases in vascular permeability and the recruitment of plasma-derived proteins into the ECM. This mechanism is particularly prominent during acute inflammatory responses or during tumorigenesis where provisional ECM formation containing fibrin, fibronectin, and other ECM proteins is observed. 10,16,34,49 Other studies have revealed how new epitopes are revealed in ECM proteins after binding to cell-surface receptors. These sites, termed receptor-induced binding sites by Plow and colleagues, 65,66 were identified in fibrinogen after its binding to the platelet integrin, αllbβ3. These sites are believed to facilitate platelet aggregation and fibrinogen polymerization to stabilize developing fibrin-platelet clots. 65,66 Thus, matricryptic sites are revealed in several instances of heterotypic binding of ECM molecules to other components of ECM or to cell-surface receptors.
In addition, matricryptic sites are revealed during ECM self-assembly or multimerization which has been observed with fibrinogen, vitronectin, and fibronectin. 10,26-33,40,49,67 As many recent studies have indicated, ECM protein multimerization is a major mechanism regulating ECM assembly. 10 In some cases, matricryptic sites may catalyze the multimerization process to stimulate the deposition of insoluble ECM. 29,49 For example, the addition of a recombinant 14-kd fibronectin fragment to intact fibronectin induces multimerization of fibronectin through a self-assembly mechanism involving covalent disulfide cross-links. 29 The disulfide-exchange reaction that participates in this multimerization event 10,29 has recently been attributed to an additional matricryptic site (ie, a Cys-X-X-Cys motif) in fibronectin which possesses disulfide isomerase activity. 33 A further example of these concepts is that monoclonal antibodies, which bind only to surface-adsorbed fibrinogen and not to soluble fibrinogen, were found to inhibit fibrin polymerization indicating that matricryptic sites are exposed and directly contribute to fibrin multimerization. 49 An important extension of these findings is that ECM multimerization increases matrix valency (perhaps by increasing the exposure of matricryptic sites) which then enhances cell-matrix interactions. 29,68
Enzymatic degradation of ECM and ECM protein denaturation are additional mechanisms that reveal matricryptic sites. Conformational changes in ECM secondary to denaturation are known to occur after proteolysis, oxidant damage, and thermal injury. 69-77 Cell types that are particularly efficient in inducing ECM denaturation during acute and chronic injury include leukocytes such as neutrophils and monocytes/macrophages (see below) and malignant tumor cells. 70,73,78,79 An excellent model of ECM denaturation is the well-described unfolding of triple helical collagen molecules that occurs after cleavage of triple-helical collagen by collagenases such as MMP-1 and MMP-8. 69,78,80 The triple helix of cleaved collagen molecules then undergoes thermal melting at 37°C. Thermal denaturation of intact collagen molecules similarly unfolds the triple helix at temperatures greater than 42°C, 69 a phenomenon that occurs in vivo after thermal injury to the skin. 76,77 Also, the unfolding of collagen renders its individual chains susceptible to broad spectrum proteases that can create biologically active collagen fragments (see below). 69
Leukocytes, such as neutrophils and macrophages, cause ECM denaturation by the action of secreted proteases (eg, elastase, collagenases, gelatinases) and oxidants. 73,78 Interestingly, neutrophils and monocytes express the integrins, αMβ2 (Mac-1), αXβ2 (p150,95), and α4β1, which can interact with conformationally modified or denatured proteins. 81-84 Although the nature of the Mac-1, p150,95, and α4β1 binding sites within denatured proteins remains unknown, the majority of proteins seem to possess them. Thus, leukocyte-derived ECM degrading agents may create leukocyte-integrin binding sites in their surrounding ECM. The ability of leukocytes to denature and degrade ECM could allow them to migrate into essentially any tissue site and may facilitate their ability to phagocytose diverse tissue or matrix debris. In related studies, we have recently found that the leukocyte integrins, α4β1, αMβ2, αXβ2, and αLRIβ3, are all capable of binding osteopontin 85 (Bayless et al, unpublished observations), an Arg-Gly-Asp (RGD)-containing protein that is induced during injury and which is secreted in substantial amounts by macrophages. This may indicate a special role for osteopontin in leukocyte functions such as migration, tissue recruitment, and phagocytosis. Thus, osteopontin, an ECM protein known to be markedly induced during tissue injury, contains multiple integrin-binding sites (and perhaps matricryptic sites) 17,59,85 capable of regulating leukocyte and other cellular behaviors during wound repair responses.
Matricryptic Sites Bind Integrins and Regulate Extracellular Matrix Assembly
Recent data suggests that heterotypic binding, multimerization, or adsorption of ECM molecules can promote the exposure of matricryptic sites with affinity for integrins. The RGD integrin- and cell-binding site of vitronectin has recently been shown to be cryptic, in that it is not exposed in plasma vitronectin unless it adsorbs to surfaces or multimerizes. 40 This property may allow the RGD site of vitronectin to be exposed only when it is needed (ie, after increases in vascular permeability and binding of vitronectin into the ECM of injured tissues). A related and interesting question is whether the RGD sites within soluble plasma fibronectin are cryptic. A number of studies suggest that this is the case because monoclonal antibodies, which bind near the RGD site, bind weakly to fibronectin in solution but strongly bind after its adsorption. 34 Also, the 120-kd RGD-containing and cell-binding domain fragment of fibronectin can induce monocyte chemotaxis whereas the intact protein does not. 37 Structural analysis shows that the RGD site is present in a flexible loop which seems to be sensitive to cell-mediated mechanical forces. 35 In contrast to the above studies, this structural analysis suggested that appropriate forces applied to fibronectin molecules may actually decrease the accessibility of the RGD site to integrin binding. The above data together suggests that the RGD sites are present within a flexible domain that could be regulated (perhaps in a reversible manner) to expose or hide the sites depending on the biological situation. Other types of molecular interactions involving fibronectin heterotypic or self-assembly binding events may induce the exposure of its RGD sites (like those described for adsorption above) such as when fibronectin covalently cross-links into fibrin matrices, binds heparan sulfate proteoglycans, binds denatured collagen, or polymerizes during fibronectin matrix assembly. 26-30,32,34-36 During this latter event, cells bind secreted fibronectin and exert tension on these molecules. This tension reveals matricryptic sites which promote fibronectin-fibronectin binding and assembly of a fibronectin matrix. 26,27 Experiments using a fibronectin-green fluorescent protein chimera have demonstrated that cell-bound fibronectin fibrils exhibit elasticity in response to changes in cell movement and shape. 28 These studies support the concept that cell-mediated mechanical forces can generate and perhaps regulate the exposure of matricryptic sites in ECM to effect ECM assembly and subsequent cellular responses. It is also well known that cell-mediated mechanical forces on ECM can regulate complex processes such as cellular morphogenesis 25 and some of these effects might be mediated by matricryptic sites.
Matricryptic RGD Sequences in Collagens and the Probable Importance of RGD as a Wound Signal
After the seminal discovery of the RGD cell-binding sequence in fibronectin and vitronectin, 86,87 it was recognized that collagens have multiple copies of this sequence. However, it became clear that cell attachment to native collagen substrates did not appear to involve RGD. Anti-integrin antibodies and RGD peptides which block the function of RGD-binding integrins failed to interfere with cell binding to native collagen substrates. 20,41 In contrast, other investigators showed that cell attachment to denatured collagen was RGD-dependent. 20,21,41 Davis 20 originally proposed that the exposure of RGD sites, which occurs during the transition from native to denatured collagen, might constitute a wound signal for cells. Furthermore, the αvβ3 integrin was shown to bind strongly to denatured collagen and minimally to native collagen in affinity chromatography experiments suggesting that the RGD sites in native collagen are cryptic. 20 In addition, the α2β1 integrin which binds native collagen showed minimal binding to denatured collagen. 20,88 These results suggested the possibility that cells might use this information as part of a wound recognition system. Considerable recent work has provided support for this idea. For example, melanoma tumor cells are unable to proliferate and form a tumor without the αvβ3 integrin which is a receptor for denatured collagen. 21 Matrix metalloprotease (MMP)-1-treated collagen was also shown to bind melanoma cell αvβ3. 21 Furthermore, during normal bone resorption by osteoclasts, it was shown that denatured collagen and αvβ3 were present at cell matrix contact sites, whereas in adjacent sites, native collagen was present. 89 These data provide direct evidence for the potential importance of integrin-denatured collagen interactions in biologically important phenomena. Local collagen denaturation can result in the generation of matricryptic RGD signals delivered to cells through the αvβ3 integrin and may potentially eliminate or alter signals delivered through α2β1 or a recently described native collagen-binding tyrosine kinase receptor. 5,6 A recent article reports that denatured type I collagen fragments induce focal contact disassembly in vascular smooth muscle cells in a manner that is not dependent on RGD sequences and that may involve α2β1. 46 The above data supports the concept that collagen-derived matricryptic sites include RGD as well as non-RGD sites. Also, a recent report shows that cells are able to bend collagen fibrils 90 raising the possibility that cell-mediated mechanical forces exerted on collagen fibrils, like those mentioned above concerning cell-fibronectin interactions, might generate collagen-derived matricryptic sites.
RGD Sites May Be a Fundamental Matricryptic Signal
Many studies throughout the years suggest that RGD sites may represent a fundamental matricryptic signal within ECM proteins. A very important unanswered question concerns the normal density of RGD integrin-binding sites in noninjured tissues. We speculate that very few exposed RGD sites exist in normal tissue and that after tissue injury, a marked increase in the density of RGD sites will occur. Thus, RGD sequences may be an important mediator in tissue repair responses along with other mediators that regulate inflammatory or wound repair phenomena. The potential sources of this RGD signal include: 1) plasma-derived proteins, fibronectin, vitronectin, and fibrinogen; 2) alteration of pre-existing ECM proteins such as collagens; and 3) proteins synthesized in the wound site such as osteopontin, fibronectin, and tenascin. The abundance of collagens, and the fact that most collagens contain multiple RGD sequences (ie, 7- type I, 11- type IV, 11- type VI), strongly suggests that these proteins may constitute a biologically significant source of RGD sequences for cellular injury responses.
An important question is whether the exposure of RGD sites within tissue injury sites is required for appropriate responses of cells to initiate and propagate the wound-repair response program. A related consideration is whether RGD sites are necessary for malignant tumor growth and progression because the tumor microenvironment is reminiscent of wounds with a prominent provisional ECM containing fibrin and other RGD-containing proteins such as fibronectin and osteopontin. 16,91 Major cellular responses within wounds include proliferation, migration, phagocytosis, as well as differentiation and apoptosis. 92 All of these responses are known to be regulated by RGD sequences and by RGD-binding integrins, such as αvβ3. 93 To illustrate this point further, one can consider the conditions used to propagate cells in tissue culture which mimic a wound environment. Two major proteins from serum that are critical to cell adhesion during growth in tissue culture are vitronectin and fibronectin, 86,87,93 both RGD-containing ECM proteins. Also, certain cell types such as endothelial cells proliferate more readily when the substrate is first coated with denatured collagen, 94 a protein containing multiple RGD sites. Denatured collagen substrates also strongly adsorb serum-derived fibronectin as well as fibronectin secreted from cells. 44 Intriguingly, heparin is also typically added to endothelial cells to enhance proliferation. 95 Thus, three components of a putative wound scaffold matrix are present (denatured collagen, fibronectin, heparin) to optimize endothelial cell growth (see later on). It would seem that optimal tissue-culture conditions include RGD-containing ECM substrates. What remains unclear is whether RGD signals are actually required for the growth of adherent normal or transformed cells. We speculate that the recruitment of RGD-containing proteins from plasma, the exposure of cryptic RGD sites from existing ECM, and the synthesis of RGD containing ECM proteins within wound sites provides evidence for an RGD signal requirement for appropriate cellular responses during tissue injury in vivo.
Other studies demonstrate the importance of RGD-mediated signaling in microvascular responses such as arteriolar vasomotor activity and the regulation of angiogenesis. 17,22,96-98 The αvβ3 integrin expressed on vascular smooth muscle cells was shown to regulate arteriolar vasodilation in response to RGD peptides 22,98 and this receptor is markedly induced on endothelial cells during angiogenesis. 96,99 Reagents which interfere with αvβ3 function can inhibit angiogenesis and induce vessel regression through an apoptotic mechanism. 97 In addition, αvβ3 has been reported to directly interact with MMP-2 on the cell surface to modulate both adhesive and proteolytic function. 100 One interesting aspect of this finding is that both αvβ3 (through RGD sites) and MMP-2 (through its fibronectin-like domain) have direct binding affinity for denatured collagen. This affinity may facilitate the interaction between αvβ3 and MMP-2 on the cell surface. Also, MMP-2 is a potent enzyme that degrades denatured collagens 70,100 and MMP-2, like other enzymes, should be capable of generating biologically active RGD-containing fragments from denatured collagen degradation. Such interactions may play an important role in the control of proteolytic balance which is a critical regulator of tissue morphogenesis and regression as well as tumorigenesis. 2,70,79,101,102
Matricryptins Induce Signals, Alter Cell Behavior, and Alter Microvascular Responses
Many studies throughout the years have strongly implicated the role of ECM breakdown in the generation of new biological signals. Enzymes that are either known to generate such fragments or which may participate in their generation include MMPs, serine proteases (ie, plasmin and neutrophil elastase), and enzymes that degrade glycosaminoglycans (ie, hyaluronidase and heparinase). 70-73,78 Here, we have proposed the name, matricryptins, to describe these biologically active fragments of ECM. Matricryptins are derived from molecular domains that are cryptic and enzymatic breakdown is required to expose the new biologically relevant activity. A series of biological activities can now be attributed to matricryptins such as effects on cell proliferation, vascular permeability, cell migration, arteriolar vasoreactivity, stimulation and inhibition of angiogenesis, ECM assembly, and focal contact stability (Table 1) ▶ .
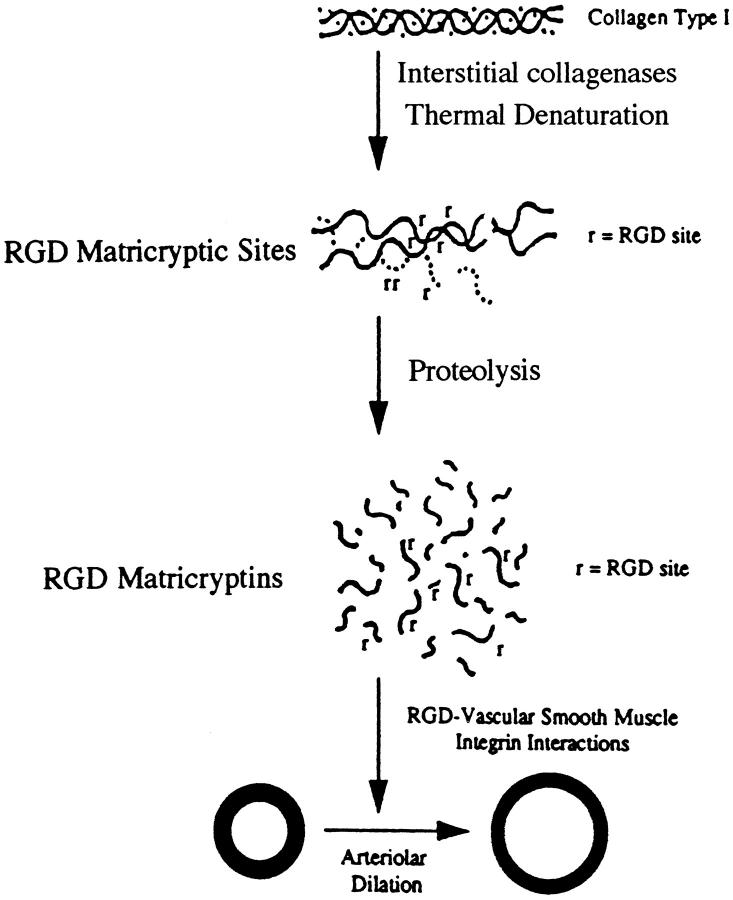
Recent work from our laboratories 22 has shown that proteolytic fragments of denatured collagen type I induce arteriolar vasodilation in a manner identical to that of synthetic RGD peptides (Figure 3) ▶ . In both cases, this vasodilatory response involved the αvβ3 integrin expressed by the arteriolar vascular smooth muscle. Blocking antibodies directed to αvβ3 were shown to inhibit these responses. Increased blood flow to injured tissues is a well-known consequence of tissue injury and may in part be controlled by this RGD matricryptin-mediated mechanism. Further studies using patch-clamp recordings have shown that soluble RGD peptides, when added to isolated vascular smooth muscle cells, decrease Ca2+ current through L-type Ca2+ channels. 98 This decreased Ca2+ current, which lowers intracellular Ca2+, is known to induce a vasodilatory response which is consistent with our results showing RGD peptide-induced vasodilation of intact arterioles. 22,103 Interestingly, this decreased Ca2+ current occurred equally well in vascular smooth muscle cells when the RGD signal was supplied in either a soluble or insoluble manner. 98 A previous study revealed an αvβ3 integrin signaling response to soluble ligands in endothelial cells. 104 In contrast, the α5β1 integrin was found to increase Ca2+ current through L-type channels in vascular smooth muscle cells, but it could only signal when its ligands were presented in an insoluble form. 98 These data suggest that αvβ3 may possess a special ability among the integrin family of receptors to signal when ligands are presented in a soluble form. This property may allow this receptor to serve as a wound or RGD sensor to detect RGD locally or after diffusion from distant sites which could be an important component in a wound recognition and response system.
Figure 3.
Generation of RGD-containing matricryptic sites and matricryptins from collagen type I regulates arteriolar vasomotor activity.
Matricryptic Sites May Participate in the Formation of a Wound Repair Scaffold
As mentioned above, matricryptic sites exist within ECM molecules such as fibrinogen and fibronectin which are major components of the provisional ECM that forms within injured tissues after increases in vascular permeability. Evidence suggests that matricryptic sites play a role in both fibrin and fibronectin matrix assembly (Table 1) ▶ and because these two molecules have affinity for each other, it is possible that they also play a role in the formation of a fibrin-fibronectin scaffold. In addition, fibronectin has the ability to selectively adsorb to denatured collagen. 44,45 This interaction has formed the basis for one of the steps in the purification of fibronectin from human plasma. 44,105 Although fibronectin does bind native collagens, it shows a markedly increased affinity for denatured collagen. 44,45 Because collagen denaturation occurs within areas of tissue injury, 70-74,78,80,102 this affinity may allow fibronectin to preferentially adsorb to these areas. An intriguing possibility is that fibronectin-denatured collagen complexes present RGD or other matricryptic sites in such a way that provides a signal unique to an injured tissue compared to a normal tissue. Further consideration suggests the possibility that heparin, released from mast cells during tissue injury, could bind fibronectin-denatured collagen complexes to stabilize this interaction or affect the presentation of matricryptic sites. Previous studies indicate that heparin increases the affinity of fibronectin for denatured collagen. 44,45 The complex of fibronectin/denatured collagen/heparin might serve as a nucleation center for the selective entrapment of molecules involved in wound repair such as growth factors, ECM proteins, proteases, and protease inhibitors. Many growth factors and ECM components have affinity for heparin or other components of this trimolecular complex. 9,17,44,58,87 Thus, the generation of denatured collagen after tissue injury could serve as a component of a nucleation center for the accumulation of repair molecules precisely within the wound site. The recruitment of plasma-derived ECM such as fibrinogen/fibrin, which also directly interacts with fibronectin, might serve as the structural scaffold (ie, fibrin clot) on which the assembly of this wound repair apparatus could occur. In support of these concepts, low-density lipoprotein uptake by macrophages was found to be markedly enhanced by the concomitant presence of fibronectin/denatured collagen/heparin complexes. 106 This data suggests that, in addition to serving as a nucleation center, the complex may directly stimulate cellular functions (eg, phagocytosis) necessary for proper wound repair responses.
Summary
In summary, it is clear that matricryptic sites and matricryptins represent an important class of biological information that is revealed to cells during tissue injury responses. One of the most significant matricryptic sites is the RGD sequence, which appears to be a cryptic site in many, if not all, ECM proteins that possess the sequence. This sequence seems to play a special role in microvascular signaling by regulating arteriolar vasoactivity as well as angiogenic responses. The RGD sequence also appears to play a fundamental role in cell phenomena critical to the repair of injured tissue such as cell proliferation, migration, survival, morphogenesis, and phagocytosis. In addition to RGD sites, many recent studies have identified new non-RGD matricryptic sites that are currently being molecularly characterized. A wide variety of biological activities can now be attributed to these matricryptic sites and include effects on cell proliferation, migration, ECM matrix assembly, development of a wound repair scaffold, arteriolar vasoreactivity, focal adhesion stability, and morphogenesis. Thus, the regulated exposure of matricryptic sites represents a fundamental step during tissue repair responses and acts in concert with other mediators to control these events.
Footnotes
Address reprint requests to George E. Davis, M.D., Ph.D., Department of Pathology and Laboratory Medicine, Texas A&M University Health Science Center, 208 Reynolds Medical Building, College Station, TX 77843-1114. E-mail: gedavis@tamu.edu.
Supported by National Institutes of Health Grants HL 59373, HL 59971 (to G. E. D.), HL 55050 (to G. A. M.), and HL 46502 (to M. J. D.).
References
- 1.Lukashev ME, Werb Z: ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol 1998, 8:437-441 [DOI] [PubMed] [Google Scholar]
- 2.Boudreau N, Bissell MJ: Extracellular matrix signalling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol 1998, 10:586-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruoslahti E: Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999, 76:1-20 [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO: Integrins: versatility, modulation and signalling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 5.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD: An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell 1997, 1:25-34 [DOI] [PubMed] [Google Scholar]
- 6.Vogel W, Gish GD, Alves F, Pawson T: The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1997, 1:13-23 [DOI] [PubMed] [Google Scholar]
- 7.Sage EH, Bornstein P: Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 1991, 266:14831-14834 [PubMed] [Google Scholar]
- 8.Bornstein P: Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin-1. J Cell Biol 1995, 130:503-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taipale J, Keski-Oja J: Growth factors in the extracellular matrix. FASEB J 1997, 11:51-59 [DOI] [PubMed] [Google Scholar]
- 10.Schwarzbauer JE, Sechler JL: Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol 1999, 11:622-627 [DOI] [PubMed] [Google Scholar]
- 11.Ryan MC, Christiano AM, Engvall E, Wewer UM, Miner JH, Sanes JR, Burgeson RE: The functions of laminins: lessons from in vivo studies. Matrix Biol 1996, 15:369-381 [DOI] [PubMed] [Google Scholar]
- 12.Iozza RV: Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 1998, 67:609-652 [DOI] [PubMed] [Google Scholar]
- 13.Yan Q, Sage EH: SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 1999, 47:1495-1506 [DOI] [PubMed] [Google Scholar]
- 14.Rittling SR, Denhardt DT: Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol 1999, 7:103-113 [DOI] [PubMed] [Google Scholar]
- 15.Crossin KL: Tenascin: a multifunctional extracellular matrix protein with a restricted distribution in development and disease. J Cell Biochem 1996, 61:592-598 [DOI] [PubMed] [Google Scholar]
- 16.Dvorak HF, Nagy JA, Berse B, Brown LF, Yeo KT, Yeo TK, Dvorak AM, van de Water L, Sioussat TM, Senger DR: Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann NY Acad Sci 1992, 667:101-111 [DOI] [PubMed] [Google Scholar]
- 17.Senger DR: Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol 1996, 149:1-7 [PMC free article] [PubMed] [Google Scholar]
- 18.French-Constant C, Van de Water L, Dvorak HF, Hynes RO: Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989, 109:903-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocher O, Kennedy SP, Madri JA: Alternative splicing of endothelial cell fibronectin mRNA in the IIICS region. Functional significance. Am J Pathol 1990, 137:1509-1524 [PMC free article] [PubMed] [Google Scholar]
- 20.Davis GE: Affinity of integrins for damaged extracellular matrix. αvβ3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun 1992, 182:1025-1031 [DOI] [PubMed] [Google Scholar]
- 21.Montgomery AM, Reisfeld RA, Cheresh DA: Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA 1994, 91:8856-8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogford JE, Davis GE, Platts SH, Meininger GA: Vascular smooth muscle αvβ3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res 1996, 79:821-826 [DOI] [PubMed] [Google Scholar]
- 23.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V: Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 1997, 277:225-228 [DOI] [PubMed] [Google Scholar]
- 24.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88:277-285 [DOI] [PubMed] [Google Scholar]
- 25.Sage EH: Pieces of eight: bioactive fragments of extracellular proteins as regulators of angiogenesis. Trends Cell Biol 1997, 7:182-186 [DOI] [PubMed] [Google Scholar]
- 26.Erickson HP: Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA 1994, 91:10114-10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K: Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 1998, 141:539-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi T, Kiehart DP, Erickson HP: Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc Natl Acad Sci USA 1999, 96:2153-2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morla A, Zhang Z, Ruoslahti E: Superfibronectin is a functionally distinct form of fibronectin. Nature 1994, 367:193-196 [DOI] [PubMed] [Google Scholar]
- 30.Hocking DC, Sottile J, McKeown-Longo PJ: Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem 1994, 269:19183-19187 [PubMed] [Google Scholar]
- 31.Hocking DC, Smith RK, McKeown-Longo PJ: A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol 1996, 133:431-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingham KC, Brew SA, Huff S, Litvinovich SV: Cryptic self-association sites in type III modules of fibronectin. J Biol Chem 1997, 272:1718-1724 [DOI] [PubMed] [Google Scholar]
- 33.Langenbach KJ, Sottile J: Identification of protein-disulfide isomerase activity in fibronectin. J Biol Chem 1999, 274:7032-7038 [DOI] [PubMed] [Google Scholar]
- 34.Ugarova TP, Zamarron C, Veklich Y, Bowditch RD, Ginsberg MH, Weisel JW, Plow EF: Conformational transitions in the cell binding domain of fibronectin. Biochemistry 1995, 34:4457-4466 [DOI] [PubMed] [Google Scholar]
- 35.Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V: Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc Natl Acad Sci USA 1999, 96:1351-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia AJ, Vega MD, Boettiger D: Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell 1999, 10:785-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark RA, Wikner NE, Doherty DE, Norris DA: Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120 kDa fibroblastic cell binding fragment. J Biol Chem 1988, 263:12115-12123 [PubMed] [Google Scholar]
- 38.Schor SL, Ellis I, Dolman C, Banyard J, Humphries MJ, Mosher DF, Grey AM, Mould AP, Sottile J, Schor AM: Substratum-dependent stimulation of fibroblast migration by the gelatin-binding domain of fibronectin. J Cell Sci 1996, 109:2581-2590 [DOI] [PubMed] [Google Scholar]
- 39.Homandberg G, Williams JE, Grant D, Schumacher B, Einstein R: Heparin-binding fragments of fibronectin are potent inhibitors of endothelial cell growth. Am J Pathol 1985, 120:327-332 [PMC free article] [PubMed] [Google Scholar]
- 40.Seiffert D, Smith JW: The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem 1997, 272:13705-13710 [DOI] [PubMed] [Google Scholar]
- 41.Pfaff M, Aumailley M, Specks U, Knolle J, Zerwes HG, Timpl R: Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type IV. Exp Cell Res 1993, 206:167-176 [DOI] [PubMed] [Google Scholar]
- 42.Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA: Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol 1986, 39:255-266 [DOI] [PubMed] [Google Scholar]
- 43.Albini A, Adelmann-Grill BC: Collagenolytic cleavage products of collagen type I as chemoattractants for human dermal fibroblasts. Eur J Cell Biol 1985, 36:104-107 [PubMed] [Google Scholar]
- 44.Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E: Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol 1982, 82:803-831 [DOI] [PubMed] [Google Scholar]
- 45.Johansson S, Hook M: Heparin enhances the rate of binding of fibronectin to collagen. Biochem J 1980, 187:521-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carragher NO, Levkau B, Ross R, Raines EW: Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlate with cleavage of pp125FAK, paxillin, and talin. J Cell Biol 1999, 147:619-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giannelli G, Pozzi A, Stetler-Stevenson WG, Gardner HA, Quaranta V: Expression of matrix metalloprotease-2-cleaved laminin-5 in breast remodeling stimulated by sex steroids. Am J Pathol 1999, 154:1193-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aumailley M, Gerl M, Sonnenberg A, Deutzmann R, Timpl R: Identification of the Arg-Gly-Asp sequence in laminin A chain as a latent cell-binding site being exposed in fragment P1. FEBS Lett 1990, 12:82-86 [DOI] [PubMed] [Google Scholar]
- 49.Zamarron C, Ginsberg MH, Plow EF: Monoclonal antibodies specific for a conformationally altered state of fibrinogen. Thromb Haemost 1990, 64:41-46 [PubMed] [Google Scholar]
- 50.Ge M, Ryan TJ, Lum H, Malik AB: Fibrinogen degradation product fragment D increases endothelial monolayer permeability. Am J Physiol 1991, 261:L283-L289 [DOI] [PubMed] [Google Scholar]
- 51.Rowland FN, Donovan MJ, Picciano PT, Wilner GD, Kreutzer DL: Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol 1984, 117:418-428 [PMC free article] [PubMed] [Google Scholar]
- 52.West DC, Hampson IN, Arnold F, Kumar S: Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228:1324-1326 [DOI] [PubMed] [Google Scholar]
- 53.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS: Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci USA 1996, 93:7832-7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW: Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol 1999, 162:4171-4176 [PubMed] [Google Scholar]
- 55.Lane TF, Ireula-Arispe ML, Johnson RS, Sage EH: SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol 1994, 125:929-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki T, Gohring W, Mann K, Maurer P, Hohenester E, Knauper V, Murphy G, Timpl R: Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteases increases its affinity for collagens. J Biol Chem 1997, 272:9237-9243 [DOI] [PubMed] [Google Scholar]
- 57.Sasaki T, Hohenester E, Gohring W, Timpl R: Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J 1998, 17:1625-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dardik R, Lahav J: Functional changes in the conformation of thrombospondin-1 during complexation with fibronectin or heparin. Exp Cell Res 1999, 248:407-414 [DOI] [PubMed] [Google Scholar]
- 59.Smith LL, Cheung H-K, Ling LE, Chen J, Sheppard D, Pytela R, Giachelli C: Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by α9β1. J Biol Chem 1996, 271:28485-28491 [PubMed] [Google Scholar]
- 60.Denda S, Muller U, Crossin KL, Erickson HP, Reichardt LF: Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin alpha8 beta1 receptor interactions with tenascin: murine alpha8 beta1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry 1998, 37:5464-5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senior RM, Griffin GL, Mecham RP: Chemotactic activity of elastin-derived peptides. J Clin Invest 1980, 66:859-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldfinger LE, Stack MS, Jones JCR: Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol 1998, 141:255-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jemmerson R: Antigenicity and native structure of globular proteins: low frequency of peptide reactive antibodies. Proc Natl Acad Sci USA 1987, 84:9180-9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spangler BD: Binding to native proteins by antipeptide monoclonal antibodies. J Immunol 1991, 146:1591-1595 [PubMed] [Google Scholar]
- 65.Zamarron C, Ginsberg MH, Plow EF: A receptor-induced binding site in fibrinogen elicited by its interaction with platelet membrane glycoprotein IIb-IIIa. J Biol Chem 1991, 266:16193-16199 [PubMed] [Google Scholar]
- 66.Ugarova TP, Budzynski AZ, Shattil SJ, Ruggeri ZM, Ginsberg MH, Plow EF: Conformational changes in fibrinogen elicited by its interaction with platelet membrane glycoprotein GPIIb-IIIa. J Biol Chem 1993, 268:21080-21087 [PubMed] [Google Scholar]
- 67.Stockman A, Hess S, Declerck P, Timpl R, Preissner KT: Multimeric vitronectin: identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem 1993, 268:22874-22882 [PubMed] [Google Scholar]
- 68.Stupack DG, Li E, Silletti SA, Kehler JA, Geahlen RL, Hahn K, Nemerow GR, Cheresh DA: Matrix valency regulates integrin-mediated lymphoid adhesion via Syk kinase. J Cell Biol 1999, 144:777-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross J: An essay on biological degradation of collagen. Hay ED eds. Cell Biology of Extracellular Matrix. 1981, :pp 217-258 Plenum Press, New York [Google Scholar]
- 70.Stetler-Stevenson WG: Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol 1996, 148:1345-1350 [PMC free article] [PubMed] [Google Scholar]
- 71.Basbaum CB, Werb Z: Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol 1996, 8:731-738 [DOI] [PubMed] [Google Scholar]
- 72.Nagase H, Woessner JF: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 73.Weiss SJ: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-376 [DOI] [PubMed] [Google Scholar]
- 74.Vissers MC, Winterbourn CC: The effects of oxidants on neutrophil-mediated degradation of glomerular basement membrane collagen. Biochim Biophys Acta 1986, 889:277-286 [DOI] [PubMed] [Google Scholar]
- 75.Hassan MS, Mileva MM, Dweck HS, Rosenfeld L: Nitric oxide products degrade chrondroitin sulfate. Nitric Oxide 1998, 2:360-365 [DOI] [PubMed] [Google Scholar]
- 76.Deno DC, McCafferty MH, Saba TM, Blumenstock FA: Mechanism of acute depletion of plasma fibronectin following thermal injury in rats: appearance of a gelatinlike ligand in plasma. J Clin Invest 1984, 73:20-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LaCelle P, Blumenstock FA, McKinley C, Saba TM, Vincent PA, Gray V: Blood-borne collagenous debris complexes with plasma fibronectin after thermal injury. Blood 1990, 75:470-478 [PubMed] [Google Scholar]
- 78.Owen CA, Campbell EJ: The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol 1999, 65:137-150 [DOI] [PubMed] [Google Scholar]
- 79.Werb Z, Vu TH, Rinkenberger JL, Coussens LM: Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS 1999, 107:11-18 [DOI] [PubMed] [Google Scholar]
- 80.Cleutjens JPM, Weber KT: Collagen degradation after myocardial infarction. Weber KT eds. Wound Healing in Cardiovascular Disease. 1995, :pp 169-177 Futura Publishing, Armonk, NY: [Google Scholar]
- 81.Davis GE: The Mac-1 and p150,95 β2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res 1992, 200:242-252 [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Plow EF: Overlapping but not identical sites in the recognition of C3bi, neutrophil inhibitory factor, and adhesive ligands by the αMβ2 integrin. J Biol Chem 1996, 271:18211-18216 [DOI] [PubMed] [Google Scholar]
- 83.Davis GE, Thomas JS, Madden S: The α4β1 integrin can mediate leukocyte adhesion to casein and denatured protein substrates. J Leukoc Biol 1997, 62:318-328 [DOI] [PubMed] [Google Scholar]
- 84.Ortlepp S, Stephens PE, Hogg N, Figdor CG, Robinson MK: Antibodies that activate β2 integrins can generate different ligand binding states. Eur J Immunol 1995, 25:637-643 [DOI] [PubMed] [Google Scholar]
- 85.Bayless KJ, Meininger GA, Scholtz JM, Davis GE: Osteopontin is a ligand for the α4β1 integrin. J Cell Sci 1998, 111:1165-1174 [DOI] [PubMed] [Google Scholar]
- 86.Pierschbacher MD, Ruoslahti E: Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309:30-33 [DOI] [PubMed] [Google Scholar]
- 87.Suzuki S, Oldberg A, Hayman EG, Pierschbacher MD, Ruoslahti E: Complete amino acid sequence of human vitronectin deduced from cDNA: similarity of cell attachment sites in vitronectin and fibronectin. EMBO J 1985, 4:2519-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santoro SA: Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell 1986, 46:913-920 [DOI] [PubMed] [Google Scholar]
- 89.Nesbitt SA, Horton MA: Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 1997, 276:266-269 [DOI] [PubMed] [Google Scholar]
- 90.Lee GM, Loeser RF: Cell surface receptors transmit sufficient force to bend collagen fibrils. Exp Cell Res 1999, 248:294-305 [DOI] [PubMed] [Google Scholar]
- 91.Senger DR, Asch BB, Smith BD, Perruzzi CA, Dvorak HF: A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells. Nature 1983, 302:714-715 [DOI] [PubMed] [Google Scholar]
- 92.Martin P: Wound healing: aiming for perfect skin regeneration. Science 1997, 276:75-81 [DOI] [PubMed] [Google Scholar]
- 93.Ruoslahti E: RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996, 12:697-715 [DOI] [PubMed] [Google Scholar]
- 94.Gimbrone MA, Jr: Culture of vascular endothelium. Prog Hemost Thromb 1976, 3:1-28 [PubMed] [Google Scholar]
- 95.Maciag T, Cerundolo J, Lisley S, Kelley PR, Forand R: An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci USA 1979, 76:5674-5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brooks PC, Clark RA, Cheresh DA: Requirement of vascular integrin αvβ3 for angiogenesis. Science 1994, 264:569-571 [DOI] [PubMed] [Google Scholar]
- 97.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 98.Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ: Modulation of calcium current in arteriolar smooth muscle by αvβ3 and α5β1 integrin ligands. J Cell Biol 1998, 143:241-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clark RA, Tonnesen MG, Gailit J, Cheresh DA: Transient functional expression of alphav beta3 on vascular cells during wound repair. Am J Pathol 1996, 148:1407-1421 [PMC free article] [PubMed] [Google Scholar]
- 100.Brooks PC, Stromblad S, Sanders LC, Aimes RT, Stetler-Stevenson WG, Quiqley JP, Cheresh DA: Localization of matrix metalloprotease MMP-2 to surface of invasive cells by the interaction with integrin αvβ3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 101.Werb Z, Chin JR: Extracellular matrix remodelling during morphogenesis. Ann NY Acad Sci 1998, 857:110-118 [DOI] [PubMed] [Google Scholar]
- 102.Moses MA, Marikovsky M, Harper JW, Vogt P, Eriksson E, Klagsbrun M, Langer R: Temporal study of the activity of matrix metalloproteinases and their endogenous inhibitors during wound healing. J Cell Biochem 1996, 60:379-386 [DOI] [PubMed] [Google Scholar]
- 103.D’Angelo G, Mogford JE, Davis GE, Davis MJ, Meininger GA: Integrin mediated reduction in vascular smooth muscle [Ca2+]; induced by RGD-containing peptide. Am J Physiol 1997, 272:H2065-H2070 [DOI] [PubMed] [Google Scholar]
- 104.Bhattacharya S, Fu C, Bhattacharya J, Greenberg S: Soluble ligands of the αvβ3 integrin mediate enhanced tyrosine phosphorylation of multiple proteins in adherent bovine artery endothelial cells. J Biol Chem 1995, 270:16781-16787 [DOI] [PubMed] [Google Scholar]
- 105.Retta SF, Ferraris P, Tarone G: Purification of fibronectin from human plasma. Methods Mol Biol 1999, 96:119-124 [DOI] [PubMed] [Google Scholar]
- 106.Falcone DJ, Mated N, Shio H, Minick CR, Fowler SD: Lipoprotein-heparin-fibronectin-denatured collagen complexes enhance cholesterol ester accumulation in macrophages. J Cell Biol 1984, 99:1266-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]