Abstract
When we evaluated the age-associated changes in autoimmune exocrinopathy in a NFS/sld murine model for primary Sjögren’s syndrome (SS), severe destructive autoimmune lesions developed in the salivary and lacrimal glands in the aged mice, compared with those observed in the younger model. We detected a decreased secretion of saliva and tear flow in the aged group. A significant increase of TUNEL+-apoptotic epithelial duct cells in the salivary glands was detected in the aged SS animal model. A higher proportion of mouse salivary gland cells bearing Fas was found in the aged group, whereas no significant changes were seen on tissue-infiltrating CD4+ T cells bearing FasL in the salivary glands from young and aged mice. We detected an increased cleavage product of organ-specific autoantigen, 120-kd α-fodrin, in the aged salivary gland tissues on immunoblotting, and an increase in serum autoantibody production against 120-kd α-fodrin by enzyme-linked immunosorbent assay. An increase in the proliferative response of splenic T cells against organ-specific autoantigen was observed, whereas nonspecific concanavalin A responsiveness was decreased in the aged mice. In addition, a decrease in Fas expression was found on splenic CD4+ T cells in the aged mice, and anti-Fas mAb-stimulated apoptosis was down-regulated on CD4+ T cells. These results indicate that age-associated dysregulation of CD4+ T cells may play a crucial role on acceleration of organ-specific autoimmune lesions in a murine model for primary SS through Fas-mediated apoptosis.
Aging is associated with a progressive decline in T cell functions, including decreased response to mitogens, soluble antigens, and production of interleukin (IL)-2, expression of IL-2R, decrease in naive and increase in memory cells, and defects in the signaling pathway. 1-5 Programmed cell death (apoptosis) is essential for normal development and for maintenance of cellular homeostasis in multicellular organisms. 6,7 In addition, apoptosis plays an important role in maintaining T cell repertoire and deletion of autoreactive T cells. 8,9 Apoptosis is regulated by a number of gene products that promote cell death or extend cell survival. 10,11 Fas ligand (FasL) mediates cell death by cross-linking Fas receptor in apoptosis-sensitive Fas+ cells. 12,13 On the other hand, it is now evident that the interaction of Fas with FasL regulates a large number of pathophysiological processes of apoptosis including autoimmune diseases. 9,14-17
Primary Sjögren’s syndrome (SS) in humans is an organ-specific autoimmune disease characterized by lymphocytic infiltration into the salivary and lacrimal glands, resulting in symptoms of dry mouth and dry eye due to insufficient secretion. 18,19 It is possible that individual T cells activated by an appropriate antigen can proliferate and form a restricted clone. 20,21 Recently, we identified 120-kd α-fodrin as an important organ-specific autoantigen in both the NFS/sld murine model for SS and in human SS patients. 22 Since it was reported that Fas expression was observed in the salivary gland cells in human SS, 23 we speculate that Fas-mediated apoptosis may contribute to tissue destruction with aging in the salivary and lacrimal glands with SS. In addition, there are no published data on the aging process in the animal model for organ-specific autoimmune diseases including SS.
The aim of this study was to evaluate the possible relationship between the Fas-mediated apoptosis and the development and acceleration of organ-specific autoimmune lesions with aging in murine SS model of NFS/sld mice.
Materials and Methods
Mice and Treatment
NFS/sld carrying the mutant gene sld 24 were bred in our own facilities, maintained in a specific pathogen-free mouse colony, and given food and water ad libitum. An animal model for primary SS was previously established in NFS/sld mutant mice. 25 Thymectomy was performed on the day 3 after birth (3d-Tx), and a total of 114 NFS/sld mice, consisting of 79 3d-Tx (females, n = 54; males n = 25) and 35 non-Tx female mice, were investigated. They were killed by cervical dislocation during time intervals of 2, 4, 6, 10, 12, 18, and 20 months of age. Five to eight mice in each age group were analyzed. The aged group consisted of 18- and 20-month-old mice and the young group was comprised of 2- and 4-month-old mice.
Histopathology
All organs were removed from the mice, fixed with 10% phosphate-buffered formalin, and embedded in paraffin. The sections (4 μm) were stained with hematoxylin and eosin. Histological grading of inflammatory lesions was done according to the modified method proposed by White and Casarett 26 as follows: a score of 1 indicates that one to five foci being composed of more than 20 mononuclear cells per focus were seen; a score of 2 indicates that more than five such foci were seen but without significant parenchymal destruction; a score of 3 indicates degeneration of parenchymal tissue; a score of 4 indicates extensive infiltration of the glands with mononuclear cells and extensive parenchymal destruction; and a score of 5 indicates that severe destructive foci with focal fibrosis, ductal dilatation, and/or fatty infiltration were seen in addition to the score 4 lesions. These slides were scored by three independent, well-trained pathologists in a blinded manner.
Measurement of Fluid Secretion
Detection of tear and saliva volume in the aged and young SS animal models of NFS/sld mice was done according to a modified method as described. 27 Five mice in each group were analyzed at 2- and 18-months of age.
In Situ End Labeling of Fragmented DNA (TUNEL)
Apoptotic cells were detected in sections using the in situ TUNEL Kit (Wako Pure Chemicals, Osaka, Japan), as described previously. 28 Briefly, paraffin-embedded sections were deparaffinized and rehydrated, and washed twice in phosphate-buffered saline (PBS). Sections were incubated with proteinase K (20 mg/ml) for 10 minutes. After washing in distilled water, these sections were incubated with 2% H2O2 in PBS to block endogenous peroxidase. Sections were then presoaked in TdT buffer (0.5 mmol/L cacodylate, 1 mmol/L CoCl, 0.5 mmol/L dithiothreitol, 0.05% bovine serum albumin, 0.15 mol/L NaCl) for 10 minutes, and incubated for 2 hours at 37°C in 25 ml of TdT solution, containing 1× terminal transferase buffer, 0.5 nmol of biotin-dUTP, and 10U of TdT (Wako Pure Chemicals). After the TdT reaction, sections were soaked in TdT blocking buffer (300 nmol/L NaCl, 30 mmol/L tri-sodium citrate-2-hydrate), incubated with horseradish peroxidase-conjugated streptavidin for 30 minutes at room temperature, and developed for 10 minutes in phosphate-buffered citrate (pH 5.8) containing 0.6 mg/ml of diaminobenzidine. Nuclei were counterstained with hematoxylin. When we used DNase-I-treated and -untreated sections of submandibular glands in non-Tx mice, almost all acinar and duct cells were TUNEL+ in DNase-I-treated sections, and were TUNEL− in untreated sections (data not shown).
Flow Cytometry
Spleen cell suspensions were stained with antibodies conjugated to phycoerythrin (anti-CD4, Cedar Lane Laboratories Ltd., Ontario, Canada; B220, Pharmingen, San Diego, CA), and fluorescein isothiocyanate (anti-CD8, Cedar Lane Laboratories Ltd.; CD3, anti-I-As, anti-CD5, Pharmingen), and analyzed with an EPICS flow cytometer (Coulter, Miami, FL). Isolated mouse salivary gland (MSG) cells (described below) were stained with biotinylated anti-Fas monoclonal antibody (mAb; Pharmingen) and fluorescein isothiocyanate-conjugated avidin (Vector Laboratories, Inc., Burlingame, CA), and analyzed by EPICS. Double-labeled surface phenotypes such as CD3/B220, CD4/FasL (Santa Cruz, Santa Cruz, CA), and CD8/FasL were analyzed. Apoptotic cells were also detected by flow cytometry with an EPICS flow cytometer (Coulter) using the Annexin V-FITC Apoptosis Detection Kit (Genzyme, Cambridge, MA).
Cell Preparation
We obtained tissue infiltrating cells from salivary gland tissues as previously described. 29 Briefly, affected submandibular glands from five mice were removed, cut into small pieces with scissors, passed through a 100-gauge stainless steel mesh, and suspended in RPMI 1640 containing 10% fetal calf serum, 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer, penicillin (100 U/ml), and streptomycin(100 μg/ml). After washing twice with medium, infiltrating cells were isolated from parenchymal cells by Ficoll-Isopaque density-gradient centrifugation. Spleen cells were aseptically prepared from 8- to10-week-old, nontreated NFS/sld female mice. To purify CD4 or CD8 single-positive T cells, we used nylon fiber for T cell selection and immunomagnetic beads (Dynal, Oslo, Norway) with anti-CD4 mAb, anti-CD8 mAb, and Mac-1 mAb (Becton Dickinson, San Jose, CA) as reported previously. 30,31 The purity of CD4 or CD8 single-positive cells was 90% or higher.
Primary Culture of Mouse Salivary Gland Cells
MSG epithelial cells were prepared as previously described. 27,32 Briefly, MSGs were minced into 1-mm 2 pieces, washed with Hanks’ balanced salt solution without Ca2+ and Mg2+, and placed in a 60-well dish containing Hanks’ balanced salt solution with 0.76 μg/ml ethylenediaminetetraacetic acid, 4.9 μg/ml l-ascorbic acid, and 4.9 μg/ml reduced glutathione. Fragments were washed with Dulbecco’s minimal essential medium/STI, and placed in a mixture of collagenase (type I, 750 U/ml) and hyaluronidase (type IV, 500 U/ml) dissolved in Dulbecco’s minimal essential medium/F12 containing 10% fetal bovine serum. The first digest suspension was passed through sterile 100-μm nylon mesh filters and were redigested for 30 minutes by the same digestion procedure and then the digest suspension was passed through a 100-μm nylon mesh filter. Adherent cells, after culture in minimal essential medium containing 10% fetal bovine serum for 24 hours at 37°C, were isolated as salivary gland epithelial cells. We confirmed that more than 95% of the cells were positively stained with anti-keratin polyclonal antibody.
Proliferation Assay
Proliferative T cell response to the 120-kd α-fodrin antigen was examined in spleen cells from 3d-Tx NFS/sld mice in a defined chronological order. Antigen-stimulated blastogenesis was measured in spleen cells from young and aged NFS/sld mice. Spleen cells were plated at 1 × 10 6 cells per well in 96-well microtiter plates in 200 μl HL-1 medium (Ventrex, Portland, ME) containing 2 mmol/L glutamine and 10 μg/ml−1 antigen. During the last 8 hours of the 72-hour culture period, 1 μCi of [3H]-thymidine was added per well, and the incorporated radioactivity was determined. Data are expressed as counts per minute per culture in triplicate.
Western Blot Analysis
Autoantigen cleavage of the 120-kd α-fodrin in the tissue homogenates from salivary glands was analyzed by immunoblotting with polyclonal antibodies to synthetic α-fodrin of the purified 120-kd antigen. 22 Tissue samples were homogenized in 20 mmol/L Tris-HCl buffer (pH 7.4), containing 5 mmol/L diisopropyl fluorophosphate, 5 mmol/L ethylenediaminetetraacetic acid, 5 mmol/L benzamidine, 2 mmol/L phenylmethyl sulfonyl fluoride, and 2 mmol/L N-ethylmaleimide. After centrifugation for 20 minutes at 12,000 rpm at 4°C, the supernatant was extracted and used for cytoplasmic protein. Pellets were homogenized in 20 mmol/L Tris-HCl buffer containing 2% Triton X-100. After centrifugation for 20 minutes at 12,000 rpm at 4°C, the supernatant was used for membrane protein.
Enzyme-Linked Immunosorbent Assay
Serum autoantibodies were detected using recombinant α-fodrin protein (JS-1). 22 After coating with the recombinant α-fodrin protein in a 96-well ELISA plate, biotinylated anti-mouse immunoglobulin G (Vector Laboratories) was added as second antibody. Measurements of JS-1 specific autoantibodies were read by automatic enzyme-linked immunosorbent assay (ELISA) reader (Flow Laboratories, McLean, VA). To detect serum antibody to DNA, the relative avidity of the serum antibodies for binding to ssDNA was measured in a solid phase ELISA. Purified ssDNA was prepared by boiling calf thymus DNA (Sigma Chemical Co., St. Louis, MO) for 10 minutes followed immediately by dilution in ice-cold borate-buffered saline. Flat bottom 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with ssDNA (50 mg/ml) overnight at 4°C in PBS, blocked at room temperature with 5% skim milk, and incubated with sera. Plates were read on an automatic ELISA reader (Flow Laboratories) at 492 nm.
Results
Pathology
Severe destructive autoimmune lesions developed in the major salivary (submandibular, parotid, and sublingual) and lacrimal glands in aged NFS/sld mice of 12 months of age or more, compared with those in the young SS model mice (Figure 1) ▶ . A higher incidence of autoimmune lesions was observed in females than in males at all ages. The severity of the lesions correlates with sex difference and aging. Inflammatory infiltrations were more marked and were accompanied by a more severe periductal fibrosis with advance of age. Multiple confluent foci of mononuclear cell infiltration and extensive parenchymal destruction were seen in the aged mice older than 12 months of age. In particular, severe inflammatory lesions with focal fibrosis, irregular dilatation of ducts, and/or fatty degeneration were frequently observed only in the aged groups of 18 and 20 months of age (Figure 2 ▶ , A and B).
Figure 1.
Mean grade of autoimmune lesions in the salivary and lacrimal glands in aging NFS/sld animal model of both sexes. We observed a significant increase in mean grade of autoimmune lesions with aging, and the predominance in female at all ages. Grading of inflammatory lesions was classified according to the modified method of White and Casarett 26 (*, P < 0.01; **, P < 0.001, Mann-Whitney U test).
Figure 2.

Representative severe destructive autoimmune lesions in the aged parotid (A) and lacrimal (B) glands of 20-month-old NFS/sld animal model, showing focal fibrosis, ductal dilatation, and fatty infiltration. H&E, ×120.
Decrease of Saliva and Tear Secretion in the Aged Mice
The average saliva and tear volume of the aged SS animal model was significantly lower than that of the young group (Figure 3) ▶ . The total amount of fluid decreased gradually with advance of age. Decrease in saliva and tear secretion probably correlates with severe inflammatory changes.
Figure 3.
Secretion of saliva and tears. The average saliva and tear volume of the aged SS model mice (18 to 20 months of age) was significantly lower than that of the young mice (2 to 4 months of age) and the total amount of fluid decreased with advancing age. Results are expressed as mean ± SEM in five mice examined per each group (*, P < 0.05; **, P < 0.005, Student’s t-test).
Age-Assocated Increase on Fas-Mediated Apoptosis
To determine the possible involvement of apoptotic cascade in tissue destruction in the SS animal model, we examined apoptotic cells in the salivary gland specimens from the aged and young SS model mice. A significant increase of TUNEL+-apoptotic epithelial duct cells in the salivary glands was detected in the aged 3d-Tx NFS/sld mice, compared with those in the young group at all ages (Figure 4) ▶ . A lesser number of TUNEL+-apoptotic acinar cells was observed, but no significant difference of TUNEL+-acinar cells was detected in between the aged and young NFS/sld mice at all ages. A higher proportion of cultured MSG cells expressing Fas in the aged mice was detected than that in the young mice on flow cytometry (Figure 5A) ▶ . No significant changes were found in the tissue-infiltrating CD4+ T cells bearing FasL in the salivary gland tissues both from the aged and the young SS model mice (Figure 5B) ▶ .
Figure 4.
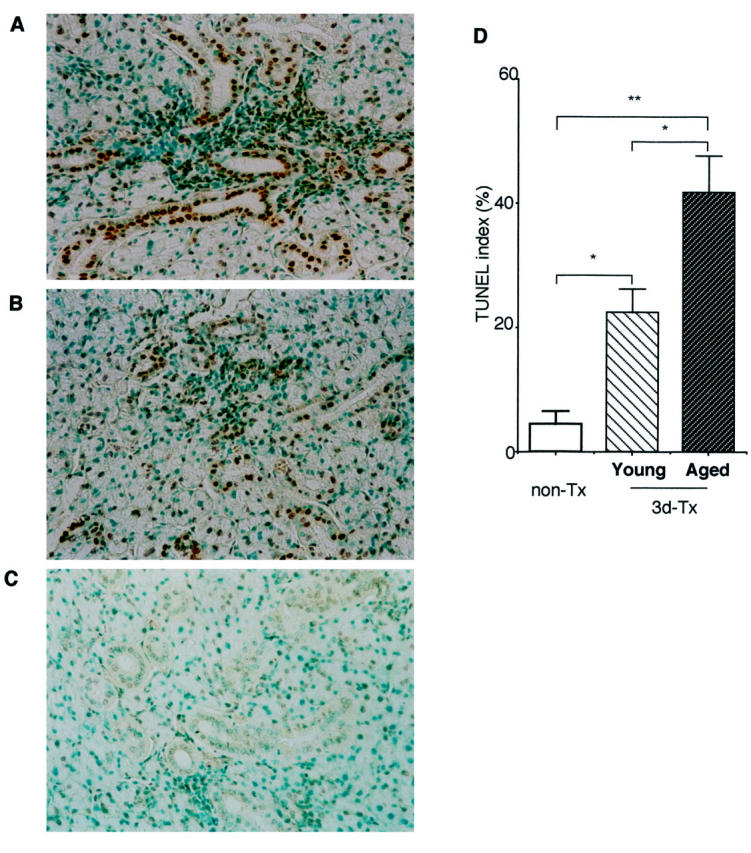
Detection of TUNEL+-apoptotic duct cells in the salivary gland sections from aged (18 to 20 months of age) (A) and young (2 to 4 months of age) 3d-Tx NFS/sld mice (B) and non-Tx NFS/sld control mice (6 months of age) (C). D: A significant increase of apoptotic epithelial duct cells was observed in the aged salivary gland tissues from 3d-Tx NFS/sld mice. The percentage of duct cells staining positively with TUNEL was enumerated using a 10 × 20-grid net micrometer disk covering an objective of area 0.16 mm2. Data were analyzed in 10 fields per section, and were expressed as mean percentage ± SD in five mice examined in each group (*, P < 0.01; **, P < 0.001, Student’s t-test).
Figure 5.
Flow cytometric analysis of Fas expression on MSG cells from aged (18 to 20 months of age) and young (2 to 4 months of age) 3d-Tx NFS/sld and FasL expression on the tissue-infiltrating lymphocytes (TIL) purified from salivary glands gated on CD4. A higher proportion of MSG cells expressing Fas was observed in the aged than that in the young mice. No significant changes were found on the tissue-infiltrating CD4+ T cells bearing FasL both from the aged and the young SS model mice. Five mice in each group were analyzed, and similar results were obtained.
Age-Associated Increase in Autoantigen Cleavage and Serum Autoantibody Production
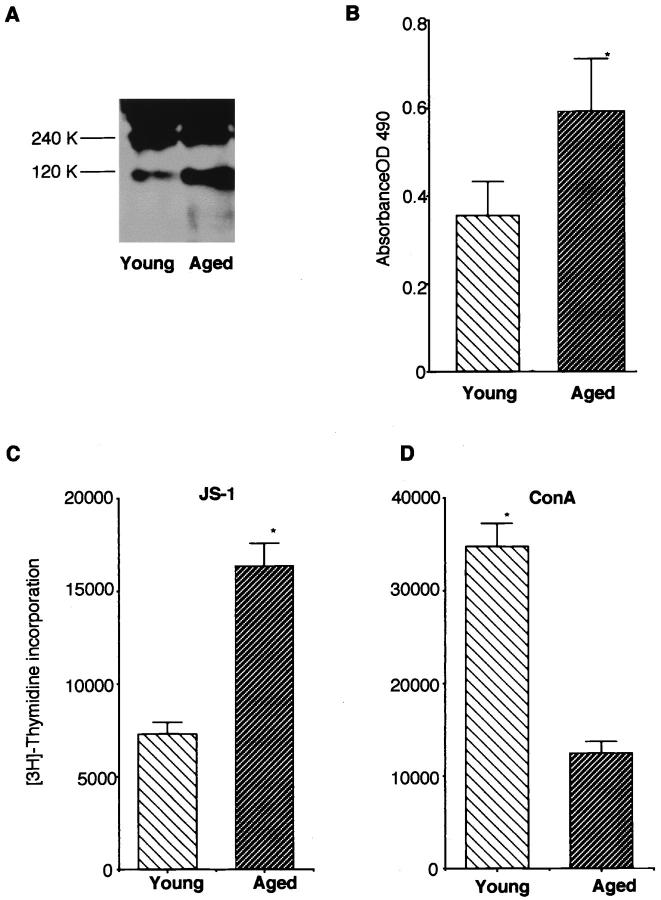
Western blot analysis demonstrated a more intense band of anti-120-kd α-fodrin in the aged salivary gland tissues than that in the young group (Figure 6A) ▶ , indicating that age-associated acceleration of autoantigen cleavage was detected in the organ. In addition, a higher titer of serum autoantibodies against 120-kd α-fodrin was detected in the aged mice as compared with those in the young mice by ELISA (Figure 6B) ▶ . When we analyzed the proliferative response of splenic T cells against organ-specific autoantigen, we found a significant increase in blastogenesis, compared with that in the young mice (Figure 6C) ▶ . In contrast, a significant decrease in nonspecific concanavalin A responsiveness of splenic T cells was detected in the aged SS model mice (Figure 6D) ▶ .
Figure 6.
A: Detection of organ-specific autoantigen, 120-kd α-fodrin, in the salivary gland tissues from aged (18 to 20 months of age) and young (2 to 4 months of age) SS model mice on Western blot analysis. Representative experiment demonstrates a more intense band of anti-120-kd α-fodrin in the salivary gland tissues from aged mice than those in young mice. Five tissue samples were examined for each group. B: A high titer of serum autoantibodies against 120-kd α-fodrin was detected in aged mice (18 to 20 months of age) compared with that in young SS model mice (2 to 4 months of age) by ELISA. Results indicate mean ± SEM values for five samples examined in each group (*, P < 0.01, Student’s t-test). C: A significant increase in proliferative response of splenic T cells against organ-specific autoantigen, 120-kd α-fodrin was detected in the aged (18 to 20 months of age), as compared with that in the young mice (2 to 4 months of age) (*, P < 0.01, Student’s t-test). D: A significant decrease in nonspecific concanavalin A responsiveness of splenic T cells was detected in the aged SS model mice (18 to 20 months of age) (*, P < 0.001, Student’s t-test). Five mice in each age group were analyzed. Data are expressed as counts per minute per culture ± SD in triplicate.
Age-Associated Decline of Immune Responses
To examine whether the autoimmune responses in the aged mice was affected by the phenotypic changes of peripheral T cells, we analyzed the surface phenotype in splenic T cells expressing Fas and FasL. A significant decrease of CD4+ T cells expressing Fas in the aged mice was observed as compared with those in the young mice, whereas no difference in CD8+ T cells expressing Fas was found (Figure 7A) ▶ . We observed no difference in FasL expression on both splenic CD4+ and CD8+ T cells (data not shown). We next investigated Fas-mediated apoptosis in freshly isolated splenic CD4+ T cells in vitro from the young and aged mice. Anti-Fas mAb-stimulated apoptosis was significantly decreased in the aged CD4+ T cells in spleen than those in the young mice (Figure 7B) ▶ suggesting that there may be a dysregulation of Fas-mediated apoptosis on activation-induced cell death in the aged SS animal model.
Figure 7.
A: Flow cytometric analysis of Fas-expressing CD4+ and CD8+ T cells freshly isolated from the spleens in young (2 to 4 months of age), and aged (18 to 20 months of age) mice. A significant decrease of CD4+ T cells expressing Fas in the aged mice was observed as compared with those in the young mice, whereas no difference was found in CD8+ T cells expressing Fas. Five samples were analyzed in each group. B: Anti-Fas mAb-stimulated apoptosis was significantly decreased in the aged CD4+ T cells in spleen than those in the young mice, but not in CD8+ T cells. Isolated CD4+ T cells were stimulated with anti-Fas mAb and analyzed for apoptotic nuclei with PI and Anexin V. Five mice in each age group were analyzed.
Discussion
Histology of age-associated autoimmune lesions in the SS animal model showed, to our surprise, severe destructive changes with inflammatory infiltration, focal fibrosis, ductal dilatation, and/or fatty degeneration, which were quite similar to those observed in the salivary and lacrimal glands of the SS patients with long-lasting duration. Thus, we believe that this SS animal model with aging is the best suitable to elucidate the mechanisms of in vivo progression toward a more aggressive disease in the organ-specific autoimmune disease in humans.
The Fas-mediated apoptosis is recognized as a major pathway for the induction of the tissue damage in autoimmune diseases. 33,34 Expression of Fas by pancreatic β cells has been shown to have a major influence on the susceptibility of tissue destruction in nonobese diabetic mice to diabetes. 35 Recently, we reported that estrogen deficiency induces severe destructive organ-specific autoimmune lesions in a murine SS model, and demonstrated an important role of Fas-mediated apoptosis toward target tissue destruction. 32 We detected a significant increase in TUNEL+-apoptotic epithelial duct cells in the salivary glands in the aged SS animal model than those in the young model. We found Fas expression on the cultured MSG cells from the aged SS model mice was significantly more augmented than those in the young model. An increased expression of Fas by MSG cells has been shown to have a major influence on the susceptibility of tissue destruction in the aged salivary glands. These results indicate that the age-associated acceleration of apoptotic cascade developed in the organ-specific autoimmune lesions in the salivary and lacrimal glands in this animal model. Indeed, we detected a significant decrease in saliva and tear flow secretion in the aged group.
Our data showed an increased cleavage product of organ-specific autoantigen, 120-kd α-fodrin, in the aged salivary gland tissues on immunoblotting and a significant increase in serum autoantibody production against 120-kd α-fodrin by ELISA. Moreover, an increase in the proliferative response of splenic T cells against organ-specific autoantigen was observed, whereas nonspecific concanavalin A responsiveness was decreased in the aged mice. It is likely that the autoreactive CD4+ T cell clone reactive with tissue-specific self-peptide is expanded during aging in the SS animal model. These results strongly suggest that age-associated dysregulation of immune functions may play a major role on acceleration of organ-specific autoimmune lesions. To examine whether the expression of Fas and FasL in aging T cells correlated with a susceptibility to apoptosis, we compared anti-Fas-induced apoptosis in T cell subsets between the aged and young SS animal model. We found that the freshly isolated CD4+ T cells from the aged SS model mice are resistant to anti-Fas-induced apoptosis. Our data show a decreased expression of Fas at basal levels in CD4+ T cell subsets from the aged SS animal model as compared with the young model. In contrast, studies on aging humans show an increase in Fas expression on T cells. 36 It was reported that there is an altered expression of genes regulating apoptosis in lymphocytes from aging humans, and that T cell subsets from aging humans show increased susceptibility to undergo anti-Fas-induced apoptosis as compared with T cell subsets from young humans. 36 The reason for this discrepancy in Fas expression levels in humans and mice is not known, but could be related to species differences. The Fas receptor cross-linking results in the death of Fas+ T cells on ligation with FasL. 37,38 This interaction plays a central role to terminate an ongoing immune response by inducing apoptosis in activated T cells. 39 Activation-induced cell death through Fas-mediated apoptosis is an important process that regulates the size and the duration of the primary immune T cell response. 40 Activation-induced cell death in T cells in vivo has been proposed to limit the expansion of an immune response by eliminating effector cells that are no longer needed.
Aging is generally associated with an increase in frequency of infection and increased incidence of cancer and autoimmune diseases. 41 One of the questions remains unresolved as to whether the cells expressing low levels of apoptotic molecules represent a subset of T cells that are normally present in young subjects, and aging represents an expansion of such a population. An increase in this population reacting with tissue specific self-peptide would be a consequence of increased production of this population or decreased death of a subset that does not express high levels of Fas/FasL. Further studies are needed to elucidate the mechanisms that T cells with decreased apoptotic molecules may represent a prolonged in vivo activation as a reflection of aging.
In conclusion, we have demonstrated, for the first time, that age-associated dysregulation of CD4+ T cells may play a crucial role in the acceleration of organ-specific autoimmune lesions in the salivary and lacrimal glands, and aging itself seems to influence both effector and target cells through Fas-mediated apoptosis in a murine model for primary SS.
Footnotes
Address reprint requests to Yoshio Hayashi, Department of Pathology, Tokushima University School of Dentistry, 3 Kuramotocho, Tokushima 770, Japan. E-mail: hayashi@dent.tokushima-u.ac.jp.
This work was supported in part by a Grant-in-Aid for Scientific Research (No. 08407057) from the Ministry of Education, Science and Culture of Japan.
References
- 1.Miller RA: The aging immune system: primers and prospectus. Science 1996, 273:70-74 [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G, Adibadeh M, Pohla H, Schaudt K: Immunosenescence: aging of the immune system. Immunol Today 1995, 16:420-422 [DOI] [PubMed] [Google Scholar]
- 3.Hodes RJ: Molecular alterations in the aging immune system. J Exp Med 1995, 182:1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ, Adler WH: Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest 1988, 81:1096-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proust JJ, Filburn CR, Harrison SA, Buchholz MA, Nordin AA: Age-related defect in signal transduction during lectin activation of murine T lymphocytes. J Immunol 1987, 139:1472-1478 [PubMed] [Google Scholar]
- 6.Nagata S, Suda T: Fas and Fas ligand: lpr and gld mutations. Immunol Today 1995, 16:39-43 [DOI] [PubMed] [Google Scholar]
- 7.Nagata S, Goldstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 8.Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 9.Bieganowska KD, Ausubel LJ, Modabber Y, Slovik E, Messersmith W, Hafler DA: Direct ex vivo analysis of activated, Fas-sensitive autoreactive T cells in human autoimmune disease. J Exp Med 1997, 185:1585-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch RJ, Nemerow GR, Han J: Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase 6b. Immunity 1997, 6:739-749 [DOI] [PubMed] [Google Scholar]
- 11.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT: Caspase-3-generated fragment of Gelsolin: effector of morphological change in apoptosis. Science 1997, 278:294-298 [DOI] [PubMed] [Google Scholar]
- 12.Brunner T, Mogll RJ, LaFace D, Yoo NJ, Mahboubl A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR: Cell-autonomous Fas(CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature 1995, 373:441-444 [DOI] [PubMed] [Google Scholar]
- 13.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A: Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995, 373:444-448 [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Terasaki S, Itoh J, Katoh H, Yonehara S, Nose M: Rheumatic disease in an MRL strain of mice with a deficit in functional Fas ligand. Arthritis Rheum 1997, 40:1054-1063 [DOI] [PubMed] [Google Scholar]
- 15.Ludgate M, Jasani B: Apoptosis in autoimmune and non- autoimmune thyroid disease. J Pathol 1997, 182:123-124 [DOI] [PubMed] [Google Scholar]
- 16.Casiano CA: Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med 1996, 184:765-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieux-Laucat F: Mutation in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995, 268:1347-1349 [DOI] [PubMed] [Google Scholar]
- 18.Daniels T, Whitcher JP: Association of patients of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren’s syndrome. Arthritis Rheum 1994, 37:869-877 [DOI] [PubMed] [Google Scholar]
- 19.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV: Sjögren’s syndrome. Proposed criteria for classification. Arthritis Rheum 1986, 29:577-585 [DOI] [PubMed] [Google Scholar]
- 20.Stamenkvic I, Stegagno M, Wright KA, Krane SM, Amento EP, Colvin RB, Duquesnoy RJ, Kurnick JT: Clonal dominance among T lymphocyte infiltrates in arthritis. Proc Natl Acad Sci USA 1988, 85:1179-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acha-Orbea H, Steinman L, McDevitt HO: T cell receptors in murine autoimmune diseases. Ann Rev Immunol 1989, 7:371-405 [DOI] [PubMed] [Google Scholar]
- 22.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y: Identification of α-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science 1997, 276:604-607 [DOI] [PubMed] [Google Scholar]
- 23.Kong L, Ogawa N, Nakabayashi T, Lin G, Souza ED, McGuff H, Guerrero D, Talal N: Fas and Fas ligand expression in salivary glands of patients with primary Sjögren syndrome. Arthritis Rheum 1997, 40:87-97 [DOI] [PubMed] [Google Scholar]
- 24.Hayashi Y, Kojima A, Hata M, Hirokawa K: A new mutation involving the sublingual gland in NFS/N mice: Partially arrested mucous cell differentiation. Am J Pathol 1988, 132:187-191 [PMC free article] [PubMed] [Google Scholar]
- 25.Haneji N, Hamano H, Yanagi K, Hayashi Y: A new animal model for primary Sjögren’s syndrome in NFS/sld mutant mice. J Immunol 1994, 153:2769-2777 [PubMed] [Google Scholar]
- 26.White SC, Casarett GW: Induction of experimental autoallergic sialadenitis. J Immunol 1974, 112:178-185 [PubMed] [Google Scholar]
- 27.Saito I, Haruta K, Shimuta M, Inoue H, Sakurai H, Yamada K, Ishimaru N, Higashiyama H, Sumida T, Ishida H, Suda T, Noda T, Hayashi Y, Tshubota K: Fas Ligand-mediated exocrinopathy resembling Sjögren’s syndrome in mice transgenic for IL-10. J Immunol 1999, 162:2488-2494 [PubMed] [Google Scholar]
- 28.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Tovka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 29.Hayashi Y, Haneji N, Hamano H, Yanagi K: Transfer of Sjögren’s syndrome-like autoimmune lesions into SCID mice and prevention of lesions by anti-CD4 and anti-T cell receptor antibody treatment. Eur J Immunol 1994, 24:2826-2831 [DOI] [PubMed] [Google Scholar]
- 30.Rudolphi A, Reimann J: Transplantation of CD4+ T cell clones into SCID mice. J Immunol Methods 1993, 158:27-36 [DOI] [PubMed] [Google Scholar]
- 31.Lea T, Vartdal F, Davies C, Ugelstad J: Magnetic monosized polymer particles for fast and specific fractionation of human mononuclear cells. Scand J Immunol 1985, 22:207-216 [DOI] [PubMed] [Google Scholar]
- 32.Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y: Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren’s syndrome through Fas-mediated apoptosis. Am J Pathol 1999, 155:173-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishio A, Katakai T, Oshima C, Kasakura S, Sakai M, Yonehara S, Suda T, Nagata S, Masuda T: A possible involvement of Fas-Fas ligand signaling in the pathogenesis of murine autoimmune gastritis. Gastroenterology 1996, 111:956-967 [DOI] [PubMed] [Google Scholar]
- 34.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Begnasco M, Testi R, Galluzzo A: Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science 1997, 275:960-963 [DOI] [PubMed] [Google Scholar]
- 35.Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA, Jr, Matis LA: The role of Fas in autoimmune diabetes. Cell 1997, 89:17-24 [DOI] [PubMed] [Google Scholar]
- 36.Increased apoptosis of T cell subsets in aging humans: altered expression of Fas(CD95), Fas ligand, Bcl-2, and Bax. J Immunol 1998, 160:1627–1637 [PubMed]
- 37.Lynch DH, Ramsdel F, Alderson MR: Fas and Fas L in homeostatic regulation of immune responses. Immunol Today 1995, 16:569-574 [DOI] [PubMed] [Google Scholar]
- 38.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH: Autocrine T cell suicide mediated by Apo-1(Fas/CD95). Nature 1995, 373:438-441 [DOI] [PubMed] [Google Scholar]
- 39.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH: Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 1995, 181:71-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parijs LV, Abbas AK: Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science 1998, 280:243-248 [DOI] [PubMed] [Google Scholar]
- 41.Makinodan T, Kay MMB: Age influence on the immune system. Adv Immunol 1980, 29:287-330 [DOI] [PubMed] [Google Scholar]