Abstract
ALK (anaplastic lymphoma kinase) is a tyrosine kinase receptor, expressed as part of the chimeric NPM-ALK protein, in anaplastic large cell lymphomas (ALCLs) exhibiting the t(2;5)(p23;q35) translocation. As a result of this translocation, the NPM (nucleophosmin) gene is fused to the portion of the ALK gene encoding its intracytoplasmic segment. In normal mouse tissues, mRNA encoding the Alk receptor has been found only in neural cells, suggesting involvement of this receptor in the development of the nervous system. The purpose of the present study was to examine the presence of ALK transcripts and protein in normal human tissues and a variety of cell lines and human tumors. Emphasis was placed on neuroblastomas because other tyrosine kinase receptors are expressed in human neuroblastomas. Fifty-six cell lines, including 29 lines of neural origin, and lymphoid and nonlymphoid tissue specimens, including 24 neuroblastomas, were investigated for ALK expression, using reverse transcriptase-polymerase chain reaction, Western blotting, and immunohistochemistry. The results confirmed that mRNA encoding ALK protein was not detectable in any normal or neoplastic hematopoietic tissue tested, except for t(2;5)-positive ALCL. The salient finding was that 13 of the 29 cell lines of neural origin and 22 of 24 neuroblastomas were found to express ALK transcripts and ALK protein. However, no correlation was evident between any known prognostic factors and the level of ALK expression.
Chromosomal translocations play an important role in the genesis of both lymphoproliferative disorders and solid tumors and often create hybrid genes that encode fusion proteins possessing oncogenic properties. 1,2 Recently, a number of investigations have been devoted to the t(2;5)(p23;q35) translocation, which is considered to be highly characteristic of anaplastic large cell lymphomas (ALCL). 3-5 This translocation fuses the NPM (nucleophosmin) gene at 5q35, to the portion of the ALK (anaplastic lymphoma kinase) gene on chromosome 2p23, which encodes the intracellular region of the protein. 6 The resulting chimeric protein consists of the amino-terminal portion of NPM, a nucleolar phosphoprotein involved in the shuttling of ribonucleoproteins between the nucleolus and the cytoplasm, 7 fused to the cytoplasmic domain of the ALK receptor tyrosine kinase. 6
Because NPM is a “housekeeping” gene, the NPM-ALK chimeric protein is constitutively expressed from the NPM promoter, leading to the ectopic overexpression of the ALK catalytic domain. 6 In addition, the NPM protein normally forms homo-oligomers, and it has been demonstrated that the NPM-ALK chimeric protein is able to form dimers both with normal NPM and with itself. 8 It is thought that NPM-ALK homodimerization mimicks ligand-induced receptor cross-linking and is thus responsible for the transphosphorylation and activation of ALK. 8 This constitutive activation of the ALK tyrosine kinase domain seems to play a role in the malignant transformation of lymphoid cells in ALCLs. 8,9,10 However, we have recently shown that genes other than NPM could provide a promoter to drive the expression of the ALK tyrosine kinase domain, inasmuch as an ALK product has been found in a case of ALCL associated with a variant (1;2)(q25;p23) translocation. 4,11,12 In addition, expression of the full-length ALK receptor protein, by a mechanism that remains to be elucidated, has been reported in a subtype of B-cell lymphomas that lack the (2;5) translocation. 13
In normal mouse tissues, mRNA encoding the Alk receptor has been reported in neural cells, 14,15 and it has been suggested that Alk plays a role in the development of the embryonic nervous system. 14 However, published studies have focused mainly on Alk expression in the mouse, and the purpose of the present study was to examine normal human tissues and a variety of human tumors and cell lines, with emphasis on neuroblastomas, for the presence of ALK transcripts and protein. Previous studies have described tyrosine kinase receptor expression in neuroblastomas. 16,17 A number of recently published studies have identified the expression of the TRK neurotrophin receptor tyrosine kinase in neuroblastoma. 18 In addition, receptors of the TRK family have been shown to correlate with the behavior of human neuroblastomas. 19 Thus we investigated the expression of ALK, which shares significant homology to the TRK receptors, in primary neuroblastomas to evaluate the prognostic significance of ALK expression.
Materials and Methods
Cell Lines
Fifty-six human neoplastic cell lines were investigated, all of which were obtained, unless otherwise indicated, from the American Type Culture Collection. Lymphoid cell lines comprised CEM, HSB-2, and Jurkat (acute T-lymphoblastic leukemias); LIB (large B-cell lymphoma, our laboratory); and SU-DHL-1 (t(2;5)-positive anaplastic lymphoma, kindly provided by Dr M. L. Cleary, Stanford University Medical Center, Stanford, CA). Nonlymphoid lines comprised 11 epithelial cell lines, 11 mesenchymal cell lines, and 29 cell lines of neural or neuroectodermal origin (Table 1) ▶ . The NB neuroblastoma cell lines (all derived at St. Jude Children’s Research Hospital) have previously been described. 20 The t(2;5)-positive SU-DHL-1 anaplastic lymphoma cell line and the Rh30 rhabdomyosarcoma cell line 6 were used, respectively, as positive controls for NPM-ALK and full-length ALK. Tumors were available as cultured cell lines or as frozen specimens of neoplasms grown in SCID or nude mice.
Table 1.
ALK Expression Analysis by RT-PCR and/or Western Blotting with Anti-ALK Antibodies in Human Cell Lines
| Cell lines | ALK Expression | ||
|---|---|---|---|
| Name | Sample | RT-PCR | Western blotting |
| Neuroectodermal | |||
| SK-N-DZ | Neuroblastoma | Pos† | Pos |
| SK-N-SH | Neuroblastoma | Pos‡ | Pos |
| SK-N-FI | Neuroblastoma | Pos† | ND |
| IMR-32 | Neuroblastoma | ND | Pos |
| NB-6 | Neuroblastoma | ND | Pos |
| NB-10 | Neuroblastoma | ND | Pos |
| NB-12 | Neuroblastoma | ND | Pos |
| NB-14 | Neuroblastoma | ND | Pos |
| NB-17 | Neuroblastoma | ND | Pos |
| NB-5 | Neuroblastoma | ND | Neg |
| NB-7 | Neuroblastoma | ND | Neg |
| NB-8 | Neuroblastoma | ND | Neg |
| NB-16 | Neuroblastoma | ND | Neg |
| SK-N-AS | Neuroblastoma | Neg† | ND |
| SK-N-MC | Neuroectodermal tumor | Pos‡ | Pos |
| SK-PN-DW | Neuroectodermal tumor | Pos† | Pos |
| PFSK-1 | Neuroectodermal tumor | Neg† | ND |
| SW 1088 | Astrocytoma | Neg† | ND |
| SW 1783 | Astrocytoma | Neg† | ND |
| A-172 | Glioblastoma | Neg† | ND |
| T98G | Glioblastoma | Neg† | ND |
| U-138MG | Glioblastoma | Neg† | ND |
| U-373MG | Glioblastoma | Neg† | ND |
| Hs 683 | Glioma | Neg† | ND |
| D283 Med | Medulloblastoma | Neg† | Neg |
| D341 Med | Medulloblastoma | Neg† | ND |
| H4 | Neuroglioma | Neg† | ND |
| Calas | Melanoma | Pos§ | Neg |
| NCI-H69 | Small cell lung carcinoma | Pos§ | Neg |
| Lymphoid | |||
| SU-DHL-1 | Anaplastic large cell lymphoma | Pos§ | Pos¶ |
| CEM | Acute T lymphoblastic leukemia | Neg§ | Neg |
| HSB-2 | Acute T lymphoblastic leukemia | Neg§ | Neg |
| Jurkat | Acute T lymphoblastic leukemia | Neg§ | Neg |
| LIB | Large B-cell lymphoma | Neg§ | Neg |
| Epithelial | |||
| NCI-H292 | Lung carcinoma (mucoepidermoid) | Neg† | ND |
| SW 13 | Adrenal cortex adenocarcinoma | Neg† | ND |
| C-41 | Cervical carcinoma | Neg† | ND |
| HT-3 | Cervical carcinoma | Neg† | ND |
| JEG-3 | Choriocarcinoma | Neg† | ND |
| JAR | Placental choriocarcinoma | Neg† | ND |
| SW 1463 | Colon adenocarcinoma | Neg† | ND |
| HCT 116 | Colon carcinoma | Neg† | ND |
| PANC-1 | Pancreatic carcinoma | Neg† | ND |
| KB | Epidermoid carcinoma | Neg† | ND |
| NCCIT | Teratocarcinoma | Neg† | ND |
| Mesenchymal | |||
| RD | Rhabdomyosarcoma | Pos† | Pos |
| Rh30 | Rhabdomyosarcoma | Pos§ | Pos |
| A-204 | Rhabdomyosarcoma | Neg† | ND |
| Hs 729T | Rhabdomyosarcoma | Neg† | ND |
| Hs 27 | Fibroblast | Neg† | ND |
| GCT | Histiocytoma | Neg† | ND |
| G-402 | Leiomyoblastoma | Neg† | ND |
| SK-UT-1B | Leiomyosarcoma | Neg† | ND |
| SW 872 | Liposarcoma | Neg† | ND |
| SW 684 | Fibrosarcoma | Neg† | ND |
| G-401 | Malignant rhabdoid tumor | Neg† | ND |
Pos means positive result; Neg means negative result; ND means not done.
† Amplification performed using primers from intracellular sequence Shu18/AShu18.
‡ Amplification performed using primers from intracellular and extracellular sequence, and dilutions performed to quantitate mRNA levels. For details see Table 2 ▶ .
§ Amplification performed using primers from intracellular sequence (ALK2S/ALK0 and ALKTK3/ALKCO2) and for extracellular sequence (ALKEC1/ALKEC2), and dilutions performed to quantitate mRNA levels. For details see Table 2 ▶ .
¶ Band of 80 kd (NPM-ALK) and not 200 kd (ALK).
Tissue Samples
All tissues were studied as fresh-frozen biopsy samples.
Lymphoid Tissues
Five nonneoplastic lymphoid tissues (four reactive lymph nodes, one tonsil), one thymoma, and 35 lymphomas of different categories were tested. The latter included eight cases of anaplastic large cell lymphoma that have been reported previously. 4,11 These cases coexpressed CD30 and EMA antigens and were of T or null phenotype. 21 In four cases, the chimeric NPM-ALK transcripts were detected using reverse transcriptase-polymerase chain reaction (RT-PCR) with 5′NPM and 3′ALK primers, as previously described, 4 and/or by immunostaining with ALK1 antibody. 11 The four remaining cases showed no evidence of the t(2;5). Ten cases of nonanaplastic large-cell lymphoma of T phenotype and six cases of B-cell lymphoma (one follicular lymphoma, five diffuse large B-cell lymphomas) were also investigated. The 11 remaining cases were lymph nodes involved in Hodgkin’s disease (seven cases of nodular sclerosing type, four cases of mixed cellularity type).
Nonlymphoid Tissues
One salivary gland, two thyroid glands, one glioblastoma, one colonic adenocarcinoma, and 24 neuroblastomas were investigated. Most of the neuroblastoma cases were obtained from the Pediatric Oncology Group (POG) Neuroblastoma Tumor Bank, after protocol approval (Table 3) ▶ . Histological analysis in each case revealed a homogeneous population of tumor cells. These comprised a cohort of 20 tumors chosen to represent the spectrum of neuroblastoma subgroups by disease stage, age, N-myc copy number, and DNA ploidy and were from uniformly treated patients. Included in this cohort were tumors with bad (N-myc amplification, age >1 year, stage III or IV, diploid DNA content in patients ≤1 year), as well as favorable (absence of N-myc amplification, age ≤1 year; stage I, II, or IVS; hyperploid DNA content in patients ≤1 year) prognostic indicators. The four remaining neuroblastoma cases were selected from the tissue bank of one of the investigators (GD).
Table 3.
Correlation between Known Neuroblastoma Prognostic Factors and ALK Expression in Primary Patient Neuroblastomas
| Patient no.* | ALK expression | Age (years)† | POG stage | EFS‡ (days) | OS§ (days) | Treat-m¶ | Status∥ | DI** | N-myc amplif†† | |
|---|---|---|---|---|---|---|---|---|---|---|
| Western blot††‡‡ | RT-PCR†† | |||||||||
| 48 | + | ND | 5.1 | 2 | 4465 | 4465 | R | A | 1 | − |
| 433 | − | ND | 0 | 4S | 34 | 3051 | F | A | 1 | − |
| 696 | − | ND | 1.5 | 3 | 2476 | 2476 | R | A | ND | − |
| 783 | + | ND | 0.5 | 3 | 2001 | 2001 | R | A | 1.5 | − |
| 952 | ++ | ND | 1.7 | 4 | 237 | 671 | F | E | 1 | − |
| 1176 | + | ND | 3.8 | 3 | 336 | 336 | F | E | 1.8 | + |
| 1200 | + | ND | 0.1 | 4S | 1789 | 1789 | R | A | 1.35 | − |
| 1343 | +++ | ND | 0.6 | 1 | 1448 | 1448 | R | A | 1.38 | − |
| 1347 | ++ | ND | 0.2 | 1 | 1359 | 1359 | R | A | 1.6 | − |
| 1368 | ++++ | ND | 0.4 | 3 | 1629 | 1629 | R | A | 1.17 | − |
| 1389 | +++ | ND | 8.8 | 4 | 572 | 572 | F | E | 1 | − |
| 1418 | ++ | ND | 0.2 | 2 | 1679 | 1679 | R | A | 1.46 | − |
| 1528 | ++ | ND | 0.3 | 4S | 290 | 290 | F | E | 1 | + |
| 1590 | −/+ | ND | 5.5 | 3 | 323 | 323 | F | E | 1.25 | − |
| 1634 | ++++ | ND | 0 | 4 | 0 | 0 | F | E | 1 | + |
| 1898 | ++ | ND | 5.7 | 2 | 1164 | 1164 | R | A | 1 | ND |
| 2072.2 | +++ | ND | 5.3 | 3 | 452 | 452 | F | E | 1.76 | + |
| 4111 | +++ | ND | 0.6 | 1 | 588 | 588 | R | A | 1.69 | − |
| 4316 | −/+ | ND | 4.2 | 4 | 324 | 324 | R | A | 1 | + |
| 4348 | ++ | ND | 1 | 4 | 420 | 420 | R | A | 1 | + |
| N1 | ND | + | ||||||||
| N2 | ND | + | ||||||||
| N3 | − | + | ||||||||
| N4 | + | + |
* Clinical prognostic data were not available for cases N1-4.
† age of 0 years indicates newborns.
‡ EFS, event-free survival.
§ OS, overall survival.
/p Treatm., response to chemotherapy; R, remission; F, failed.
∥ A, alive; E, expired.
** DI, DNA index.
†† ND, not done.
‡‡ (−), No ALK expression; the ALK expression level was scored from (−/+) to (+++).
Immunostaining
ALK proteins were detected on frozen sections or cytospin preparations by monoclonal antibody ALK1, which recognizes the intracellular domain of ALK, as previously described. 11
RNA Extraction and cDNA Preparation
Total RNA was obtained from frozen cell pellets (10 × 10 6 cells) or from 20 5-μm-thick frozen sections of tumor fragments, using the RNeasy Midi kit (Qiagen SA, Courtaboeuf, France). Reverse transcription was performed at 42°C on either 1 μg total RNA, using an ALK specific oligonucleotide primer (see below) and AMV reverse transcriptase for 90 minutes, as recommended by the enzyme manufacturer (Boehringer Mannheim France SA, Meylan, France), or on 5 μg total RNA, using an oligo(dT) primer and Superscript II RT (Stratagene, La Jolla, CA).
RT-PCR Analysis
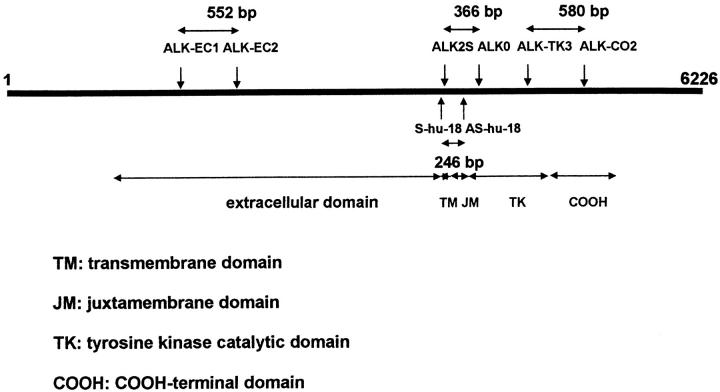
cDNA quality was checked either by amplifying a 461-bp fragment of β-actin, between primers ACT1: 5′ TCA TGT TTG AGA CCT TCA A 3′ and ACT2: 5′ GTC TTT GCG GAT GTC CAC G 3′, or a product of 450 bp, using G3PDH PCR primers (Clontech, Palo Alto, CA). The presence of ALK mRNA was investigated using primer pairs chosen from the ALK cDNA sequence 13 (Figure 1) ▶ :
Figure 1.
Positions of the primers used to investigate the presence of ALK mRNA are shown on a schematic representation of the ALK cDNA sequence. The three primer pairs Shu18-AShu18, ALK2S-ALK0, and ALKTK3-CO2 amplify fragments of the segment encoding the ALK intracytoplasmic domain, and the primer pair ALKEC1-ALKEC2 amplifies a fragment of the extracellular portion of the ALK mRNA.
ALK2S: 5′ GCT GAG CAA GCT CCG CAC CTC GAC 3′ (position 4148–4171) and ALK0: 5′ CCC GCC ATG AGC TCC AGC AGG ATG 3′ (position 4513–4490 of the full-length normal ALK cDNA; clone RMS17–2), giving a 366-bp fragment of the segment encoding the juxtamembrane and tyrosine kinase domains.
ALK-TK3: 5′ ATG GAC CCA CCC AAG AAC TGC CC 3′ (position 4953–4975) and ALK-CO2: 5′ CAG TAC AGC TTC CCT CCA GCC CC 3′ (position 5532–5510), which amplify a 580-bp fragment containing the sequence encoding the carboxy-terminal residues of the kinase catalytic domain and a portion of the carboxy-terminal tail, the region previously used as an immunogen to generate the monoclonal antibody ALK1. 11
S-hu-18 (5′ AAG CAC CAG GAG CTG CAA 3′) (position 4095–4112) and AS-hu-18 (5′ GCT TGG GTC GTT GGG CAT 3′) (position 4340–4323), chosen to span the intron located between the exons encoding the juxtamembrane domain and the amino-terminal portion of the kinase domain of human ALK to distinguish unequivocally products derived from genomic DNA, as opposed to the cDNA created by reverse transcription. These primers yield a PCR product of 246 bp, using human ALK cDNA as a template.
ALK-EC1: 5′ CCA TCT CCT TCT CCT GAT TAT TTT 3′ (position 1611–1634) and ALK-EC2: 5′ CAC TGC AGA CAA GCT GGG GTT 3′ (position 2162–2142), yielding a 552-bp PCR fragment of the extracellular portion of the ALK mRNA.
The strategy consisted of a two-step PCR amplification, using the same primers. The first round was performed on total cDNA (corresponding to 0.2 μg of total RNA), using an automated thermal cycler (Hybaid; TR3 Omnigene, Teddington, UK), with 300 μmol/L of each primer, 0.25 U of Goldstar DNA polymerase (Eurogentec, Liège, Belgium), and 500 μmol/L deoxynucleotide triphosphates in PCR buffer containing 1.5 mmol/L MgCl2 in a final volume of 25 μl. After an initial denaturation of 2 minutes at 95°C, 30 cycles of amplification were performed as follows: 95°C for 45 seconds, 68°C for 45 seconds, and 72°C for 20 seconds. The second amplification was performed under the same conditions, using 1 μl of the first-round PCR product as a template. PCR products were electrophoresed on 1.2% agarose gels and visualized with ethidium bromide staining.
PCR Sensitivity for ALK mRNA Detection
To test the sensitivity of our PCR method with the ALK2S and ALK0 primers, we used as the target a purified ALK cDNA fragment containing the sequence located between this primer pair. We then performed a two-step amplification with the ALK2S and ALK0 primers, using serial dilutions of this cDNA of known molecular concentration (measured by spectrophotometric analysis). Results were analyzed by electrophoresis on an agarose gel.
Semiquantitative RT-PCR Analysis of ALK mRNA in Cell Lines and Neuroblastoma Cases
Semiquantitation of the level of specific cDNA targets in six cell lines and four neuroblastomas was performed by limiting dilution (Table 2) ▶ . Tenfold sample dilutions were prepared in Tris-EDTA buffer containing salmon sperm DNA (5 μg/ml) as a carrier, and each dilution was submitted to a two-stage PCR, using the ALK2S and ALK0 primers, followed by agarose gel analysis. We could thus determine the highest dilution giving a positive amplification, and this was compared to the dilution range of the two reference cell lines (Rh30 and SU-DHL-1).
Table 2.
Quantitation of ALK mRNA, Detection of ALK Protein, and in Vitro Kinase Assays
| RT-PCR* | Western blotting anti-ALK (kd) | In vitro tyrosine kinase assay | Immunostaining for ALK protein | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell lines | Intracytoplasmic region | Extracellular region | Proteins immuno- precipitated with | ||||||
| Type | Name | hu18×AShu18 | ALK2S×ALK0 | ALKTK3×CO2 | ALKEC1×EC2 | ALK1 (kd) | antiP-Tyr (kd) | ||
| Neuroectodermal | |||||||||
| Neuroectodermal | SK-N-MC | Pos | ++++ | Pos | +++ | 200 | (200) | Neg | (Pos) |
| Neuroblastoma | SK-N-SH | Pos | +++++ | Pos | ++++ | 200 | 200 | (140) | (Pos) |
| Small cell carcinoma | NCl-H69 | +++ | Pos | Pos | Neg | Neg | 175 | Neg | |
| Melanoma | Calas | ++ | Pos | Pos | Neg | Neg | 175 | Neg | |
| Lymphoid | |||||||||
| ALCL | SU-DHL-1 | +++++ | Pos | Neg | 80 | 80 | 80 | Pos | |
| Mesenchymal | |||||||||
| Rhabdomyosarcoma | Rh30 | +++++ | Pos | ++++ | 200 | 200 | (200) | Pos |
Pos means positive result; Neg means negative result; blanks denotes not done. Results in parentheses indicate weak reactions.
* +++++ and ++++, high ALK mRNA level using semiquantitative assay; +++ and ++, low ALK mRNA level using semiquantitative assay
Western Blotting Studies
Cell pellets containing 5 × 10 6 cells, or small fragments of solid tumors were prepared as previously described. 11 Cytoplasmic extracts containing 20 μg protein (or 100 μg for neuroblastoma cases), as determined by Bradford assay, 22 were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 7.5% gel under reducing conditions. Proteins were transferred to Immobilon (polyvinylidene difluoride) membranes (Millipore UL, Watford, UK) by semidry electroblotting, and the membranes were then blocked by incubation in phosphate-buffered saline containing 5% nonfat milk and 0.1% Tween 20 for 30 minutes at 37°C. The membranes were then incubated for 30 minutes at room temperature with ALK1, with rabbit polyclonal anti-ALK#11, 15 or with a negative control antibody (MR12, a mouse anti-rabbit Ig antibody, prepared in the author’s laboratory). After a wash in phosphate-buffered saline containing 0.1% Tween 20, sites of antibody binding were detected by incubation with horseradish peroxidase-conjugated goat anti-mouse (diluted 1:1000) or anti-rabbit (1:500) immunoglobulins (Dako, Glostrup, Denmark), before chemiluminescent detection with Amersham ECL reagents (Amersham International, Little Chalfont, UK).
In Vitro Kinase Assay
Fragments of solid tumors or cell pellets (5 × 10 6 cells) from the CEM, Jurkat, HSB-2, LIB, Calas, NCI-H69, SK-N-SH, SK-H-MC, Rh30, and SU-DHL-1 cell lines were suspended in the lysis buffer, as previously described. 23
After a brief spin, the precleared lysate was added to 25 μl of protein G-Sepharose preloaded with either monoclonal anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) or antibody ALK1 and rotated at 4°C for 1 hour. The Sepharose beads were washed three times in lysis buffer and twice in kinase buffer (20 mmol/L HEPES (pH 7.4), 0.1% Brij 96) before incubation for 15 minutes in kinase buffer containing 10 mmol/L MnCl2, 10 mmol/L NaF, 1 mmol/L Na3(VO4), and 5 μCi [γ-32P]ATP (Amersham International). The reaction was stopped by the addition of 2× SDS sample buffer and separated on a 10% gel by SDS-PAGE. The gels were then dried and subjected to autoradiography.
Sequential Immunoprecipitation and Western Blotting Studies
Supernatants from the SU-DHL-1 anaplastic large cell lymphoma cell line and the two neuroblastoma cell lines SK-N-MC and SK-N-SH were prepared using the tyrosine kinase assay lysis buffer as described above. After preclearing with protein G-Sepharose, the lysates were rotated for 2 hours at 4°C with protein G-Sepharose preloaded with one of the following antibodies: ALK#11, ALK1, or anti-phosphotyrosine. After washing, the pellets were resuspended in 2× SDS sample buffer and resolved by SDS-PAGE. Proteins were electrophoretically transferred to Immobilon and stained using one of the following antibodies: ALK#11, ALKc (kindly provided by B. Falini), 24 anti-phosphotyrosine, or MR12, as described above.
Results
RT-PCR Analysis (Figure 1 ▶ , Tables 1 and 2 ▶ ▶ )
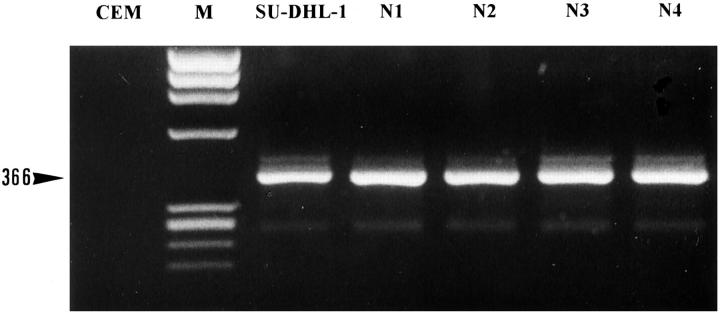
Positive amplification was detected as expected in the four cases of ALCL and the SU-DHL-1 cell line that carried the t(2;5) anomaly, using only the two primer pairs (see Materials and Methods) corresponding to the ALK cytoplasmic domain. The rhabdomyosarcoma cell lines (Rh30, RD) known to express full-length ALK showed positive amplification for both extracytoplasmic and extracellular ALK domains. However, five neuroblastoma lines, four neuroblastoma cases (N1, N2, N3 and N4) (Figure 2) ▶ , the small cell lung carcinoma cell line NCI-H69, and the melanoma cell line Calas also gave positive results with these primers. No amplification was obtained from any of the lymph nodes, normal tissues, or other lymphomas studied.
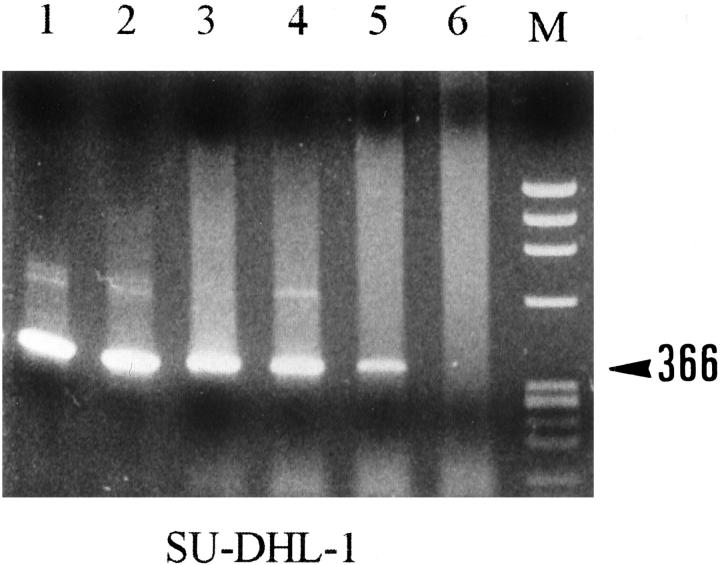
Figure 2.
RT-PCR analysis with the ALK2S and ALK0 primers of four neuroblastoma cases. M, size marker; CEM, T-cell line, negative control; SU-DHL-1, t(2;5)-positive lymphoma cell line, positive control; N1 to N4, neuroblastoma cases. As expected, a band of 366 bp was detected in the SU-DHL-1 cell line, but also in all of the neuroblastoma cases.
Semiquantitative RT-PCR Analysis
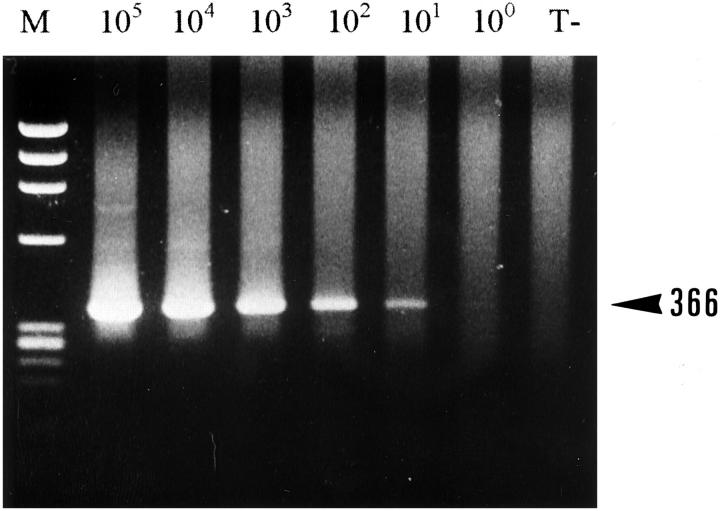
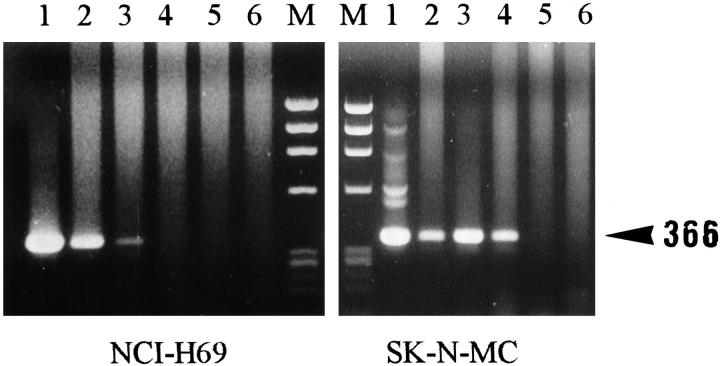
It was possible to detect approximately 10 molecules of cDNA (Figure 3) ▶ when the ALK2S and ALK0 primers (corresponding to sequences from the juxtamembrane and catalytic domains of the ALK protein) were used in a two-stage PCR method. Semiquantitative RT-PCR analysis for ALK mRNA with this primer pair (Table 2) ▶ showed that the SK-N-SH and SK-N-MC (Figure 4) ▶ neuroectodermal cell lines expressed levels of ALK mRNA comparable to those found in the t(2;5)-positive SU-DHL-1 (Figure 5) ▶ and the Rh30 cell lines. Intermediate or low levels were found in the NCI-H69 (small cell carcinoma) (Figure 4) ▶ and Calas (melanoma) cell lines (Table 2) ▶ , and the four neuroblastoma tumors tested showed intermediate to high ALK mRNA levels.
Figure 3.
Assessment of PCR sensitivity using ALK2S and ALK0 primers. A two-step PCR with the ALK2S and ALK0 primers was performed on 10 5 to 100 molecules from a target ALK cDNA. T−, negative control (no cDNA template); M, size marker. Note that a signal is detected with 10 molecules of target cDNA (366 bp).
Figure 4.
Semiquantitative titration of ALK mRNA from the NCI-H69 and SK-N-MC cell lines, with PCR using the ALK2S and ALK0 primers. Semiquantitation of total ALK mRNA from the NCI-H69 and SK-N-MC cell lines was performed by limiting dilutions. Tenfold dilutions of NCI-H69 and SK-N-MC cDNAs were prepared, and each dilution was submitted to a two-stage PCR with the ALK2S and ALK0 primers. The greatest dilution showing a positive amplification was analyzed by agarose gel electrophoresis and compared with the results obtained with the SU-DHL-1 cell line (Figure 5) ▶ . Lanes 1–6, left: 10-fold dilutions (log10) of cDNA from NCI-H69; lanes 1–6, right: 10-fold dilutions (log10) of cDNA from SK-N-MC cell lines. M, size marker. Note that with the SK-N-MC cell line the positive signal disappeared after the fourth dilution, whereas with the NCI-H69 cell line the signal disappeared earlier, after the third dilution.
Figure 5.
Semiquantitative titration of ALK mRNA using the SU-DHL-1 cell line, with PCR using the ALK2S and ALK0 primers. Semiquantitation of total ALK mRNA from the SU-DHL-1 cell line was performed by limiting dilutions. Tenfold dilutions of SU-DHL-1 cDNA were prepared, and each dilution was submitted to a two-stage PCR with the ALK2S and ALK0 primers. The greatest dilution showing a positive amplification was analyzed by agarose gel electrophoresis. These dilutions were used as a reference for comparison to the results obtained using the cell lines investigated in the study (see Materials and Methods). Lanes 1–6: 10-fold dilutions (log10) of cDNA from SU-DHL-1 cell line. M, size marker. Note that signal extinction is seen with a 10 6 dilution (lane 6).
Semiquantitative RT-PCR analysis of Rh30, SK-N-SH, and SK-N-MC cell lines for ALK mRNA using primers for the ALK extracellular region (ALK-EC1 and ALK-EC2) yielded comparable results (Table 2) ▶ . The slightly lower sensitivity achieved with this primer pair was interpreted as reflecting the greater sequence encompassed by these primers and the mRNA polyA tail.
Immunostaining
Two neuroectodermal cell lines (SK-N-SH and SK-H-MC) (Table 2) ▶ and one of the four cases of neuroblastoma (N4) (Figure 6) ▶ showed weak immunostaining on both cryostat and paraffin sections. Although ALK is a receptor anchored through the membrane, the staining seemed to be cytoplasmic, as already reported in normal cells in the central nervous system. 11 In contrast, as expected from previous results, the t(2;5)-positive SU-DHL-1 cell line, the four cases of t(2;5)-positive ALCLs, and the Rh30 rhabdomyosarcoma cell line (expressing full-length ALK protein) were all clearly positive for ALK protein.
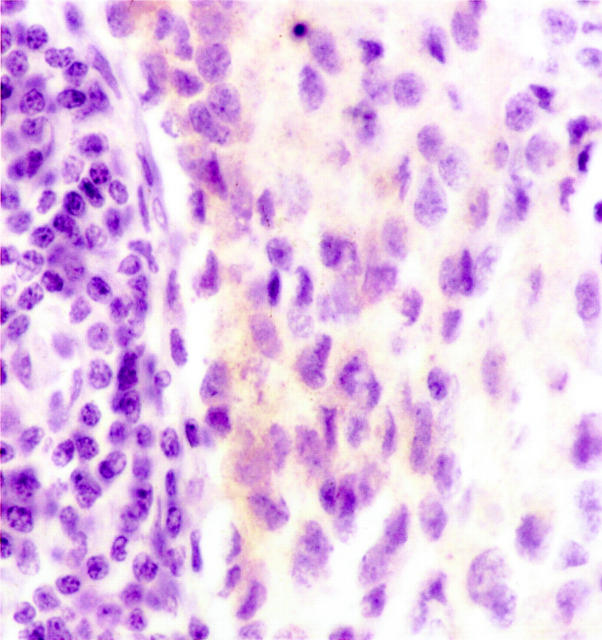
Figure 6.
Immunohistochemical demonstration of ALK proteins in paraffin sections from a case of neuroblastoma (N4), using the monoclonal antibody ALK1. Neoplastic cells show a weak staining that seems to be restricted to the cytoplasm. This staining is unexpected, ALK being a receptor that should be anchored through the membrane, but membrane staining is probably much easier to see with strong rather than weak immunohistochemical labeling.
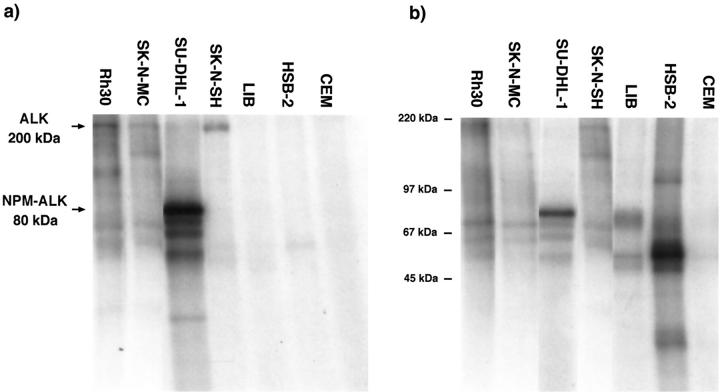
Western Blotting for ALK Proteins (Tables 1 and 2) ▶ ▶
Bands of 200 kd (ALK) and 80 kd (NPM-ALK) were found, as expected, in lysates of, respectively, the Rh30 and SU-DHL-1 cell lines (Table 2 ▶ and Figure 7 ▶ ). Full-length 200-kd ALK receptor protein was also detected in one neuroectodermal cell line and nine neuroblastoma cell lines (Table 1) ▶ , two of which are shown in Figure 7 ▶ . The 200-kd full-length receptor was also observed in 19 neuroblastoma cases (Table 3 ▶ and Figure 8 ▶ ). No correlation was evident between ALK receptor expression levels and known neuroblastoma prognostic indicators such as N-myc amplification, age of patient, stage, or DNA content (Table 3) ▶ .
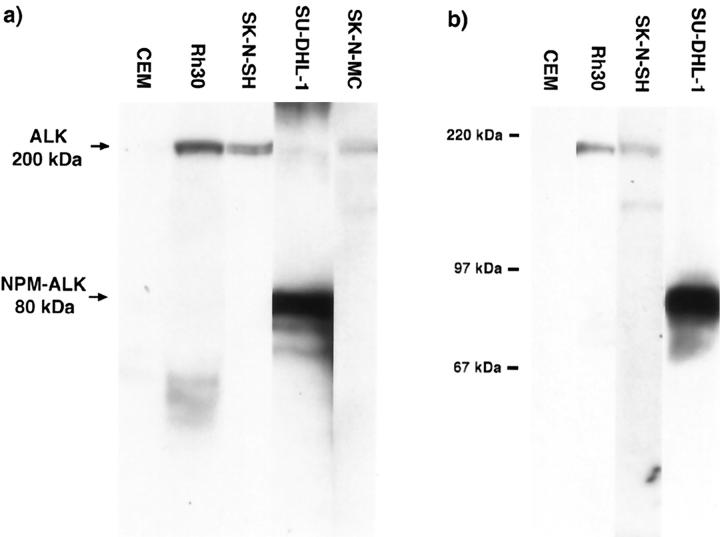
Figure 7.
Western blotting of cell lines, using monoclonal (ALK1) (a) or polyclonal (anti-ALK 35 11) (b) anti-ALK antibodies. ALK proteins of 200 kd are seen in the SK-N-SH and SK-N-MC neuroblastoma cell lines, corresponding in size to the full-length protein found in the Rh30 rhabdomyosarcoma cell line. A band of 80 kd, representing NPM-ALK, is detected in the t(2;5)-positive SU-DHL-1 cell line, and no ALK proteins are detected in the CEM negative control. Molecular weight markers are indicated.
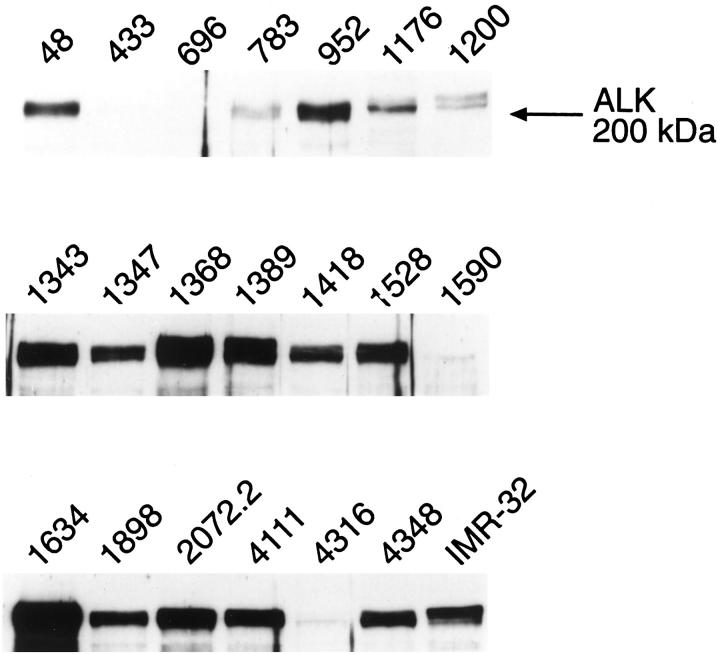
Figure 8.
Western blotting for ALK protein in primary neuroblastoma samples. The patient numbers correspond to those shown in Table 3 ▶ . IMR-32, a human neuroblastoma cell line, was included as a positive control for ALK expression. Note that the ALK glycoprotein often migrates as a doublet, presumably reflecting differential glycosylation. Equal amounts (100 μg) of total cellular proteins, as determined by Bradford assay, 23 were loaded in the individual sample lanes. The levels of ALK expression can be seen to vary markedly among the various specimens.
ALK protein was not detected in any cell lines of lymphoid or epithelial origin.
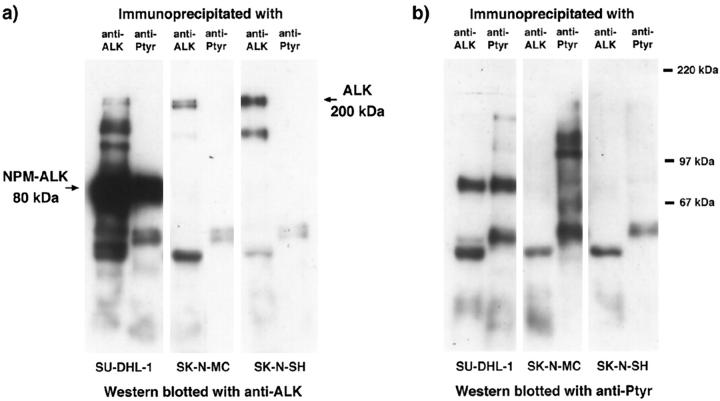
In Vitro Tyrosine Kinase Assay (Table 2) ▶
As expected, proteins immunoprecipitated from the t(2;5)-positive SU-DHL-1 cell line with either anti-ALK or anti-phosphotyrosine and subjected to this assay showed a strong band of 80 kd, representing autophosphorylated NPM-ALK (Figure 9) ▶ . A strong 200-kd band representing autophosphorylated ALK was detected in proteins immunoprecipitated from the Rh30 and SK-N-SH cell lines with anti-ALK (Figure 9a) ▶ . A 200-kd protein was also weakly present in anti-phosphotyrosine immunoprecipitates (Figure 9b) ▶ from these cell lines. Proteins immunoprecipitated from the SK-N-MC cell line with anti-ALK (but not with anti-phosphotyrosine) showed a weak phosphorylated 200-kd band (Figure 9a) ▶ . No phosphorylated proteins were detected by the in vitro kinase assay in ALK immunoprecipitates of the CEM, HSB-2, Jurkat, or LIB lymphoid cell lines.
Figure 9.
In vitro tyrosine kinase assay of proteins immunoprecipitated with anti-ALK (a) or anti-phosphotyrosine (b). As expected, autophosphorylated NPM-ALK (in the SU-DHL-1 cell line) and full-length ALK (in the Rh30 cell line) are detected in both the ALK and phosphotyrosine immunoprecipitates. A band of 200 kd is seen in the anti-ALK immunoprecipitates from the SK-N-SH and SK-N-MC cell lines, but only a very weak 200-kd band is detected in the anti-phosphotyrosine precipitate of SK-N-SH cells. No phosphorylated proteins are observed in anti-ALK immunoprecipitates from the CEM, HSB-2, and LIB cell lines. In contrast, anti-phosphotyrosine precipitates from the LIB cell line show weak bands of 56, 75, and 78 kd, and in the HSB-2 cell line a prominent band of 60 kd is seen.
Sequential Immunoprecipitation and Western Blotting Assays
Phosphorylated 80-kd NPM-ALK protein was detected in both the ALK and the phosphotyrosine immunoprecipitates from the SU-DHL-1 cell line (Figure 10) ▶ . In contrast, the 200-kd ALK protein present in the SK-N-MC and SK-N-SH cell lines could only be detected by Western blotting of immunoprecipitates obtained with anti-ALK (Figure 10a) ▶ but not with anti-phosphotyrosine immunoprecipitates (Figure 10a) ▶ . This indicated that the full-length protein present in these cells was not strongly phosphorylated. In keeping with this interpretation, no reactivity was observed when anti-ALK immunoprecipitates from these two neuroectodermal cell lines were Western blotted with anti-phosphotyrosine (Figure 10b) ▶ . Phosphorylated proteins other than ALK, however, were present in the phosphotyrosine immunoprecipitates from these two neuroectodermal cell lines (Figure 10b) ▶ . Comparable results were obtained using rabbit anti-ALK#11 to either immunoprecipitate ALK or to detect ALK proteins by Western blotting (not shown).
Figure 10.
Sequential immunoprecipitation and Western blotting of ALK proteins in neuroblastoma cell lines. Proteins immunoprecipitated with anti-ALK or with anti-phosphotyrosine antibodies were Western blotted using either antibody ALKc (a) or phosphotyrosine (b). With use of antibody ALKc, the 80-kd NPM-ALK protein was detected in both ALK and phosphotyrosine immunoprecipitates of the SU-DHL-1 cell line. Although ALK proteins were present in the ALK immunoprecipitates of the SK-N-MC and SK-N-SH cell lines, these proteins were not detected in the phosphotyrosine immunoprecipitates of these cell lines. With the use of the anti-phosphotyrosine antibody, 80-kd NPM-ALK protein was detected in both ALK and phosphotyrosine immunoprecipitates of the SU-DHL-1 cell line. However, no phosphorylated ALK proteins were observed in immunoprecipitates of the two neuroblastoma cell lines. Comparable results were obtained using the polyclonal anti-ALK#11 reagent (data not shown).
Discussion
ALK is a novel protein-tyrosine kinase receptor that shares a high degree of homology with leukocyte tyrosine kinase (LTK), a member of the insulin receptor subfamily. 6,14,15 The normal function of ALK and its ligand remain to be determined.
Recent studies using Northern blotting analysis, in situ hybridization, and/or immunohistochemistry suggest that ALK expression is normally restricted to the nervous system in both the human and mouse. 11,14,15 To date, there is little information on the presence of ALK mRNA in normal lymphoid tissues, although spleen, thymus, peripheral blood leukocytes, B-lymphoblastoid cell lines, and various t(2;5)-negative leukemia-lymphoma cell lines have been found to be negative for the ALK mRNAs 6 or ALK proteins. 3,4,11 It is therefore generally accepted that the ALK promoter is silent in normal lymphoid cells and that the expression of a truncated ALK protein under the control of the NPM promoter occurs ectopically in malignant lymphoid cells bearing the t(2;5)(p23;q35) translocation.
The results from the present study are consistent with this belief because, with the exception of ALCLs, mRNAs encoding for ALK proteins were not detectable in any normal or neoplastic hematopoietic tissue tested. The most interesting finding was the detection of ALK mRNA and ALK protein in cell lines and tumors of neural origin. In the SK-N-SH and SK-N-MC neuroectodermal cell lines, and in the Rh30 rhabdomyosarcoma line (used as a positive control for full-length ALK expression), ALK mRNAs were detected using primers corresponding to both the cytoplasmic and the extracellular domains of the receptor (see Table 2 ▶ and Figure 1 ▶ ). Semiquantitative RT-PCR indicated that the levels of ALK mRNAs were comparable to those found in the Rh30 cell line. This evidence for the expression of ALK in these cell lines was confirmed by positive immunohistochemical staining (albeit weak) and by Western blotting, which revealed a 200-kd band corresponding to full-length ALK protein. The latter technique also revealed ALK protein in eight other neuroblastoma or neuroectodermal lines that were not studied by RT-PCR. Thus evidence was obtained for ALK expression in neuroblastoma cells by three independent techniques (RT-PCR, Western blotting, and immunohistochemistry). These data are in agreement with recent reports of ALK mRNAs and ALK protein in parts of the central and peripheral nervous system in both embryonic and adult mice. 14,15
Although in vitro kinase assays of proteins immunoprecipitated with anti-ALK from lysates of the SK-N-SH, SK-N-MC, and Rh30 cell lines revealed phosphorylation of full-length ALK, sequential immunoprecipitation followed by immunoblotting (anti-ALK followed by anti-phosphotyrosine or vice versa) provided no evidence of significant levels of endogenously autophosphorylated ALK. This is in contrast to the high level of constitutive phosphorylation of NPM-ALK observed here and in previous reports. 8,9 These results suggest that full-length ALK protein may not be enzymatically active (and hence may not be involved in oncogenic transformation) in these cell lines and support the view that its presence may be a physiological rather than an oncogenic phenomenon.
We have demonstrated for the first time the presence of ALK mRNA and/or ALK protein in fresh primary neuroblastomas (22/24 cases). However, no correlation between known neuroblastoma prognostic factors and the level of ALK expression was identified. This is not the first report of tyrosine kinase receptor expression in neuroblastomas. 16-18 The expression of TRKA and TRKC has been shown to correlate with a favorable patient outcome, whereas the expression of TRKB is associated with unfavorable, aggressive tumors. 18,19,25 Highly malignant neuroblastomas that express TRKB also produce its cognate ligand, BDNF, and it therefore appears that these tumor cells are stimulated to grow in an autocrine fashion. The mechanisms by which TRKA and TRKC expression contributes to a better prognosis in neuroblastoma are unclear, although it is thought that receptor activation by their ligands, NGF and NT3, respectively, may produce a more differentiated tumor cell phenotype. Consistent with this hypothesis, overexpression of TRKA in neuroblastoma cell lines enhances NGF-induced differentiation both in vitro and in vivo. 26 These data suggest the possibility that other neural-specific receptor tyrosine kinases could also be important in the development and progression of neuroblastoma. In this study, the expression in many human neuroblastomas of ALK, a receptor tyrosine kinase with significant homology to the TRK receptors, suggests that this neural-specific receptor may play an as yet unidentified role in the natural history of these tumors.
Acknowledgments
We thank Xiaoli Cui for excellent technical assistance, Susan Ragsdale for providing the NB neuroblastoma cell lines, and Dr. Susan Cohn of the POG Neuroblastoma Biology Committee and Tumor Bank for supplying human neuroblastoma tumor samples for analysis and for reviewing the data concerning ALK expression and neuroblastoma prognosis before its publication. We are also grateful to Dr. Brunangelo Falini for kindly providing the antibody ALKc.
Footnotes
Address reprint requests to Dr. Georges Delsol, Laboratoire d’Anatomie Pathologique, Centre Hospitalo-Universitaire Purpan, Place du Dr. Baylac, 31059 Toulouse Cedex, France. E-mail: delsol.g@chu-toulouse.fr.
Supported by the Projet Hospitalier de Recherche Clinique (PHRC98), the GELA (Groupe d’Etude des Lymphomes de l’Adulte), the Ligue Contre le Cancer de la Haute Garonne, the Ligue Contre le Cancer de l’Aveyron, the Ligue Contre le Cancer du Gers, the Leukemia Research Fund (grant number 9646, UK), National Cancer Institute grant CA 69129 (SWM), Cancer Center Support (CORE) grant CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital.
References
- 1.Rabbitts TH: Chromosomal translocations in human cancer. Nature 1994, 372:143-149 [DOI] [PubMed] [Google Scholar]
- 2.Look AT: Oncogenic transcription factors in the human acute leukemias. Science 1997, 278:1059-1064 [DOI] [PubMed] [Google Scholar]
- 3.Shiota M, Fujimoto J, Taneka M, Satoh H, Ichinohasama R, Abe M, Nakano M, Yamamoto T, Mori S: Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood 1994, 84:3648-3652 [PubMed] [Google Scholar]
- 4.Lamant L, Meggetto F, Al Saati T, Brugières L, Bressac de Paillerets B, Dastugue N, Bernheim A, Rubie H, Terrier-Lacombe MJ, Robert A, Brousset P, Rigal F, Schlaifer D, Shiota M, Mori S, Delsol G: High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, RT-PCR and P80 immunostaining. Blood 1996, 87:284-291 [PubMed] [Google Scholar]
- 5.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Chauky A, Brugières L, Terrier-Lacombe MJ, Pulford K, Sabatini I, Pileri S, Mason DY, Delsol G: Morphological and phenotypic spectrum of ALK1-positive anaplastic, Ki-1+ large cell lymphoma. Blood 1998, 91:2076-2084 [PubMed] [Google Scholar]
- 6.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 7.Borer RA, Lehner CF, Eppenberger HM, Nigg EA: Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989, 56:379-390 [DOI] [PubMed] [Google Scholar]
- 8.Bischof D, Pulford K, Mason DY, Morris SW: Role of the nucleophosmin (NPM) portion of the non-Hodgkin’s lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol 1997, 17:2312-2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T: Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996, 93:4181-4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, Morris SW: Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 1997, 90:2901-2910 [PubMed] [Google Scholar]
- 11.Pulford K, Lamant L, Morris SW, Butler L, Wood K, Stroud D, Delsol G, Mason DY: Detection of ALK and NPM-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89:1394-1404 [PubMed] [Google Scholar]
- 12.Mason DY, Pulford KAF, Bischof D, Kuefer MU, Butler LH, Lamant L, Delsol G, Morris SW: Nucleolar localization of the NPM-ALK tyrosine kinase is not required for malignant transformation. Cancer Res 1998, 58:1057-1062 [PubMed] [Google Scholar]
- 13.Delsol G, Lamant L, Mariamé B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, Al Saati T, Ceretti DP, Morris SW, Mason DY: A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood 1997, 89:1483-1490 [PubMed] [Google Scholar]
- 14.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T: Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997, 14:439-449 [DOI] [PubMed] [Google Scholar]
- 15.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP: ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 1997, 14:2175-2188 [DOI] [PubMed] [Google Scholar]
- 16.Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM: Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res 1992, 52:1364-1368 [PubMed] [Google Scholar]
- 17.Borrello MG, Bongarzone I, Pierotti MA, Luksch R, Gasparini M, Collini P, Pilotti S, Rizzetti MG, Mondellini P, De Bernardi B, Di Martino D, Garaventa A, Brisigotti M, Tonini GP: trk and ret proto-oncogene expression in human neuroblastoma specimens: high frequency of trk expression in non-advanced stages. Int J Cancer 1993, 54:540-545 [DOI] [PubMed] [Google Scholar]
- 18.Brodeur GM, Nakagawara A, Yamashiro DJ, Ikegaki N, Liu XG, Azar CG, Lee CP, Evans AE: Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neuro-Oncol 1997, 31:49-55 [DOI] [PubMed] [Google Scholar]
- 19.Brodeur GM, Maris JM, Yamashiro DJ, Hogarty MD, White PS: Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol 1997, 19:93-101 [DOI] [PubMed] [Google Scholar]
- 20.Lahti JM, Valentine M, Xiang J, Jones B, Amann J, Grenet J, Richmond G, Look AT, Kidd VJ: Alterations in the PITSLRE protein kinase gene complex on chromosome 1p36 in childhood neuroblastoma. Nature Genet 1994, 7:370-375 [DOI] [PubMed] [Google Scholar]
- 21.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 22.Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 23.Pulford K, Falini B, Cordell J, Rosenwald A, Ott G, Mûller-Hermelink HK, MacLennan KA, Lamant L, Carbone A, Campo E, Mason DY: Biochemical detection of novel anaplastic lymphoma kinase proteins in tissue sections of anaplastic large cell lymphoma. Am J Pathol 1999, 154:1657-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falini B, Bigerna B, Fizzoti M, Pulford K, Pileri SA, Delsol G, Carbone A, Paulli M, Magrini U, Menestrina F, Giardini R, Pilotti S, Mezzelani A, Ugolini B, Billi M, Pucciarini A, Pacini R, Pelicci PG, Flenghi L: ALK expression defines a distinct group of T/null lymphomas (ALK lymphomas) with a wide morphological spectrum. Am J Pathol 1998, 153:875-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahlman S, Hoehner JC: Neurotrophin receptors, tumor progression and tumor maturation. Mol Med Today 1996, 2:432-438 [DOI] [PubMed] [Google Scholar]
- 26.Matsushima H, Bogenmann E: Expression of trkA cDNA in neuroblastomas mediates differentiation in vitro and in vivo. Mol Cell Biol 1993, 13:7447-7456 [DOI] [PMC free article] [PubMed] [Google Scholar]